Abstract
Background
A novel technique of continuous transversus abdominis plane block (TAPB) has been reported to be beneficial to patients undergoing abdominal surgery because it can significantly relieve postoperative pain. The aim of our study is to compare this novel technique with a traditional technique of continuous epidural analgesia (EA).
Methods
We conducted our meta-analysis in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Only randomized controlled trials (RCTs) that compared the efficacy of continuous TAPB and continuous EA to relieve postoperative pain were included. Patients were classified by nationality (Chinese, non-Chinese) for the subgroup analysis.
Results
Nine RCTs with 598 patients were included in our study. Pain levels measured by visual analog scale (VAS) scores at rest on postoperative day 1 were equivalent for continuous TAPB groups and continuous EA groups in non-Chinese and Chinese patients. The TAPB groups experienced a lower rate of hypotension, sensorimotor disorder, and nausea compared with the continuous EA group within 48 hours after surgery.
Conclusion
Continuous TAPB and continuous EA are equally effective in relieving postoperative pain at rest 24 hours after surgery, but EA was associated with more side effects such as hypotension, nausea, and sensorimotor disorder.
Keywords: Nerve block, postoperative pain, postoperative analgesia, enhanced recovery after surgery, hypotension, sensorimotor disorder
Introduction
Epidural analgesia (EA) was once one of the most commonly used methods of postoperative analgesia because it resulted in lower visual analog scale (VAS) pain scores and fewer cardiopulmonary complications.1 Many novel analgesia techniques after surgery are now hot topics such as TAPB and wound infusion of analgesics after surgery.2 EA is still a commonly used method to relieve postoperative pain, although there is an incidence of inadequate analgesia after surgery, which ranges from 28% to 32%.3,4
Transversus abdominis plane block (TAPB) is a novel local anesthesia technique that provides analgesia to the abdominal wall that was first introduced in 2001.5 Since then, it has been widely used by anesthetists because it is simple to perform under ultrasound guidance, and it is not associated with some common side effects.6 However, single-shot TAPB was reported to provide analgesia less than 24 hours after abdominal surgery7–10 and there is only limited evidence showing that single-shot TAPB, alone with multimodal analgesia, was beneficial to relieve postoperative pain.11–15
A recent RCT performed by Kadam and Field16 showed that continuous TAPB resulted in less rescue analgesic consumption and lower VAS pain scores after abdominal surgery. Niraj et al.17 showed that intermittent boluses of TAPB resulted in no obvious advantage compared with intermittent boluses of EA after abdominal surgery in 2011. Many studies have been conducted to investigate the efficacy of continuous TAPB for postoperative analgesia compared with continuous EA, but the outcomes remain unclear and controversial.18–21 Thus, we aimed to collect all published studies that reported the comparison of both methods that were used for postoperative analgesia and systematically reviewed them to investigate whether continuous TAPB is better for postoperative pain compared with the traditional gold standard of continuous EA.
Methods and materials
We conducted our systematic review and meta-analysis of efficacy of continuous TAPB with continuous epidural analgesia in patients undergoing abdominal surgery according to the rules of PRISMA.22 We have registered our study protocol on PROSPERO (CRD number: 42019142824). Because this was a meta-analysis of previously published articles, ethics approval was not required.
Inclusion and exclusion criteria
The inclusion criteria for the articles was as follows: (1) Study is an RCT; (2) investigated the efficacy of continuous TAPB for postoperative analgesia compared with continuous EA; (3) patients were scheduled for elective abdominal surgery with normal coagulation and renal function; and (4) patient age >18 and <85 years. The exclusion criteria were as follows: (1) Study is not an RCT; (2) data could not be extracted; (3) studies including emergency surgeries; (4) contraindications to multimodal analgesia; (5) infection of the puncture site; (6) analgesic dependence; (7) chronic pain; or (8) coagulopathy, major psychiatric illness, or spinal abnormality.
Search strategies and screen of articles
We performed comprehensive searches of PubMed, Cochrane Library, EMBASE, China National Knowledge Network, and Wanfang databases using the key words TAPB, nerve block, abdominal wall block, transversus abdominal wall block, epidural anaesthesia, epidural anesthesia, epidural analgesia, epidural injection, and epidural drug administration. Search strategies differed in different databases, and we present detailed information about the search strategies in the supplementary files. We set a language restriction for English and Chinese, and the last search for studies was completed in July 2019. Searches were re-run just before the final analyses and any further studies that were identified were retrieved for inclusion. Unpublished studies were not be sought.
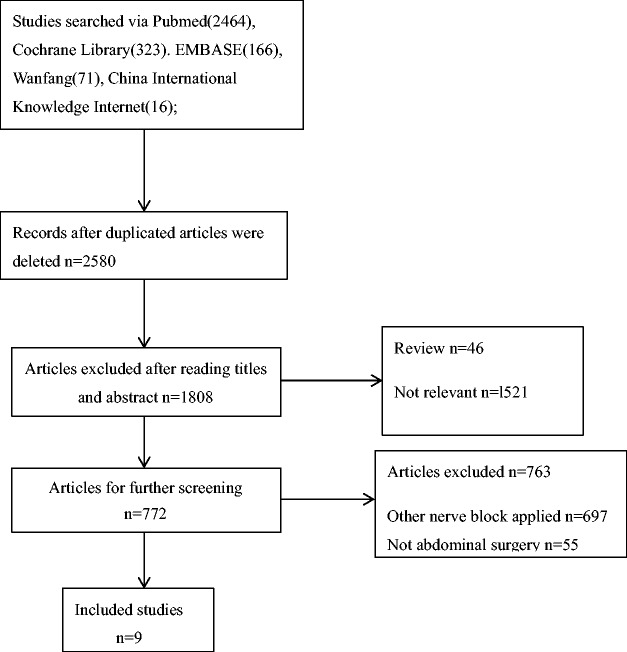
Initially, our searches identified with 3040 publications, and only 9 studies were included because most of the publications did not meet our inclusion criteria. Two reviewers (Xiangbo Liu and Fei Peng) independently screened all the articles that were located and disagreements were resolved by a third reviewer (Cehua Ou). Detailed information about the study screening is shown in Figure 1.
Figure 1.
Flowchart of the article screening process.
Data extraction and risk of bias assessment
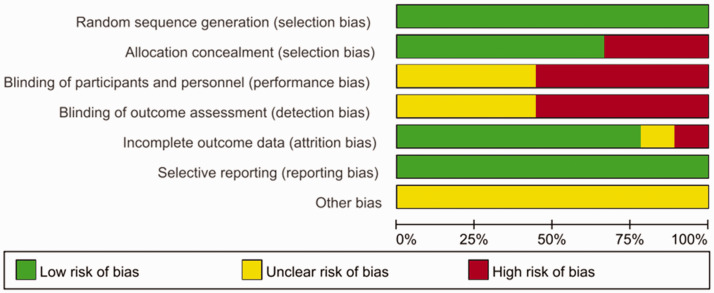
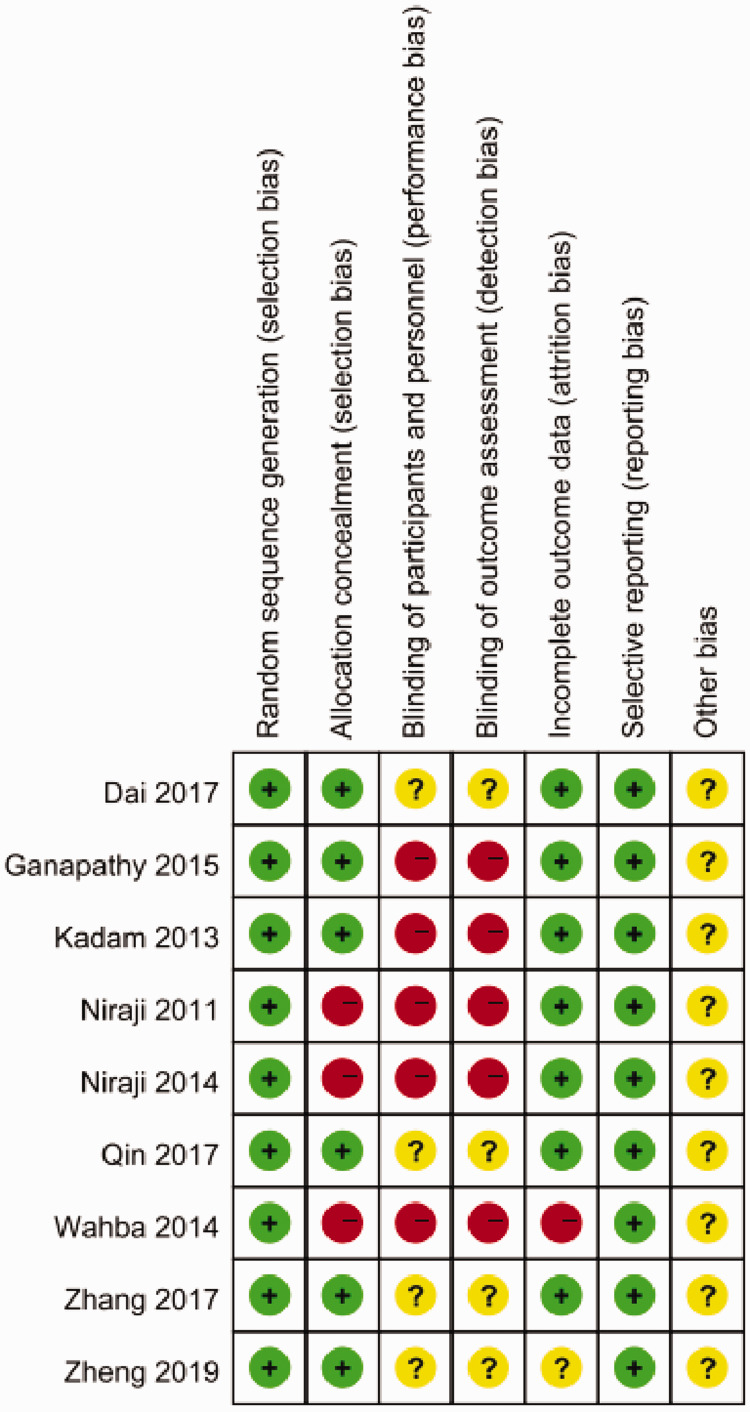
The following information was collected from these studies: (1) First authors’ names and publication year; (2) type of surgery and American Society of Anesthesiologists (ASA) status anesthesia methods; (3) numbers of patients (male/female); (4) postoperative analgesia technique; and (5) primary and secondary outcomes of these studies. Characteristics of the included studies are shown in Table 1. The risk of bias was assessed using the review authors’ judgments about each risk of bias item based on the Cochrane Collaboration Risk of Bias Tool. The risk of bias graph and risk of bias summary were presented as follows (Figure 2; Figure 3) . The risk of bias assessment was performed using Review Manager (RevMan) version 5.3.5 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014), and the studies were shown to have a high risk of bias regarding blinding of participants and assessors. However, these five studies were also included in a meta-analysis by Baeriswyl et al.,19 who had attempted to contact all the authors of these five studies, and two of them17,21 provided the data requested. Thus, we extracted these data from Baeriswyl et al.’s article directly.
Table 1.
Characteristics of the included studies.
| Study | Patients allocation (M/F) and age (years) | Surgery, ASA status, anesthesia technique and postoperative analgesia technique | TAP and epidural block methods | Outcomes |
|---|---|---|---|---|
| Ganapathy2015 | Group T 24 (8/16) Age 58±12.2 yearsGroup E 26 (10/16) Age 61.7±10.8 years | 1. Abdominal laparotomy;2. ASA I–III; 3. General anesthesia; 4. Acetaminophen, naproxen, gabapentin, and either TAPB PCA or epidural PCA | 1. Ultrasound-guided bilateral continuous block through the subcostal approach, 0.2% ropivacaine 30 mL followed by 0.35% ropivacaine 4–5 mL/hour for 72 hours.2. Epidural analgesia bupivacaine 0.25% 5 mL followed by 0.1% bupivacaine + hydromorphone 10 µg/mL, 8 mL/hours for 72 hours between T7 and T9. Boluses of hydromorphone were used as rescue analgesia. | 1. Primary outcome was the pain score on coughing 24 hours after surgery.2. Secondary outcomes were pain scores from 24 to 72 hours, intraoperative and postoperative opioid consumption, time to onset of bowel movement, and complications relating to analgesic techniques. |
| Niraj2011 | Group T 27 (18/9) Age 64±12 yearsGroup E 31 (20/11) Age 64±12 years | 1. Abdominal laparotomy; 2. ASA I–III; 3. General anesthesia4. Acetaminophen, tramadol IV and either TAPB PCA or epidural PCA | 1. Ultrasound-guided bilateral continuous block through the subcostal approach, 0.375% levobupivacaine 1.25 mg/kg followed by 0.25% levobupivacaine at a rate of 8–10 mL/hour for 72 hours.2. Epidural analgesia bupivacaine 0.25% 20 mL followed by 0.125% bupivacaine +fentanyl 2µg/mL, 8–12 mL/hour for 72 hours with a 2 mL bolus available every 30 minutes between T7 and T9. Boluses of morphine were used as rescue analgesia. | 1. Primary outcome was the pain score on coughing at 8, 24, 48, and 72 hours after surgery.2. Secondary outcomes were VAS score for pain at rest, postoperative nausea, tramadol usage, and patient satisfaction at 72 hours. |
| Niraj2014 | Group T 35 (19/16)Age 66.7±12.6 yearsGroup E 35 (22/13)Age 64.4±16.2 years | 1. Abdominal laparoscopic; 2. ASA I–III; 3. General anesthesia4. Acetaminophen, diclofenac, tramadol IVand either TAPB PCA or epidural PCA | 1. Ultrasound-guided bilateral continuous block through intercostoiliac approach, 0.375% ropivacaine 20 mL followed by ropivacaine 0.2% at a rate of 8 mL/hour for 72 hours.2. Epidural analgesia ropivacaine 0.2% 8–15 mL bolus followed by ropivacaine 0.2% , 5–15 mL/hour for 72 hours between T9 and T11.Boluses of morphine were used for rescue analgesia. | 1. The primary outcome was the VAS score for pain on coughing at 24 hours after surgery.2. Secondary outcome measures included visual analog pain scores at rest and on coughing, postoperative nausea scores, tramadol consumption, and patient satisfaction at 48 hours. |
| Kadam2013 | Group T 22 (14/8)Age 63.8±10.1 yearsGroup E 19 (14/5)Age 60.9±13.2 years | 1. Abdominal laparotomy; 2. ASA I–III; 3. General anesthesia; 4. Acetaminophen, IV PCA of fentanyl and either TAPB PCA or epidural PCA | 1. Ultrasound-guided bilateral continuous block through intercostoiliac approach, bupivacaine 0.25% 20 mL followed by a 15-mL bolus of bupivacaine 0.25% every 8 hours for 48 hours.2. Epidural analgesia bupivacaine 0.125% 10 mL followed by bupivacaine 0.125%, 6–8 mL/hours for 48 hours between T7 and T9.Boluses of fentanyl were used for rescue analgesia. | 1. Primary outcome was the VAS score for pain on coughing.2. Secondary outcomes were complications relating to analgesic techniques, rescue medication required, and patients’ satisfaction with analgesic techniques. |
| Wahba2014 | Group T 22 (11/11)Age 66.4±4.8 yearsGroup E 22 (10/12)Age 66.3±5.4 years | 1. Abdominal laparotomy; 2. ASA III; 3. General anesthesia4. IV PCA of morphine and either TAPB PCA or epidural PCA | 1. Ultrasound-guided bilateral continuous block through intercostoiliac approach, bupivacaine 0.25% 20 mL followed by a 15-mL bolus of bupivacaine 0.25% every 8 hours for 48 hours.2. Epidural analgesia bupivacaine 0.125% 10 mL followed by bupivacaine 0.125%, at a rate of 6 mL/hours for 48 hours between T9 and T11.Boluses of dezocine were used for rescue analgesia. | 1. The primary outcome was morphine consumption at day 1–2 and the time to first request for morphine.2. The secondary outcome was VAS score for pain on coughing and at rest, postoperative sedation, vital signs, and complications relating to analgesic techniques. |
| Zheng2019 | Group T 60 (0/60)Age 33.5±3.5 yearsGroup E 60 (0/60)Age 32.8±3.8 years | 1. Cesarean section delivery; 2. ASA I–II; 3. Combined spinal anesthesia4. Dolantin IV and either TAPB PCA or epidural PCA | 1. Ultrasound-guided bilateral continuous block through intercostoiliac approach, ropivacaine 0.375% 3 mL/kg followed by ropivacaine 0.15% at a rate of 2 mL/hour for 48 hours.2. Epidural analgesia ropivacaine 0.75% 1.5 mL followed by ropivacaine 0.15%, at a rate of 4 mL/hour for 48 hours between T9 and T11. | The observational index was the VAS score for pain 2 hours, 6 hours, 12 hours, 24 hours, 36 hours, and 48 hours after surgery, consumption of analgesics within 48 hours and complications relating to analgesic techniques. |
| Qin2017 | Group T 35 (22/13)Age 59.7±8.5 yearsGroup E 36 (21/15)Age 58.0±11.4 years | 1. Abdominal laparoscopic; 2. ASA I–III; 3. General anesthesia4. Either TAPB PCA or epidural PCA | 1. Ultrasound-guided bilateral continuous block through intercostoiliac approach, ropivacaine 0.375% 2.5 mg/kg followed by ropivacaine 0.2% at a rate of 6–8 mL/hour for 48 hours.2. Epidural analgesia lidocaine 2% 2 mL followed by a bolus of 1.5–2.5 mL ropivacaine 0.375% every 1–1.5 hours during surgery. Ropivacaine 0.15% at a rate of 4 mL/hour for 48 hours after surgery between T7 and T9. | The observational index was the VAS score for pain 2 hours, 6 hours, 12 hours, and 24 hours, 36 hours, and 48 hours after surgery, patients’ analgesic satisfaction within 48 hours and complications relating to analgesic techniques. |
| Dai2017 | Group T 29 (10/19)Age 52.9±10.8 yearsGroup E 30 (14/16)Age 56.1±12.0 years | 1. Abdominal laparoscopic; 2. ASA I–III 3. General anesthesia4. Either TAPB PCA or epidural PCA | 1. Ultrasound-guided bilateral continuous block through the intercostoiliac approach, ropivacaine 0.375% 0.3 mL/kg followed by a bolus of 0.1 mL/kg ropivacaine 0.375% every 2 hours. Ropivacaine 0.2% at a rate of 0.1 mL/kg/hour for 48 hours.2. Epidural analgesia ropivacaine 0.375% 8–10 mL followed by a bolus of 4–5 mL ropivacaine 0.375% every 1–1.5 hours during surgery. Ropivacaine 0.2% at a rate of 3–4 mL/hour for 48 hours after surgery between T9 and T11.Boluses of dezocine were used for rescue analgesia. | The observational index was the VAS score for pain 6 hours, 12 hours, 24 hours, and 48 hours after surgery, patients’ analgesic satisfaction within 48 hours and complications relating to analgesic techniques. |
| Zhang2017 | Group T 25 (0/25)Age 22–40 yearsGroup E 60 (0/60)Age 22–40 yearsGroup E+T 25 (0/25)Age 22–40 years | 1. Cesarean section delivery; 2. ASA I–II; 3. Combined spinal anesthesia; 4. Either TAPB PCA or epidural PCA | 1. Ultrasound-guided bilateral continuous block through the subcostal approach, ropivacaine 0.375% 200 mL followed by a 2-mL bolus of ropivacaine 0.375% every 15 minutes at a rate of 2 mL/hour for 48 hours. 2. Epidural analgesia with 2-mL bolus of ropivacaine 0.375% every 15 minutes at a rate of 2 mL/hour for 48 hours between T7 and T9. | The observational indexes were VAS, BCS, and Ramsay sedation score 2 hours, 6 hours, 12 hours, 24 hours, and 48 hours after surgery; consumption of analgesics within 48 hours; and complications relating to analgesic techniques. |
Group T: Patients underwent continuous transversus abdominis plane block; Group E: Patients underwent continuous epidural analgesic.
Group E+T: Patients underwent a single shot of TAPB and continuous epidural analgesic.
TAPB, transversus plane block; PCA, patient-controlled analgesic; PCIA, patient-controlled intravenous analgesic; IV, intravenous; ASA, American Society of Anesthesiologists; BCS: Bruggrmann Comfort Scale.
Figure 2.
Risk of bias graph for the included studies.
Figure 3.
Risk of bias summary for the included studies.
Outcomes and data synthesis
The primary outcome of our study was the VAS score for pain at rest on postoperative day 1, and the secondary outcomes were the incidence of VAS scores for pain at rest on postoperative day 2, VAS scores for pain at movement on postoperative day 1 and day 2, length of hospital stay, time to ambulation, the incidence of hypotension, nausea, and sensorimotor disorder within 48 hours after surgery. The median and interquartile range were used for mean and standard deviation approximations, as follows: the mean was estimated as being equivalent to the median and the standard deviation was approximated to be the interquartile range divided by 1.35, or the range divided by 4.23 We also performed a subgroup analysis by classifying patients as Chinese or non-Chinese.
We pooled data from all these studies and calculated the relative risk (RR) and the 95% confidence interval (CI) for all the dichotomous outcomes and weighted mean differences for continuous data using RevMan 5.3. Estimate of RRs and mean differences were performed using a random-effects model, which provides an appropriate estimate of the data with a relatively wide range of CI when the outcomes are statistically heterogeneous. We conducted heterogeneity tests to estimate inconsistencies across all these studies using Q test and I2 test. Publication bias was conducted by using a funnel plot to determine whether there was a bias toward publication. Sensitivity analyses were performed using RevMan 5.3 to estimate any impact of study quality on the outcome.
Results
Among the nine RCTs that were included 17,18,20,21,24–28, two focused on cesarean section surgery,20,24 while the other seven focused on gastrointestinal surgery.17,18,21,25–28 Four studies included Chinese patients20,24–26 and the other five studies included non-Chinese patients.17,18,21,27,28 All authors combined the postoperative analgesia with general anesthesia except for two studies.20,24
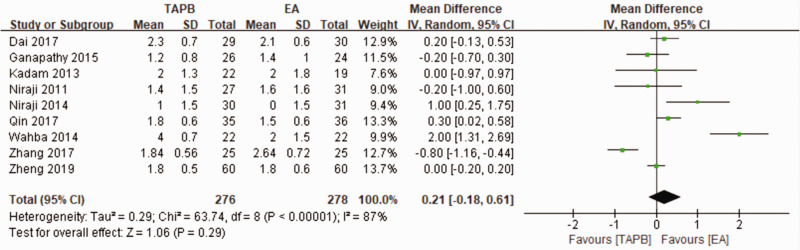
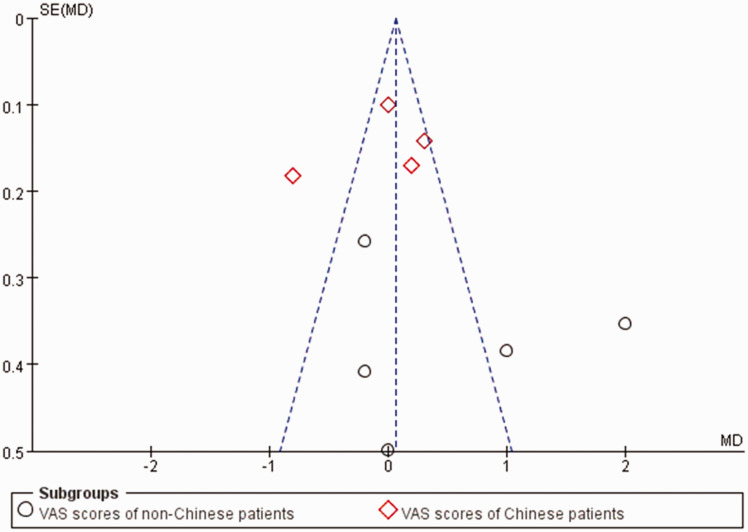
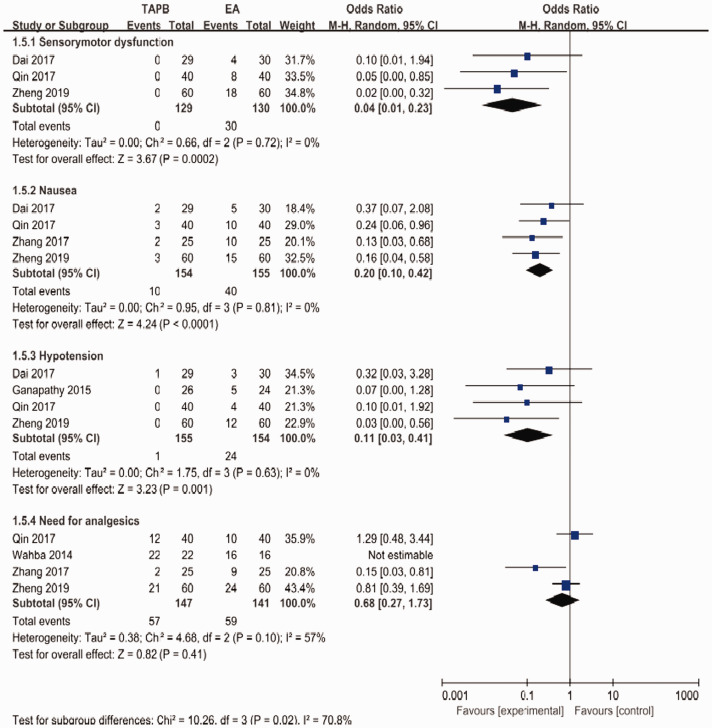
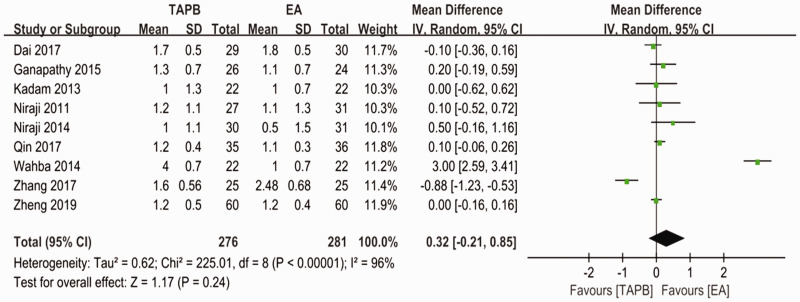
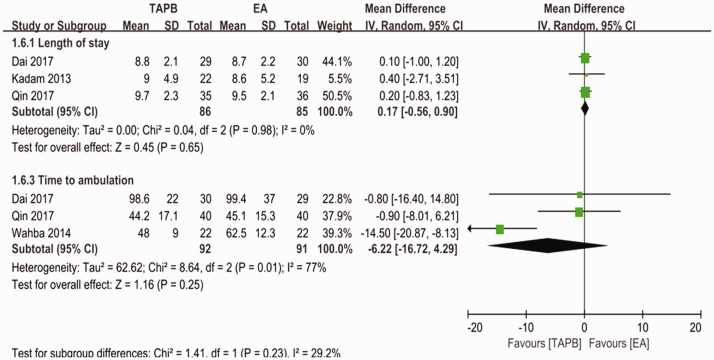
For the primary outcome, pain levels measured using VAS scores at rest on postoperative day 1 were equivalent for the continuous TAPB and continuous EA groups in non-Chinese patients (mean difference: 0.53; 95% confidence interval [CI]: −0.37 to 1.42; I2 = 87%) and in Chinese patients (mean difference: −0.06; 95% CI: −0.47 to 0.34; I2 = 88%) (Figure 4). We used a funnel plot to evaluate the publication bias. A slightly nonsymmetrical funnel plot (Figure 5) showed a publication bias may exist. The subgroup analysis cannot account for the existing heterogeneity, while the following sensitivity analysis has shown the source of the heterogeneity. For the secondary outcomes, other side effects related to analgesia techniques or complications after surgery were also assessed carefully and reported as secondary outcomes, which showed that the TAPB group experienced a lower rate of hypotension (relative risk: 0.11; 95% CI: 0.03 to 0.41; I2 = 0%; p = 0 .001), sensorimotor disorder (relative risk: 0.04; 95% CI: 0.01 to 0.23; I2 = 0%; p = 0 .0002), and nausea (relative risk: 0.2; 95% CI: 0.10 to 0.42; I2 = 0%; p < 0 .0001) compared with the continuous EA group within 48 hours after surgery (Figure 6). Other secondary outcomes showed no significant differences between the two groups (Figure 7; Figure 8; Figure 9).
Figure 4.
Forest plot of comparison between TAPB and EA for the VAS scores at rest on postoperative day 1.
TAPB, transversus abdominis plane block; EA, epidural analgesia; VAS, visual analog scale.
Figure 5.
Funnel plot to evaluate the publication bias.
Figure 6.
Forest plot to compare between TAPB and EA for sensorimotor dysfunction, nausea, hypotension, and the need for analgesics.
TAPB, transversus abdominis plane block; EA, epidural analgesia.
Figure 7.
Forest plot to compare between TAPB and EA for VAS scores at rest on postoperative day 2.
TAPB, transversus abdominis plane block; EA, epidural analgesia; VAS, visual analog scale.
Figure 8.
Forest plot to compare between TAPB and EA for length of hospital stay and time to ambulation.
TAPB, transversus abdominis plane block; EA, epidural analgesia.
Figure 9.
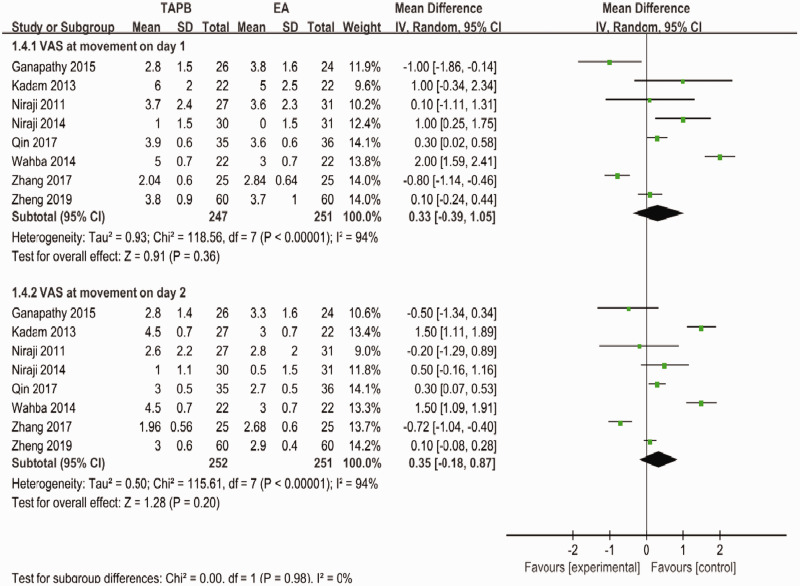
Forest plot to compare between TAPB and EA for VAS scores at movement on days 1 and 2.
TAPB, transversus abdominis plane block; EA, epidural analgesia; VAS, visual analog scale.
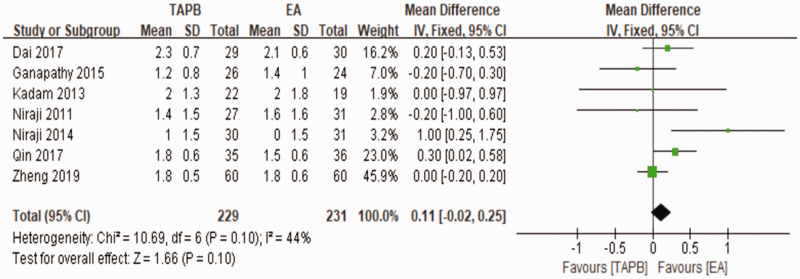
A sensitivity analysis was performed to investigate the source of the existing heterogeneity. We excluded one or two studies each time and reanalyzed the rest to see whether the I2 change significantly compared with the original analysis. A significant change in I2 was seen when we excluded Wahba and Kamal28 and Zhang et al.,20 and the result of the primary outcome showed a mean difference of 0.14 (95% CI: −0.08 to 0.35; I2 = 44%) (Figure 10).
Figure 10.
Sensitivity analysis for the primary outcome.
Discussion
Kadam and Field16 showed that continuous TAPB resulted in less rescue analgesic consumption and lower VAS pain scores after abdominal surgery. In 2011, Niraj et al.17 showed that intermittent TAPB boluses resulted in no obvious advantage compared with intermittent EA boluses after abdominal surgery. Thus, there has been no clear conclusion until now.
Our study systematically analyzed the analgesic efficacy and side effects of continuous TAPB compared with continuous epidural analgesia in patients undergoing abdominal surgery. Based on the nine RCTs with 598 participants, the outcomes showed that both techniques that were used in patients who underwent abdominal surgery were associated with equivalent VAS pain scores at rest or movement on postoperative days 1 and 2, rescue consumption of narcotics, length of hospital stay, and time to ambulation, while side effects related to analgesia techniques such as nausea, hypotension, and sensorimotor disorders were significantly reduced in the continuous TAPB group compared with the EA group. There are several studies that investigated single-shot TAPB and EA for postoperative analgesia, but dynamic analgesia after surgery is a crucial factor for Enhanced Recovery after Surgery (ERAS).29 Thus, from this point of view, we strongly recommended using continuous TAPB for postoperative analgesia.
Continuous TAPB, which has a safe and effective profile, is widely used to block the anterolateral abdominal wall nerve and the lower intercostals nerve to provide postoperative analgesia for patients. In addition, continuous epidural nerve block produces a continuous analgesic effect by administering local anesthetic continuously into the epidural cavity to block the nerve root. The causes of postoperative hypotension, sensorimotor disorder, and nausea in the two groups were analyzed, which may be related to the continuous amount of local anesthetic and the use of extra analgesic drugs in the two groups. However, there are no techniques without drawbacks. Both techniques have a failure or inadequate analgesia rate of around 30%.4,17 Ultrasound-guided continuous TAPB is always difficult to perform in basic level hospitals while continuous EA has rare but life-threatening complications such as spinal hematoma and damage to the spinal cord.30 The benefits and risks of both techniques should be weighed under certain conditions, and the technique that is more beneficial to the patients should be chosen.
It should be emphasized that there are inconsistencies between the included studies, which contributed to the existing heterogeneity. For the different anesthetic techniques, patients’ conditions, and surgery types, these factors all increased the heterogeneity, which we cannot control.
Limitations
There were some limitations in our study. First, the total number of patients in these nine RCTs is relatively small, but the clear and practical search strategies for comprehensive searches of five official databases, definite inclusion and exclusion criteria, and rigorous consideration of data may compensate for this limitation. Second, the existing publication bias may be a threat to the quality of our meta-analysis. Third, some observational indexes, such as time to first bowel sound and time to passing flatus, are also important factors that may impact the patients’ prognosis, but only a few articles on postoperative analgesia have reported these observational indexes among patients with continuous TAPB and continuous EA. Thus, further studies should focus on these observational indexes. Fourth, when we performed our study, we wanted to complete a subgroup analysis based on different medications that were used in different studies. However, we found that the medications for TAPB and EA were different among the included studies, and thus, we could not perform this subgroup analysis. The use of different medications may have resulted from the physicians’ personal preferences. Fifth, the type and amount of postoperative opioid consumption is an important parameter because it may also have an impact on the VAS score, and we thought about this question when we were performing our study. However, we found that the opioids that were consumed postoperatively varied significantly among different studies because of the different types of surgery or possibly because of the physicians’ personal preferences. For example, we included studies that reported about patients who underwent cesarean section and laparoscopic surgery, and the postoperative pain level is likely different among patients who underwent these two types of surgery. Thus, opioid consumption after surgery was significantly different.
Conclusion
The results of this meta-analysis showed that both continuous TAPB and continuous EA could provide equivalent analgesia in patients after abdominal surgery. Because patients in the continuous TAPB group experienced a lower rate of hypotension, sensorimotor disorder, and nausea within 48 hours after abdominal surgery, we recommend the continuous TAPB technique for postoperative analgesia after abdominal surgery, if possible.
Acknowledgements
We thank Xiangbo Liu and Fei Peng for screening the articles, data synthesis, and data collection; Xiangbo Liu, Fei Peng, and Guo Mu for their assistance with the study design and preparing the primary manuscript; and Cehua Ou for his funding support and help with editing this article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
Our study was supported by the Foundation of Health Commission of SiChuan Province (16PJ567).
ORCID iD
References
- 1.Moraca RJ, Sheldon DG, Thirby RC. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg 2003; 238: 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawal N. Epidural technique for postoperative pain: Gold standard no more? Reg Anesth Pain Med 2012; 37: 310–317. [DOI] [PubMed] [Google Scholar]
- 3.Ready LB. Acute pain: Lessons learned from 25,000 patients. Reg Anesth Pain Med 1999; 24: 499–505. [DOI] [PubMed] [Google Scholar]
- 4.Varadhan KK, Neal KR, Dejong CH, et al. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: A meta-analysis of randomized controlled trials. Clin Nutr 2010; 29: 434–440. [DOI] [PubMed] [Google Scholar]
- 5.Rafifi AN. Abdominal field block: A new approach via the lumbar triangle. Anaesthesia 2001; 56: 1024–1026. [DOI] [PubMed] [Google Scholar]
- 6.Baeriswyl M, Kirkham KR, Kern C, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane block in adult patients: A meta-analysis. Anesth Analg 2015; 121: 1640–1654. [DOI] [PubMed] [Google Scholar]
- 7.Walter CJ, Maxwell-Armstrong C, Pinkney TD, et al. A randomised controlled trial of the efficacy of ultrasound-guided transversus abdominis plane (TAP) block in laparoscopic colorectal surgery. Surg Endosc 2013; 27: 2366–2372. [DOI] [PubMed] [Google Scholar]
- 8.McDonnell JG, O’Donnell B, Curley G, et al. The analgesic efficacy of transversus abdominis block after abdominal surgery: A prospective randomized controlled trial. Anesth Analg 2007; 104: 193–197. [DOI] [PubMed] [Google Scholar]
- 9.Niraj G, Searle A, Mathews M, et al. Analgesic efficacy of ultrasound-guided transversus abdominis plane block in patients undergoing open appendectomy. Br J Anaesth 2009; 103: 601–605. [DOI] [PubMed] [Google Scholar]
- 10.Belavy D, Cowlishaw PJ, Howes M, et al. Ultrasound-guided transversus abdominis plane block for analgesia after Caesarean delivery. Br J Anaesth 2009; 103: 726–730. [DOI] [PubMed] [Google Scholar]
- 11.Abdallah FW, Chan VW, Brull R. Transversus abdominis plane block: A systematic review. Reg Anesth Pain Med 2012; 37: 193–209. [DOI] [PubMed] [Google Scholar]
- 12.Carney J, McDonnell JG, Ochana A, et al. The transversus abdominis plane block provides effective postoperative analgesia in patients undergoing total abdominal hysterectomy. Anesth Analg 2008; 107: 2056–2060. [DOI] [PubMed] [Google Scholar]
- 13.El-Dawlatly AA, Turkistani A, Kettner SC, et al. Ultrasound-guided transversus abdominis plane block: description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. Br J Anaesth 2009; 102: 763–767. [DOI] [PubMed] [Google Scholar]
- 14.Hebbard P, Fujiwara Y, Shibata Y, et al. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care 2007; 35: 616–617. [PubMed] [Google Scholar]
- 15.McDonnell JG, Curley G, Carney J, et al. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: A randomized controlled trial. Anesth Analg 2008; 106: 186–191. [DOI] [PubMed] [Google Scholar]
- 16.Kadam VR, Field JB. Ultrasound-guided continuous transverse abdominis plane block for abdominal surgery. J Anaesthesiol Clin Pharmacol 2011; 27: 333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niraj G, Kelkar A, Jeyapalan I, et al. Comparison of analgesic efficacy of subcostal transversus abdominis plane blocks with epidural analgesia following upper abdominal surgery. Anaesthesia 2011; 66: 465–471. [DOI] [PubMed] [Google Scholar]
- 18.Niraj G, Kelkar A, Hart E, et al. Comparison of analgesic efficacy of four-quadrant transversus abdominis plane (TAP) block and continuous posterior TAP analgesia with epidural analgesia in patients undergoing laparoscopic colorectal surgery: An open-label, randomised, non-inferiority trial. Anaesthesia 2014; 69: 348–355. [DOI] [PubMed] [Google Scholar]
- 19.Baeriswyl M, Zeiter F, Piubellini D, et al. The analgesic efficacy of transverse abdominis plane block versus epidural analgesia: A systematic review with meta-analysis. Medicine (Baltimore) 2018; 97: e11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang GL, Rn Y, Meng MH, et al. Effect of ultrasound-guided continuous transverse abdominis block on postoperative pain after cesarean section. Shandong Medical Journal 2017; 57: 84–86. (in Chinese) DOI: 10.3969/j.issn.1002-266X.2017.37.029. [Google Scholar]
- 21.Kadam VR, Van Wijk RM, Moran JL, et al. Epidural versus continuous transversus abdominis plane catheter technique for postoperative analgesia after abdominal surgery. Anaesth Intensive Care 2013; 41: 476–481. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009; 339: b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 24.Zheng JL, Zhong Q. Ultrasound-guided continuous transversus abdominis plane block and continuous epidural analgesia after cesarean section. Journal of Military Surgeon in Southwest China 2019; 21: 31–35. (in Chinese) DOI: 10.3969/j.issn.1672-7193.2019.01.010. [Google Scholar]
- 25.Qin CS, Lin YN, Liu JC. Ultrasound-guided continuous transversus abdominis plane block in patients undergoing laparoscopic colorectal ultrasound-guided continuous transverse abdominal muscle block in laparoscopic colorectal surgeries. Journal of Minimally Invasive Medicine 2016; 11: 664–668. (in Chinese) DOI: 10.11864/j.issn.1673.2016.05.02. [Google Scholar]
- 26.Dai Y, Sun XD, Liu JC. Comparison of analgesic efficacy of transversus abdominis plane block and epidural analgesia after laparoscopic colorectal surgery. Journal of Guangxi Medical University 2017; 34: 1213–1216. (in Chinese) DOI: 10.16190/j.cnki.45-1211/r.2017.08.027. [Google Scholar]
- 27.Ganapathy S, Sondekoppam RV, Terlecki M, et al. Comparison of efficacy and safety of lateral-to-medial continuous transversus abdominis plane block with thoracic epidural analgesia in patients undergoing abdominal surgery: A randomised, open-label feasibility study. Eur J Anaesthesiol 2015; 32: 797–804. [DOI] [PubMed] [Google Scholar]
- 28.Wahba SS, Kamal SM. Analgesic efficacy and outcome of transversus abdominis plane block versus low thoracic-epidural analgesia after laparotomy in ischemic heart disease patients. J Anesth 2014; 28: 517–523. [DOI] [PubMed] [Google Scholar]
- 29.Elhassan A, Elhassan I, Elhassan A, et al. Perioperative surgical home models and enhanced recovery after surgery. J Anaesthesiol Clin Pharmacol 2019; 35: S46–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pumberger M, Memtsoudis SG, Stundner O, et al. An analysis of the safety of epidural and spinal neuraxial anesthesia in more than 100,000 consecutive major lower extremity joint replacements. Reg Anesth Pain Med 2013; 38: 515–519. [DOI] [PubMed] [Google Scholar]