Abstract
Objective
Many studies have shown that the C1562T polymorphism in the matrix metalloproteinase (MMP)-9 gene promoter is associated with susceptibility to ischemic stroke (IS), but the association between them remains controversial. Our objective was to explore the relationship between MMP9 C1562T polymorphism and susceptibility to IS in the Chinese population.
Methods
We conducted a database search of Wanfang, China Science and Technology Journal database, China National Knowledge Infrastructure, Medline, Embase, PubMed and Springerlink through September 2019. Meta-analysis was performed using Stata15.0 software (StataCorp LP, College Station, TX, USA).
Results
Thirteen articles were included, including 3,996 patients and 3,815 controls. Among the Chinese population, the results showed no significant difference for the allele model (T vs. C; odds ratio = 1.05, 95%CI: 0.80–1.37). Significant differences were found in the dominant model (TT+TC vs. CC; odds ratio = 2.94, 95%CI: 1.58–5.45) and in the recessive model (TT vs. TC+CC; pooled OR = 0.81, 95%CI: 0.66–0.99). Neither the homozygous model or heterozygous model was significant.
Conclusion
We identified a correlation between MMP-9 C1562T polymorphism and IS in the Chinese population; the TT+TC genotype may increase the risk of IS.
Keywords: Ischemic stroke, polymorphism, MMP-9, matrix metalloproteinase-9, meta-analysis, dominant model
Introduction
Ischemic stroke (IS) is one of the most common cerebrovascular diseases. It is caused mainly by occlusion of the blood supply, resulting in ischemia and hypoxia of cerebral cells, which leads to neurological impairment and high disability and mortality.1 In 2016, the number of people with cardiovascular diseases in China exceeded 93 million, 24,098,000 of whom had IS. Compared with 1990, this represents an increase of almost 174%. In the same period, the number of deaths increased by nearly 333,000, an increase close to 84%, and the disease burden was 16.216 million person-years.2 IS is a polygenic disease caused by a variety of environmental and genetic factors, and these genetic factors play an important role in the pathophysiology of IS. Inflammation is a key pathogenic mechanism of atherosclerosis, which ultimately leads to stroke by promoting the formation of atherosclerotic plaques, the development of unstable plaques, and plaque rupture.3
Matrix metalloproteinase 9 (MMP-9), also known as gelatinase B, is a type of matrix metalloproteinase secreted by monocytes, neutrophils, and vascular endothelial cells.4 Mainly through the degradation of type IV and V collagen, it causes the destruction of extracellular matrix and basement membrane and then causes vascular injury. The collagen fiber in the fiber cap of an atherosclerotic plaque can be degraded by MMP-9 to become thinner, resulting in unstable plaques. A C1562T polymorphism (−1562C>T) is present in the gene encoding MMP-9 and MMP-9 is involved in the pathological process of atherosclerosis, including extracellular matrix degradation, inflammatory cell infiltration, and plaque rupture.5 MMP-9 is a zinc ion-dependent endopeptidase involved in many biological reactions, such as human growth and development, and is related to the advanced cortical function of some nervous systems. Two days after a stroke, MMP-9 content was shown to be significantly higher in ischemic lesions than in non-ischemic lesions.6 Therefore, MMP-9 plays an important role in the process of IS and reperfusion injury after stroke. Some studies have shown that a high level of MMP-9 is found not only in ischemic tissues, but also in the ischemic penumbra, and thus is related to the progression of IS.7
Many studies have shown that MMP-9 participates in the formation, migration, rupture, and disintegration of atherosclerotic plaques, cerebral ischemia-reperfusion injury, hemorrhage transformation after cerebral infarction, and neuronal apoptosis,8–12 which is closely related to the occurrence and development of stroke. The C → T functional polymorphism exists at residue 1562 of the MMP9 gene promoter. The polymorphism produces promoter genotypes with in low (C/C) or high (C/T, T/T) activity, resulting in decreased or increased expression of MMP-9.13 When the C allele is replaced with the T allele, gene transcription is enhanced, protein synthesis and release are increased, and extracellular matrix degradation is promoted; this mutation and its aftermath are the main cause of atherosclerotic plaque formation, rupture, and reperfusion injury after cerebral ischemia, and it is the molecular mechanism underlying cerebral vascular infarction.14 The C1562T polymorphism in MMP9 is related to the pathogenesis of IS, the study of which allows us to better understand the pathogenesis and biological indicators of IS. At present, many studies have explored the relationship between serum MMP-9 level, MMP9 gene promoter C1562T polymorphism, and IS. However, the results have not been consistent and there is no clear consensus on this relationship. Therefore, the aim of this study was to summarize and analyze the relationship between MMP9 gene C1562T polymorphism and IS.
Methods
Ethical approval
Ethical approval for this study was deemed unnecessary because we analyzed only previously published articles.
Literature retrieval
The target of our literature search was case–control studies on the association between C1562T gene polymorphism in the MMP9 promoter and IS in the Chinese population. The keywords “matrix metalloproteinase 9” or “MMP-9” in combination with “gene” or “polymorphism” as well as “stroke” or “cerebral infarction” were used. The China Science and Technology Journal database, China Wanfang database, and China National Knowledge Infrastructure (CNKI) database were searched to obtain the relevant Chinese literature. The above keywords were also used to search in the databases of PubMed, Medline, Springerlink, and Embase to obtain articles published in English. The retrieval time was from the establishment of each database to September 2019.
Literature inclusion and exclusion criteria
The inclusion criteria were as follows: (1) the study investigated the correlation between C1562T gene polymorphism of MMP9 promoter and IS among the Chinese population; (2) case–control study; (3) the distribution of genotypes in the control group satisfied Hardy–Weinberg equilibrium (HWE) with P > 0.05; (4) the distribution frequency of alleles and genotypes in the case and control groups was reported in the study; (5) Chinese studies were included in the core journals of Peking University Library or the key magazine of China technology.
The exclusion criteria were as follows: (1) duplicate publications and those from which we could not extract statistical content; (2) studies that did not conform to HWE in the control group; and (3) articles with a Newcastle–Ottawa scale (NOS)15 quality score <6.
Evaluation of the quality of the literature and data extraction
In accordance with the NOS,15 the full text of the articles was carefully read and evaluated in terms of quality, with low quality articles scoring <6 stars and high quality articles scoring >6 stars; only articles scoring ≥6 stars were included. In line with a uniform quality criterion, the evaluation was made independently by two evaluators who extracted the document materials and then cross checked the results. When the assessment diverged between evaluators, discrepancies were resolved by discussion or by a third party. The extracted data included the number of MMP9 C1562T genotypes in both cases and controls, author, publication date, country, and ethnic origin.
Statistical methods
Meta-analysis was carried out using Stata 15.0 statistical software (StataCorp LP, College Station, TX, USA). Odds ratios (OR) and 95%CI, as the effect size, were calculated to present the results of the meta-analysis. The Q-test was used to test the heterogeneity of the results; If I2 ≥ 50% or P ≤ 0.05, the random effects model was used; if I2 < 50% and P > 0.05, there was no heterogeneity, so the fixed effects model was used for data consolidation. The Z-test was used to test the significance of the pooled OR value. This meta-analysis included an evaluation of publication bias, and the standard was whether the funnel plot was symmetrical or not. Funnel plots used the standard error of each study’s log(OR) to map its OR value. If a funnel plot is asymmetric, it may indicate publication bias. Egger’s test was also used to test publication bias.
Results
Basic information of the retrieved articles
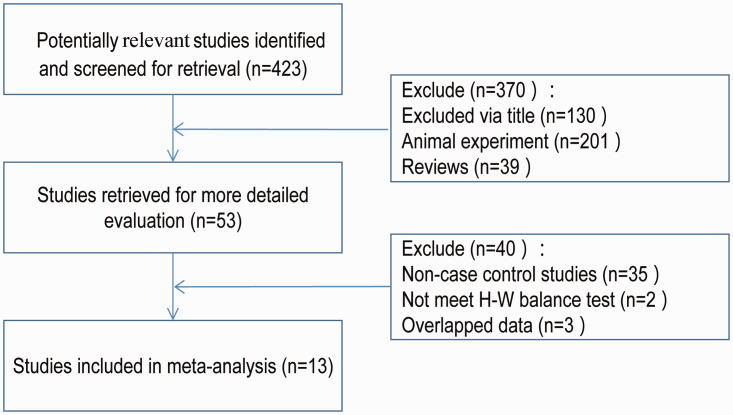
According to the inclusion and exclusion criteria, 13 articles16–28 were included in this meta-analysis. There were 3,996 patients in the IS (case) group and 3,815 patients in the control group. The specific literature screening process is shown in Figure 1. The characteristics and genotype distribution frequency of the study, together with the results of HWE test in the control group, are shown in Table 1. The results of the quality evaluation of the literature is shown in Table 2.
Figure 1.
A PRISMA flow diagram of the study selection process. H-W = Hardy–Weinberg.
Table 1.
Characteristics of included studies for C1562T polymorphism in MMP9 gene.
| Reference | Country | Control source | Cases/controls (n) |
Genotype: case |
Genotype: control |
Genotyping method | Sex ratio (male cases/controls) | Age, years (case/control) | HWE | NOS score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | CC | CT | TT | |||||||||
| Zhou et al., 200816 | China | HB | 101/114 | 87 | 14 | 0 | 99 | 13 | 2 | PCR-RFLP | 46/51 | 61.6 ± 11.5/59.6 ± 10.9 | 0.060 | 7 |
| Zhang et al., 200817 | China | HB | 114/80 | 95 | 16 | 3 | 63 | 15 | 2 | PCR-RFLP | 82/54 | 66.0 ± 10.2/64.0 ± 13.6 | 0.350 | 7 |
| Zhou and Liu, 200918 | China | PB | 70/60 | 46 | 22 | 2 | 50 | 8 | 2 | PCR-RFLP | 40/35 | 57.7 ± 10.2/54.2 ± 9.4 | 0.090 | 7 |
| Hou et al., 200919 | China | PB | 57/84 | 46 | 10 | 1 | 66 | 18 | 0 | PCR-RFLP | 39/52 | 68.0 ± 10.0/63.0 ± 9.0 | 0.270 | 7 |
| Shi et al., 201020 | China | HB | 224/112 | 186 | 38 | 0 | 92 | 20 | 0 | PCR-RFLP | 123/54 | 64.5 ± 10.9/66.9 ± 10.9 | 0.300 | 7 |
| Liu et al., 201121 | China | HB | 232/235 | 181 | 48 | 3 | 204 | 29 | 2 | PCR-RFLP | 146/144 | 61.0 ± 12.5/60.9 ± 9.9 | 0.400 | 7 |
| Li et al., 201322 | China | HB | 302/308 | 252 | 50 | 0 | 271 | 37 | 0 | PCR-RFLP | 165/140 | 65.7 ± 9.9/63.2 ± 8.2 | 0.260 | 7 |
| Yue et al., 201423 | China | HB | 284/226 | 227 | 50 | 7 | 195 | 28 | 3 | PCR-RFLP | 163/124 | 67.5 ± 13.3/67.9 ± 11.7 | 0.100 | 7 |
| Hao et al., 201524 | China | HB | 317/317 | 44 | 59 | 214 | 9 | 66 | 242 | PCR-RFLP | 180/180 | 62.1 ± 10.3/62.5 ± 9.9 | 0.090 | 8 |
| Zhao et al., 201525 | China | HB | 335/335 | 48 | 64 | 223 | 10 | 71 | 254 | PCR-RFLP | 194/194 | 63.7 ± 9.4/64.5 ± 9.2 | 0.080 | 8 |
| Lee AF (2018)26 | China | HB | 300/300 | 201 | 95 | 4 | 221 | 76 | 3 | PCR-RFLP | 163/124 | 59.7 ± 12.6/58.7 ± 11.7 | 0.200 | 7 |
| Li et al., 201827 | China | HB | 1274/1258 | 1002 | 241 | 31 | 1041 | 202 | 15 | PCR-RFLP | 890/823 | 66.9 ± 10.6/65.6 ± 9.1 | 0.150 | 8 |
| Liu et al., 201628 | China | HB | 386/386 | 300 | 79 | 7 | 296 | 83 | 7 | PCR-RFLP | 267/265 | 62.1 ± 9.9/61.9 ± 9.8 | 0.670 | 7 |
MMP9, matrix metalloproteinase 9 gene; HB, hospital-based; PB, population-based; PCR-RFLP, identified by restriction fragment length polymorphism-PCR; HWE, Hardy–Weinberg equilibrium; NOS, Newcastle-Ottawa Scale (study quality).
Table 2.
Results of quality evaluation of literature.
| Reference | Case selection |
Comparability between groups |
Exposure factor measurement |
NOS score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Blind method | 7 | Response rate | ||
| Zhou et al., 200816 | * | * | * | * | * | * | ? | * | ? | 7 |
| Zhang et al., 200817 | * | * | * | * | * | * | ? | * | ? | 7 |
| Zhou and Liu, 200918 | * | * | * | * | * | * | ? | * | ? | 7 |
| Hou et al., 200919 | * | * | * | * | * | * | ? | * | ? | 7 |
| Shi et al., 201020 | * | * | * | * | * | * | ? | * | ? | 7 |
| Liu et al., 201121 | * | * | * | * | * | * | ? | * | ? | 7 |
| Li et al., 201322 | * | * | * | * | * | * | ? | * | ? | 7 |
| Yue et al., 201423 | * | * | * | * | * | * | ? | * | ? | 7 |
| Hao et al., 201524 | * | * | * | * | * | * | ? | * | * | 8 |
| Zhao et al., 201525 | * | * | * | * | * | * | ? | * | * | 8 |
| Lee AF (2018)26 | * | * | * | * | * | * | ? | * | ? | 7 |
| Li et al., 201827 | * | * | * | * | * | * | ? | * | * | 8 |
| Liu et al., 201628 | * | * | * | * | * | * | ? | * | ? | 7 |
Quality criteria: 1 = case identification appropriate; 2 = case representativeness; 3 = source of the control clear; 4 = control group chosen properly; 5 = controls the most important confounding factors; 6 = control other confounding factors; 7 = same exposure determination method; *, yes; ?, unclear; NOS, Newcastle–Ottawa Scale.
Meta-analysis results
Comparison of alleles
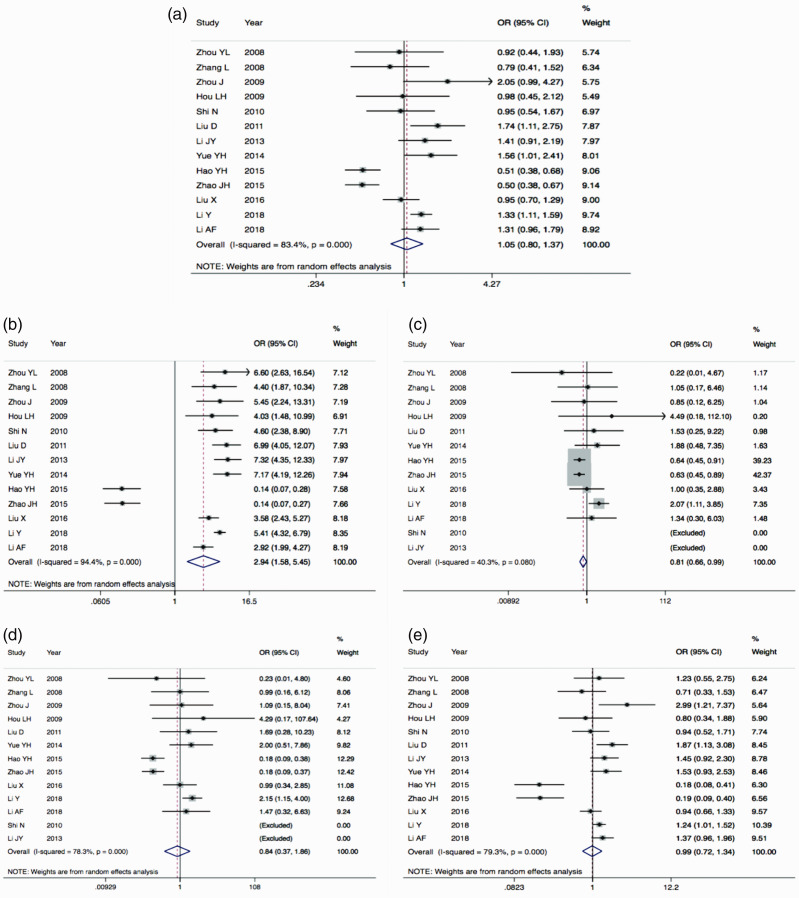
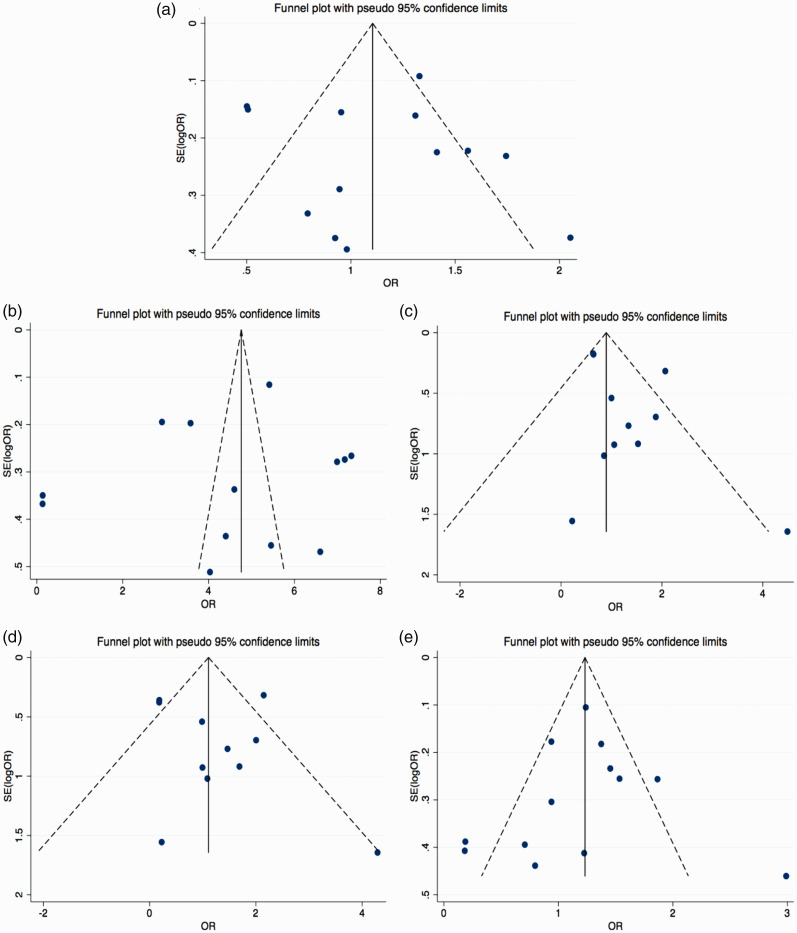
The major results of the meta-analysis are shown in Table 3 and Figure 2. With I2 = 83.4% and P < 0.05, the T allele was compared with the C allele, indicating that there was significant difference in heterogeneity among the studies. Thus, the random effects model was used. There was no significant difference in the combination of OR = 1.05 (95%CI: 0.80–1.37). This suggested that the risk of IS was not associated with the frequency of MMP9 C1562T alleles. The funnel plot was symmetrical (Figure 3a). The results of Egger’s test demonstrated a P-value > 0.05, indicating that publication bias was well controlled and the reliability of the conclusion was high.
Table 3.
Results of meta-analysis for MMP9 C1562T polymorphism and ischemic stroke risk.
| Genetic model | n | OR | 95%CI | P | I2 (%) | P for heterogeneity | Model | Publication bias |
|---|---|---|---|---|---|---|---|---|
| Allelic | 13 | 1.05 | 0.80–1.37 | 0.732 | 83.4 | 0.000 | REM | 0.781 |
| Dominant | 13 | 2.94 | 1.58–5.45 | 0.001 | 94.4 | 0.000 | REM | 0.621 |
| Recessive | 11 | 0.81 | 0.66–0.99 | 0.040 | 40.3 | 0.080 | FEM | 0.111 |
| Homozygous | 11 | 0.84 | 0.37–1.86 | 0.661 | 78.3 | 0.000 | REM | 0.574 |
| Heterozygous | 13 | 0.99 | 0.72–1.34 | 0.926 | 79.3 | 0.000 | REM | 0.769 |
MMP9, matrix metalloproteinase 9 gene; OR, odds ratio; 95%CI, 95% confidence interval; REM, random effects model; FEM, fixed effects model.
Figure 2.
Forest plot for the five genetic models: (a) allelic model, (b) dominant model, (c) recessive model, (d) homozygous model, and (e) heterozygous model. The size of each box for an individual study represents the OR of the study and its 95%CI; the red dotted line represents the pooled OR position; and the diamond represents the 95% confidence interval for merging OR. OR, odds ratio; 95%CI, 95% confidence interval.
Figure 3.
Funnel plot for the five genetic models: (a) allelic model, (b) dominant model, (c) recessive model, (d) homozygous model, and (e) heterozygous model. SE, standard error; OR, odds ratio; 95%CI, 95% confidence interval.
Dominant genetic model
In the dominant genetic model (TT+TC vs. CC), genotypes TT+TC were used as the exposure factor and genotype CC as the non-exposure factor. The heterogeneity test showed that the difference was significant (P < 0.05; Table 3), so the random effects model was used. The results indicated a significant difference in the combination of OR = 2.94 (95%CI: 1.58–5.45; P = 0.001); that is, the frequency of the TT+TC genotype of the MMP9 C1562T locus was higher in Chinese patients with IS than in the control group. The funnel plot was basically symmetrical (Figure 3b). Egger’s test demonstrated a P-value > 0.05, which indicated that the publication bias was well controlled and the reliability of the conclusion was high.
Recessive genetic model
In the recessive genetic model (TT vs. TC+CC), genotype TT was used as the exposure factor and genotype TC+CC as the non-exposure factor. The heterogeneity test showed I2 = 40.3% and P > 0.05, indicating that there was no significant difference in heterogeneity among the studies. Thus, the fixed effects model was used. With an OR = 0.81 (95%CI: 0.66–0.99), the difference was significant (P = 0.040). As the upper limit of the 95%CI of the OR was close to 1, a conclusion of statistical significance could be drawn after looking at the sensitivity results. The funnel plot was basically symmetrical (Figure 3c). Egger’s test demonstrated a P-value > 0.05, indicating that publication bias was not evident.
Homozygous genetic model
In the homozygous genetic model (TT vs. CC), genotype TT was used as the exposure factor and genotype CC as the non-exposure factor. The heterogeneity test showed an I2 = 78.3% and P < 0.05, indicating significant differences in heterogeneity among the studies. Thus, the random effects model was used. With OR = 0.84 (95%CI: 0.37–1.86), the difference was not significant. In other words, the frequency of the TT genotype at the MMP9 C1562T locus in Chinese patients with IS was not greater than that in the control group. The funnel plot was basically symmetrical (Figure 3d). Egger’s test demonstrated a P-value > 0.05, which indicated that publication bias was not evident.
Heterozygous genetic model
In the heterozygous genetic model (TC vs. CC), genotype TC was used as the exposure factor and genotype CC as the non-exposure factor. The heterogeneity test showed an I2 = 79.3% and P < 0.05, indicating significant differences in heterogeneity among the studies. Thus, the random effects model was used. With OR = 0.99 (95%CI: 0.72–1.34), the difference was not significant. That is, the frequency of the TC genotype at the MMP9 C1562T locus in Chinese patients with IS was not higher than that in the control group. The funnel plot was basically symmetrical (Figure 3e). Egger’s test again showed that publication bias was not evident.
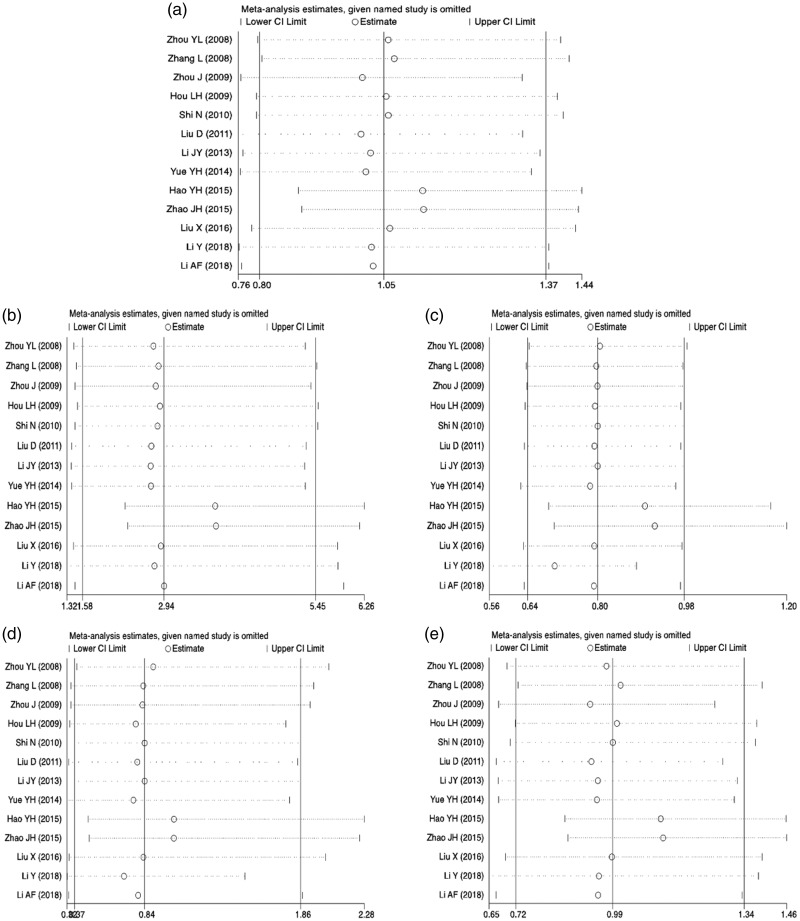
Sensitivity analysis
Each study was excluded one by one and analyzed by meta-analysis (Figure 4). The results showed that in the recessive gene genetic model, after removal of two articles that reported a large number of cases, the results changed significantly and the conclusion was different (Figure 4c). Therefore, the recessive gene model could not be concluded. The results for the allele model and the other three models showed no significant changes in the combined effect, indicating that the 16 articles included were stable.
Figure 4.
Sensitivity analysis plot for the five genetic models: (a) allelic model, (b) dominant model, (c) recessive model, (d) homozygous model, and (e) heterozygous model. 95%CI, 95% confidence interval.
Discussion
With characteristics of high morbidity, disability, and mortality, stroke is a main cause of death in the Chinese population; IS endangers the health and quality of life of patients, and brings a heavy burden to patients, their families, and society. Although the diagnosis and treatment of IS are diverse, the disability and mortality rate have not decreased effectively. The MMP9 gene is located in the chromosome 20q12.2-13.1 region and contains 13 exons and 12 introns. MMP-9, also known as gelatinase B, is an important member of the matrix metalloproteinase family. It can degrade and reshape extracellular matrix to promote the aggregation and migration of vascular endothelial and smooth muscle cells; it can also regulate cell proliferation and apoptosis, participating in the pathophysiological process of vascular response and neurovascular regeneration and remodeling.29 MMP-9 is released when neurons, astrocytes, oligodendrocytes, and microglia are injured by ischemia. Furthermore, free radicals and inflammatory molecules released from ischemic injury can activate MMP-9. The increase in free radicals and inflammatory molecules is related to complications such as neuronal injury, apoptosis, oxidative stress, interfering oxidative DNA repair, cerebral edema, and post-infarction hemorrhage caused by increased permeability of the blood–cerebrospinal fluid barrier.30,31 As the most common single nucleotide polymorphism in the MMP9 gene, −1562C>T is associated with cardiovascular diseases such as coronary heart disease.32 However, the relationship between MMP9 C1562T polymorphism and IS has not yet been determined. Therefore, in this study, we conducted a meta-analysis of the relationship to draw accurate and objective conclusions about this relationship.
In accordance with the strict inclusion criteria, this meta-analysis included 13 articles,16–28 consisting of 3,996 patients and 3,815 controls, all in the Chinese population. We found a correlation between MMP9 C1562T polymorphism and IS in the Chinese population, mainly in the dominant genetic model. In this model, the combined OR value was 2.94, and the difference was statistically significant. In addition, the sensitivity analysis showed that the results were stable. We found no significant differences in the allele model or the homozygous and heterozygous genetic models. In the recessive genetic model, we found a significant difference, but the sensitivity analysis showed that the difference was not significant after removal of two important studies; therefore, we could not draw a conclusion on this. The results of publication bias indicated that the funnel plots of each model were basically symmetrical. Furthermore, the results of Egger’s test showed that P-values were >0.05, indicating no publication bias. The heterogeneity test showed that the I2 values of the models were >50% except for the recessive genetic model (I2 = 40.3%), indicating that there was heterogeneity among the studies. In a meta-analysis that included 14 studies, He et al.33 argued that the C1562T polymorphism of MMP9 was associated with the risk of IS in the Chinese population. In that study population, the T allele and TT and TC genotypes increased the risk of IS. However, the conclusion of the current study is inconsistent with that report, but our study is more convincing as we excluded a control study that did not conform to HWE,34 and included more high-quality studies conducted in the Chinese population.
This study had some limitations. First, only published studies were included in this meta-analysis; therefore, there may be publication bias. Second, we found moderate heterogeneity among the studies in most genetic models. Third, the effects of gene linkage and gene–environment interaction on IS were not analyzed.
Overall, the MMP9 C1562T polymorphism was associated with IS, and the dominant genotypes (TT+TC) may increase the risk of IS in the Chinese population. However, the relationship between genotype and IS needs to be further studied in a larger population, so that the effects of gene–gene and gene–environment interactions can be considered.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
ZhongXin Xu https://orcid.org/0000-0003-1575-9063
References
- 1.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Circulation 2013; 8: 870–947. [DOI] [PubMed] [Google Scholar]
- 2.Li YC, Liu SW, Zeng XY, et al. Report on burden of cardiovascular diseases from 1990 to 2016 in China. Chin Circul J 2019; 34: 729–740. [Google Scholar]
- 3.Gorgui J, Gasbarrino K, Georgakis MK, et al. Circulating adiponectin levels in relation to carotid atherosclerotic plaque presence, ischemic stroke risk, and mortality: a systematic review and meta-analyses. Metabolism 2017; 69: 51–66. [DOI] [PubMed] [Google Scholar]
- 4.Sheu JR, Fong TH, Liu CM, et al. Expression of matrix metalloproteinase-9 in human platelets: regulation of platelet activation in in vitro and in vivo studies. Brit J Pharmacol 2010; 143: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamarina NA, McMillan WD, Shively VP, et al. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery 1997; 122: 264–271. [DOI] [PubMed] [Google Scholar]
- 6.Clark AW, Krekoski CA, Bou SS, et al. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett 1997; 238: 53–56. [DOI] [PubMed] [Google Scholar]
- 7.Morancho A, Rosell A, García-Bonilla L, et al. Metalloproteinase and stroke infarct size: role for anti-inflammatory treatment? Ann N Y Acad Sci 2010; 1207: 123–133. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg GA, Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology 1997; 48: 921–926. [DOI] [PubMed] [Google Scholar]
- 9.Power C, Henry S, Del Bigio MR, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol 2003; 53: 731–742. [DOI] [PubMed] [Google Scholar]
- 10.Wells JE, Biernaskie J, Szymanska A, et al. Matrix metalloproteinase (MMP)-12 expression has a negative impact on sensorimotor function following intracerebral haemorrhage in mice. Eur J Neurosci 2015; 21: 187–196. [DOI] [PubMed] [Google Scholar]
- 11.Abilleira S, Montaner J, Molina CA, et al. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg 2003; 99: 65–70. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Sabín J, Delgado P, Abilleira S, et al. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke 2004; 35: 1316–1322. [DOI] [PubMed] [Google Scholar]
- 13.Cao JP, Lee X, Ma Y, et al. Study of protective effects of Ginkgolide B on myocardial ischemia reperfusion injury in rats. Prev Treat Cardio Cereb Vasc Dis 2016; 16: 8–10. [Google Scholar]
- 14.Zhang X, Cao X, Xu X, et al. Correlation between the −1562C/T polymorphism in the matrix metalloproteinase-9 gene and hemorrhagic transformation of ischemic stroke. Exp Ther Med 2015; 9: 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 16.Zhou YL, Jin XP, Zhu M, et al. Relationship between matrix metalloproteinase-9 polymorphism and acute cerebral infarction. J Clin Neurol 2008; 41: 97–101. [Google Scholar]
- 17.Zhang L, Zhang YD, Lee JM, et al. Relationship between the gene polymorphism of matrix Metalloproteinase-9/C 1562T and ischemic stroke. J Clin Neurol 2008; 21: 425–427. [Google Scholar]
- 18.Zhou J, Liu J. Matrix Metalloproteinase -9 polymorphism (C1562T) in patients with cerebral infarction. China Journal of Modern Medicine 2009; 19: 1829–1831. [Google Scholar]
- 19.Hou LH, Liu XE, Lee H, et al. Polymorphism of matrix Metalloproteinase-9 related to cerebral infarction. Chinese Remedies & Clinics 2009; 9: 575–577. [Google Scholar]
- 20.Shi N, Wang L, Song LY, et al. A study of the association between polymorphism of MMP-9 gene and atherosclerosis and cerebral infarction. Chin J Clin Neurosci 2011; 19: 476–480. [Google Scholar]
- 21.Liu D, Yang J, Zhang GW, et al. Study on polymorphisms of matrix Metalloproteinase-2 and -9 genes of stroke patients. Chin J Geriatr Heart Brain Vessel Dis 2011; 13: 901–904. [Google Scholar]
- 22.Li JY, Wu HR, Li HX, et al. Association between gene polymorphism of ALOX5AP, COX2, MMP-9 and ischemic stroke. Med J Qilu 2013; 28: 321–324. [Google Scholar]
- 23.Yue YH, Bai XD, Zhang XN, et al. Correlation between serum matrix Metalloproteinase-9 levels and its gene −1562C>T polymorphism to ischemic stroke subtypes in Uyghur nationality. Chin J Arterioscler 2014; 22: 55–60. [Google Scholar]
- 24.Hao Y, Tian S, Sun M, et al. Association between matrix metalloproteinase gene polymorphisms and development of ischemic stroke. Int J Clin Exp Pathol 2015; 8: 11647–11652. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao JH, Xu YM, Xing HX, et al. Associations between matrix metalloproteinase gene polymorphisms and the development of cerebral infarction. Genet Mol Res 2016; 14: 19418–19424. [DOI] [PubMed] [Google Scholar]
- 26.Lee AF, Zhang YC, Lee YF, et al. Study on relationship between matrix Metalloproteinase-9 1562 gene polymorphism and acute cerebral infarction in 300 cases. Chongqing Med 2018; 47: 52–55. [Google Scholar]
- 27.Li Y, Chen L, Yao S, et al. Association of polymorphisms of the matrix Metalloproteinase 9 gene with ischaemic stroke in a Southern Chinese population. Cell Physiol Biochem 2018; 49: 2188–2199. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Zhu R, Li Q. Association of MMP-9 gene polymorphism and ischemic stroke: evidence from a case-control study to a meta-analysis. Int J Exp Med 2016; 10: 18844–18852. [Google Scholar]
- 29.Manickam V, Tiwari A, Jung JJ, et al. Regulation of vascular endothelial growth factor receptor 2 trafficking and angiogenesis by Golgi localized t-SNARE syntaxin 6. Blood 2010; 117: 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamashita T, Abe K. Therapeutic approaches to vascular protection in ischemic stroke. Acta Med Okayama 2011; 65: 219–223. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Candelario-Jalil E, Thompson JF, et al. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J Neurochem 2010; 112: 134–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones GT, Tromp G, Kuivaniemi H, et al. Meta-analysis of genome-wide association studies for abdominal aortic aneurysm identifies four new disease-specific risk loci. Circ Res 2017; 120: 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He T, Wang J, Wang XL, et al. Association between the matrix Metalloproteinase-9 rs3918242 polymorphism and ischemic stroke susceptibility: a meta-analysis. J Stroke Cerebrovasc Dis 2017; 26: 1136–1143. [DOI] [PubMed] [Google Scholar]
- 34.Nie SW, Wang XF, Tang ZC. . Correlations between MMP-2/MMP-9 promoter polymorphisms and ischemic stroke. Int J Clin Exp Med 2014; 7: 400–404. [PMC free article] [PubMed] [Google Scholar]