Abstract
The objective of this study was to investigate the feasibility of using sonoelastography to depict Achilles tendon stiffness after platelet-rich plasma injection and eccentric exercise for chronic Achilles tendinopathy, and to correlate sonoelastography findings with clinical outcome up to 12 months after treatment. Forty-five Achilles tendons from 45 patients (33 males, 12 females; mean age 51 years) were examined using sonoelastography and ultrasound at baseline, 4–6 weeks, 6 months and 12 months post-treatment. The strain ratio (between Achilles tendon and Kager's fat) during sonoelastography was obtained. The proportion of tendons with hypoechogenicity and neovascularity were documented. Clinical outcomes were assessed by the Victorian Institute of Sport Assessment-Achilles questionnaire and correlated with sonographic findings. The Victorian Institute of Sport Assessment-Achilles improved significantly from 38.4 (±14.1) at baseline, 77.2 (±12.5) at 6 months (p < 0.001) to 81.2 (±10.8) at 12 months (p < 0.001). The strain ratio values were 2.16 (±1.02) at baseline, 2.03 (±0.67) at 4–6 weeks, 1.81 (±0.62) at 6 months and 1.19 (±0.34) at 12 months with a significant reduction observed at 6 months (p = 0.006) and 12 months (p < 0.001). At 12-month evaluation, none of the tendons regained a normal echotexture. Strain ratio demonstrated a moderately good inverse correlation with Victorian Institute of Sport Assessment-Achilles (r = −0.610, p<0.001) while B-mode and Doppler ultrasound did not show a significant correlation (r = −0.041, p = 0.817, and r = −0.116, p = 0.514). Achilles tendon stiffness shows moderately good correlation with clinical symptom at 12-month post-treatment. Sonoelastography using strain ratio could be a promising ancillary tool for monitoring Achilles tendon healing after treatment.
Keywords: Sonoelastography, ultrasound, tendinopathy, tendon stiffness, treatment
Introduction
Achilles tendinopathy is a common and often debilitating condition, reportedly accounting for 5–18% of all running injuries in the athletic population.1 Histologically, it is characterized by collagen fibre disorganization, mucoid and lipoid degeneration, as well as extensive cellular, vascular and neural proliferation.2 These alterations are believed to reduce the elasticity and stiffness of the tendon, and compromise the load-bearing capacity. This may ultimately lead to partial tearing or complete rupture.3 A number of treatment options are currently available, ranging from rehabilitation regimens to surgery. Among these, the local injection of platelet-rich plasma (PRP) and eccentric loading exercise programme are emerging treatments. Several available clinical studies have evaluated changes in tendon morphology and vascularity using ultrasound (US) after PRP injections. Results have shown only little or no structural improvement at short-to-midterm (6–14 months) follow-up while the data on tendon vascularity changes are conflicting.4–6 Furthermore, these variables demonstrated poor correlation with clinical outcome. In a two-year prospective study evaluating the diagnostic and prognostic value of US and magnetic resonance (MR) imaging in the assessment of Achilles tendon disorders, Khan et al.7 reported that conventional US is not useful in monitoring Achilles tendon healing as US abnormalities persist even after patients have made good functional recovery. The authors concluded that MR imaging can be an effective alternative to conventional US. However, MR imaging is costly and of limited availability.
Sonoelastography (SE) is a non-invasive US-based technique that evaluates the mechanical properties of tissues. SE is based on the principle that tissue displacement in response to external compression produces ‘strain’ within the tissue and that the strain is smaller in harder tissue than in softer tissue.8 Recent studies reported that SE supplement with strain ratio can quantitatively evaluate tendon stiffness and thereby discriminate symptomatic and asymptomatic Achilles tendons.9,10 In addition, evaluation of Achilles tendon ruptures revealed significantly increased softness in areas of fibre disruption that became progressively stiffer with healing.11,12 These studies suggest a potential application of SE for the diagnosis and monitoring of treatment of Achilles tendon injuries. The primary objective of this study was to evaluate whether SE can be used to evaluate Achilles tendon stiffness following percutaneous PRP injection and exercise therapy. The secondary objective was to correlate the SE findings with clinical outcome at 12 months after treatment.
Materials and methods
This study was performed in accordance with the Declaration of Helsinki. The study was approved by Monash University Human Research Ethics Committee – approval: CF12/3597 – 2012001708. Written informed consent was obtained from each participant. Between January 2013 and January 2014, consecutive patients who were referred to our centre for PRP treatment of mid-substance Achilles tendinopathy were enrolled. All patients had previously undergone conservative treatments such as non-steroidal anti-inflammatory drugs, laser therapy, shock wave, local administration of corticosteroids with unsatisfactory outcome (i.e. six months of persisting symptoms after treatment). Suitability for inclusion was evaluated by referring clinician and musculoskeletal radiologist. The diagnosis of mid-substance Achilles tendinopathy comprised a history of increasing pain on loading activities for a minimum duration of six months, pain on palpation at a level 2–6 cm above the Achilles tendon insertion, and confirmation on US of local tendon thickening (maximum antero-posterior (AP) thickness in transverse plane > 6 mm) with hypoechogenicities and irregular fibre orientation.13 In patients who presented with bilateral symptoms, the more severely affected side was examined. Patients with systemic disease, partial tear (discrete intratendinous defect), full-thickness tear or complete rupture of the Achilles tendon were excluded. Additionally, patients who sustained a previous rupture or had undergone surgery on the Achilles tendon were excluded. At baseline, demographic and anthropometric data (gender, age, BMI) were obtained.
Pre-treatment evaluation
All participants were asked to complete the Victorian Institute of Sport Assessment for Achilles (VISA-A) questionnaire. The VISA-A questionnaire is a validated clinical rating scale that evaluates the symptoms and dysfunction of the Achilles tendon.14 The VISA-A scores range from 0 to 100. The perfect VISA-A score for an asymptomatic individual is 100 points, denoting maximum activity and no pain while 0 denoting no activity and maximum pain or impairment. A lower score indicates greater severity of Achilles tendinopathy.
Sonographic assessments (B-mode US, colour Doppler (CD) imaging and SE) were performed using the Philips iU22 US scanner (Philips Healthcare, Bothell, WA) equipped with a L17-5 MHz high resolution linear transducer. Longitudinal and transverse B-mode US scans of the affected Achilles tendon were performed. A standardized scanning protocol with optimized B-mode scanning parameters such as depth, frequency and focal zone was used to ensure reproducibility of results obtained. The maximum AP thickness of the Achilles tendon was measured in the transverse plane.15 The measurements were repeated once (two sets of readings were obtained for each parameter) and the mean used in the analyses. For the purpose of reproducibility, the distance between the thickest point of the Achilles tendon and its insertion on calcaneus was recorded. CD US was used to examine intratendinous vascularity using low pulse repetition frequencies (455 Hz) and low wall filter settings (36 Hz) to detect low velocity blood flow. The CD gain was set marginally below the artefact threshold.16 The B-mode US and CD imaging abnormalities were graded according to the previously validated criteria (Table 1).6,17
Table 1.
B-mode ultrasound and colour Doppler imaging grading system
| Imaging mode | Grading scale |
|---|---|
| B-mode ultrasound6 | |
| Normal | Parallel fibres with homogeneous echogenicity of the Achilles tendon |
| Mild | Disorganized, hypoechogenicity in less than one-third of the Achilles tendon |
| Moderate | Disorganized, hypoechogenicity in between one-third and two-thirds of the tendon |
| Severe | Disorganized, hypoechogenicity in greater than two-thirds of the tendon |
| Colour Doppler ultrasound17 | |
| 0 | No visible intratendinous vessels |
| 1+ | One or two small intratendinous vessels |
| 2+ | Several (more than two) irregular vessels throughout the tendon |
| 3+ | Florid vascularity (neovessels present in more than half of the Achilles tendon included in the sampling box) |
The SE was performed by applying light repetitive compression over the Achilles tendon (with the hand-held transducer) in the longitudinal plane. We measured the relative stiffness along the tendon fibres in the longitudinal plane instead of across the fibres in the transverse plane as the longitudinal plane has been proven to be more reproducible than the transverse plane.18 Transducer pressure was applied vertically, perpendicular to the Achilles tendon and was adjusted according to the real-time visual indicator for compression on the right side of the screen (a green compression bar indicates quality sonoelastogram with appropriate tissue deformation, whereas a grey bar denotes excessive or too little pressure). The availability of this pressure indicator gives instant feedback to the operator regarding the amount of pressure applied. This helps to reduce variability and ensure consistency during image acquisition. For each Achilles tendon, at least three sonoelastograms were obtained by the operator. The strain ratio (strain value of the Achilles tendon to the adjacent Kager's fat) was measured on the longitudinal plane of the middle Achilles tendon section where Kager's fat is present, using a manually applied ellipsoid region of interest (ROI) (Figure 1). The ROI was first placed in the centre of the Achilles tendon. An equally sized area of Kager's fat (reference tissue or denominator) was then included and the strain ratio was determined using the QLab analysis software.18 The strain ratio was calculated in triplicate for each tendon and the mean used in analyses. In addition to semi-quantitative strain ratio measurement, the mean pixel intensity of the colour histograms for each Achilles tendon sonoelastogram was analysed using the method described previously by Wu et al.19 The ImageJ software (version 1.42q; National Institutes of Health, Bethesda, MD, USA) was used. A ROI was selected (using the freehand selection tool) with its border covering the Achilles tendon in the field of view. In the program, each pixel was separated into red, green and blue components (colour intensity range 0–255). The program calculated the mean pixel values of each colour components within the ROI. For each Achilles tendon, the mean values of the red, green and blue components, respectively, in the three selected sonoelastograms were used for analysis. The higher value defined a greater colour intensity.
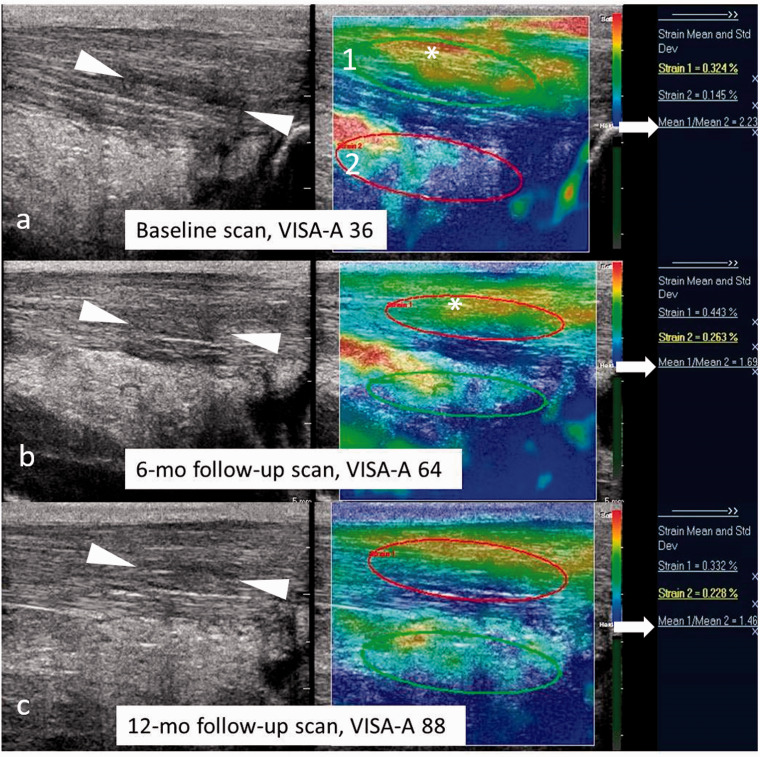
Figure 1.
Baseline, 6- and 12-month follow-up US and sonoelastogram of chronic Achilles tendinopathy in a 58-year-old male treated with PRP injection. (a) Longitudinal B-mode US (left image) at baseline shows thickened tendon with hypoechoic areas of mucoid degeneration and intratendinous delamination (arrowheads). Corresponding sonoelastogram (right) displays red-yellow color-coded tendon consistent with softening of the Achilles tendon fibres (asterisk). The strain ratio (Achilles tendon strain, 1/Kager's fat strain, 2) was calculated as 2.23 (arrow). (b) Longitudinal B-mode US (left image) at six months shows partial resolution of the hypoechoic areas seen at baseline US (arrowheads in (b)). Corresponding sonoelastogram (right image) reveals area of soft tendinopathy on the baseline sonoelastogram has reduced and area of intermediate stiffness (yellow-green, asterisk) is present, likely consistent with stiffer, scar tissue formation during the healing process. The strain ratio measured 1.69 (arrow). (c) Longitudinal B-mode US (left image) at 12 months shows persistent thickening of the Achilles tendon with intratendinous hypoechogenicities (arrow heads) despite good clinical recovery of the patient (VISA: 88). There was a further reduction in strain ratio score (1.46, arrow) corresponding with an improvement in clinical outcome. VISA-A: Victorian Institute of Sport Assessment for Achilles.
PRP preparation and administration
Approximately 10 ml of blood was aspirated from the brachial vein at the antecubital fossa to fill a collecting tube (Sarstedt, citrate containing). The collecting tube underwent 7 minutes of centrifugation at 2000 r/min. Thereafter, a 3 ml syringe was filled with the buffy coating and plasma supernatant for injection. A 25G needle was used to inject 2 ml of lignocaine into the subcutaneous tissues. Using longitudinal US guidance, a 5 cm 22G needle was passed into the deep surface of the Achilles. The needle was withdrawn and simultaneously gentle pressure exerted on the syringe to fill the hypoechoic, tendinopathic areas. Multiple passes were made until the syringe was empty.
Patients were advised to minimize physical activity for 48 hours after treatment and to avoid full loading of the affected limb during the following two weeks. Thereafter, a rehabilitation programme composed of eccentric training and stretching (with extended knees and flexed knees) as described by Alfredson et al.20 was recommended daily for at least three months, during which a gradual return to sport or routine activity was encouraged.
Follow-up
All the patients were scheduled for follow-up at 4–6 weeks, 6 months and 12 months after the initial injection. At follow-up, the VISA-A questionnaire and sonographic assessment were repeated. Sonographic changes in Achilles tendon thickness, echotexture, vascularization and pain and VISA-A functional scores were recorded. An improvement in VISA-A of at least 25 points from baseline was considered clinically significant.7 A second injection was given at 4–6 weeks following the initial treatment. Patients were offered a third injection at six-month follow-up if there was no significant improvement in clinical symptoms (i.e. <25 points improvement in VISA-A scores).
Injection procedures were performed by the experienced musculoskeletal radiologist (DC). Sonographic examinations were performed by a board certified sonographer (CCO) with more than 10 years' experience in musculoskeletal US imaging and three years' experience in SE. Both the radiologist and sonographer were blinded to the VISA-A results. The static and cine loop images were documented on digital video disks and interpreted by the same sonographer three months after the final image acquisition (to reduce possible recall bias).
Statistical analysis
Statistical analyses were performed using SPSS version 21.0 software package (SPSS Inc., Chicago, Illinois, USA). Descriptive statistics were used to summarize the characteristics of the participants, including mean and standard deviations for the continuous variables and counts and percentages for the categorical variables. The paired sample t-test was used to compare the continuous, normally distributed data such as Achilles tendon thickness, strain ratio scores and red/green/blue pixels intensity at each follow-up time point in relation to baseline, while the Wilcoxon's sign rank test was used to analyse the changes in VISA-A scores over time. The association between the categorical data (the proportion of B-mode and CD tendon grading) at each time point was compared using the Chi square test. The correlation between the sonographic findings and VISA-A scores at 12-month follow-up was assessed using the Spearman's correlation coefficient. For all analysis, a p-value of < 0.05 was considered statistically significant.
Results
Forty-five patients (33 males, 12 females), mean age 51 (±10.3; range 31–74) years were entered into the study. The mean symptom duration was 25.5 (±15.5; range 6–60) months. All patients were assessed at 4–6 weeks' follow-up. At 12-month follow-up, 34 of the 45 (75.6%) patients were available for evaluation. The progress of the patients in the study is shown in Figure 2. Twenty-eight patients had two injections while six others had three injections (mean number of injections 2.18).
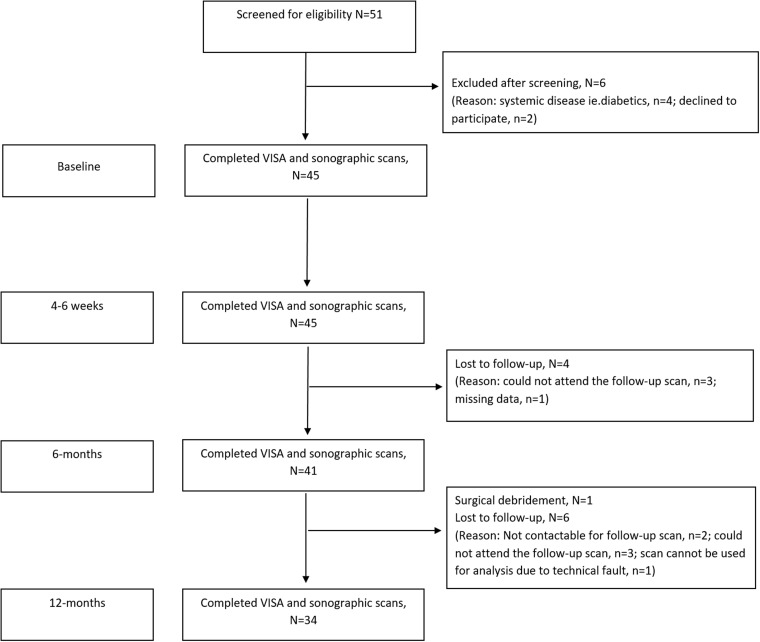
Figure 2.
Flow diagram of the study participant through the study. At baseline, 45 patients were entered into the study. All the patients were evaluated at 4–6 weeks' time point. Four patients were lost to follow-up at 6-month post-injection while another six were lost to follow-up at the 12-month time point. One patient underwent surgical debridement of degenerative Achilles tendon tissue after the 6-month follow-up. Therefore, 34 of the 45 patients were analysed at the final (12 months) evaluation. VISA-A: Victorian Institute of Sport Assessment for Achilles.
The VISA-A improved significantly from 38.4 (±14.1) at baseline, 77.2 (±12.5) at 6 months (p < 0.001) to 81.2 (±10.8) at 12 months (p < 0.001). The strain ratio values were 2.16 (±1.02) at baseline, 2.03 (±0.67) at 4–6 weeks, 1.81 (±0.62) at 6 months and 1.19 (±0.34) at 12 months with a significant reduction observed at 6 months (p = 0.006) and 12 months (p < 0.001) (Figure 3).
Figure 3.
Line chart showing the progression of clinical (Victorian Institute of Sport Assessment-Achilles, VISA-A) and sonoelastographic (SE) data (strain ratio scores) over 12-month post therapy. SE using strain ratio demonstrates progressive stiffening (lower strain ratio scores) of the treated Achilles tendons correlating with improvement in clinical outcome (higher VISA-A) up to 12-month time point. VISA-A: Victorian Institute of Sport Assessment for Achilles.
At the 12-month evaluation, none of the tendons regained normal echotexture. Neovessels were present in 14 Achilles tendons (14/34: 41.2%) despite a significant improvement in VISA-A scores. The strain ratio demonstrated a significant, moderately good correlation with VISA-A (r = −0.610, p < 0.001) while B-mode and CD US did not show a significant correlation (r = −0.041, p = 0.817, and r = −0.116, p = 0.514).
The variations in VISA-A, strain ratio scores, tendon AP thickness and sonoelastogram pixel values (red, green and blue colour components) between baseline and each time points are presented in Table 2. There was a significant reduction in red colour pixels (soft tendinopathic areas) and increase in blue colour pixels (stiff areas) at 6 months (p = 0.003, p < 0.001, respectively) and 12 months (p < 0.001 for both red and blue pixels) time points compared to baseline. At baseline, all tendons showed B-mode US abnormalities (Table 3) while intratendinous vascularity was present in 95.6% (43/45) of tendons (Table 4). The proportion of Achilles tendons with neovascularity was significantly reduced at 6 months (p < 0.001) and 12 months (p < 0.001) whereas a significant change in the distribution of tendons with hypoechogenicity was only observed at 12 months in comparison to baseline (p < 0.001).
Table 2.
Clinical, ultrasound and sonoelastography findings in patients treated with ultrasound-guided platelet-rich plasma injection at different time points
| Variable | Baseline | 4–6 weeks | 6 months | 12 months | p-valuea | p-valueb | p-valuec |
|---|---|---|---|---|---|---|---|
| VISA-A* | 38.4 (± 14.1) | 40.6 (± 12.7) | 77.2 (± 12.5) | 81.2 (± 10.8) | 0.065 | <0.001 | <0.001 |
| AP thickness (cm) | 0.94 (± 0.27) | 0.92 (± 0.26) | 0.88 (± 0.26) | 0.86 (± 0.25) | 0.093 | 0.003 | <0.001 |
| Strain ratio | 2.16 (± 1.02) | 2.03 (± 0.67) | 1.81 (± 0.62) | 1.19 (± 0.34) | 0.089 | 0.006 | <0.001 |
| Achilles tendon red | 97.2 (± 14.0) | 95.3 (± 13.6) | 92.6 (± 17.2) | 83.06 (± 26.5) | 0.104 | 0.003 | <0.001 |
| Achilles tendon green | 136.6 (± 11.9) | 140.2 (± 11.2) | 138.8 (± 11.5) | 155.84 (± 23.9) | 0.089 | 0.071 | 0.198 |
| Achilles tendon blue | 88.7 (± 20.3) | 90.7 (± 22.3) | 102.1 (± 25.9) | 114.56 (± 31.6) | 0.304 | <0.001 | <0.001 |
VISA-A: Victorian Institute of Sport Assessment for Achilles.
Data are means ( ± standard deviation).
Baseline versus 4–6 weeks.
Baseline versus six months.
Baseline versus 12 months.
Statistically significant at p < 0.05 (paired sample t-test was used unless stated otherwise. *Wilcoxon signed rank test).
Table 3.
B-mode ultrasound features (hypoechogenicity) of the Achilles tendons in 45 patients treated with ultrasound-guided platelet-rich plasma injection at different time points
| Variable | Baseline | 4–6 weeks | 6 months | 12 months | p-valuea | p-valueb | p-valuec |
|---|---|---|---|---|---|---|---|
| Ultrasound abnormalities | |||||||
| Mild | 2 (4.4) | 2 (4.4) | 5 (12.2) | 8 (23.5) | 1.0 | 0.251 | 0.016 |
| Moderate | 12 (26.7) | 14 (31.1) | 11 (26.8) | 17 (50.0) | 0.642 | 0.986 | 0.017 |
| Severe | 31 (68.9) | 29 (64.5) | 25 (61.0) | 9 (26.5) | 0.906 | 0.442 | <0.001 |
| p-value* | 0.896 | 0.401 | <0.001 | ||||
Parentheses indicate percentage.
Baseline versus 4–6 weeks.
Baseline versus six months.
Baseline versus 12 months.
Denotes p-value when the abnormalities were pooled.
Statistically significant at p < 0.05 (Chi square test).
Table 4.
Doppler ultrasound features of the Achilles tendons in 45 patients treated with ultrasound-guided platelet-rich plasma injection at different time points
| Variable | Baseline | 4–6 weeks | 6 months | 12 months | p-valuea | p-valueb | p-valuec |
|---|---|---|---|---|---|---|---|
| Absent (0) | 2 (4.4) | 3 (6.7) | 9 (22.0) | 20 (58.8) | 1.0 | 0.015 | <0.001 |
| Present | 43 (95.6) | 42 (93.3) | 32 (78.0) | 14 (41.2) | |||
| 1+ | 6 (13.4) | 8 (17.8) | 19 (46.3) | 9 (26.5) | 0.561 | 0.001 | 0.078 |
| 2+ | 10 (22.2) | 21 (46.6) | 10 (24.4) | 5 (14.7) | 0.069 | 0.812 | 0.399 |
| 3+ | 27 (60.0) | 13 (28.9) | 3 (7.3) | 0 (0) | 0.020 | <0.001 | <0.001 |
| p-value* | 0.130 | <0.001 | <0.001 |
Parentheses indicate percentage.
Baseline versus 4–6 weeks.
Baseline versus six months.
Baseline versus 12 months.
Denotes p-value when the overall tendon gradings were pooled.
Statistically significant at p < 0.05 (Chi square test).
Discussion
The present study demonstrated progressive reduction in strain ratio scores of the Achilles tendon up to one year following percutaneous PRP injection and eccentric exercise loading programme (Figure 3), and moderately good correlation between these data and clinical outcome. A significant inverse correlation between strain ratio and VISA-A scores (r = −0.610, p < 0.001) at 12-month evaluation indicates that a lower strain ratio (stiffer Achilles tendon properties) was associated with an improvement in clinical manifestations of Achilles tendinopathy (higher VISA-A). Our observations support previous studies that the Achilles tendons showed progressive stiffening during healing process correlating with improvement of clinical symptoms.11,21,22 The injured Achilles tendons exhibit soft properties while the healing tendons gradually improved stiffness. This may be related to the proposed therapeutic effect of PRP in stimulating regeneration of tendon. It has been hypothesized that following PRP activation, the release of growth factors from the highly concentrated platelets in the Achilles tendon injury zone recruits reparative cells and gradually repairs the damaged collagen of the tendon.23 Following this, there is a gradual decline in tendon vascularity and cellularity.23 Consistent with this theory is the finding that the significant improvement in clinical, vascular and mechanical stiffness in our patients occurred after 4–6 weeks' time point following the PRP injection. Recently, the semi-quantitative strain ratio measurements has been proven to be a reliable method with good inter- and intra-operator reliability.10 Taken together with previous findings, our results show that semi-quantitative strain ratio measurement may complement the conventional US imaging in assessing whether a tendon is healing normally (regaining stiffer properties) or whether there is a delay in the healing process. This may in turn allow clinicians to better assess the affected Achilles tendon by helping ascertain suitability of patients to return to activities after tendon injuries.
Preliminary studies of chronic Achilles tendinosis and lateral epicondylitis have used SE to demonstrate tendon softening compared with healthy controls.24,25 In two separate studies, Klauser et al.26,27 provided encouraging histological evidence that pathological changes of the tendon lead to changes in their properties in which diseased or injured tendons exhibit softer characteristic features than the healthy tendons. The soft tendon properties could be potential parameters that were involved in the mechanisms of development and progression of tears if left untreated. Tendons are tissues responsive to mechanical load at a cellular and molecular level. Different exercise loading programmes such as eccentric or heavy slow resistance aim to improve symptoms by inducing changes in tendon properties including stiffness.28 Previous prospective cohort studies reported that a 12-week eccentric exercise programme for treatment of Achilles tendinopathy resulted in increased collagen synthesis with type III collagen replaced by stronger and stiffer type I collagen.29 As a result, tendon mechanical stiffness may partially recover.30 Our observation of progressive reduction in strain ratio scores (increased stiffness of the Achilles tendon) and clinical improvement are likely due to stiffer collagen tissue formation during the tendon remodelling and healing. Also, decrease in Achilles tendon thickness may be another sign of tendon regeneration, as the ground substance decreases and collagen fibres become more closely packed and aligned like in normal tendons. The significant improvement in tendon echotexture we observed only after 12-month treatment (Table 3) suggests that realignment of collagen bundles may take up to a year or even longer.30
Despite improvement in clinical symptoms, we observed incomplete resolution of intratendinous hypoechogenicity and neovascularity at 12 months compared to baseline. Our results support previous studies that following PRP treatment, B-mode and CD US abnormalities of the Achilles tendon do not necessarily correlate with clinical improvement.6,7 Similar discordant findings have also been reported with MR imaging as the follow-up modality.31 This suggests that the source of patients' symptoms is more complex than can be ascribed to morphological and vascular abnormalities. In fact, it has been reported that clinical symptoms of pain associated with tendinopathy are attributable to biochemical factors such as substance P and calcitonin gene-related peptide which cause neurogenic inflammation.32
This study has several limitations. The study suffered from small sample size with a dropout rate of 22.2% (10/45). This might have influenced our conclusions and also affected the statistical power of the results. Lack of a placebo or control group does not allow definitive conclusions to be drawn regarding the effectiveness of PRP and rehabilitation. While this study seems to demonstrate a positive clinical outcome of PRP (and rehabilitation) in treating refractory Achilles tendinopathy, other randomized control studies have reported that PPR injection is no more effective than placebo.5,33 However, the aim of this study was not to evaluate the effectiveness of PRP treatment but to explore whether SE could demonstrate increased mechanical stiffness of the Achilles tendon following PRP therapy. The issue of efficacy of PRP remains to be proven. Our study did not analyse the correlation of longitudinal changes between strain ratio and VISA-A over time. We acknowledge that this is crucial for clinical relevance. Future studies evaluating the potential correlation between change of strain ratio and change of clinical outcome scores are currently under way. Previous literature has shown that tendons exhibit structural and mechanical adaptations in response to various physical activities.34,35 We did not standardize or record the activity levels of all participants during the immediate period prior to US examinations. We do not have data whether the number of injections given after baseline assessments or previous treatments received by patients influence US assessments and clinical outcome. This should be further investigated.
Conclusion
The SE appears able to depict Achilles tendon stiffness up to one year following PRP injection and rehabilitation treatment. The stiffness of the Achilles tendon shows better correlation with clinical symptoms compared to B-mode and CD US findings. SE using strain ratio can be a promising ancillary tool for monitoring Achilles tendon healing after treatment. However, future larger scale randomized controlled studies with longer follow-up are warranted to confirm these preliminary findings.
Acknowledgements
The authors wish to thank Mr Greg Lammers for his invaluable assistance and all the participants for their enthusiasm and commitment to the study.
Contributors
CCO
– Substantial contributions to study conception and design, data acquisition and data analysis.
– Drafting the article and revising it critically for important intellectual content.
– Final approval of the version to be published.
MS, DC
– Substantial contributions to study conception and design, data analysis, data interpretation.
– Revising manuscript critically for important intellectual content.
– Final approval of the version to be published.
PM, MAP, MC, DJ, NV
– Substantial contributions to study design, data acquisition and data analysis.
– Revising manuscript for important intellectual content.
– Final approval of the version to be published.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval
Ethics approval was obtained from Monash Research Ethics Committee (CF12/3597 –2012001708).
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor
Not applicable.
Permission obtained in writing from patient or any person whose photo is included for publishing their photographs and images
Yes. A written permission was obtained from the patient prior to submitting this manuscript for publication. All the images were anonymized.
References
- 1.Jarvinen TA, Kannus P, Maffulli N, et al. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin 2005; 10: 255–266. [DOI] [PubMed] [Google Scholar]
- 2.Maffulli N, Kenward MG, Testa V, et al. Clinical diagnosis of Achilles tendinopathy with tendinosis. Clin J Sport Med 2003; 13: 11–15. [DOI] [PubMed] [Google Scholar]
- 3.Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol 2010; 108: 670–675. [DOI] [PubMed] [Google Scholar]
- 4.Abate M, Verna S, Di Gregorio P, et al. Sonographic findings during and after platelet rich plasma injections in tendons. Muscles Ligaments Tendons J 2014; 4: 29–34. [PMC free article] [PubMed] [Google Scholar]
- 5.de Vos RJ, Weir A, Tol JL, et al. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med 2011; 45: 387–392. [DOI] [PubMed] [Google Scholar]
- 6.Finnoff JT, Fowler SP, Lai JK, et al. Treatment of chronic tendinopathy with ultrasound-guided needle tenotomy and platelet-rich plasma injection. PM R 2011; 3: 900–911. [DOI] [PubMed] [Google Scholar]
- 7.Khan KM, Forster BB, Robinson J, et al. Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. Br J Sports Med 2003; 37: 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klauser AS, Faschingbauer R, Jaschke WR. Is sonoelastography of value in assessing tendons?. Semin Musculoskelet Radiol 2010; 14: 323–333. [DOI] [PubMed] [Google Scholar]
- 9.Ahn KS, Kang CH, Hong SJ, et al. Ultrasound elastography of lateral epicondylosis: clinical feasibility of quantitative elastographic measurements. AJR Am J Roentgenol 2014; 202: 1094–1099. [DOI] [PubMed] [Google Scholar]
- 10.Ooi CC, Schneider ME, Malliaras P, et al. Diagnostic performance of axial-strain sonoelastography in confirming clinically diagnosed Achilles tendinopathy: comparison with B-mode ultrasound and color Doppler imaging. Ultrasound Med Biol 2015; 41: 15–25. [DOI] [PubMed] [Google Scholar]
- 11.Tan S, Kudas S, Ozcan AS, et al. Real-time sonoelastography of the Achilles tendon: pattern description in healthy subjects and patients with surgically repaired complete ruptures. Skeletal Radiol 2012; 41: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 12.Zhang LN, Wan WB, Wang YX, et al. Evaluation of elastic stiffness in healing Achilles tendon after surgical repair of a tendon rupture using in vivo ultrasound shear wave elastography. Med Sci Monit 2016; 22: 1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung JL, Griffith JF. Sonography of chronic Achilles tendinopathy: a case-control study. J Clin Ultrasound 2008; 36: 27–32. [DOI] [PubMed] [Google Scholar]
- 14.Robinson JM, Cook JL, Purdam C, et al. The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med 2001; 35: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredberg U, Bolvig L, Andersen NT, et al. Ultrasonography in evaluation of Achilles and patella tendon thickness. Ultraschall Med 2008; 29: 60–65. [DOI] [PubMed] [Google Scholar]
- 16.Boesen AP, Boesen MI, Torp-Pedersen S, et al. Associations between abnormal ultrasound color Doppler measures and tendon pain symptoms in badminton players during a season: a prospective cohort study. Am J Sports Med 2012; 40: 548–555. [DOI] [PubMed] [Google Scholar]
- 17.Lind B, Ohberg L, Alfredson H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc 2006; 14: 1327–1332. [DOI] [PubMed] [Google Scholar]
- 18.Drakonaki EE, Allen GM, Wilson DJ. Real-time ultrasound elastography of the normal Achilles tendon: reproducibility and pattern description. Clin Radiol 2009; 64: 1196–1202. [DOI] [PubMed] [Google Scholar]
- 19.Wu CH, Chang KV, Mio S, et al. Sonoelastography of the plantar fascia. Radiology 2011; 259: 502–507. [DOI] [PubMed] [Google Scholar]
- 20.Alfredson H, Pietila T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med 1998; 26: 360–366. [DOI] [PubMed] [Google Scholar]
- 21.Chen XM, Cui LG, He P, et al. Shear wave elastographic characterization of normal and torn Achilles tendons: a pilot study. J Ultrasound Med 2013; 32: 449–455. [DOI] [PubMed] [Google Scholar]
- 22.Busilacchi A, Olivieri M, Ulisse S, et al. Real-time sonoelastography as novel follow-up method in Achilles tendon surgery. Knee Surg Sports Traumatol Arthrosc 2016; 24: 2124–2132. [DOI] [PubMed] [Google Scholar]
- 23.Kaux JF, Crielaard JM. Platelet-rich plasma application in the management of chronic tendinopathies. Acta Orthop Belg 2013; 79: 10–15. [PubMed] [Google Scholar]
- 24.De Zordo T, Chhem R, Smekal V, et al. Real-time sonoelastography: findings in patients with symptomatic Achilles tendons and comparison to healthy volunteers. Ultraschall Med 2010; 31: 394–400. [DOI] [PubMed] [Google Scholar]
- 25.De Zordo T, Lill SR, Fink C, et al. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. AJR Am J Roentgenol 2009; 193: 180–185. [DOI] [PubMed] [Google Scholar]
- 26.Klauser AS, Miyamoto H, Tamegger M, et al. Achilles tendon assessed with sonoelastography: histologic agreement. Radiology 2013; 267: 837–842. [DOI] [PubMed] [Google Scholar]
- 27.Klauser AS, Pamminger M, Halpern EJ, et al. Extensor tendinopathy of the elbow assessed with sonoelastography: histologic correlation. Eur Radiol 2017; 27: 3460–3466. [DOI] [PubMed] [Google Scholar]
- 28.Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes: a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med 2013; 43: 267–286. [DOI] [PubMed] [Google Scholar]
- 29.Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med 2004; 38: 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact 2006; 6: 181–190. [PubMed] [Google Scholar]
- 31.Owens RF, Jr, Ginnetti J, Conti SF, et al. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot Ankle Int 2011; 32: 1032–1039. [DOI] [PubMed] [Google Scholar]
- 32.Alfredson H, Cook J. A treatment algorithm for managing Achilles tendinopathy: new treatment options. Br J Sports Med 2007; 41: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Jonge S, de Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med 2011; 39: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 34.Cook JL, Kiss ZS, Ptasznik R, et al. Is vascularity more evident after exercise? Implications for tendon imaging. AJR Am J Roentgenol 2005; 185: 1138–1140. [DOI] [PubMed] [Google Scholar]
- 35.Tardioli A, Malliaras P, Maffulli N. Immediate and short-term effects of exercise on tendon structure: biochemical, biomechanical and imaging responses. Br Med Bull 2012; 103: 169–202. [DOI] [PubMed] [Google Scholar]