Abstract
Severe cases of coronavirus disease 2019 (COVID-19) cannot be adequately managed with mechanical ventilation alone. The role and outcome of extracorporeal membrane oxygenation (ECMO) in the management of COVID-19 is currently unclear. Eight COVID-19 patients have received ECMO support in Shanghai with seven with venovenous (VV) ECMO support and one veno arterial (VA) ECMO during cardiopulmonary resuscitation. As of March 25, 2020, four patients died (50% mortality), three patients (37.5%) were successfully weaned off ECMO after 22, 40, and 47 days support, respectively, but remain on mechanical ventilation. One patient is still on VV ECMO with mechanical ventilation. The partial pressure of oxygen/fractional of inspired oxygen ratio before ECMO initiation was between 54 and 76, and all were well below 100. The duration of mechanical ventilation before ECMO ranged from 4 to 21 days. Except the one emergent VA ECMO during cardiopulmonary resuscitation, other patients were on ECMO support for between 18 and 47 days. In conclusion, ensuring effective, timely, and safe ECMO support in COVID-19 is key to improving clinical outcomes. Extracorporeal membrane oxygenation support might be an integral part of the critical care provided for COVID-19 patients in centers with advanced ECMO expertise.
Keywords: coronavirus disease 2019, extracorporeal membrane oxygenation, China
Coronavirus disease 2019 (COVID-19) has spread rapidly in China and many other countries since its outbreak due to its person-to-person transmission and its highly contagious nature. The majority of COVID-19 patients suffer mild symptoms and recover completely. However, approximately 14% of cases are severe and 5% are critical with mortality estimates of 2.3%–3.83%.1–3 The role of extracorporeal membrane oxygenation (ECMO) in the management of COVID-19 is unclear at this point. It has been used in some patients with COVID-19 in China, but detailed information is unavailable.4–7 Extracorporeal membrane oxygenation may have a role in the management of some patients with COVID-19 who have refractory hypoxemic respiratory failure. However, much about the virus is unknown, including the natural history, incidence of late complications, viral persistence, or the prognoses in different subsets of patients.8
The World Health Organization (WHO) guidance document includes a statement to consider referring patients with refractory hypoxemia despite lung-protective ventilation in settings with access to expertise in ECMO support.9 Similarly, the United States Center for Disease Control provides interim guidance (https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html) for clinical management of COVID-19 patients with and without acute respiratory distress syndrome (ARDS).10 Where ECMO expertise is available, ECMO should be considered according to the standard management algorithm for ARDS in supporting patients with viral lower respiratory tract infection. However, there is little worldwide experience with using ECMO to support COVID-19 patients. A recent Euro Extracorporeal LifeSupport Organization adult ECMO-COVID-19 patients survey verified the use of ECMO for COVID-19 in Europe: nine in England, two in German, three in Belgium, 18 in France, 10 in Spain, one in Sweden, one in Poland, one in Czech, and 14 in Italy.11
As of March 22, 2020, 404 COVID-19 cases have been confirmed in Shanghai, China, because the first patient was confirmed on January 20, 2020.4 All patients were admitted to a COVID-19-designated hospital, Shanghai Public Health Clinical Center. Extracorporeal membrane oxygenation support has been integrated in the comprehensive COVID-19 management by multidisciplinary teams led by critical care physicians. We aim to share our experiences, core principles, outcomes, and pitfalls in establishing and managing ECMO in critically ill COVID-19 patients and provide guidance on this technique and the resources and expertise required from our initial experience in Shanghai, China.
Materials and Methods
Shanghai has extensive experience in epidemic prevention and control following the H7N9 crisis in 2013.12 Since the outbreak of COVID-19 in Wuhan, Hubei Province, in December 2019, Shanghai has closely monitored the progress of COVID-19, registered all available ECMO equipment from local medical centers, and immediately assembled a multidisciplinary COVID-19 ECMO expert team. All confirmed COVID-19 patients were admitted to negative pressure patient units at Shanghai Public Health Clinical Center and managed according to the severity of disease.
To maintain the highest quality of ECMO management, a three member ECMO team with one physician perfusionist, one critical care physician, and one pulmonologist, are available at all times to oversee ECMO management, participate in clinical evaluation and treatment, and communicate with the Shanghai COVID-19 ECMO Expert Team for guidance. A total of 12 ECMO units from Shanghai’s major hospitals were enlisted to ensure the needs of critically ill COVID-19 patients, with four units stored outside the hospital as backup. To ensure consistency and standardization among ECMO team members, the ECMO team developed the “Shanghai ECMO Support for COVID-19 Guideline” which was incorporated into the “Shanghai Expert Consensus on Comprehensive Treatment of Coronavirus Disease 2019.”13
Extracorporeal Membrane Oxygenation Environmental Requirement
All COVID-19 ECMO patients should be placed in a single negative pressure intensive care unit room. Pipeline oxygen and air supply must be available. There should be at least two power outlets dedicated to ECMO and equipped with uninterruptible power supply (UPS), which can support ECMO at full capacity for at least 30 minutes in the case of power failure. Extracorporeal membrane oxygenation equipment should be plugged into the UPS and should not be shared with any other electronic devices. The ECMO water tank and monitors may share a power outlet with other equipment.
Extracorporeal Membrane Oxygenation Equipment and Disposables
The same brand ECMO machine and disposables should be utilized to ensure consistency and safety. The number of ECMO equipment should be maintained at the current number of critically ill COVID-19 patients in the hospital + 2. The number of ECMO kits and cannulas should be maintained at the current number of critically ill COVID-19 patients in the hospital + 4. Activated coagulation time (ACT) cartridges should be calculated based on every 4 hours monitoring per patient and at least 1 week’s supply should be available.
Transferred ECMO equipment from other hospitals should be tested for the air and oxygen connections before use. The ECMO centrifugal pump should be examined to ensure that it is able to operate for at least 30 minutes after power loss. The ECMO cart should have a full oxygen cylinder tank with adjustable flow meters (provides continuous oxygen supply for 2 hours at 5 L/minute). Each ECMO unit should have a manual hand crank, four metal pipeline clamps, four plastic pipeline clamps, a tube of ultrasonic coupling agent, a flashlight, and pipeline straps. All ECMO disposables, equipment, and disinfectant kits should be placed in the ECMO cart and placed within 50 meters of ECMO patients. Disposables should be replaced immediately.
Extracorporeal Membrane Oxygenation Indications and Timing
To avoid organ damage by prolonged hypoxia, standard ARDS treatment should include protective lung ventilation, optimal positive end expiratory pressure, pharmaceutical paralysis, lung recruitment, and prone positioning. If the patient does not improve as indicated by either partial pressure of oxygen (PaO2)/fractional of inspired oxygen (FiO2) <100 mmHg or power of hydrogen (PH) <7.25 and partial pressure of carbon dioxide >60 mmHg over 6 hours despite optimal mechanical ventilation, ECMO should be considered. Because some severe COVID-19 patients progress very quickly, ECMO should be immediately established if any of the following criteria are met after failure of aggressive ventilation management and worsening clinical condition: 1) PaO2/FiO2 <50 mmHg for more than 1 hour. 2) PaO2/FiO2 <80 mmHg for more than 2 hours, and 3) existence of uncompensated respiratory acidosis with PH <7.2 for more than 1 hour.
Infection Control in Extracorporeal Membrane Oxygenation Management
During ECMO cannulation, replacement of oxygenators, and other invasive procedures, level 3 infection control precautions should be exercised including the use of Powered Air-Purifying Respirators (PAPR), protective suites, disposable sterile surgical gowns, and three layers of sterile gloves. During routine ECMO rounding, invasive procedures and airway exposure should be avoided. Routine ECMO rounding generally does not require PAPR usage.
Extracorporeal Membrane Oxygenation Establishment
Except for emergency veno arterial (VA) ECMO cannulation during cardiopulmonary resuscitation, all patients should be placed on venovenous (VV) ECMO for the purpose of correcting hypoxia. Before instituting VV ECMO, hemodynamics should be evaluated with point-of-care ultrasound and treated with positive inotropes and vasopressors to maintain hemodynamic stability. The internal jugular vein should be cannulated with a 17Fr arterial cannula, and the femoral vein should be accessed with a 21Fr venous cannula under real-time ultrasound guidance with the Seldinger technique. The tip of the internal jugular vein cannula (Perfusion Cannula; Maquet Cardiopulmonary GmbH, Antalya, Turkey) should be positioned at the junction between right atrium and superior vena cava. The tip of the femoral vein cannula (Drainage Cannula; Maquet Cardiopulmonary GmbH, Antalya, Turkey) should be advanced into the right atrium approximately 1 cm beyond the inferior vena cava junction and avoid cannula tip contacting the interatrial septum using echocardiographic guidance.
The ECMO circuit is prefilled with crystalloid, and the oxygen saturation probe should be routinely placed on the drainage and perfusion cannulas. FiO2 is initially set to 100%. Extracorporeal membrane oxygenation (Maquet Cardiopulmonary GmbH, Rastatt, Germany) starts from 1 L/minute blood flow and increases 1 L/minute every 30 seconds until the maximum flow is achieved. Peripheral pulse oxygen saturation and mixed venous oxygen saturation are monitored. Cannulas are secured after verification by echocardiography and surface markers are identified. Then the ECMO flow and sweep rate should be adjusted to maintain peripheral capillary oxygen saturation >90% and mixed venous oxygen saturation >70%. If ECMO flow is inadequate and cannula position seems appropriate per echocardiography imaging, volume should be given (Figure 1).
Figure 1.
A coronavirus disease 2019 patient is supported with extracorporeal membrane oxygenation and mechanical ventilation support.
Extracorporeal Membrane Oxygenation Management Goal
The patient’s hemoglobin level should be maintained above 11 g/dl throughout the ECMO support. Indices including peripheral arterial oxygenation, ECMO premembrane oxygenation, postmembrane oxygenation, percutaneous oxygenation, and mixed venous oxygenation should be used to determine the patient’s oxygen supply and demand balance. Gastrointestinal dysfunction and increased abdominal pressure in COVID-19 patients may directly affect ECMO flow and cause instability.
Extracorporeal Membrane Oxygenation Anticoagulation Management
Extracorporeal membrane oxygenation patients should be anticoagulated using regular heparin followed by a maintenance infusion titrated between 2 and 20 unit/kg/hour to target ACT 180–200 seconds and partial thromboplastin time 50–80 seconds. If heparin resistance is suspected and antithrombin deficiency III activity is low, fresh frozen plasma should be given. In addition, heparin-induced thrombocytopenia (HIT) should be monitored and treated accordingly. When there is significant thrombosis on the ECMO oxygenator, increase in fibrinolysis (D dimer > 10 µg/ml), decrease in fibrinogen (FIB < 1.5 g/L), and thromboelastogram (TEG) signs of hyperfibrinolysis, the ECMO circuit should be replaced. In addition, tranexamic acid should be started (10–20 mg/kg over 3 hours followed by a 1,000 mg, 1–2 mg/kg/hour daily infusion for 2–3 days) and FIB replaced at 1–2 g/days until FIB >1.5 g/L. If there is significant bleeding or need of invasive procedures, heparin may be reduced or suspended for a maximum period of 24 hours.
Point-of-Care Ultrasound During Extracorporeal Membrane Oxygenation
Point-of-care ultrasound should be performed daily. Ultrasonography of the lungs, heart, abdomen, and vasculature is extremely valuable in this patient population for a multitude of purposes. Lung ultrasound is used to assess indicators of pneumothorax, pleural effusion, lung consolidation, and interstitial changes. Cardiac ultrasound is used to evaluate ejection fraction, pulmonary artery pressure, chamber size, right ventricular/left ventricular function, pericardial effusion, and inferior vena cava diameter. Abdominal ultrasound can help assess bowel peristalsis as well as obstruction, and vascular ultrasound can detect deep venous thrombosis.
Protective Lung Ventilation on Extracorporeal Membrane Oxygenation
The main purpose of mechanical ventilation on ECMO is to protect the lungs and avoid damage to the right heart. An aggressive strategy of protective lung ventilation sets FiO2 <40%, tidal volume (Vt) 2–4 ml/kg (ideal body weight), plateau pressure <25 cm H2O, and respiratory rate (RR) 8–10 times/minute. If the plateau pressure exceeds 25 cm H2O, Vt should be reduced by 1 ml/kg. Pressure control mechanical ventilation is the most commonly used ventilator mode before weaning ECMO. If patient’s oxygenation is inadequate, ECMO malfunction should be considered before adjusting ventilator parameters. Sedation and paralysis should be considered in COVID-19 patients with anxiety and high oxygen demand status.
Chest computed tomography (CT) is not routinely performed as transport is considered a patient safety issue. Respiratory mechanics, lung ultrasound, and electrical impedance imaging (electrical impedance tomography [EIT]) are alternative monitoring methods for lung mechanics. Hypercapnia should be initially managed by increasing the VV ECMO sweep rate. During weaning, the RR should be adjusted accordingly, but RR should generally be less than 14.
Awake Extracorporeal Membrane Oxygenation
Coronavirus disease 2019 patients usually develop refractory hyperthermia and other high oxygen consumption states even with effective support. Considering the great difficulty with infection control and unstable pulmonary status in COVID-19 patients, we do not recommend ECMO support without endotracheal intubation.
Continuous Renal Replacement Therapy While on Extracorporeal Membrane Oxygenation
Indications for continuous renal replacement therapy (CRRT) during ECMO are as follows: serum creatinine ≥354 μmol/L; urine volume <0.3 ml/kg/hour >24 hours; refractory metabolic acidosis with PH <7.2 or HCO3− <15 mmol/L; severe electrolyte disturbances including K ≥6.5 mmol/L; volume overload, such as conjunctival edema, pulmonary edema, congestive heart failure, pleural, and abdominal effusion; sepsis; and multiple organ failure. The above indications could be expanded depending on the severity of COVID-19, and early intervention is advocated. If CRRT equipment is capable of recognizing the ECMO pressure range, the CRRT access line is connected to the port on the ECMO oxygenator, and the CRRT return line is connected to the port between the pump and the oxygenator. Two modes of CRRT are used, continuous venous to venous hemodiafiltration (CVVHDF) and continuous venous to venous hemofiltration (CVVH). To improve dialysis efficiency, CVVH is preferred. If the patient is hypercoagulable or not on anticoagulation, CVVHDF or CVVH combined with CVVHDF could be used. The CRRT dose is usually 28–32 ml/kg/hour for 10–12 hours.
Extracorporeal Membrane Oxygenation Complications
Mouth, nose, and throat bleeding should be controlled by decreasing anticoagulation in addition to ear nose throat consultation. To reduce infections, laboratory testing and blood draws should be minimized. Extracorporeal membrane oxygenation cannulation components need covering with anti-infection transparent dressing with daily inspection and regular disinfection.
Coronavirus disease 2019 patients commonly suffer from flatulence. If abdominal flatulence results in elevated abdominal pressure, the femoral vein drainage can be seriously reduced during ECMO support. If nasal lactulose and enema are ineffective in treatment, traditional Chinese medicine may be considered including nasal administration of 50 ml dachengqi decoction twice a day.
Caution must be taken to avoid ECMO cannula displacement and accidental decannulation. The ECMO circuit should be properly secured to the bedside with avoidance of dropping the equipment to the ground. Bedside nursing staff must be trained to manage ECMO emergencies such as power loss and air/oxygen loss.
Extracorporeal Membrane Oxygenation Weaning Procedures and Standards
Patients with improved chest x-rays, chest CT, arterial blood gas, and respiratory mechanics may attempt ECMO weaning. The sweep to flow ratio is maintained at 1:1, and ECMO flow gradually reduced to 2.5 L/minute while continuing the same mechanical ventilation parameters. With the ECMO flow maintained at 2.5 L/minute, the ECMO sweep is gradually reduced until there is complete cessation of the sweep. We observe the patient for 6–12 hours after each adjustment. If the patient condition worsens or oxygenation levels are not sustained during the weaning, ECMO parameters are restored to previous settings. To take patients off ECMO, the following criteria must be maintained for 24–48 hours at ECMO flow rates of 2.5 L/minute without sweep: hemodynamic stability; improvements in chest x-ray, chest CT, EIT, or lung ultrasonography; PaO2/FiO2 >150 mmHg, PCO2 ≤50 mmHg, and RR ≤20; body temperature <38°C; Murray index: 2–3; hematocrit >35% (Figure 2).
Figure 2.
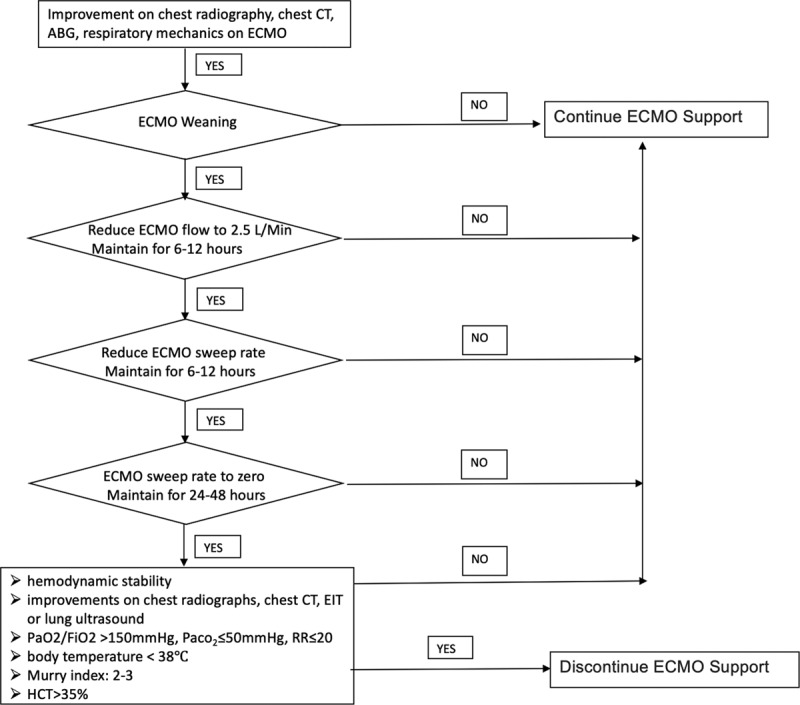
Flowchart of ECMO weaning protocol in COVID-19 patients. ABG, arterial blood gas; COVID-19, coronavirus disease 2019; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; EIT, electrical impedance tomography; FiO2, fractional of inspired oxygen; HCT, hematocrit; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; RR, respiratory rate.
Results
Extracorporeal membrane oxygenation was necessary for 8 out of the 16 critically ill COVID-19 patients in whom severe hypoxia could not be reversed with optimized mechanical ventilation.
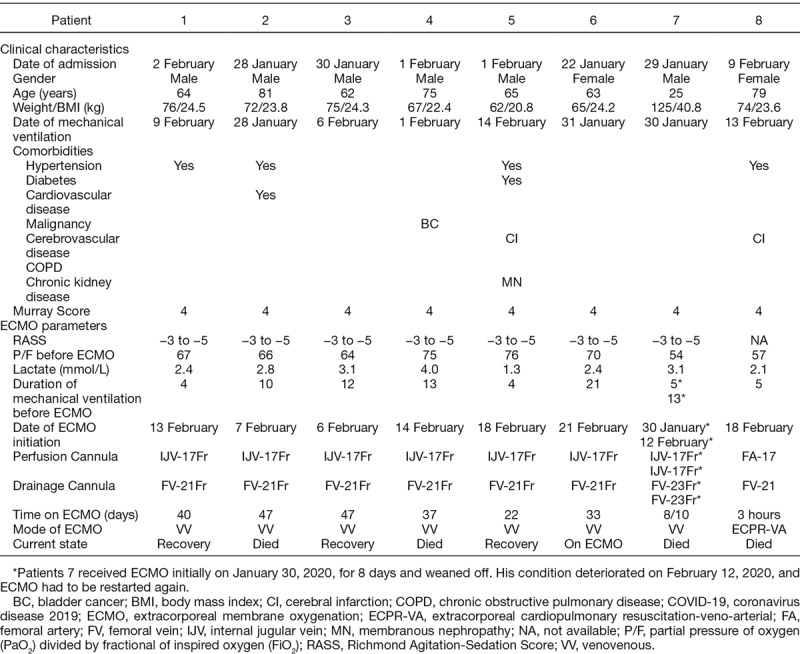
On January 30, 2020, the first VV ECMO for a COVID-19 patient with severe hypoxia that could not be reversed by aggressive mechanical ventilation was established. To date, eight COVID-19 patients have received ECMO: seven with VV ECMO support and one patient was emergently placed on VA ECMO during cardiopulmonary resuscitation. As of March 25, 2020, four patients died (50% mortality). Three patients were successfully (37.5%) weaned off ECMO after 22, 40, and 47 days support, respectively, but remain on mechanical ventilation. One patient is still on VV ECMO with mechanical ventilation. Patient 7 received ECMO initially on January 30, 2020, for 8 days and weaned off. His condition deteriorated on February 12, 2020, and ECMO had to be restarted again. He died after 10 days of ECMO support the second time.
Two patients were female, and six were male. The age range was from 25 to 81 years old. Four patients had hypertension, and two patients had cerebral infarct medical history. The PaO2/FiO2 ratio before ECMO initiation were between 54 and 76, and all were well below 100. The duration of mechanical ventilation before ECMO ranged from 4 to 21 days. Except the one emergent VA ECMO during cardiopulmonary resuscitation, other patients were on ECMO support for between 18 and 47 days (Table 1).
Table 1.
COVID-19 Patients’ Data Who Had Received ECMO Support as of March 25, 2020 in Shanghai, China
Discussion
Extracorporeal membrane oxygenation has been used clinically in Shanghai for nearly 19 years. Cross-training, academic exchange, as well mutual support among perfusionists, critical care physicians, pulmonologists, cardiologists, and emergency medicine physicians have created a solid foundation for the successful implementation of ECMO programs in this area during crises. Extracorporeal membrane oxygenation support in the prevention and control of the highly pathogenic avian influenza H7N8 in 2013 provided our team valuable experience. The Shanghai ECMO Clinical Quality Control Registry in 2018 properly prepared ECMO-capable hospitals and departments for the timely enlisting of equipment and ECMO experts during the COVID-19 crisis.14
Diffuse pulmonary edema and hyaline membrane formation are the main pathologic features in COVID-19 patients.15 Hypoxia can progress rapidly, and optimal mechanical ventilation might not be enough to correct for patients in critical status. Traditional ECMO indications, or the standards as adopted in the extracorporeal membrane oxygenation for severe acute respiratory distress syndrome study,16 may lead to prolonged hypoxia and multiple organ failure in these patients. Therefore, we recommend early establishment of ECMO when mechanical ventilation is insufficient to correct hypoxia in COVID-19 patients. Our indications are consistent with the latest version of the COVID-19 Diagnosis and Treatment Guidelines (Version 7).17
The safety and success of ECMO in the Shanghai Public Health Clinical Center were challenged by many factors including, but not limited to, strict infection control requirements, unfamiliar team members in new environments, and slow communication and feedback in isolation wards. Lessons learned from prior crisis experiences including equipment check before use, management of gas and electrical emergency hazards, availability and location of ECMO disposables, and proactive alarm management to avoid disturbances to unfamiliar healthcare providers were invaluable for our team and the hospital.
It is understood that ECMO does not provide direct support for organs other than the lungs or heart beyond increasing systemic oxygen delivery and mitigating ventilator-induced lung injury. A substantial proportion of critically ill patients with COVID-19 appear to have developed cardiac arrhythmias or shock, but it is unknown how many have or will develop refractory multiorgan failure, for which ECMO may be of more limited use.8 More data on the mechanism of death and disease are required to determine whether ECMO is appropriate to offer to COVID-19 patients. However, it appears that the damage and duration of lung injuries in COVID-19 are extensive, and prolonged ECMO support might be required as shown in our study.
Extracorporeal membrane oxygenation is highly resource consumptive, and many COVID-19 affected countries might not be able to afford this expensive technology. In less well-resourced countries, many more lives will be saved by ensuring that oxygen and pulse oximetry are widely available. Confirmation of the diagnosis, appropriate quarantine and oxygen therapy alone might save many lives with minimal cost. Mechanical ventilation or, for those most likely to benefit, ECMO needs to be evaluated for benefits/risks on a case-by-case basis.8
In conclusion, ensuring effective, timely, and safe ECMO support in COVID-19 is key to improving clinical outcomes. Extracorporeal membrane oxygenation support might be an integral part of the critical care provided for COVID-19 patients in centers with advanced ECMO expertise.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
This work was supported by the Science and Technology Commission Shanghai Municipality, Emergency Science and Technology Projects, No. 20411950501.
References
- 1.World Health Organization: Coronavirus disease (COVID-2019) situation reports. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/Situation-reports/. Accessed on March 23, 2020.
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu ZY, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Gu SC, Wu XJ, Xia JG, Zhang Y, Zhan QY. Extracorporeal membrane oxygenation support in 2019 novel coronavirus disease: Indications, timing and implementation. Chin Med J 2020. doi: 10.1097/CM9.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: The potential role of extracorporeal membrane oxygenation. JAMA 2020. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected—interim guidance. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed February 11, 2020.
- 10.Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed March 25, 2020.
- 11.EURO ELSO. European Survey on ECMO in adult COVID-19 pts at 20/03/20. Available at: https://www.euroelso.net/covid-19/covid-19-survey/. Accessed on March 25, 2020.
- 12.Huang L, Zhang W, Yang Y, et al. Application of extracorporeal membrane oxygenation in patients with severe acute respiratory distress syndrome induced by avian influenza A (H7N9) viral pneumonia: National data from the Chinese multicentre collaboration. BMC Infect Dis 201818: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanghai Clinical Treatment Expert Group for Corona Virus Disease 2019: Comprehensive treatment and management of corona virus disease 2019: expert consensus statement from Shanghai. Chin J Infect Dis 2020. 38: Epub ahead of print. doi: 10.3760/cma.j.issn.1000-6680.2020.0016. [Google Scholar]
- 14.Zhao Y, Wei L, Wang J, et al. Report on quality control and supervision of extracorporeal membrane oxygenation in Shanghai 2018. Chin J Extra Corpor By 20194: 198–201. [Google Scholar]
- 15.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020. pii: S2213-2600(20)30076-X. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet: Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018378: 1965–1975. [DOI] [PubMed] [Google Scholar]
- 17.Office of the National Health and Health Commission. Diagnosis and treatment of new coronavirus pneumonia (Pilot version 7). Available at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed March 23, 2020.


