Abstract
The present study compared high-fat style diet (HFSD)-induced renal nerve-dependent dysregulation of the baroreflex control of renal sympathetic nerve activity (RSNA) in ovary-intact and ovariectomized (OVX) rats. Female rats received a normal diet (ND) or a HFSD for 10 weeks prior to the acute study. The rats were anesthetized; RSNA and heart rate (HR) were measured. Acute bilateral renal denervation was performed, and baroreflex gain curves were constructed from the baroreflex changes in RSNA to vasopressor and vasodepressor drugs. Cardiopulmonary baroreflex control of RSNA was assessed by acute saline volume expansion (VE). Mean blood pressure was elevated in the OVX-HFSD rats compared to the HFSD group reaching significance on week 6 of the experimental study (P < 0.01). Adiposity index and creatinine clearance were significantly greater in all HFSD rats compared to their ND counterparts. Fractional excretion of sodium rose initially in all HFSD rats but was normalized towards the end of the study although absolute sodium excretion remained high. In the acute study, baroreflex gain curve sensitivity (A2) of RSNA was similarly decreased in both the HFSD and OVX-HFSD rats by 88% (P < 0.005) and 94% (P < 0.001) respectively compared to their control counterparts, but was normalized following bilateral renal denervation. VE-reduced RSNA in ND and OVX-ND rats by 55% and 52% (both P < 0.001) respectively, but did not alter RSNA in both HFSD and OVX-HFSD female rats. Following bilateral renal denervation, HFSD and OVX-HFSD rats exhibited 37% (P < 0.01) and 24% (P < 0.01) reduction in RSNA respectively. These findings demonstrate that although obesity-induced impairment of baroreflex control of RSNA occurred similarly in HFSD and OVX-HFSD rats, mean blood pressure was increased only in the ovarian hormones deprived-group suggesting that ovarian hormones could have modulatory role on other mechanisms that regulate blood pressure in female obesity.
Impact statement
Over activation of renal sensory nerve in obesity blunts the normal regulation of renal sympathetic nerve activity. To date, there is no investigation that has been carried out on baroreflex regulation of renal sympathetic nerve activity in obese ovarian hormones deprived rat model, and the effect of renal denervation on the baroreflex regulation of renal sympathetic nerve activity. Thus, we investigated the role of renal innervation on baroreflex regulation of renal sympathetic nerve activity in obese intact and ovariectomized female rats. Our data demonstrated that in obese states, the impaired baroreflex control is indistinguishable between ovarian hormones deprived and non-deprived states. This study will be of substantial interest to researchers working on the impact of diet-induced hypertension in pre- and postmenopausal women. This study provides insight into health risks amongst obese women regardless of their ovarian hormonal status and may be integrated in preventive health strategies.
Keywords: Baroreflex, obesity, ovariectomy, renal denervation, renal sympathetic nerve activity, high-fat diet
Introduction
Obesity in humans could lead to progressive renal injury associated with hypertension and an inappropriate activation of renal and muscle sympathetic nerve activities.1–3 Obesity is commonly associated with the excessive increase of visceral adipose tissue especially adipocyte size.4,5 Amongst women, most, but not all, gain weight and accumulate central abdominal fat after the menopause,6 and reports have indicated that menopause-related central obesity is an enhanced cardiovascular risk factor.7,8 Previous animal9,10 and human studies11 reported that females were protected from hypertension until they reached menopause. On the other hand, a chronically elevated blood pressure was strongly associated with increased body mass index (BMI) in young obese women, indicating that the protection from hypertension might be restricted to lean pre-menopausal women.12–14
The mechanisms responsible for the increase in blood pressure in obese postmenopausal women are complex and varied. There is a complex relationship between obesity, menopausal status and sympathetic nerve activity (SNA), which has been suggested as playing a key role in the development of hypertension. Importantly, in obesity, SNA is modulated at least in part by the regional distribution of adipose tissue which is primarily in subcutaneous depots in pre-menopausal women compared to visceral deposition after the menopause.15 Indeed, a rise in visceral adiposity in postmenopausal women is associated with a sympatho-excitation.16–18 Similarly, renal fat deposition has been shown to be predictive of hypertension.19 Therefore, abdominal obesity together with ectopic fat deposition plays a crucial role in the pathophysiology of hypertension.
It has been established that obesity-induced renal injury is strongly linked to the genesis of secondary hypertension.20 Heightened renal sympathetic nerve activity (RSNA) in obesity stimulates renin secretion and renal sodium reabsorption that leads to the progression and maintenance of hypertension.21,22 On the other hand, renal afferent nerves can initiate a sympatho-excitation which results in an increase in peripheral vascular resistance and blood pressure.23,24 Indeed, the renal sensory innervation may play an important role in determining sympathetic output which has been reported to be increased in both human and animal models of obesity.25,26 The mechanism for the increase in renal sympathetic output in these animal models of obesity appears to be due to the blunting of the arterial and cardiopulmonary baroreflexes resulting from an inflammatory response.25,26
To date, the effect of loss of ovarian function on obesity-induced dysregulation of the baroreflex control of renal sympathetic nerve activity has not been fully elucidated. The present study was designed to compare obesity-induced renal nerve-dependent dysregulation of arterial and cardiopulmonary baroreflex control of renal sympathetic nerve activity in presence and absence of ovarian hormones states. This was done by undertaking studies in HFSD fed-ovariectomized and ovary-intact female rats in which the kidneys were acutely denervated.
Materials and methods
Animals and treatments
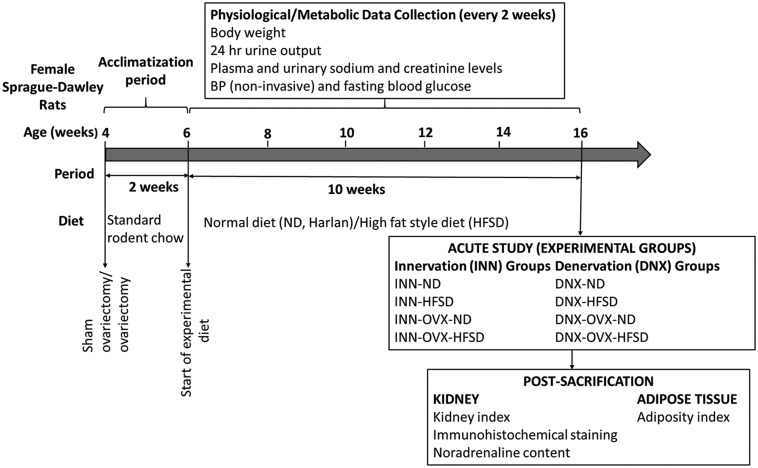
All experimental procedures were approved by the Faculty of Medicine Institutional Animal Care and Use Committee, University of Malaya (FOM IACUC). Female Sprague Dawley rats (n = 48, 3 weeks old) with an initial body weight of 80–100 g were purchased from Invivos Pte. Ltd, Singapore and were housed in standard cages in a temperature- and humidity-controlled room (21–23°C and 40–60% relative humidity) with a 12 h: 12 h light–dark cycle, food and water available ad libitium. Female rats were either sham-operated (n = 32) or bilaterally ovariectomized (n = 24) at four weeks of age.27 After induction of anesthesia (mixture of 5 mg kg−1 of xylazine and 50 mg kg−1 of ketamine intraperitoneally; Xylazil-20, Troy Laboratories Pty Limited, Australia; Ketamil, Troy Laboratories Pty Limited, Australia), short dorso-lateral incisions were made. The ovaries were gently exteriorized, separated from the uterus, and removed. The muscle and skin layers were then individually sutured. Diclofenac sodium (Fenac, L.B.H. Laboratory, Thailand) and ceftriaxone sodium (Trixone, L.B.H. Laboratory, Thailand) was injected intramuscularly at a dose of 25 mg kg−1 each to provide further analgesia and to prevent bacterial infection respectively. For sham- operated rats, the same procedure was applied, except their ovaries were left intact. After an acclimatization period of 2 weeks with standard rodent chow (Gold Coin Sdn. Bhd., Penang, Malaysia), the rats (6 weeks old) were randomly assigned to four groups: (1) sham-operated ovary-intact animals receiving normal diet (ND) (2) sham-operated ovary-intact animals receiving high-fat style diet (HFSD) (3) ovariectomized animals (OVX) receiving ND and (4) ovariectomized animals (OVX) receiving HFSD. At the end of week 10, animals underwent the acute terminal studies with each group further divided into two subgroups, consisting of rats in which their renal nerves were retained (INN) or subjected to bilateral renal denervation (DNX). The experimental design of the study is summarized in Figure 1 and the experimental groups are as follows with each group consisting of six rats:
Figure 1.
Experimental design. The animals were allocated to eight different groups. ND: normal diet; HFSD: high-fat style diet; OVX: ovariectomized; INN: innervated; DNX: denervated; BP: blood pressure.
Group 1: INN-ND Group 5: INN-OVX-ND
Group 2: INN-HFSD Group 6: INN-OVX-HFSD
Group 3: DNX-ND Group 7: DNX-OVX-ND
Group 4: DNX-HFSD Group 8: DNX-OVX-HFSD
Diet protocol
Normal diet was purchased from Teklad Research Diet (Harlan laboratories, Madison, WI, USA) whereas HFSD was locally purchased and composed of six highly palatable and energy dense diets consisting of chocolate bars, salami, smoked chicken, vanilla wafer, buttered cakes and Ritz crackers. The HFSD in the present study is quite similar to the diet in other studies28,29 except that it comprised of local substitutes that were readily and commercially available. Daily rotation of each of the six items constituting the HFSD was different in each week throughout the study. The diets were given in the same quantities and at the same time for all the groups of rats. The composition of the diets is as follows:
| Composition of diet | Normal diet | High-fat style diet |
|---|---|---|
| Protein (% kcal) | 22 | 32.3 |
| Fat (% kcal) | 12 | 42.6 |
| Carbohydrate (% kcal) | 66 | 27.8 |
| Sodium (g/100 g) | 0.2 | 0.6 |
| Total gross energy with calories (kcal g−1) | 3 | 3.4 |
Physiological data collection
Rats were kept individually in metabolic cages for 24 h on weeks 0, 2, 4, 6, 8 and 10. Urine output over a period of 24 h was measured. Blood samples (400–500 μL) were collected via the tail vein in heparinized microcentrifuge tubes (Eppendorf, USA; heparin 5000 IU/mL, Leo Pharmaceuticals, Denmark). The blood samples were centrifuged at 3000 r/min for 5 min to extract the plasma. Creatinine (Plate reader; BioTek Instruments, Winooski, VT, USA) and sodium (Flame photometer; Corning 410 C, Hastead, Essex, UK) concentrations were measured in both plasma and urine. At the end of week 10, the acute terminal studies were performed.
Measurement of blood pressure and fasting blood glucose
Blood pressure was determined by the tail-cuff method (CODA, Kent Scientific Corporation, Torrington, CT, USA) on weeks 0, 2, 4, 6, 8 and 10. A day after, experimental animals were fasted for 12 h and glucose levels were determined in aliquots of blood, obtained by tail prick, using a glucometer (Accu-Chek®Performa, Roche Diagnostics, Germany).
Acute study
Detection of proestrus phase of estrus cycle prior to acute study
Both ovary-intact and ovariectomized rats underwent the acute terminal studies. The estrus cycle of each ovary-intact rat was first established and animals underwent the acute studies during the proestrus (high levels of estrogen). For determination of proestrus phase, the genital area was carefully exposed and 10 μL of saline (0.9% NaCl) was introduced into their vagina. Thereafter, vaginal fluid was collected as previously described.30 The presence of round epithelial cells with defined nuclei in the vaginal smear when visualized under the microscope was taken as an indicator of proestrus phase.
Surgical procedure for acute study
Overnight fasted ovary-intact and ovariectomized rats were anesthetized with 1 mL of α-chloralose-urethane mixture (250 and 16.5 mg mL−1 respectively, intraperitoneally) and maintained with supplemental doses of the anesthetic (0.05 mL, intravenously) every 30 min. A tracheostomy was performed in which a polypropylene catheter (1.98 mm ID) was inserted into the trachea to facilitate mechanical ventilation. The left femoral vein was cannulated in order to permit the continuous saline infusion (0.9% NaCl, 3 mLh−1) and maintenance doses of anesthesia as well as the administration of drugs. The left femoral artery was cannulated and the cannula connected to a pressure transducer (P23 ID Gould Statham Instrument, Nottingham, UK) coupled to a computerized data acquisition system (PowerLab®; AD Instrumentation, Sydney, Australia) to measure mean arterial pressure (MAP) and heart rate (HR). The urinary bladder was cannulated through a small abdominal incision for drainage of urine.
Using a retroperitoneal incision to expose the left kidney and a dissecting microscope, a section of the left renal nerve bundle was placed on fine bipolar stainless steel wire electrodes. A successful renal nerve signal was verified visually on the computer screen and by an audible sound using an audio amplifier. Once adequate renal nerve recording was obtained, the nerve bundle was fixed on the electrodes with dental glue (Klasse 4 Dental, Augsburg, Germany). Renal sympathetic nerve activity (RSNA) was amplified with a gain of 100,000 by setting the low- and high-pass filters at 1.0 and 0.1 kHz, respectively. The rats were then allowed to stabilize for 2 h before commencing the baroreflex gain curve and volume expansion studies.
Acute bilateral renal denervation
A flank incision was made retroperitoneally to expose the right kidney and all the visible renal nerves were dissected and smeared with 10% phenol in absolute alcohol. Thereafter, the left kidney was exposed and the renal nerve bundle was dissected and placed on the recording electrodes. Denervation of the afferent renal nerves of the left kidney was achieved by mechanically crushing the nerve bundle at a point between the electrodes and the kidney using surgical forceps as previously described.25,26 This approach intercepted most afferent nerve trafficking from the kidney, but enabled recording of the sympathetic nerve activity by the electrodes. Additionally, it is expected that sympathetic nerve traffic would not be transmitted across the damaged section of the renal nerve bundle into the kidney effectively denervating the organ. The nerve bundle was then fixed on the electrodes with dental glue. In the innervated animals, recordings were obtained from the left kidney with both the left and right renal nerves be left intact. The baroreflex gain curve and volume expansion studies were initiated 2 hours after the completion of acute bilateral renal denervation.
Baroreflex gain curve and volume expansion studies
High-pressure baroreflex regulation of RSNA was assessed via intravenous administration of vasoactive drugs. Phenylephrine and sodium nitroprusside (50 μg in 0.2 mL of saline for each) was infused at 18 mL h−1 over 40 s to increase and decrease arterial pressure respectively. Variables were permitted to return to baseline levels after each drug administration. Baroreflex gain curves for RSNA were generated from which the baroreflex parameters, namely range of the curve (A1), sensitivity coefficient of the slope over which the baroreceptor operated (A2), mid-point blood pressure (A3) and minimal point of the curve (A4) were derived.
After completion of the baroreflex gain curve study, animals were allowed to recover for 30 min before initiation of the volume expansion study. Rats were infused with saline for 30 min at a rate of 0.25 mL per 100 g body weight per min via the femoral vein. MAP and RSNA basal readings were recorded for 5 min which were taken as 100%. The percentage reduction of these variables was determined at 5 min intervals over a 30 min period. At the completion of the study, sham operated (ovary-intact) and ovariectomized rats were euthanized via intravenous administration of an overdose of α-chloralose-urethane anesthetic. A background noise level was recorded 20 min later and the magnitude of the noise signal was removed from all RSNA measurements taken during the acute study. Kidneys and total abdominal fat were excised and weighed for determination of kidney and adiposity index respectively. Thereafter, the kidneys were either snap frozen in liquid nitrogen (N2) and stored at −80°C for noradrenaline measurement or fixed in 10% buffered formalin solution for later immunohistochemical staining.
Measurement of kidney and adiposity indices
Both kidneys were rinsed in phosphate buffered saline (PBS), patted dry and weighed for determination of kidney index (100 × kidney weight/body weight). Total abdominal adipose tissue was also carefully patted dry and weighed for determination of adiposity index (100 × total abdominal fat weight/body weight).
Noradrenaline
Immunohistochemical detection of renal noradrenaline
Kidneys were fixed in 10% buffered formalin solution (Sigma Aldrich, Germany) and processed for paraffin embedding. After deparaffinization, tissue sections (5 μm) were incubated in 0.01 M citrate buffer (10 min, 100°C) for antigen retrieval. The tissue sections were rinsed in PBS, followed by incubation in 3% hydrogen peroxide for 1 h to quench the endogenous peroxidase activity, and blocked with 5% BSA. Thereafter, the sections were incubated overnight with primary rabbit anti-noradrenaline antibody (Abcam, Cambridge, USA) at a dilution of 1:200 in 5% BSA. After washing in PBS, the sections were incubated with biotinylated secondary polyclonal goat anti-rabbit antibodies (Santa Cruz Biotechnology, Santa Cruz, California, USA) at a dilution of 1:400 in 5% BSA for 1 h at room temperature. The sections were then incubated in an avidin-biotinylated-peroxidase complex system (ImmunoCruz ABC Staining, Santa Cruz Biotechnology, Texas, USA) and immunoreactivity was visualized with 3,3′-diaminobenzidine tetrahydrochloride and hydrogen peroxide. Finally, the slides were lightly counterstained with hematoxylin and analyzed under a microscope. Evaluation of immunohistochemical labelling was carried out using double-blinded observers.
Measurement of renal noradrenaline
Kidneys were snap frozen in liquid nitrogen (N2) and stored at −80°C until further analysis. On the day of noradrenaline measurement, the frozen kidneys were thawed and homogenized over ice in PBS. After centrifugation of the homogenates at 1500g for 10 min, noradrenaline concentrations in the supernatants were measured using a commercial ELISA kit (Elabscience Biotechnology, Wuhan, China).
Calculations
The data obtained from the metabolic studies permitted the calculations of renal excretory and hemodynamic functions. Urine flow rate (UFR) was calculated using the following formula: V/T × BW, where V was the volume of urine, T the time period over which urine was collected, and BW the body weight of the rat. Absolute sodium excretion (UNaV) was calculated as UNa × UFR, in which UNa was the concentration of urinary sodium. The clearance of creatinine (CrCl) was utilized as an approximate measure of glomerular filtration rate, CrCl = UCr × UFR/PCr, in which UCr and PCr were the concentrations of creatinine in urine and plasma respectively. Fractional excretion of sodium (FENa) was calculated as CNa/CrCl, in which CNa was clearance of sodium.
Statistical analysis
Data were expressed as means ± S.E.M., and graphical as well as statistical analyses were undertaken using IBM SPSS statistical software package (IBM SPSS Statistics for Windows; Version 23.0, IBM Corporation, Armonk, NY, USA). Body weight, renal functional assessment parameters, non-invasive mean blood pressure and fasting blood glucose were analyzed using univariate three-way repeated measures ANOVA with the application of the Greenhouse–Geisser correction if assumption of Mauchy’s test of sphericity is violated, followed by a Bonferroni post hoc test with statistical significance defined as P < 0.05 for the comparison between groups. Data in Tables 1 and 2 as well as renal noradrenaline content were analyzed using univariate three-way ANOVA whilst sympatho-inhibitory responses to volume expansion were analyzed using one-way ANOVA followed by a Bonferroni post hoc test with statistical significance defined as P < 0.05 for the comparison between groups. The baroreflex gain curve for RSNA was generated by quantifying the four-parameter logistic regression equation: [RSNA= A1/(1 + exp (A2 (MAP-A3))) + A4], in which A1 represents range of the curve, A2 is baroreflex sensitivity, A3 is midpoint blood pressure and A4 indicates the minimum point achieved for RSNA. [Gmax = (−A1 × A2/4)] equation was utilized to calculate the maximal gain or slope (Gmax) and this was analyzed using univariate three-way ANOVA followed by a Bonferroni post hoc test.
Table 1.
Basal values of renal sympathetic activity (RSNA), mean arterial pressure (MAP) and heart rate (HR) obtained in innervated (INN) and denervated (DNX), normal diet (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX) during acute study.
|
Basal values |
INN-ND | INN-HFSD | INN-OVX-ND | INN-OVX-HFSD | DNX-ND | DNX-HFSD | DNX-OVX-ND | DNX-OVX-HFSD |
|---|---|---|---|---|---|---|---|---|
| RSNA (mVs−1) | 13 ± 3 | 14 ± 3 | 14 ± 5 | 14 ± 1 | 11 ± 1 | 15 ± 9 | 14 ± 1 | 12 ± 2 |
| MAP (mmHg) | 98 ± 10 | 113 ± 5 | 98 ± 4 | 121 ± 5# | 95 ± 6 | 111 ± 2 | 94 ± 10 | 109 ± 4 |
| HR (bpm) | 250 ± 9 | 336 ± 16 | 241 ± 11 | 280 ± 7 | 381 ± 174 | 293 ± 24# | 205 ± 15 | 240 ± 12 |
Data were analyzed using univariate three-way ANOVA followed by Bonferroni's multiple comparisons post hoc test. Data are expressed as mean ± SEM (n = 6 per group).
#P < 0.05 vs. OVX-ND (each respective innervated or denervated group).
Table 2.
Baroreflex gain curve parameters for renal sympathetic nerve activity (RSNA): (a) range of the curve (A1), (b) baroreflex sensitivity (A2), (c) midpoint blood pressure (A3) and (d) minimal point of the curve obtained in innervated (INN) and denervated (DNX), normal diet (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX) during baroreflex gain curve study.
|
Baroreflex parameters |
INN-ND | INN-HFSD | INN-OVX-ND | INN-OVX-HFSD | DNX-ND | DNX-HFSD | DNX-OVX-ND | DNX-OVX-HFSD |
|---|---|---|---|---|---|---|---|---|
| A1 (mV s−1) | 49 ± 7 | 72 ± 15 | 40 ± 10 | 107 ± 14####δ | 24 ± 6 | 53 ± 5 | 49 ± 4 | 41 ± 7ββββ |
| A2 (mV s−1 mmHg−1) | 1.17 ± 0.19 | 0.14 ± 0.02*** | 1.41 ± 0.14 | 0.08 ± 0.02#### | 1.02 ± 0.23 | 0.89 ± 0.25 α | 1.52 ± 0.31 | 1.14 ± 0.30βββ |
| A3 (mmHg) | 88 ± 4 | 110 ± 8 | 100 ± 9 | 111 ± 4 | 93 ± 8 | 108 ± 9 | 83 ± 8 | 103 ± 11 |
| A4 (mV s−1) | 83 ± 10 | 82 ± 16 | 78 ± 4 | 104 ± 23 | 97 ± 11 | 97 ± 3 | 86 ± 5 | 84 ± 4 |
Data were analyzed using univariate three-way ANOVA followed by Bonferroni's multiple comparisons post hoc test. Data are expressed as mean ± SEM (n = 6 per group).
***P < 0.005 vs. INN-ND; ####P < 0.001 vs. INN-OVX-ND; δP < 0.05 vs. INN-HFSD; αP < 0.05 vs. INN-HFSD; βββP < 0.005 vs. INN-OVX-HFSD.
Results
Body weight
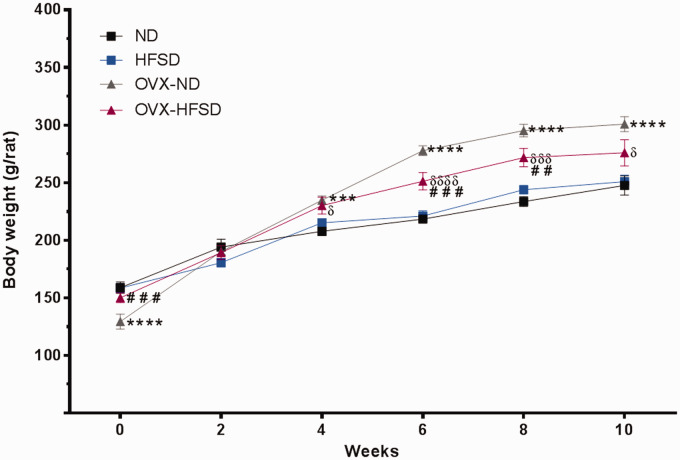
Figure 2 illustrates the body weights in the different experimental groups over the period of study. At the start of the study, OVX-HFSD rats exhibited a slightly higher body weight, of some 16%, compared to the OVX-ND rats (P < 0.005) while OVX-ND rats exhibited slightly lower body weight, of some 19%, compared to the ND rats (P < 0.001). OVX-ND rats exhibited a greater total body weight gain starting from fourth week which persisted throughout the study when compared to the corresponding female rats with intact ovaries (ranging from P < 0.005 to P < 0.001). Similarly, OVX-HFSD rats began to gain weight compared to HFSD rats beginning from fourth week which persisted throughout the study (ranging from P < 0.05 to P < 0.001). However, after 10 weeks of feeding, the body weight of the ovary-intact HFSD rats as well as that of OVX-HFSD rats did not differ from that of ovary-intact ND rats and OVX-ND rats respectively. Of note, OVX-HFSD rats exhibited higher body weight compared to HFSD rats at week 6 (P < 0.001) and 8 (P < 0.005).
Figure 2.
Body weight of normal diet (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX). Data are expressed as mean ± SEM (n = 12 per group). ***P < 0.005 or ****P < 0.001 vs. normal diet fed rats (ND); ##P < 0.01 or ###P < 0.005 vs. OVX-ND; δP < 0.05 or δδδP < 0.005 or δδδδP < 0.001 vs. high-fat style diet fed rats (HFSD). (A color version of this figure is available in the online journal.)
Adipose weight and adiposity indices
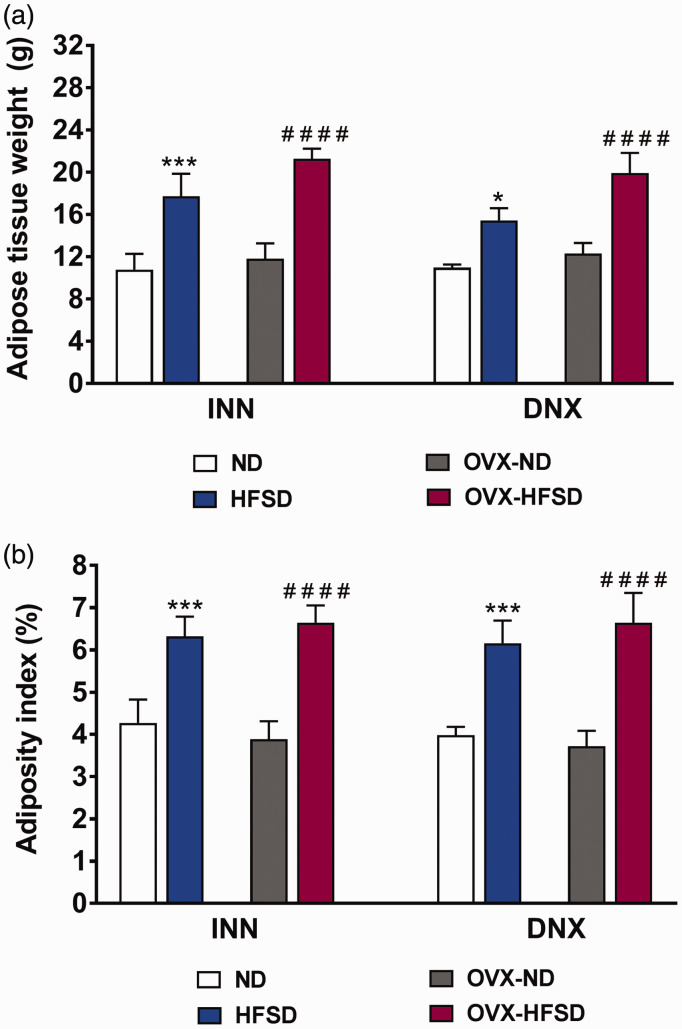
After 10 weeks of HFSD, total abdominal adipose tissue and adiposity index were greater by 80% (INN, P < 0.001), 62% (DNX, P < 0.001) and 71% (INN, P < 0.001), 78% (DNX, P < 0.001), respectively, in the OVX-HFSD rats compared to the OVX-ND rats (Figure 3(a) and (b)). A similar trend was observed in the ovary-intact rats. The total abdominal adipose tissue and adiposity index were greater by 64% (INN, P < 0.005), 41% (DNX, P < 0.05) and 48% (INN, P < 0.005), 54% (DNX, P < 0.005), respectively, in the HFSD fed rats compared to the ND fed rats. Ovariectomy did not lead to excessive fat accumulation in HFSD rats in comparison to the corresponding ovary intact HFSD fed rats.
Figure 3.
Adipose tissue weight (a) and adiposity index (b) of innervated (INN) and denervated (DNX), normal diet (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX). Data are expressed as mean ± SEM (n = 6 per group). *P < 0.05 or ***P < 0.005 vs. ND (each respective innervated or denervated group); ####P < 0.001 vs. OVX-ND (each respective innervated or denervated group). (A color version of this figure is available in the online journal.)
Physiological assessment and hemodynamic parameters
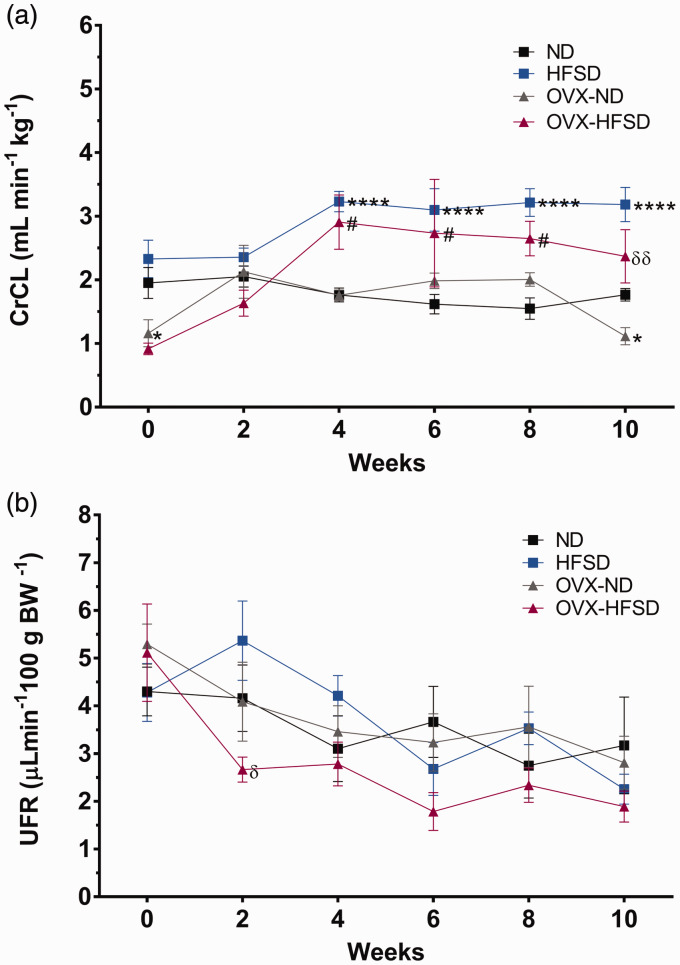
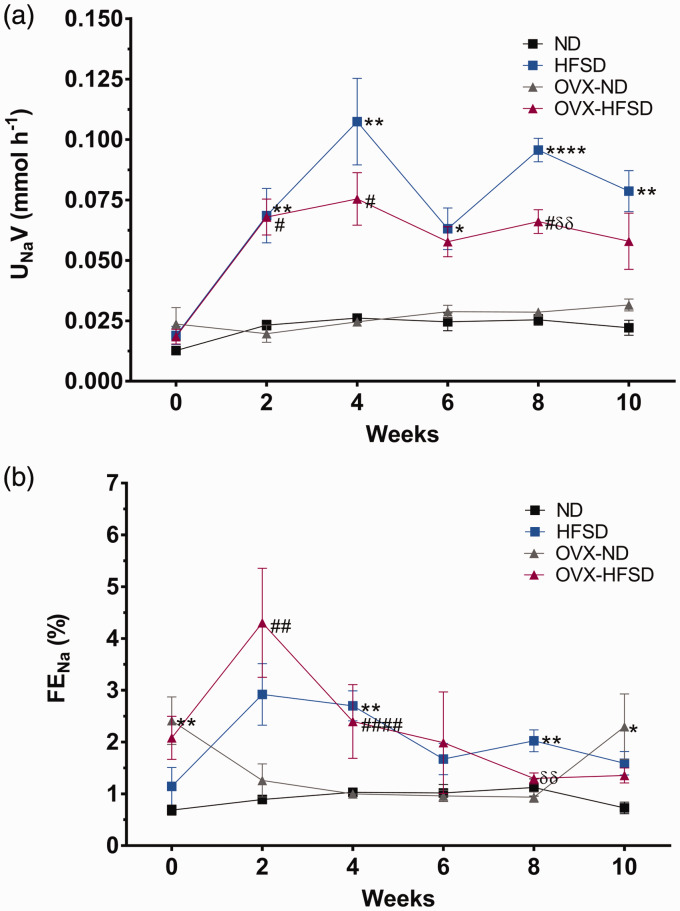
Figure 4(a) and (b) and Figure 5(a) and (b) illustrate the renal functional assessments in the different experimental groups over the period of study. Creatinine clearance (Figure 4(a)) was lower in OVX-ND rats compared to ND rats on week 0 by 40% (P < 0.05). Creatinine clearance was increased significantly beginning week 4 until week 10 in HFSD (P < 0.001), while beginning week 4 until week 8 in OVX-HFSD rats (P < 0.05), compared to their corresponding ND groups. By contrast, there was no significant change in the urine flow rate (UFR) (Figure 4(b)) across all experimental groups. Sodium excretion was markedly increased in all groups fed the HFSD throughout the 10 weeks period (Figure 5(a)). Absolute excretion of sodium was increased significantly on week 2 until 10 (ranging from P < 0.05 to P < 0.001) in HFSD rats compared to their corresponding ND counterparts. Similarly, absolute excretion of sodium was increased significantly on week 2, 4 and 8 in OVX-HFSD rats (P < 0.05) respectively compared to their corresponding OVX-ND rats. Fractional excretion of sodium (FENa) did not differ between the OVX-HFSD and OVX-ND and similarly, between HFSD and ND groups before initiation of diet (Figure 5(b)). FENa was higher on week 0 in OVX-ND rats compared to ND rats (P < 0.01). After initiation of the diet, FENa rose sharply on the second week in OVX-HFSD rats (P < 0.01) compared to their corresponding ND rats. Similarly, HFSD rats also showed an abrupt increase in FENa on the second week, but could not be statistically distinguished compared to their corresponding ND rats. However, FENa began to normalize towards the end of the study in both OVX-HFSD and HFSD rats even though absolute sodium excretion remained high during this period (Figure 5(b)).
Figure 4.
Creatinine clearance (a) and urinary flow rate (b) of normal diet (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX). Data are expressed as mean ± SEM (n = 12 per group). ****P < 0.001 vs. normal diet fed rats (ND); #P < 0.05 vs. OVX-ND; δP < 0.05 or δδP < 0.01 vs. high-fat style diet fed rats (HFSD). CrCl: creatinine clearance; UFR: urinary flow rate. (A color version of this figure is available in the online journal.)
Figure 5.
Absolute excretion of sodium (a) and fractional excretion of sodium (b) of normal diet (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX). Data are expressed as mean ± SEM (n = 12 per group). *P < 0.05, **P < 0.01 or ****P < 0.001 vs. normal diet fed rats (ND); #P < 0.05, ##P < 0.01 or ####P < 0.001 vs. OVX-ND; δδP < 0.01 vs. high-fat style diet fed rats (HFSD). UNaV: absolute excretion of sodium; FENa: fractional excretion of sodium. (A color version of this figure is available in the online journal.)
Kidney weight and indices
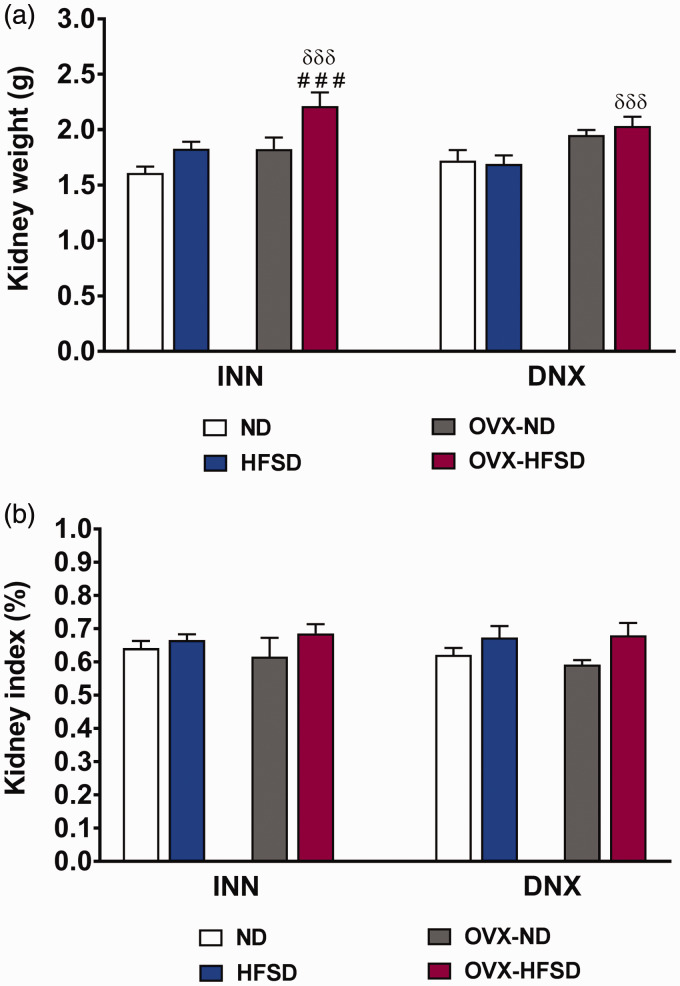
Figure 6(a) and (b) shows kidney weights were significantly increased in INN-OVX-HFSD rats compared to INN-OVX-ND and INN-HFSD rats by 21% (P < 0.001) and 21% (P < 0.005) respectively, and similarly increased in DNX-OVX-HFSD rats by 20% (P < 0.005) compared to DNX-HFSD rats. However, kidney indices were similar across all innervated as well as denervated experimental groups.
Figure 6.
Kidney weight (a) and kidney index (b) of innervated (INN) and denervated (DNX), normal diet (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX). Data are expressed as mean ± SEM (n = 6 per group). ###P< 0.005 vs. OVX-ND (each respective innervated or denervated group); δδδP < 0.005 vs. HFSD (each respective innervated or denervated group). (A color version of this figure is available in the online journal.)
Fasting blood glucose and blood pressure profile
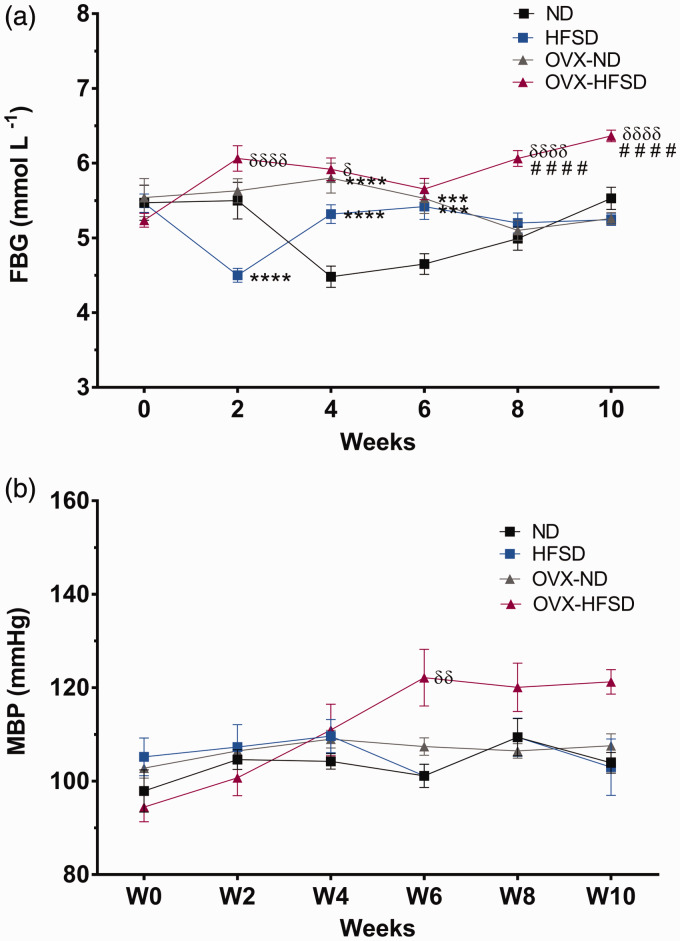
Figure 7(a) shows the fasting blood glucose (FBG) of all the experimental groups. Fasting blood glucose (FBG) was significantly increased in OVX-HFSD compared to HFSD rats especially beginning week 8 (ranging from P < 0.05 to P < 0.001). There was no meaningful trend in the FBG in the rest of the experimental groups.
Figure 7.
Fasting blood glucose (FBG) (a) and mean blood pressure (MBP) (b) of normal diet (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX). Data are expressed as mean ± SEM (n = 12 per group). ***P < 0.005 or ****P < 0.001 vs. ND, ####P < 0.001 vs. OVX-ND, δP < 0.05, δδP < 0.01 or δδδδP < 0.001 vs. HFSD. FBG: fasting blood glucose; MBP: mean blood pressure. (A color version of this figure is available in the online journal.)
Figure 7(b) shows the mean blood pressure of the experimental groups of which the OVX-HFSD exhibited an increasing trend towards the end of the experimental study reaching significance on week 6 (P < 0.01) as compared to HFSD rats. Mean blood pressure was similar across the HFSD, OVX-ND and ND groups.
Acute studies
Basal hemodynamic parameters and RSNA
Table 1 displays the basal values of MAP, integrated RSNA and HR during the initial stage of acute experiment following anesthesia prior to challenge with vasoconstrictor and vasodilator drugs. Basal RSNA was similar across all innervated and denervated experimental groups. INN-OVX-HFSD rats exhibited a higher (P < 0.05) baseline MAP by 23% (121 ± 5 mmHg vs. 98 ± 4 mmHg) compared to their ND counterparts. Nevertheless, basal MAP in INN-HFSD rats was not significantly different as compared to that of their corresponding ND rats. Basal MAP was not statistically different across all denervated experimental groups. Basal HR could not be distinguished statistically across all innervated and denervated experimental groups except the value was slightly higher (P < 0.05) in DNX-HFSD rats compared to DNX-OVX-ND rats.
Baroreflex gain curve parameters of RSNA
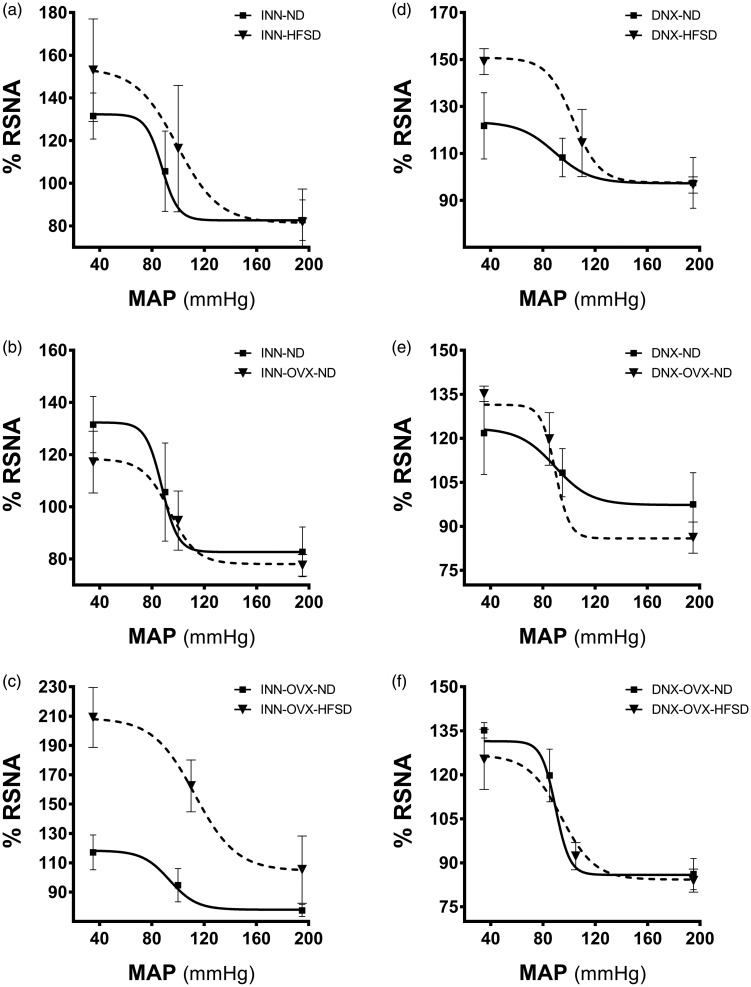
Table 2 shows the baroreflex gain curve parameters of RSNA and these parameters (A1, A2, A3, A4) were derived from the baroreflex gain curves as displayed in Figure 8. The range of the curve (A1) in INN-OVX-HFSD rats was significantly increased by 168% (P < 0.001; 107 ± 14 mV s−1 vs. 40 ± 10 mV s−1) and 49% (P < 0.05; 107 ± 14 mV s−1 vs. 72 ± 15 mV s−1) when compared to INN-OVX ND (Figure 8(c)) and INN-HFSD rats respectively. There was no significant difference in A1 values between INN-HFSD and INN-ND rats (Figure 8(a)). Denervation in the OVX-HFSD (DNX-OVX-HFSD) rats elicited a significantly (P < 0.001) lower A1 values compared to those of the INN-OVX-HFSD rats, by 161%. However, there were no statistical differences in A1 values in the DNX-HFSD (Figure 8(d)) and DNX-OVX-HFSD rats (Figure 8(f)) when compared to their denervated ND counterparts. A1 value has no effect on normal diet fed rats (ND and OVX-ND) neither in innervated (Figure 8(b) and (e)) nor denervated groups. The sensitivity of the baroreflex (A2) in both INN-HFSD rats and INN-OVX-HFSD rats was significantly depressed by 88% (P < 0.005) and 94% (P < 0.001) respectively when compared to their ND counterparts. By contrast, A2 values in both denervated HFSD fed groups (DNX-HFSD and DNX-OVX-HFSD) was significantly increased when compared to INN-HFSD (P < 0.05) and INN-OVX-HFSD (P < 0.005) respectively. Although the magnitude of A2 values in INN-OVX-HFSD rats was more depressed than INN-HFSD rats, there is no statistical difference in A2 values between these groups of rats. Furthermore, there were no statistical differences in A2 values between the DNX-HFSD and DNX-OVX-HFSD rats when compared to their denervated ND counterparts. A2 value also has no effect on normal diet fed rats (ND and OVX-ND) neither in innervated nor denervated groups. HFSD and OVX-HFSD has no meaningful effect on the midpoint of blood pressure (A3) value and the minimum point to which RSNA could be reached (A4).
Figure 8.
Baroreflex gain curves for RSNA in innervated (INN) and denervated (DNX) normal (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX) (n = 6 per group).
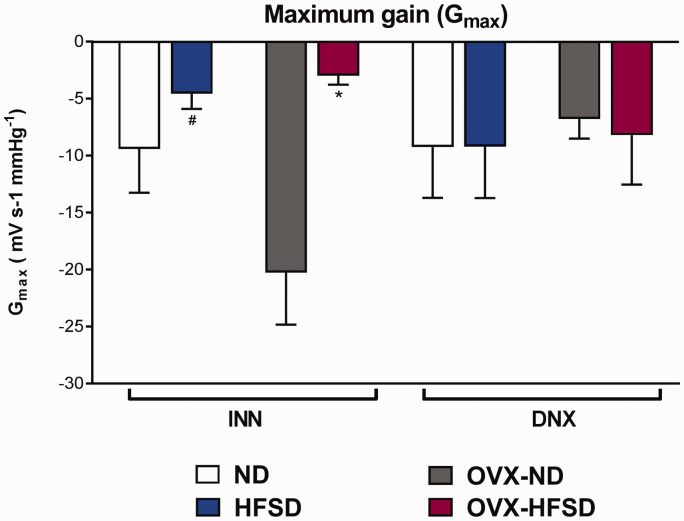
Maximum slope or gain of RSNA
Figure 9 displays the maximal gain of the RSNA baroreflex curves. Maximum gain was significantly lower (both P < 0.05) in INN-OVX-HFSD and INN-HFSD animals compared to their control ND counterparts by 86% (−2.0 ± 0 mVs−1 mmHg−1 vs. −14.5 ± 4 mVs−1 mmHg−1) and 84% (−2.5 ± 1 mVs−1 mmHg−1 vs. −15.5 ± 4 mVs−1 mmHg−1). Ovariectomy in the ND rats (OVX-ND) has no meaningful effect on the maximum gain of RSNA neither in innervated nor denervated groups. There was no significant difference in the maximum slope of RSNA between INN-HFSD and INN-OVX HFSD rats. Similarly, renal denervation of both groups of HFSD fed rats (D-HFSD and D-OVX-HFSD) could not be distinguished from their denervated ND counterparts.
Figure 9.
Maximal gain of the RSNA baroreflex gain curves in innervated (INN) and denervated (DNX), normal diet (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX). Data are expressed as mean ± SEM (n = 6 per group). *P < 0.05 vs. INN-OVX-ND. #P < 0.05 vs. INN-OVX-ND. (A color version of this figure is available in the online journal.)
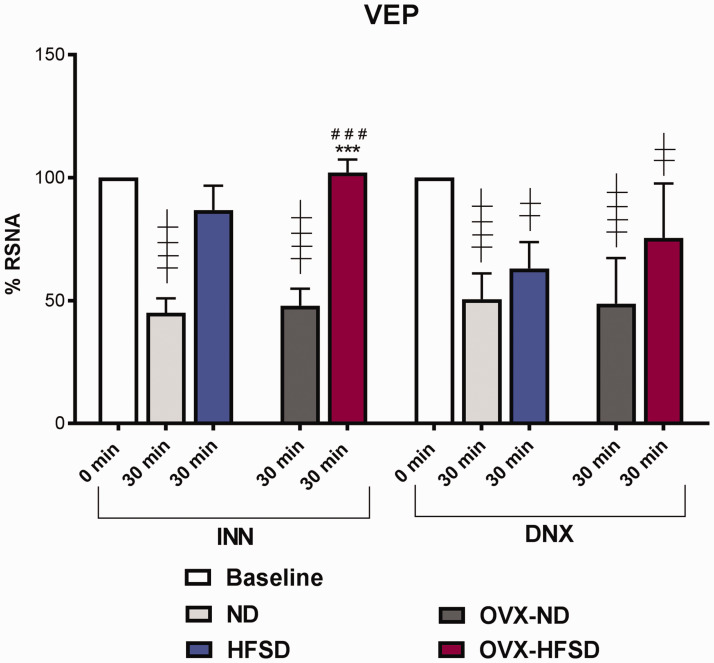
Volume expansion studies
Figure 10 illustrates the percentage reduction in RSNA after infusion of acute saline load. Innervated ND and OVX-ND rats elicited a significant (P < 0.001) renal sympatho-inhibition after the infusion of acute saline load of some 55% and 52% respectively from the baseline value. By contrast, the INN-HFSD and INN-OVX-HFSD groups of rats did not exhibit a meaningful renal sympatho-inhibition from the baseline value after 30 min of volume expansion and the degree of impairment of cardiopulmonary baroreceptors to acute volume load between these groups could not be distinguished statistically. Nevertheless, in both HFSD fed rats subjected to renal denervation (DNX-HFSD and DNX-OVX-HFSD), there was a significant (both P < 0.01) reduction in RSNA, of 37% and 24% respectively from the baseline value, in which those magnitudes of reduction could not be distinguished statistically compared to their corresponding denervated ND rats (DNX-HFSD and DNX-OVX-ND). Likewise, renal denervation of ND (DNX-ND) and OVX-ND (DNX-OVX-ND) resulted in a significant decrease in RSNA by 49% (P < 0.001) and 51% (P < 0.001) respectively from the baseline values. The magnitude of reduction of RSNA in DNX-HFSD and DNX-OVX-HFSD rats after the infusion of acute saline load were statistically indistinguishable from that obtained in their ND counterparts. Of note, degree of renal sympatho-inhibition in INN-OVX-HFSD rats was significantly different compared to INN-ND and INN-OVX-ND by 127% (P < 0.05) and 113% (P < 0.001) respectively.
Figure 10.
Renal sympathoinhibition reflex after 30 min of acute saline volume load of innervated (INN) and denervated (DNX) normal (ND) and high-fat style diet (HFSD) fed rats with and without ovariectomy (OVX) in volume expansion (VEP) study. Data are expressed as mean ± SEM (n = 6 per group). Baseline RSNA was taken as 100% at 5 min prior to the execution of the acute saline infusion. ***P< 0.005 vs. INN-ND (30 min); ###P< 0.005 vs. INN-OVX-ND (30 min); ††††P< 0.001 vs. INN (0 min); ††P< 0.01 vs. DNX (0 min); ††††P< 0.001 vs. DNX (0 min). (A color version of this figure is available in the online journal.)
Renal noradrenaline
Immunohistochemical detection of renal noradrenaline
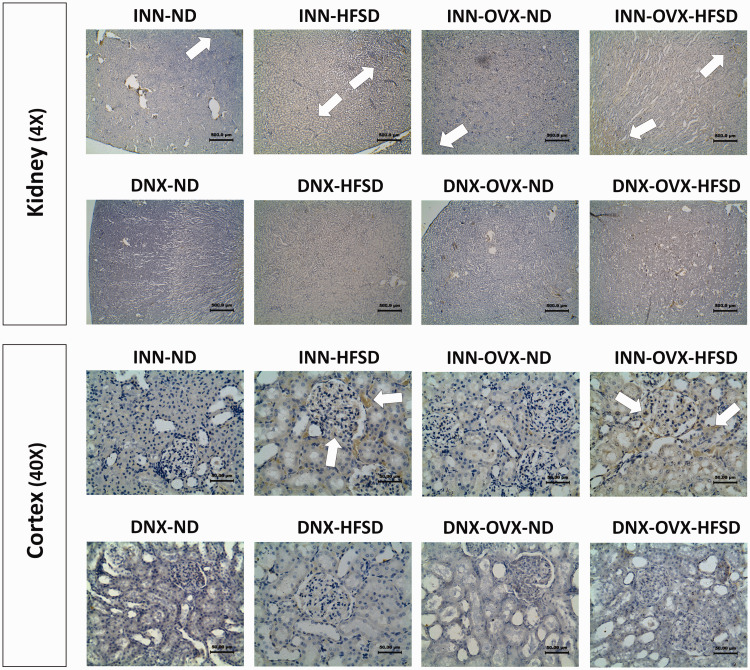
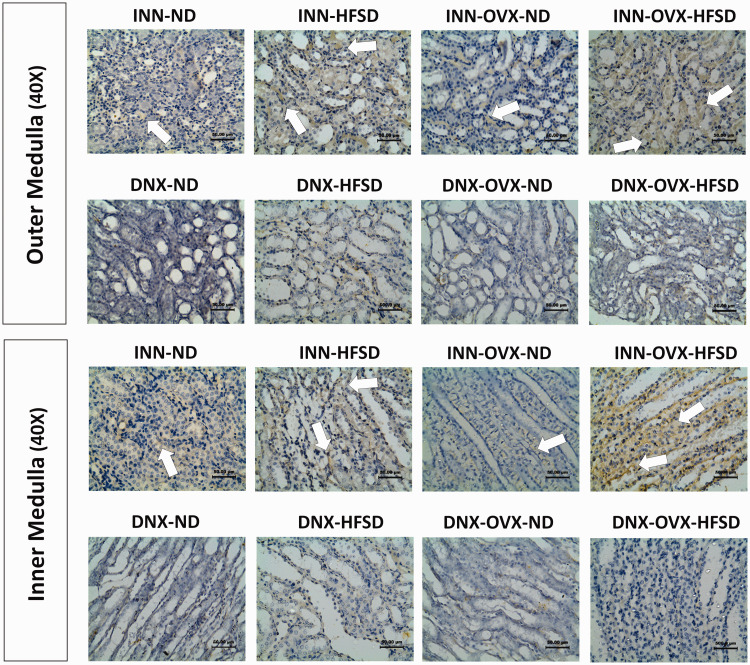
Formalin-fixed paraffin-embedded sections of rat kidney were stained for immunoreactivity to noradrenaline. Figures 11 and 12 show specific staining for noradrenaline in the renal tissue and cortex region, and outer as well as inner medulla regions, respectively. Renal staining for noradrenaline was strongest in the inner and outer medulla regions of samples from both groups of innervated HFSD and OVX-HFSD fed rats where the sympathetic nerve endings are densely located. The presence of noradrenaline was also detected in renal cortex of samples from innervated OVX-HFSD rats, and slightly to a lesser degree in renal cortex from innervated HFSD rats. There was relatively little staining in these regions of renal samples from the other experimental groups (INN-ND and INN-OVX-ND). All renal sections from denervated experimental groups showed relatively little staining.
Figure 11.
Immunohistochemical staining of noradrenaline in kidneys (4× magnification) and cortex region (40× magnification) of innervated (INN) and denervated (DNX), normal diet (ND) and high-fat style diet (HFSD) fed rats, with and without ovariectomy (OVX) (n = 5 per group). White arrow, noradrenaline. (A color version of this figure is available in the online journal.)
Figure 12.
Immunohistochemical staining of renal noradrenaline in the outer medulla (40× magnification) and inner medulla (40× magnification) regions of innervated (INN) and denervated (DNX), normal diet (ND) and high-fat style diet (HFSD) fed rats, with and without ovariectomy (OVX) (n = 5 per group). White arrow, noradrenaline. (A color version of this figure is available in the online journal.)
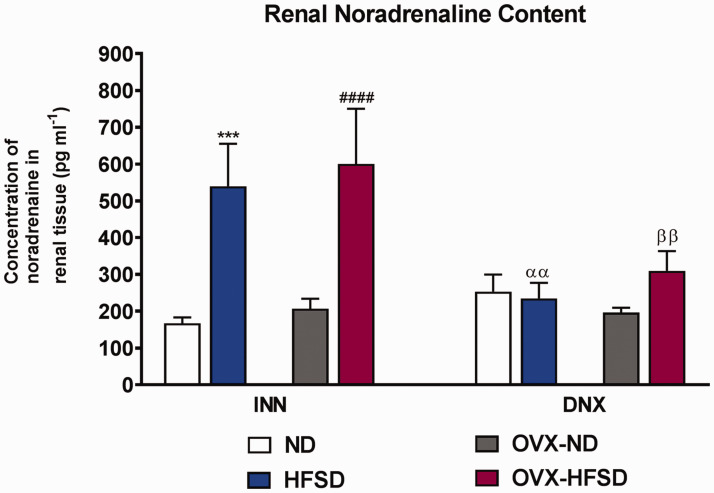
Quantification of renal noradrenaline
Figure 13 shows the renal noradrenaline contents in renal tissues from innervated and denervated ND and HFSD fed rats with and without ovariectomy. The concentration of noradrenaline in renal tissue was significantly higher in both innervated HFSD fed groups of rats by 223% (P < 0.005) and 190% (P < 0.001) in INN-HFSD and INN-OVX-HFSD rats respectively compared to their innervated ND control rats. By contrast, the renal concentration of noradrenaline could not be statistically distinguished across all denervated experimental groups. Nevertheless, renal noradrenaline content was significantly decreased in DNX-HFSD as well as DNX-OVX-HFSD rats compared to INN-HFSD (P < 0.01; 56%) and INN-OVX-HFSD rats (P < 0.01; 48%) respectively. Of note, renal noradrenaline content between INN-HFSD and INN-OVX-HFSD rats was indistinguishable statistically.
Figure 13.
Noradrenaline contents in kidneys of innervated (INN) and denervated (DNX), normal diet (ND) and high-fat style diet (HFSD) fed rats, with and without ovariectomy (OVX). Data are expressed as mean ± SEM (n = 5 per group). ***P < 0.005 vs. INN-ND, ####P < 0.001 vs. INN-OVX-ND; ααP < 0.01 vs. INN-HFSD; ββP < 0.01 vs. INN-OVX-HFSD. (A color version of this figure is available in the online journal.)
Discussion
The purpose of the present study was to test the hypothesis that renal nerve-dependent obesity-induced dysregulation of the low- and high-pressure baroreflex control of RSNA is altered in ovarian hormones deprived rat model. To this end, ovariectomized female rats were fed a HFSD for 10 weeks and the impact on the baroreflex regulation of RSNA when the renal innervation was intact and following its removal was assessed. There were two major findings. Firstly, ovariectomy did not impact on the normal regulation of RSNA by the high- and low-pressure baroreceptors of the cardiovascular system in rats fed a normal diet. Secondly, HFSD loading caused renal nerve-dependent dysregulation of the low- and high-pressure baroreflex control of RSNA in both ovary-intact and ovariectomized female rats to a similar degree. These findings would appear to indicate that female sex hormones do not modulate firstly, the normal baroreflex sensitivity in normal female rats and secondly, impairment of the baroreflex sensitivity in obese female rats. Although the present findings did not support the notion that ovarian hormones have a modulatory effect on the dysregulation of baroreflex control of RSNA in female obesity, there was a propensity for increased BP in the ovarian hormones deprived-obese female rats. Moreover, insulin resistance was present in the ovariectomized obese group as reflected in the high fasting blood glucose.
It is suggested that ovariectomized mice are more prone to obesity as a result of fat accumulation due to the lack of hormonal protection.31 However, our data indicated that ovariectomy alone did not lead to fat accumulation in animals fed the ND at the end of the experimental period; furthermore, no difference in fat mass was observed between OVX-ND and ND groups. This data is in consistent with other study in C57BL/6 mice.32 In the present study, we used HFSD or more popularly known as cafeteria diet to induce obesity in our experimental animals. Exposure of the HFSD which contains 43% fat induces the human metabolic syndrome-like phenotype more robustly than a high-fat diet (HFD).29,33 Furthermore, high energy density and palatability of this type of diet is well known to induce hyperphagia which could not be observed in rodents fed with high-fat diet.29,34–36 Our data indicated that ovariectomized rats had significantly higher body weight gains than did ovary-intact rats irrespective of diet types. Although we failed to measure food consumption, other studies have shown that ovariectomy produces hyperphagia and increases gain in body weight due to increased gain in both lean and fat mass.37,38 Despite the fact that ovariectomized animals gained more body weight in the current study, animals fed the HFSD gained more abdominal fat mass which may be a more adequate metric for obesity than body weight.39 When fed the HFSD, our study showed that OVX-HFSD animals gained more abdominal fat mass which is comparable to the ovary-intact group, suggesting that rats with normal estrous cycle are not protected from excessive fat accumulation when fed the HFSD. However, Ludgero-Correia et al. showed that ovariectomy with high-fat diet consumption led to a significant accumulation of body fat, even when compared to ovary-intact mice fed a high-fat diet.32 The reason for the discrepancy is not obvious but we may suppose that the difference in diet could contribute, HFSD (cafeteria diet) in the present study as opposed to high-fat diet in their study.32 On the other hand, fasting blood glucose was elevated in OVX-HFSD rats which strongly suggests that estrogen deprivation reduces insulin sensitivity. Studies have been demonstrated that estrogen regulates insulin sensitivity and glucose metabolism through central and peripheral actions.40
In the present study, no difference in renal dysfunction was observed between the HFSD fed ovary-intact and ovariectomized female rats. Both groups seemed to exhibit glomerular hyperfiltration which could be associated with obesity and insulin resistance.41–45 Initiation of HFSD feeding in both ovariectomized and ovary-intact groups led to an abrupt and sustained increase in absolute sodium excretion throughout the study which could be ascribed to the higher sodium content in the diet that possibly masked a sodium retention. The sodium content in the HFSD was threefold higher than the normal diet (0.6 g/100 g vs. 0.2 g/100 g). Nevertheless, fractional sodium excretion in HFSD-fed rats which was high at the initiation of the diet declined towards the end of the study indicating an underlying sodium retention. The increase in renal tubular sodium reabsorption in this study was similar to that reported in other studies.25,26,46,47 Possible explanations for the increased sodium reabsorption include activation of the renin-angiotensin-aldosterone system48 together with a direct action of the renal sympathetic nerves on the proximal tubular epithelial cells.49,50 The present study points to an increase in RSNA in view of the enhanced noradrenaline immuno-staining observed in the renal cortex, inner and outer medullary region in the HFSD fed rats.
In the present study, we did not uncover hormonally mediated differences in baroreflex control of RSNA in rats fed a normal diet or a HFSD. Of note, we assessed baroreflex control of RSNA when ovary-intact animals were in the proestrus phase since it has been shown that the gain and maximum of baroreflex control of RSNA are elevated during proestrus when the estrogen level is high.51 Our data, however, showed that normal diet fed ovary-intact and ovariectomized rats had similar high-pressure and low-pressure RSNA baroreflex sensitivity at the end of a 10-week study and this is consistent with previous reports.52–54 In contrast, it has been shown that ovariectomy reduces baroreflex sensitivity in rats and there are experimental evidence to suggest a facilitatory role for estrogen in the modulation of baroreflex function.51,55–57 Furthermore, it has been reported that the suppression of baroreflex sensitivity observed 2–3 weeks after ovariectomy is reversed with estrogen replacement.57–60 It is unlikely that progesterone contributes to the alteration of baroreflex sensitivity following ovariectomy since a neurosteroid metabolite, allopregnanolone, reduces the gain of baroreflex control of RSNA and RSNA baroreflex maximum.61 The reason for the difference between our results and those results on the effects of ovariectomy on cardiovascular regulation is not obvious, nevertheless the observed variability may be explained, in part, by the differing times following ovariectomy at which these studies are performed.51 Indeed, based on telemetry study, the initial reduction of spontaneous baroreflex sensitivity observed following ovariectomy returns towards basal values 5–6 weeks later and this effect is not due to recovery of estrogen levels.51
Dysregulation of the baroreflex control of RSNA was observed in both the ovary-intact and ovariectomized HFSD rats and this finding complements previous studies in obese male Sprague Dawley rats.25,26 The dysregulation of the arterial baroreflex control of RSNA and decline in maximal gain of the RSNA baroreflex curves in the HFSD female rats which was partially re-established following bilateral renal denervation confirms an important role of renal afferent nerve signaling in the resetting of baroreceptor activity in obesity.25,26 This aberrant renal sensory input to the brain may be critical in causing dysfunction of cardiovascular homeostasis and contribute to hypertension in obesity.25,26 Furthermore, the present data revealed an attenuated renal sympatho-inhibitory response to volume expansion in HFSD fed female rats, which was to some degree restored after bilateral renal denervation. This observation suggests that the normal function of the cardiopulmonary baroreflex has been altered through a mechanism that involves the renal afferent nerves and is supported by previous studies in male Sprague-Dawley25,26 as well as Wistar rats62 fed a high-fat diet. Impairment of this reflex can influence extracellular fluid volume and eventually blood pressure in the HFSD rats in the present study.63 Alternatively, it could be argued that the high dietary sodium rather than the high fat intake could in part affect the baroreflex mechanisms since the HFSD contains 0.6% sodium. However, this is unlikely since Huang et al.64 showed that the baroreflex gain curve parameters for RSNA in Wistar male rats fed with high sodium diet (3.0%) for seven weeks were very similar to those fed with normal sodium diet (0.3%).
What was unexpected was that the impairment of the arterial and the cardiopulmonary baroreflex control of RSNA did not differ between the ovary-intact and ovariectomized female rats fed the HFSD. The failure to observe a difference in the baroreflex control of RSNA between the central obese ovary-intact and ovariectomized rats may be due to the timing of the RSNA baroreflex study considering that RSNA baroreflex is estrus-cycle dependent.65 However, measures were taken to ensure that the baroreflex study in ovary-intact animals was performed during proestrus, when estrogen levels were high. Alternatively, the HFSD may alter ovarian function since other study has shown that high-fat diet for 18 weeks in rats accelerates ovarian follicle development and rate of follicular loss leading to premature ovarian failure.66 Notwithstanding, the HFSD feeding period in our study was much shorter than that study, 10 weeks as opposed to 18 weeks, and accordingly may somewhat have lesser effect on the ovary of intact rats in our study. Indeed, it has been shown that high-fat diet feeding of rats for 13 weeks interferes with the normal estrus cycle but does not cause significant changes in serum estradiol levels.67 The present findings seemed to be contrary to the hypothesis that female sex hormones are cardioprotective in both humans and experimental animals. Estrogens may influence factors that profoundly impact the development of diet-induced hypertension including sympatho-excitation, RAS activation and inflammation.68 However, these findings may not necessarily refute the importance of visceral fat that when present in female in significant amounts due to excessive intake of fat could diminish the modulatory role of ovarian hormones, specifically estrogen, in the dysregulation of RSNA baroreflexes.
Interestingly, as opposed to the data obtained with baroreflex control of RSNA, our results indicated that ovariectomized HFSD rats had significantly decreased insulin sensitivity as compared to ovary-intact HFSD rats suggesting that the metabolic and autonomic regulative effects of estrogens can be dissociated. Corroborating data from clinical and experimental studies indicate that estrogens have beneficial effect on insulin action and glucose homeostasis through the activation of the estrogen receptor α.27,69 Our present findings also showed that although baroreflex and renal dysfunction occurred similarly in both the HFSD and OVX-HFSD groups, the tendency to develop hypertension was observed only in the estrogen deprived HFSD-fed rats. These findings may indicate that under the influence of estrogen, other blood pressure regulative mechanisms are shifted towards cardiovascular protective pathways to counteract the detrimental effects of baroreflex and renal dysfunction on BP in the ovary intact obese group. Indeed, estrogen is known to have a role in cardiovascular protection by modulating vasodilator and vasoconstrictor pathways, including the renin-angiotensin aldosterone and the endothelin systems.40 Furthermore, development of hypertension in the OVX-HFSD could also occur through insulin resistance.70
Limitations
We acknowledge several limitations in the present study. The data derived from the acute studies were obtained under anesthesia and may not exactly reflect the situations in conscious animals. Nevertheless, since measurements were undertaken under similar conditions, internal comparisons for most variables would be acceptable although validity may be limited for comparisons between groups. Second, we only assessed sympathetic nerve activity to one vascular bed, i.e. the kidney. It remains unknown whether these results are applicable to other tissues and organs, such as skin, muscle and heart. Third, we have performed ovariectomy in very young female rats although menopause (loss of ovarian follicular function) coincides with aging. The reason for performing this procedure in very young female rats is to negate the aging factor when the RSNA baroreflexes were assessed during the acute study at the end of the experimental diet. Aging per se, is known to cause a series of alterations in the innate mechanisms that regulate cardiovascular function leading to increased risk of cardiovascular disease.71,72 Since ovariectomy is known to aggravate the impairment of cardiac and functional effects of aging,73 there is a plausibility that ovariectomy if performed in mature female rats would have led to a greater obesity-induced dysfunction of the RSNA baroreflexes. Fourth, we do not rule out the contribution of renal afferent nerve activity to the overall integrated renal sympathetic nerve activity signal recorded which would differ between the intact and bilaterally denervated groups. However, it is expected that their contribution would be minimal since their overall firing rate is low compared to that originating from the sympathetic nerves. Further, we have set the low pass filter at 100 Hz on the amplifier which would filter the afferent renal nerve impulses from the signal.
Conclusions
In conclusion, the present study showed that the baroreflex dysfunction and impairment of neurohumoral control of the kidney were not modulated by estrogen in female obesity. Disruption of cardiovascular homeostatic mechanisms and neurohumoral control of the kidney would contribute to the development of hypertension. Since blood pressure seemed to be elevated only in the ovarian hormones deprived obese female rats, we hypothesize that other mechanisms that regulate blood pressure are shifted to cardiovascular protective pathways in obese female rats with normal estrous cycle. Our findings also suggest that in female obesity, there is a break down in the protective effects of ovarian hormones on some of the mechanisms that regulate blood pressure and thus provide insight into the existence of health risks amongst obese women regardless of their ovarian hormonal status.
ACKNOWLEDGMENTS
A certified statistician, Dr. Mahmoud Danaee from Department of Social Preventive Medicine, Faculty of Medicine, Universiti Malaya is gratefully acknowledged due to his assistance in analyzing the data.
Authors’ contributions
YS, EJJ, RH, MAS, NAA participated in the conception, design of the experiments and YS, EJJ, RH, MAS, MA, NA wrote the manuscript. YS performed the experiments as well as collected the data and MK performed acquisition of images for the immunohistochemical detection in a blind manner. YS, EJ, MA, MAS and NA participated in the analysis, and the interpretation of the data. All authors contributed to manuscript revision, read and approved the submitted version.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that the present study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors fully acknowledge the Fundamental Research Grant Scheme (FRGS) grant (FP006-2017A) and University Malaya Research Fund Special Assistance (BKS) grant (BKS072-2017) provided by the Universiti Malaya, Malaysia.
ORCID iD
Nor Azizan Abdullah https://orcid.org/0000-0003-0963-2373
References
- 1.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 2002; 106:2533–6 [DOI] [PubMed] [Google Scholar]
- 2.Serra A, Romero R, Lopez D, Navarro M, Esteve A, Perez N, Alastrue A, Ariza A. Renal injury in the extremely obese patients with normal renal function. Kidney Int 2008; 73:947–55 [DOI] [PubMed] [Google Scholar]
- 3.Shen WW, Chen HM, Chen H, Xu F, Li LS, Liu ZH. Obesity-related glomerulopathy: body mass index and proteinuria. Clin J Am Soc Nephrol 2010; 5:1401–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimeno RE, Klaman LD. Adipose tissue as an active endocrine organ: recent advances. Curr Opin Pharmacol 2005; 5:122–8 [DOI] [PubMed] [Google Scholar]
- 5.Heber D. An integrative view of obesity. Am J Clin Nutr 2010; 91:280s–3s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CJ, Wu CH, Yao WJ, Yang YC, Wu JS, Lu FH. Relationships of age, menopause and central obesity on cardiovascular disease risk factors in Chinese women. Int J Obes Relat Metab Disord 2000; 24:1699–704 [DOI] [PubMed] [Google Scholar]
- 7.Pasquali R, Casimirri F, Pascal G, Tortelli O, Morselli Labate A, Bertazzo D, Vicennati V, Gaddi A. Influence of menopause on blood cholesterol levels in women: the role of body composition, fat distribution and hormonal milieu. Virgilio menopause health group. J Intern Med 1997; 241:195–203 [DOI] [PubMed] [Google Scholar]
- 8.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr 1997; 65:855–60 [DOI] [PubMed] [Google Scholar]
- 9.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension (Hypertension) 2001; 37:1199–208 [DOI] [PubMed] [Google Scholar]
- 10.Iams SG, Wexler BC. Inhibition of the development of spontaneous hypertension in SH rats by gonadectomy or estradiol. J Lab Clin Med 1979; 94:608–16 [PubMed] [Google Scholar]
- 11.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension (Hypertension) 2005; 45:522–5 [DOI] [PubMed] [Google Scholar]
- 12.Chen SC, Lo TC, Chang JH, Kuo HW. Variations in aging, gender, menopause, and obesity and their effects on hypertension in Taiwan. Int J Hypertens 2014; 2014:515297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharabi Y, Grotto I, Huerta M, Grossman E. Susceptibility of the influence of weight on blood pressure in men versus women: lessons from a large-scale study of young adults. Am J Hypertens 2004; 17:404–8 [DOI] [PubMed] [Google Scholar]
- 14.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002; 162:1867–72 [DOI] [PubMed] [Google Scholar]
- 15.Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO expert consultation on waist circumference and Waist-Hip ratio. Eur J Clin Nutr 2010; 64:2–5 [DOI] [PubMed] [Google Scholar]
- 16.Hogarth AJ, Graham LN, Corrigan JH, Deuchars J, Mary DA, Greenwood JP. Sympathetic nerve hyperactivity and its effect in postmenopausal women. J Hypertens 2011; 29:2167–75 [DOI] [PubMed] [Google Scholar]
- 17.Munoz J, Derstine A, Gower BA. Fat distribution and insulin sensitivity in postmenopausal women: influence of hormone replacement. Obes Res 2002; 10:424–31 [DOI] [PubMed] [Google Scholar]
- 18.Trikudanathan S, Pedley A, Massaro JM, Hoffmann U, Seely EW, Murabito JM, Fox CS. Association of female reproductive factors with body composition: the Framingham heart study. J Clin Endocrinol Metab 2013; 98:236–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham heart study. Hypertension 2011; 58:784–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campese VM, Mitra N, Sandee D. Hypertension in renal parenchymal disease: why is it so resistant to treatment? Kidney Int 2006; 69:967–73 [DOI] [PubMed] [Google Scholar]
- 21.Hall JE. The kidney, hypertension, and obesity. Hypertension (Hypertension) 2003; 41:625–33 [DOI] [PubMed] [Google Scholar]
- 22.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 2014; 7:75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ditting T, Tiegs G, Veelken R. Autonomous innervation in renal inflammatory disease-innocent bystander or active modulator? J Mol Med 2009; 87:865–70 [DOI] [PubMed] [Google Scholar]
- 24.Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–6 [PubMed] [Google Scholar]
- 25.Khan SA, Sattar MZ, Abdullah NA, Rathore HA, Abdulla MH, Ahmad A, Johns EJ. Obesity depresses baroreflex control of renal sympathetic nerve activity and heart rate in Sprague Dawley rats: role of the renal innervation. Acta Physiol (Oxf) 2015; 214:390–401 [DOI] [PubMed] [Google Scholar]
- 26.Khan SA, Sattar MZA, Abdullah NA, Rathore HA, Ahmad A, Abdulla MH, Johns EJ. Improvement in baroreflex control of renal sympathetic nerve activity in obese Sprague Dawley rats following immunosuppression. Acta Physiol (Oxf) 2017; 221:250–65 [DOI] [PubMed] [Google Scholar]
- 27.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009; 150:2109–17 [DOI] [PubMed] [Google Scholar]
- 28.Muntzel MS, Al-Naimi OA, Barclay A, Ajasin D. Cafeteria diet increases fat mass and chronically elevates lumbar sympathetic nerve activity in rats. Hypertension (Hypertension) 2012; 60:1498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011; 19:1109–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 2001; 74:435–40 [DOI] [PubMed] [Google Scholar]
- 31.Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J 2009; 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludgero-Correia A, Jr., Aguila MB, Mandarim-de-Lacerda CA, Faria TS. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition (Nutrition) 2012; 28:316–23 [DOI] [PubMed] [Google Scholar]
- 33.Lalanza JF, Caimari A, del Bas JM, Torregrosa D, Cigarroa I, Pallàs M, Capdevila L, Arola L, Escorihuela RM. Effects of a post-weaning cafeteria diet in young rats: metabolic syndrome, reduced activity and low anxiety-like behaviour. PloS One 2014; 9:e85049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafat A, Murray B, Rumsey D. Energy density in cafeteria diet induced hyperphagia in the rat. Appetite 2009; 52:34–8 [DOI] [PubMed] [Google Scholar]
- 35.Esposito K, Nappo F, Giugliano F, Di Palo C, Ciotola M, Barbieri M, Paolisso G, Giugliano D. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am J Clin Nutr 2003; 78:1135–40 [DOI] [PubMed] [Google Scholar]
- 36.Martire SI, Holmes N, Westbrook RF, Morris MJ. Altered feeding patterns in rats exposed to a palatable cafeteria diet: increased snacking and its implications for development of obesity. PloS One 2013; 8:e60407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Heiman ML. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am J Physiol Endocrinol Metab 2001; 280:E315–E22 [DOI] [PubMed] [Google Scholar]
- 38.Jiang JMY, Sacco SM, Ward WE. Ovariectomy-induced hyperphagia does not modulate bone mineral density or bone strength in rats. J Nutr 2008; 138:2106–10 [DOI] [PubMed] [Google Scholar]
- 39.Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, Xiang AH, Watanabe RM. A better index of body adiposity. Obesity (Silver Spring) 2011; 19:1083–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 2018; 14:185–201 [DOI] [PubMed] [Google Scholar]
- 41.Bosma RJ, van der Heide JJ, Oosterop EJ, de Jong PE, Navis G. Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int 2004; 65:259–65 [DOI] [PubMed] [Google Scholar]
- 42.Nerpin E, Riserus U, Ingelsson E, Sundstrom J, Jobs M, Larsson A, Basu S, Arnlov J. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care 2008; 31:1550–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaglione R, Ganguzza A, Corrao S, Parrinello G, Merlino G, Dichiara MA, Arnone S, D’Aubert MD, Licata G. Central obesity and hypertension: pathophysiologic role of renal haemodynamics and function. International Journal of Obesity and Related Metabolic Disorders: journal of the International Association for the Study of Obesity 1995; 19:403–9 [PubMed] [Google Scholar]
- 44.Tomaszewski M, Charchar FJ, Maric C, McClure J, Crawford L, Grzeszczak W, Sattar N, Zukowska-Szczechowska E, Dominiczak AF. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 2007; 71:816–21 [DOI] [PubMed] [Google Scholar]
- 45.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2007; 2:550–62 [DOI] [PubMed] [Google Scholar]
- 46.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 2010; 285:17271–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chagnac A, Herman M, Zingerman B, Erman A, Rozen-Zvi B, Hirsh J, Gafter U. Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant 2008; 23:3946–52 [DOI] [PubMed] [Google Scholar]
- 48.Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 2016; 311:F555–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 2011; 1:731–67 [DOI] [PubMed] [Google Scholar]
- 50.Healy V, Thompson C, Johns EJ. The adrenergic regulation of proximal tubular Na(+)/H(+) exchanger 3 in the rat. Acta Physiol (Oxf) 2014; 210:678–89 [DOI] [PubMed] [Google Scholar]
- 51.Goldman RK, Azar AS, Mulvaney JM, Hinojosa-Laborde C, Haywood JR, Brooks VL. Baroreflex sensitivity varies during the rat estrous cycle: role of gonadal steroids. Am J Physiol Regul Integr Comp Physiol 2009; 296:R1419–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Middlekauff HR, Park J, Gornbein JA. Lack of effect of ovarian cycle and oral contraceptives on baroreceptor and nonbaroreceptor control of sympathetic nerve activity in healthy women. Am J Physiol Heart Circ Physiol 2012; 302:H2560–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab 2009; 297:E85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol (Lond) 2009; 587:2019–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He XR, Wang W, Crofton JT, Share L. Effects of 17beta-estradiol on sympathetic activity and pressor response to phenylephrine in ovariectomized rats. Am J Physiol 1998; 275:R1202–8 [DOI] [PubMed] [Google Scholar]
- 56.Mohamed MK, El-Mas MM, Abdel-Rahman AA. Estrogen enhancement of baroreflex sensitivity is centrally mediated. Am J Physiol 1999; 276:R1030–7 [DOI] [PubMed] [Google Scholar]
- 57.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res 2000; 879:105–14 [DOI] [PubMed] [Google Scholar]
- 58.Saleh TM, Connell BJ, Saleh MC. Acute injection of 17beta-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Auton Neurosci 2000; 84:78–88 [DOI] [PubMed] [Google Scholar]
- 59.Saleh TM, Connell BJ. 17beta-estradiol modulates baroreflex sensitivity and autonomic tone of female rats. J Auton Nerv Syst 2000; 80:148–61 [DOI] [PubMed] [Google Scholar]
- 60.Saleh TM, Connell BJ. Role of oestrogen in the Central regulation of autonomic function. Clin Exp Pharmacol Physiol 2007; 34:827–32 [DOI] [PubMed] [Google Scholar]
- 61.Heesch CM, Foley CM. CNS effects of ovarian hormones and metabolites on neural control of circulation. Ann N Y Acad Sci 2001; 940:348–60 [DOI] [PubMed] [Google Scholar]
- 62.Wong PSK, Johns EJ. Effect of acute saline volume loading on renal sympathetic nerve activity in anaesthetised fructose-fed and fat-fed rats. J Auton Nerv Syst 1999; 75:60–9 [DOI] [PubMed] [Google Scholar]
- 63.Johns EJ. The neural regulation of the kidney in hypertension and renal failure. Exp Physiol 2014; 99:289–94 [DOI] [PubMed] [Google Scholar]
- 64.Huang C, Yoshimoto M, Miki K, Johns EJ. The contribution of brain angiotensin II to the baroreflex regulation of renal sympathetic nerve activity in conscious normotensive and hypertensive rats. J Physiol (Lond) 2006; 574:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi Z, Brooks VL. Leptin differentially increases sympathetic nerve activity and its baroreflex regulation in female rats: role of oestrogen. J Physiol (Lond) 2015; 593:1633–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, Liu WJ, Fu YC. Obesity accelerates ovarian follicle development and follicle loss in rats. Metab Clin Exp 2014; 63:94–103 [DOI] [PubMed] [Google Scholar]
- 67.Hussain MA, Abogresha NM, Hassan R, Tamany DA, Lotfy M. Effect of feeding a high-fat diet independently of caloric intake on reproductive function in diet-induced obese female rats. Arch Med Sci 2016; 12:906–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015; 116:991–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep 2004; 6:180–5 [DOI] [PubMed] [Google Scholar]
- 70.Roza NA, Possignolo LF, Palanch AC, Gontijo JA. Effect of long-term high-fat diet intake on peripheral insulin sensibility, blood pressure, and renal function in female rats. Food Nutr Res 2016; 60:28536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation 2003; 107:490–7 [DOI] [PubMed] [Google Scholar]
- 72.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation 2003; 107:139–46 [DOI] [PubMed] [Google Scholar]
- 73.Novensa L, Novella S, Medina P, Segarra G, Castillo N, Heras M, Hermenegildo C, Dantas AP. Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERalpha/ERbeta balance in female mice. PloS One 2011; 6:e25335. [DOI] [PMC free article] [PubMed] [Google Scholar]