Abstract
Peripheral artery disease (PAD) is a major health problem and is caused by atherosclerosis in arteries outside the heart leading to impaired blood flow. The presence of diabetes significantly increases the likelihood of having worse outcomes in PAD, and the molecular mechanisms involved are poorly understood. Hyperglycemia in diabetes activates the nuclear factor-kappa B (NF-κB) pathway, and chronic inflammation in diabetes is associated with vascular complications. Ischemia also activates NF-κB signaling that is important for perfusion recovery in experimental PAD. We hypothesized that prolonged exposure of endothelial cells to high glucose in diabetes impairs ischemic activation of the NF-κB pathway and contributes to poor perfusion recovery in experimental PAD. We assessed the effect of high glucose and ischemia on canonical and non-canonical NF-κB activation in endothelial cells and found both conditions activate both pathways. However, exposure of endothelial cells to high glucose impairs ischemia-induced activation of the canonical NF-κB pathway but not the non-canonical pathway. We probed an array of antibodies against signaling proteins in the NF-κB pathway to identify proteins whose phosphorylation status are altered in endothelial cells exposed to high glucose. Protein kinase C beta (PKCβ) was among the proteins identified, and its role in impaired ischemia-induced activation of NF-κB during hyperglycemia has not been previously described. Inhibition of PKCβ improves ischemia-induced NF-κB activation in vitroand in vivo. It also improves perfusion recovery in diabetic mice following experimental PAD. Thus, in diabetes, PKCβ phosphorylation contributes to impaired ischemic activation of NF-κB and likely a mechanism contributing to poor PAD outcomes.
Impact statement
Diabetes worsens the outcomes of peripheral arterial disease (PAD) likely in part through inducing chronic inflammation. However, in PAD, recovery requires the nuclear factor-kappa B (NF-κB) activation, a known contributor to inflammation. Our study shows that individually, both ischemia and high glucose activate the canonical and non-canonical arms of the NF-κB pathways. We show for the first time that prolonged high glucose specifically impairs ischemia-induced activation of the canonical NF-κB pathway through activation of protein kinase C beta (PKCβ). Accordingly, inhibition of PKCβ restores the ischemia-induced NF-κB activity both in vitroin endothelial cells and in vivoin hind limbs of type 1 diabetic mice and improves perfusion recovery after experimental PAD. Thus, this study provides a mechanistic insight into how diabetes contributes to poor outcomes in PAD and a potential translational approach to improve PAD outcomes.
Keywords: Nuclear factor-kappa B, ischemia, hyperglycemia, diabetes, protein kinase C beta, peripheral arterial disease
Introduction
Peripheral artery disease (PAD) is a chronic disease of blood vessels that affects approximately 12 million people in the United States and over 200 million people worldwide.1There are two classic clinical presentations of PAD, intermittent claudication (lower extremity pain with ambulation relieved by rest) and critical limb ischemia (CLI, pain at rest that may be associated with ulceration or gangrene). Risk factors for development of PAD are the same for development of coronary artery disease and include smoking, diabetes, hypertension, and hyperlipidemia.2However, diabetes and smoking account for 80% of the risk of developing PAD. Moreover, individuals with PAD and diabetes are five times more likely to develop CLI.2
Prior studies by our lab and others have shown that in preclinical models of PAD (experimental PAD), diabetes impairs postischemic angiogenesis and perfusion recovery.3Although some studies have provided mechanistic insight into how diabetes may impair perfusion recovery in experimental PAD, the molecular mechanisms involved remains poorly understood.3Hyperglycemia is a key metabolic derangement in diabetes. It is also known that inflammation plays a key role in many of the pathologic processes associated with diabetes complications.4NF-κB is a key transcription factor involved in inflammation,4and studies have shown that high glucose can activate NF-κB signaling.5,6The NF-κB transcription factors bind to their target DNA sequences as dimers composed of the members of the NF-κB family. These members include RelA (also named p65), RelB, c-Rel, NF-κB1 (p50/p105), and NF-κB2 (p52/p100), which form various combinations of dimers to transactivate target genes. The p105 and p100 proteins are precursors to p50 and p52 subunits, respectively.7,8Inducer-mediated canonical activation of NF-κB is accomplished by phosphorylation and degradation of Iκ-Bα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha) leading to nuclear localization of the NF-κB complex that binds to the IκB sequence of the target genes.9In a non-canonical activation, RelB/p52 dimer complex is activated.10The p100 protein functions in similar manner as IκBα to inhibit RelB nuclear translocation. An inducible processing of p100 whereby the C-terminal of the p100 is proteolyzed yields the p52 subunit, and the RelB/p52 dimers translocate to the nucleus to function as transcription factors.
Therefore, it is possible that the NF-κB pathway may be involved in the impaired perfusion recovery observed following experimental PAD in the context of diabetes. Interestingly, studies have also shown that ischemia activates NF-κB,11,12and this signaling pathway is a key regulator of arteriogenesis, collateral formation as well as tissue perfusion following experimental PAD.13However, how high glucose activation of the NF-κB pathway in diabetes impacts activation of the same pathway by ischemia is not clear. Moreover, whether the canonical and non-canonical pathways play differing roles in postischemic perfusion recovery is not known. We hypothesized that prolonged exposure of endothelial cells to high glucose in diabetes leads to chronic activation of the NF-κB pathway that impairs ischemia-induced activation of the pathway and contributes to poor perfusion recovery following experimental PAD. We conducted a series of in vitroand in vivostudies to test this hypothesis.
Materials and methods
Animal studies
All experiments were conducted according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Sciences Center. Mice (C57BL/6J, Catalog # 000664, and C57BL/6J-Ins2Akita/J, Catalog # 003548) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). The Ins2Akita is a well-studied model of type 1 diabetes.14,15Mice had free access to water and standard rodent chow. The PKCβ inhibitor, ruboxistaurin (Rbx; LY333531, Cat# HY-10195B, MedChemExpress, Princeton, NJ, USA), was administered to mice at 1 mg/kg/day by oral gavage starting 5 days prior to induction of hind limb ischemia (HLI) and continued through the study.16
HLI or experimental PAD
HLI was induced by unilateral ligation and excision of the femoral artery as previously described.17Control mice were matched for strain, sex, and age. Diabetes was confirmed in mice by Hba1c measurement (Hba1c >8) prior to the experiments. Both experimental and control groups had similar glycemic exposure as determined by HbA1c. Ischemia was confirmed by monitoring postsurgical blood flow in the ischemic and contralateral non-ischemic limbs by Laser Speckle Contrast Imaging (Perimed AB, Sweden) as described previously.18,19Measurements of perfusion recovery were performed immediately after HLI surgery, (day 0) and then on day 3, and once weekly for 2 weeks.
Cell cultures
Pooled human vascular endothelial cells (HUVECs) between passage 4 to 7 were used (Cell Applications, Cat # 200p-05n, San Diego, CA, USA). Cells were grown in endothelial cell growth medium (ECGM, Cell Applications, Inc., San Diego, CA, USA) supplemented with 10% fetal bovine serum (FBS, Cat # 10437028, Gibco-Thermo Fisher Scientific, Waltham, MA, USA) under standard culture conditions (20% O2) or 24 h in ischemic conditions (2% O2without FBS, Supplemental Material). Ischemia was simulated in vitroas previously described by culturing cells in ECGM without serum and in 2% oxygen (BioSpherix, Lacona).20Hyperglycemic conditions were created by supplementing the ECGM with 25 mM D-glucose (normal glucose concentration 5 mM D-glucose). Inhibition of PKCβ was achieved by addition of Rbx at 20 nm/L, 30 min before adding high D-glucose to ECGM.16
Protein analysis
Cell and tissue lysates were prepared as described previously.21,22Nuclear extracts were prepared using a commercially available kit (NE-PER, ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Protein expression was measured by Western blot analysis using anti-IκBα (Cat#9242 CST), anti-phospho-IκB (Cat#9246, CST), anti-phospho-p65 (Cat#3036, CST), anti-p65 (Cat# 3033 CST), anti-RelB antibody (Cat#4922 CST), anti-cRel antibody (Cat#12707 CST), anti-pSer661-PKCβ (Cat# DBOA15543, Vita Scientific), and anti-PKCβ1/2 Cat# 46809 (Cell Signaling Technology, Danvers, MA, USA) primary antibodies. Western blot gel images were captured by Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Quantification of the protein bands on blots were performed by Scion Image software (Torrance, CA, USA) and Image Studio Lite version 5.2 (Li-COR Biotechnology, Lincon, Nebraska). Abundance of phosphoproteins was determined as the ratio of band intensity of phosphoprotein to the total protein. For loading control, antibody to β-actin was used. For blots involving non-ischemic and ischemic samples, Ponceau S-stained protein bands in the respective lanes were used as loading controls since ischemia alters expression of β-actin and many typical loading controls.
Phosphoprotein array
A high throughput platform of antibodies array was used to detect differences in phosphorylation of signaling molecules involved in the NF-κB pathway (NF-κB Phospho Antibody Array, Cat# PNK215, Full Moon Biosystems, Sunnyvale, CA, USA). Cell extracts from HUVEC exposed to either 25 mM L-glucose or 25 mM D-glucose were analyzed for the phosphoprotein levels according to the manufacturer’s suggested protocol. Fluorescence signals were recorded and digitized using a microarray scanner and analyzed by manufacturer’s recommendations using ImageJ.23Following background corrections, the signals were normalized against the median intensity of all the experimental spots on the array. The fold change in phosphorylation was calculated by dividing the intensity of the phosphorylated spot by the signal intensity of corresponding non-phosphorylated spot for each protein. Differential expression between the 25 mM L-glucose and 25 mM D-glucose samples was calculated by dividing the phosphorylation ratio of the 25 mM D-glucose with that of the 25 mM L-glucose control. Significant expression was taken as greater than 1.4- or less than 0.6-fold. This experiment was carried out with three samples each per experimental group, and the array contained six spots for each antibody.
Statistical analysis
All measurements are expressed as mean ± SEM. Statistical comparisons between two groups (e.g. treated vs. untreated) were performed by t-test; where more than two groups were involved, we used one-way analysis of variance. A Pvalue of <0.05 was considered statistically significant.
Data and resource availability
The data-sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Results
Ischemia activates canonical and non-canonical pathways of NF-κB in endothelial cells
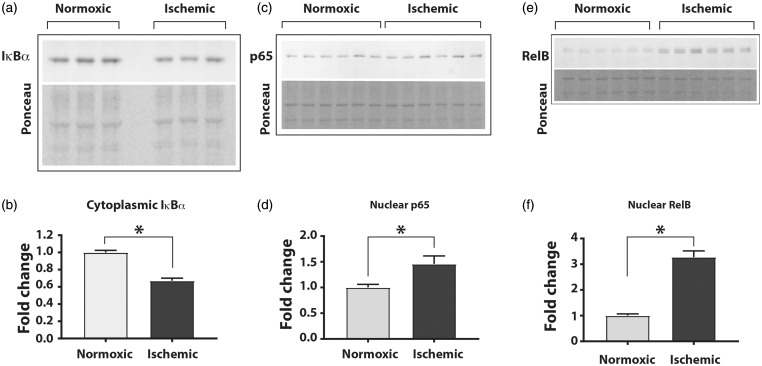
We first determined whether ischemia induces the canonical or non-canonical pathway of NF-κB activation in cultured HUVEC. Cultured cells were exposed for 24 h to either normal or ischemic conditions followed by preparation of cytoplasmic and nuclear fractions. Western blot analyses of the cytoplasmic fraction showed that compared to the cells grown under normal conditions, the cells exposed to ischemia had a significant decrease in IκBα protein, an indicator of the activated canonical NF-κB pathway (Figure 1(a) and (b); fold change normoxic to ischemic; 1.0 ± 0.02 vs. 0.67 ± 0.03, n = 6, P < 0.05). Analyses of the cell lysates from cells exposed to ischemia showed no change in p65 expression (Supplemental Figure 1a and b) but a significant increase in nuclear abundance of p65 subunit of the NF-κB (Figure 1(c) and (d); fold change normoxic to ischemic 1.0 ± 0.06 vs. 1.45 ± 0.15, n = 6, P< 0.05). Additionally, expression of total RelB, a component of the non-canonical NF-κB pathway, was significantly increased in cell lysates (Supplemental Figure 1a and b, bottom left panel) and also in the nuclear fractions of the cells exposed to ischemia (Figure 1(e) and (f); fold change normoxic to ischemic 1.0 ± 0.16 vs. 3.27 ± 0.5, n = 6, P < 0.01). However, the total level of c-Rel was not changed in ischemic cells (Supplemental Figure 1a and b). Thus, ischemia induced activation of both canonical and the non-canonical pathways of the NF-κB in cultured endothelial cells.
Figure 1.
Ischemia activates the canonical and non-canonical NF-κB pathways in HUVEC. Cells were exposed to either normoxia (21% O2) or ischemia (2% oxygen and nutrient starvation) for 24 h followed by isolation of cytoplasmic and nuclear fractions. (a) The cytoplasmic fractions were analyzed for expression of IκBα by Western blots using specific antibody, and (b) signals were quantified using densitometry by measuring the ratio of band intensity to total protein in the corresponding lanes of the Ponceau S-stained blots. (c) Western blots of the nuclear fractions were probed with anti-p65/RelA antibody and (d) signal quantified by ratio of p65 band intensity to total protein in the lanes, (e) Western blot for non-canonical pathway using an anti-RelB antibody and (f) quantitation of the signal intensity. The total protein from the respective lanes of Ponceau S-stained blots was used. Unpaired t-test with Welch’s correction and two-tailed distribution was used for statistical calculations. Asterisks denote P < 0.05, and n= 6 to 12 in each group.
Short-term exposure to high glucose activates both canonical and non-canonical NF-κB pathways
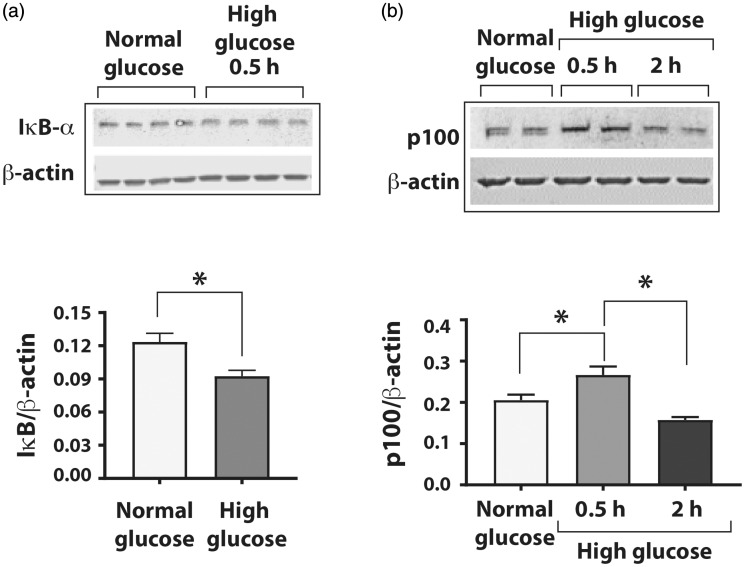
Hyperglycemia is known to cause inflammatory changes in endothelial cells through activation of the NF-κB pathways.24However, the duration of exposure necessary for activation varies in different studies.25,26We assessed the effects of short and prolonged exposures of HUVECs to high glucose on the activation of canonical and non-canonical NF-κB pathways. Western blot analyses showed that compared to the HUVEC grown in normal glucose, the cells grown in high glucose concentration for 0.5 h had significantly decreased IκBα levels (Figure 2(a); IκBα/β-actin 0.120 ± 0.004 vs. 0.100 ± 0.003, n = 4, P < 0.05), which was restored after 2 h (Supplemental Figure 2). Exposure of HUVEC to high glucose decreased the p100 processing (a measure of non-canonical NF-kB pathway activation) at 0.5 h but significantly increased the degradation of p100 at 2 h compared to the cells in normal glucose (Figure 2(b); p100/β-actin 0.20 ± 0.01 in normal glucose vs. 0.27 ± 0.01 in high D-glucose at 0.5 h vs. 0.16 ± 0.00 in high D-glucose at 2 h, n = 4, P < 0.05). Thus, high glucose activates both canonical and non-canonical NF-κB pathway with the activation of the canonical pathway preceding the non-canonical pathway.
Figure 2.
High glucose activates both canonical and non-canonical NF-κB pathways in HUVEC. Cells were exposed to normal D-glucose (5 mM), high D-glucose (25 mM), or high L-glucose (25 mM) for varying durations, and cell lysates were prepared for Western blot analyses for either degradation of IκBα (the canonical pathway) or of p100 (non-canonical pathway) using specific antibodies. Signals generated by antibody to β-actin were used for normalizing the results. (a) Western blot of total lysates from endothelial cells exposed to normal D-glucose or high D-glucose for 0.5 h showing signals for IκBα and β-actin. Changes in protein levels of IκBα in cells exposed to high D-glucose are presented as ratio of IκBα/β-actin. (b) High glucose exposure induced the non-canonical NF-κB pathway by degradation of p100 subunit after 2 h of high D-glucose exposure. The graph shows reduced p100 on the Western blot after 2 h of high D-glucose exposure. Asterisks denote P < 0.05, and n= 5 to 14 in each group.
Prolonged high glucose stimulation impairs ischemia-induced IκBα phosphorylation and degradation in endothelial cells
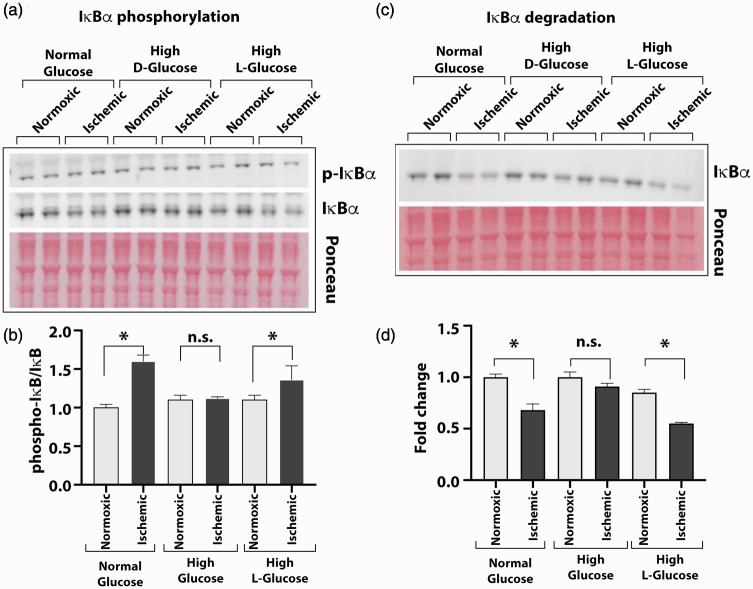
To determine the impact of high glucose on ischemia-induced NF-κB activation, we assessed the extent of IκBα phosphorylation and degradation. HUVEC cultures were grown for three days in normal D-glucose, high D-glucose, or in metabolically inert high L-glucose and subjected to ischemia for 24 hours. Analyses of cellular lysates by Western blots showed a significant increase in the phosphorylation of IκBα protein (pIκBα) from ischemic cells cultured in normal glucose and those in high L-glucose but not in the lysates from the ischemic cells cultured in high D-glucose (Figure 3(a) and (b); pIκBα/IκBα in normal glucose 1.59 ± 0.08; high L-glucose 1.36 ± 0.17, high D-glucose 1.0 ± 0.05, n = 4, P < 0.05).
Figure 3.
High glucose exposure attenuates the ischemia-induced NF-κB activation. HUVEC were grown in normal (5 mM) or high D-glucose (25 mM) containing culture medium before exposing them to ischemia and analyzing cell lysates for IκBα phosphorylation and degradation to measure the activation of NF-κB. Control cells were also grown in high concentration of metabolically inert L-glucose (25 mM) in these experiments. (a) Western blots of phosphorylated (p-IκBα) and unphosphorylated (IκBα) forms of IĸBα protein from cells grown in normal, high D-glucose, or high L-glucose with normal (normoxic) or ischemic treatments. (b) Intensity of protein bands on Ponceau S-stained blots was used for loading control. Protein quantification of IĸBα was done by densitometry, and results are presented in the bar graph as the ratio of p-IκBα to unphosphorylated form. (c) Degradation of IκBα was tested by Western blot of the HUVEC cultures in normal or high glucose conditions using specific antibody. Signals from Ponceau S-stained the blot were used for loading controls. (d) The ratio of IκBα to total protein is presented in the bar graph. Pair-wise comparisons were done between normoxic and ischemic samples in each group for statistical analyses. Asterisks denote P < 0.05, and n.s. denotes not significant; n = 5 to 14 in each group. (A color version of this figure is available in the online journal.)
Consistent with the above findings, IκBα levels decreased in ischemic cells grown in normal D-glucose and high L-glucose but not in high D-glucose (Figure 3(c) and (d); fold change normoxic vs. ischemic 0.68 ± 0.06 in normal glucose, 0.55 ± 0.03 in high L-glucose and 0.91 ± 0.03 in high D-glucose, n = 3–4, P < 0.05). These results suggest that hyperglycemic condition impairs the phosphorylation and degradation of IκBα, thus impairing the ischemia-induced activation of the NF-κB pathway in endothelial cells.
Prolonged high glucose stimulation impairs canonical NF-κB pathway activity but not the non-canonical NF-κB pathway activity in endothelial cells
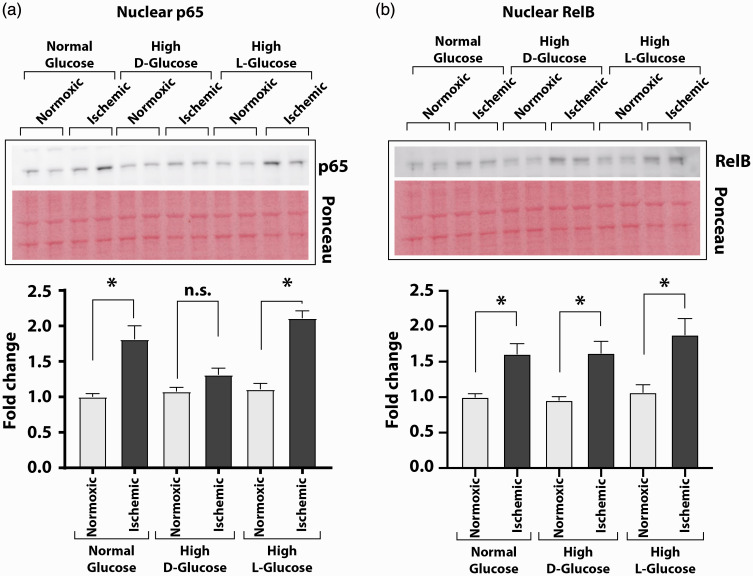
We next examined whether prolonged high glucose exposure dampens the activation of both the canonical and the non-canonical NF-κB pathway. Western blots analyses showed significant increase of p65 subunit (the canonical pathway) in the nuclear extracts from ischemic cells grown either in normal D-glucose or in high L-glucose but not in high D-glucose (Figure 4(a); nuclear p65/Ponceau: ischemic to non-ischemic 1.81 ± 0.19 vs. 1.00 ± 0.05 in normal D-glucose, 2.11 ± 0.10 vs. 1.11 ± 0.08 in high L-glucose and 1.31 ± 0.09 vs. 1.08 ± 0.06 in high D-glucose, n = 6–8, P < 0.05). However, analysis of nuclear RelB subunit (the non-canonical pathway) was increased in all three condition (Figure 4(b); nuclear RelB/Ponceau: ischemic to non-ischemic 1.61 ± 0.15 vs. 1.00 ± 0.05 in normal D-glucose, 1.62 ± 0.17 to 0.96 ± 0.05 in high D-glucose, 1.88 ± 0.23 vs. 1.07 ± 0.11 in high L-glucose, n = 6–7, P < 0.05). Thus, hyperglycemic conditions impair ischemia-induced activation of the canonical NF-κB pathway but not the non-canonical pathway in endothelial cells.
Figure 4.
High D-glucose condition impairs the canonical pathway of ischemia-induced NF-κB activation. Nuclear fractions from HUVEC grown in normal or high D-glucose containing medium were analyzed by Western blotting for enrichment of NF-κB subunits, and quantified results of densitometry are presented in bar graphs. (a) Compared to the nuclear extracts of the normoxic cells, the nuclear fractions of the ischemic cells contained significantly greater amounts of p65 subunit of the NF-κB when grown in normal D-glucose. The p65 subunit did not accumulate in the nuclear fractions of ischemic cells grown in high D-glucose. Nuclear enrichment of p65 was also seen in the ischemic cells grown in high L-glucose-containing medium. (b) RelB subunit was significantly more enriched in the nuclear fraction of the ischemic HUVEC grown in normal D-glucose, high L-glucose, or high D-glucose than in the nuclear fractions of the normoxic cells. Results in the graphs are from densitometry of Western blot signals and are presented as the ratio of target protein signal to Ponceau S-stained total protein signal on blots. Asterisks denote P < 0.05, and n.s. denotes not significant; n= 4 to 6 per group. (A color version of this figure is available in the online journal.)
High glucose exposure causes hyperphosphorylation of PKCβ in non-ischemic and ischemic endothelial cells
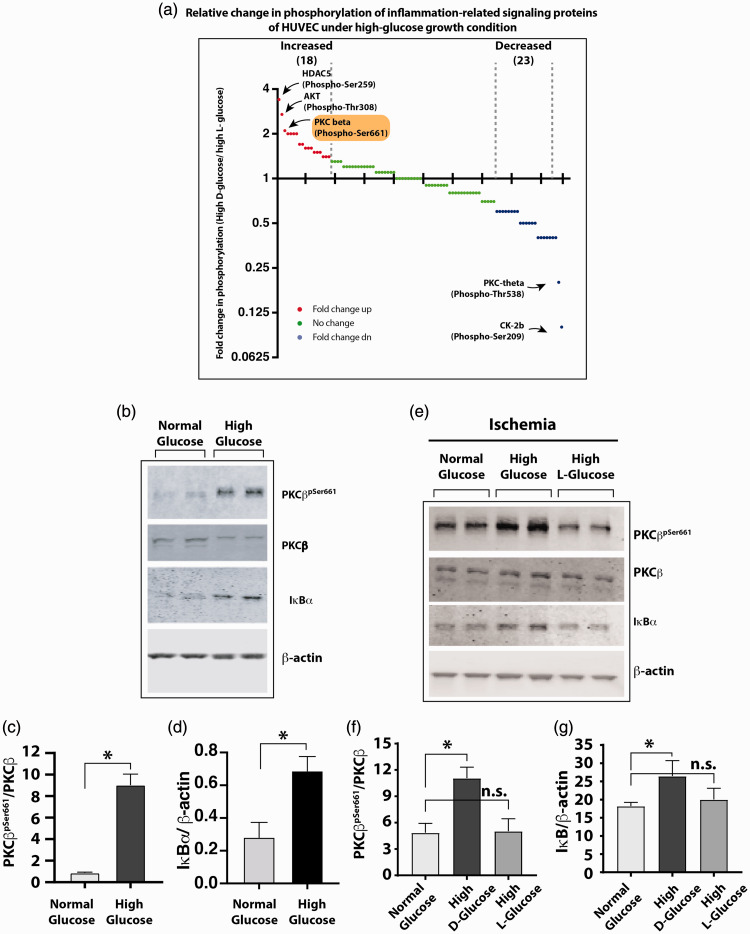
To further understand how hyperglycemia attenuates ischemia-induced NF-κB activation in HUVECs, we used a high-density array of 215 antibodies to compare the phosphorylation states of proteins involved in NF-κB activation. We used lysates from HUVECs that were grown in either high L-glucose or high D-glucose for three days. Comparison of the protein phosphorylation showed that phosphorylation of several signaling proteins was increased in the high D-glucose-grown cells, while phosphorylation of several other proteins was decreased (Figure 5(a)). Histone deacetylase 5 (HDAC5pSer259), protein kinase B (AKTpThr308), and PKCβpSer661ranked among the highest phosphorylated proteins under the high D-glucose conditions. A literature-based analysis identified PKCβ to be implicated in hyperglycemic toxicity and endothelial dysfunction.27,28Hence, we selected PKCβ for a detailed study. Western blot comparisons of PKCβpSer661in the lysates from HUVECs confirmed increased phosphorylation of PKCβ under high D-glucose growth condition (Figure 5(b) and (c); PKCβpSer661/PKCβ in normal glucose vs. high glucose 0.85 ± 0.16 vs. 8.76 ± 1.85, n = 4, P < 0.01). This increase in PKCβpSer661coincided with increased IκBα protein (Figure 5(b) and (d); IκBα/β-actin, 0.28 ± 0.05 in normal D-glucose vs. 0.69 ± 0.04 in high D-glucose, n = 4, P < 0.01) likely because of reduced degradation of IκBα protein.
Figure 5.
(a) Antibody array-based screening of relative change in phosphorylation state of proteins involved in the NF-κB activation pathway. Glass slides with printed panel of 215 antibodies were used to detect the relative abundance of total and phosphorylated proteins in HUVEC grown in either high (25 mM) L-glucose or D-glucose for 3 days. Cell lysates were overlaid on antibody panels and signals were detected using fluorescent-tagged secondary antibody. Digitized signals were analyzed as described in Materials and methods section. The graph shows fold change in the phosphorylated protein signals as a ratio of phosphorylated/total signal from high D-glucose- to high L-glucose-grown cells. The top three greatest increase in phosphorylation is labeled on the graph. PKCβ (phospho-Ser661) signal was among the highest increase signals. (b) Western blots of HUVEC grown in high D-glucose and normal D-glucose analyzed for phosphorylated PKCβ (PKCβpSer661) and accumulation of IκBα. (c) High glucose enhances phosphorylation of Serine-661 residue of PKCβ as measured by the ratio of PKCβpSer661to PKCβ. (d) High glucose impairs degradation of IκBα; the IκBα protein was significantly increased in high D-glucose-grown cells. (e) Western blots showing phosphorylated and total PKCβ and IκBα in high D-glucose-grown HUVEC subjected to ischemia. (f) PKCβ was increased in high D-glucose-grown cells with ischemia as measured by PKCβpSer661/PKCβ ratio. (g) The IκBα levels also were significantly increased in ischemic cells grown in high D-glucose. Asterisks denote P < 0.05, and n.s. denotes not significant; n= 4 to 6 per group. (A color version of this figure is available in the online journal.)
HUVEC: human vascular endothelial cell; HDAC5: histone deacetylase 5; PKC: protein kinase C; AKT: protein kinase B.
We next analyzed whether hyperphosphorylation of PKCβpSer661is associated with impaired ischemia-induced NF-κB activation in endothelial cells following prolonged high glucose stimulation. Lysates from HUVECs exposed to ischemia in the presence of normal D-glucose, high D-glucose, or high L-glucose were analyzed by Western blotting. There was increased PKCβpSer661in the high D-glucose-treated cells compared to the lysates from normal D-glucose or high L-glucose-treated cells (Figure 5(e) and (f); PKCβpSer661/PKCβ, 4.85 ± 1.06 in normal glucose vs. 11.07 ± 0.72 in high D-glucose and 5.03 ± 0.71 in high L-glucose, n = 3–4, P < 0.05). Moreover, this was associated with higher levels of IκBα, consistent with impaired activation of the NF-κB pathway (Figure 5(e) and (g); IκBα/β-actin, 18.21 ± 1.03 in normal glucose vs. 26.47 ± 2.46 in high D-glucose, and 20.02 ± 1.78 in high L-glucose, n = 3–4, P < 0.05). These results suggest that high D-glucose induced phosphorylation of PKCβ on the Ser661 residue and impaired ischemia-induced NF-κB activation.
Inhibition of PKCβpSer661restores ischemic activation of NF-κB under hyperglycemia
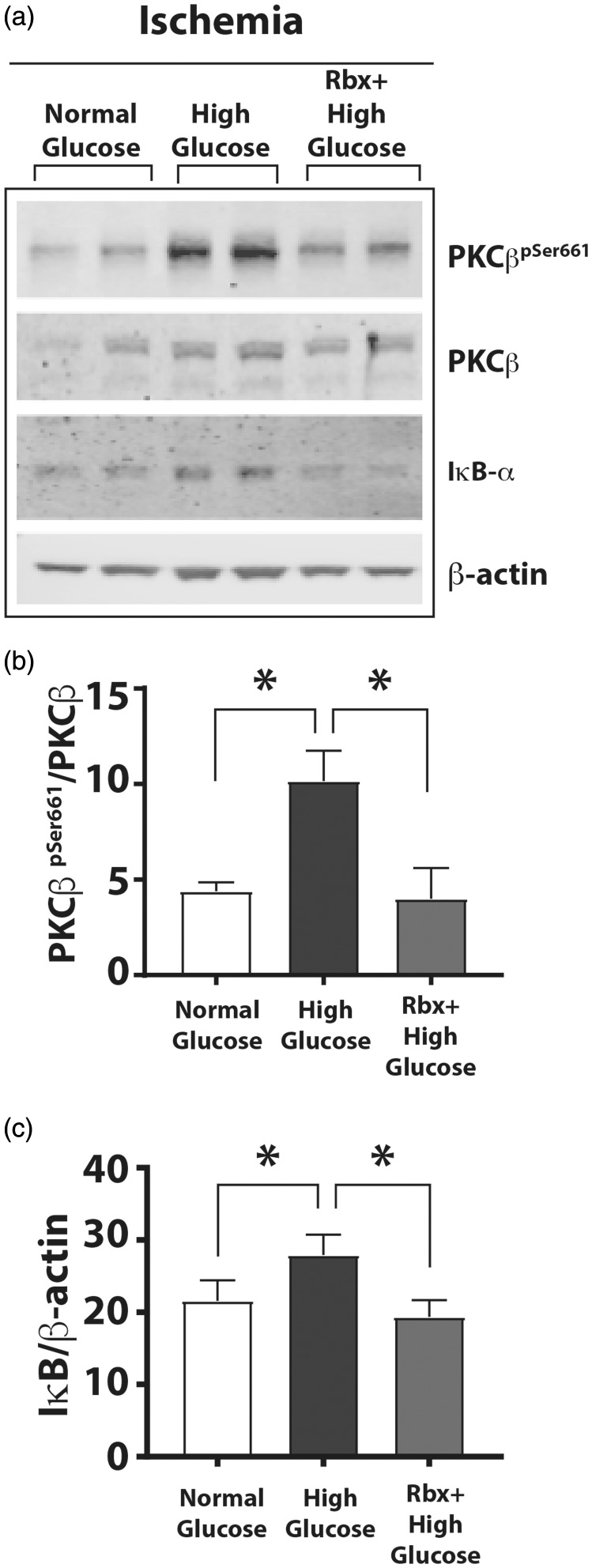
We hypothesized that inhibiting the phosphorylation of PKCβ would restore impaired IκBα degradation and augment NF-κB activation. To test this hypothesis, we cultured HUVEC in normal D-glucose or high D-glucose medium in the presence or absence of a specific inhibitor of PKCβ, Rbx (20 nM Rbx)29and subjected the cells to ischemia. Compared to the cells grown in normal D-glucose plus ischemia, cells grown in high D-glucose plus ischemia showed significantly increased PKCβpSer661(Figure 6(a) and (b); PKCβpSer661/PKCβ 4.40 ± 0.45 in normal D-glucose plus ischemia vs. 10.16 ± 1.56, P = 0.0001 in high D-glucose plus ischemia, n = 3–4, P < 0.05). The PKCβpSer661levels in high L-glucose plus ischemia were not different from those in normal glucose plus ischemia (data not shown). However, in cells grown in high D-glucose medium in the presence of Rbx, the PKCβpSer661levels reverted to normal levels (Figure 6(a) and (b), PKCβpSer661/PKCβ 4.40 ± 0.45 in normal D-glucose plus ischemia vs. 4.00 ± 0.80 in high D-glucose plus ischemia and Rbx, n = 3–4, P < 0.05).
Figure 6.
A PKCβ inhibitor Rbx decreased phosphorylation of Ser661 residue of PKCβ and increased IκBα degradation. HUVEC were grown in normal, high D-glucose, or high D-glucose + Rbx with ischemia for 24 h. (a) Western blots of HUVEC grown in different glucose concentrations with ischemia. (b) Autophosphorylated PKCβpSer661band showed greater intensity in cell lysates with high D-glucose that was decreased in the cells grown in the high D-glucose + Rbx (20 nM). (c) The IκBα levels (IκBα/β-actin) in the corresponding samples were increased in high D-glucose but were significantly reduced in the cells treated with Rbx. Asterisks denote P < 0.05, and n.s. denotes not significant; n= 4 to 6 per group.
PKCβ: protein kinase C beta; Rbx: ruboxistaurin.
We also analyzed the activation of the NF-κB pathway by measuring abundance of IκBα protein in these lysates. Although IκBα protein levels were increased in cells grown in high D-glucose plus ischemia, treatment of cells with Rbx significantly reduced the accumulation of IκBα in cells grown in high D-glucose plus Rbx to the same levels as normal glucose plus ischemia or high L-glucose plus ischemia (Figure 6(c); IκBα/β-actin, 21.57 ± 2.86 in normal glucose plus ischemia vs. 27.93 ± 1.63 in high D-glucose plus ischemia and 19.34 ± 1.35 in high D-glucose plus ischemia plus Rbx, n = 3, P < 0.05). Thus, PKCβ inhibitor Rbx decreases hyperglycemia-induced PKCβpSer661phosphorylation and restores the activation of NF-κB through the canonical IκBα degradation. These results suggest that hyperphosphorylation of PKCβpSer661under hyperglycemic conditions impairs the activation of canonical NF-κB.
Inhibition of PKCβ phosphorylation on Ser661 induces activation of NF-κB and improves perfusion recovery in mice with type 1 diabetes following experimental PAD
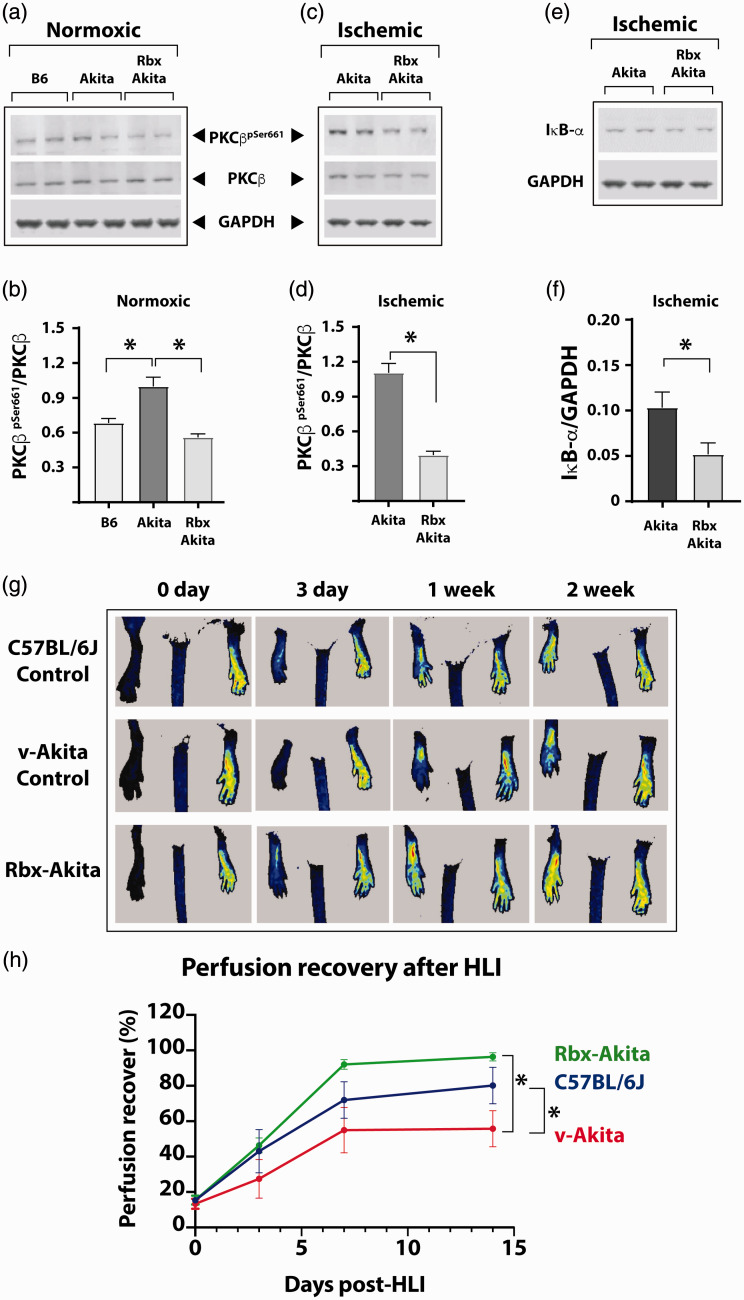
Given our in vitrofindings, we tested whether the mechanism of impaired NF-κB activation from increased PKCβpSer661levels under hyperglycemic conditions was also operative in vivoand whether inhibition of PKCβ by Rbx would ameliorate the impaired NF-κB activation and improve perfusion recovery in hyperglycemic diabetic mice following experimental PAD. We first compared PKCβ phosphorylation status in the gastrocnemius muscle from non-ischemic hind limbs of non-diabetic and diabetic mice. The Akita mice with type 1 diabetes have increased PKCβpSer661, and Rbx administration decreased this phosphorylation of PKCβ (Figure 7(a) and (b); PKCβpSer661/PKCβ, 0.67 ± 0.03 in non-diabetic C57BL/6J vs. 1.00 ± 0.06 in diabetic Akita mice and 0.56 ± 0.02 in Akita treated with Rbx, n = 5, P < 0.01).
Figure 7.
PKCβ inhibition restores NF-κB activation in hyperglycemic diabetic Akita mice after ischemia. (a) Following an HLI (three days postsurgery), Western blots of the gastrocnemius muscle homogenates from the contralateral non-ischemic hind limb were performed. (b) Quantification of the protein bands showed increased basal levels of PKCβpSer661in untreated Akita mice compared to the WT C57BL/6J mice. Homogenates from Rbx-treated Akita mice showed significantly decreased PKCβpSer661levels. (c) Western blots of ischemic limb of untreated and Rbx-treated Akita mice. (d) The protein levels of phosphorylated PKCβ (PKCβpSer661) were similar to those of non-ischemic limb samples but significantly decreased in the ischemic limb of Rbx-treated Akita mice. (e) Activation of the NF-κB canonical pathway was measured by Western blots of IκBα protein in ischemic hind limbs of untreated Akita and Rbx-treated Akita mice. (f) The IκBα levels were significantly greater in samples from Akita mice without treatments. In Rbx-treated ischemic limbs of Akita mice, the IκBα levels significantly dropped similar to the PKCβpSer661. Asterisks denote P < 0.05, and n.s. denotes not significant; n= 4 to 6 per group. (g) Recovery of blood perfusion after induced HLI in WT (C57BL/6J), vehicle-treated Akita (v-Akita), and Rbx-treated Akita (Rbx-Akita) mice viewed by laser speckle imaging immediately after surgery (day 0), day 3, 1 week, and 2 weeks after surgery. (h) Blood flow in the hind limb was measured in each mouse at multiple time points after induction of ischemia. The extent of perfusion in the operated limb is compared to that of the perfusion in the unoperated limb in the same mouse and expressed as a percentage as shown. Zero on X-axis indicates perfusion immediately after surgery, and n = 5 to 8 mice in each group; asterisks denote P < 0.05. (A color version of this figure is available in the online journal.)
PKCβ: protein kinase C beta; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; Rbx: ruboxistaurin; HLI: hind limb ischemia.
Next, experimental PAD was induced in diabetic Akita mice treated with Rbx or vehicle daily five days prior to the surgery. Western blot analyses of the ischemic gastrocnemius muscle of the untreated and the treated mice showed that mice treated with Rbx had decreased PKCβpSer661(Figure 7(c) and (d), PKCβpSer661/PKCβ, 1.10 ± 0.06 in ischemia untreated vs. 0.39 ± 0.02 in ischemia Rbx, treated, n = 5, P < 0.05). Moreover, the inhibition of PKCβpSer661by Rbx also reduced the abundance of IκBα (Figure 7(e) and (f), IκBα/glyceraldehyde 3-phosphate dehydrogenase, 0.10 ± 0.02 in ischemic untreated vs. 0.05 ± 0.01 ischemic Rbx treated, n = 5–6, P < 0.05), a sign of restoring the NF-κB activity in the hind limb muscles. Thus, similar to our in vitroresults with cultured HUVEC, inhibition of PKCβ phosphorylation (PKCβpSer661) by Rbx induces NF-κB pathway (IκBα degradation) in the hind limb tissues of hyperglycemic Akita mice.
Inhibition of PKCβ enhances postischemic perfusion in PAD
We measured the effect of inhibition of PKCβ by Rbx on the perfusion recovery in ischemic limbs of diabetic mice. Perfusion recovery was compared to that of non-diabetic mice and diabetic mice treated with vehicle. Serial monitoring of perfusion over two-week period by laser speckle contrast imaging showed no difference in perfusion immediately postsurgery between the control non-diabetic, the diabetic Akita treated with vehicle, and the Akitas treated with Rbx (Figure 7(g) and (h), % perfusion recovery in control non-diabetic 15.31 ± 1.55% vs. 13.36 ± 2.85% in vehicle-treated Akitas vs. 15.48 ± 2.52% in Rbx-treated Akitas, n = 5–8, P > 0.05). Analysis of perfusion recovery over two weeks showed vehicle-treated Akita as expect had impaired perfusion recovery compared to the non-diabetic C57BL/6J controls; however, Rbx-treated Akitas showed comparable perfusion recovery to the non-diabetic C57BL/6J mice (Figure 7(g) and (h); % perfusion recovery at week 2, 80.11 ± 10.29% non-diabetic C57BL/6J vs. 55 ± 10.17% in vehicle-treated Akitas and 96.35 ± 2.30% in Akitas treated with Rbx, n = 5–8 and P < 0.05).
Together, these results suggest that during limb ischemia, the NF-κB is induced by activation of both canonical and non-canonical pathway. However, hyperglycemia impairs the activation of the canonical pathway through increased phosphorylation of PKCβ. Inhibition of PKCβ improves the impairment of the canonical pathway and improves the postischemic limb perfusion in diabetic mice following experimental PAD. Thus, PKCβ is a critical factor in regulating the postischemic NF-κB activity in diabetes.
Discussion
Diabetes contributes to poor outcomes in peripheral arterial disease (PAD),22,30,31but the molecular mechanisms involved are not well understood. Chronic activation of NF-κB signaling is a key inflammatory process in diabetes.32–35NF-κB signaling also plays a key role in normal cellular functions including survival.36Mice with genetic deficiency in NF-κB activation in the vascular bed show aberrant endothelial function and poor postischemic perfusion recovery.13However, how hyperglycemia and chronic activation of NF-κB in diabetes affects the beneficial NF-κB signaling required for postischemic perfusion recovery in experimental PAD has not been studied. In this study, we have shown that in the endothelial cells, hyperglycemic conditions impair ischemia-induced NF-κB activation likely through enhancing the phosphorylation of Ser661 residue on PKCβ. Inhibition of PKCβ by Rbx restored ischemia-induced NF-κB activity both in vitroand in vivoand improved the perfusion recovery in diabetic mice after experimental PAD.
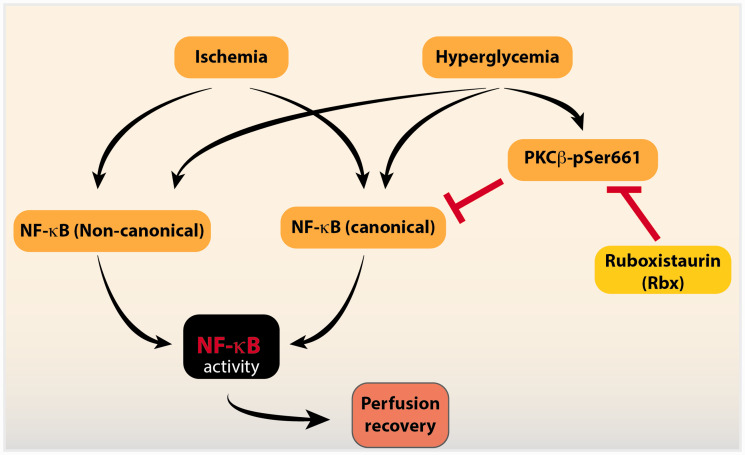
The NF-κB pathway may be activated via two major pathways termed the canonical and the non-canonical pathways that typically enhance the nuclear localization of p65-p50 or RelB/p52 dimer subunits, respectively. Analyses of phosphorylation and degradation of IκBα in the cytoplasmic compartment as well as enrichment of both p65 and RelB subunits in the nuclear fractions suggested that both the canonical and non-canonical pathways were activated in ischemic HUVEC. Moreover, upon short-term exposure of HUVECs to high D-glucose, both canonical and non-canonical pathways of NF-κB were also activated. Although the high glucose-mediated activation of the NF-κB pathway has been known,37our study reveals a novel finding that ischemia-induced NF-κB activation was attenuated in vitroand in vivoin the context of prolonged high glucose exposure. This finding was unexpected since either ischemia or high glucose conditions are capable of inducing the canonical and non-canonical pathways. Although the canonical pathway is activated by inflammatory signals and the non-canonical pathway by developmental signals, a great degree of crosstalk between these pathways has been reported38whereby these pathways can exert mutual restraint on each other. Thus, in our studies, prior activation of the NF-κB pathways by high D-glucose appears to asymmetrically attenuate ischemic activation of the canonical NF-κB pathway (Figure 8).
Figure 8.
Interaction between ischemia and hyperglycemia-induced signaling in NF-κB activation and perfusion recovery in PAD. Both ischemia and hyperglycemia induce signaling to activate NF-κB that is required for perfusion recovery. However, chronic hyperglycemic conditions attenuate the ischemia-induced activation of the canonical NF-κB pathway and contribute to poor perfusion recovery, through the effects of increased phosphorylation of PKCβ (PKCβ-pSer661). Inhibition of PKCβ activity by the specific inhibitor ruboxistaurin (Rbx) restores the canonical NF-κB pathway and improves perfusion recovery in diabetic mice after PAD. (A color version of this figure is available in the online journal.)
PKCβ: protein kinase C beta; NF-κB: nuclear factor-kappa B.
Hyperglycemia in diabetes contributes to endothelial cells dysfunction through various mechanisms including generation of reactive oxygen species, advanced glycation end products, and activation of PKC.39,40Activation of the NF-κB pathway entails a cascade of phosphorylation events performed by several protein kinases. A comparison of phosphorylated proteins from HUVEC grown either in normal or high D-glucose using a phosphoantibody arrays identified increased phosphorylation of a number of protein and HDAC5pSer259, AKTpThr308, and PKCβpSer661ranked among the highest phosphorylated proteins. HDAC5 binds to myocyte enhancer factor-2 transcription factor to repress genes involved in cardiac myocyte hypertrophy. Our review of the literature showed HDAC5pSer259is downstream of p65 activation in NF-κB signaling41thus less likely to explain the impaired NF-kB activation we are studying. Additionally, although there is evidence that AKT activation could be upstream of NF-κB signaling, AKT activation has been associated with anti-apoptotic and pro-proliferation effects on endothelial cells42and, therefore, less likely to be the mechanism contributing to impaired perfusion recovery. Lastly, in high glucose, activation of PKCα and PKCβ isoforms is associated with adverse effects on recovery from ischemic cardiac injury28and increased apoptosis of brain microvascular endothelial cells.43PKC is a serine/threonine kinase that can modulate the functions of other proteins by phosphorylating them.44High glucose is known to increase expression and activation of PKCβ and PKCδ isoforms.45The PKCβ1 isoform contains a conserved Serine-661 residue in the C-terminal region that is autophosphorylated and determines the subcellular localization of the kinase.46Prolonged activation of PKCβ in cancer cells is associated with impaired NF-κB activation by TNF.47Administration of the inhibitors of PKCβ in diabetic mice ameliorates diabetic complications in the retinal and kidney.48,49Hence, assessment of the role of PKCβ phosphorylation in impaired ischemia-induced NF-κB activation was favored. The increased PKCβpSer661is associated with impaired activation of NF-κB in HUVEC and in ischemic hind limbs of diabetic mice. However, treatment with Rbx a specific inhibitor of PKCβ50reduced the phosphorylation of Serine-661 of PKCβ, and this was associated with restored ischemia-induced NF-κB activation in the endothelial cells as well as in the hind limbs of treated diabetic mice. Moreover, compared to the wild-type (WT) mice, the untreated Akita mice showed an impaired perfusion in ischemic limbs, and this was improved in Rbx-treated Akita mice. Interestingly, Rbx has been investigated as potential therapy in several disease conditions resulting from diabetes complications including diabetic peripheral neuropathy, retinopathy, nephropathy, and macular edema.51,52Recent large animal studies also show that Rbx may have therapeutic efficacy in preclinical diseases models not resulting from diabetes complications.53Our result showing that postischemic perfusion recovery in diabetic mice treated with Rbx was superior to that of non-diabetic mice is consistent with possible effects of Rbx beyond diabetes-related complications.
Lastly, although ischemia activates both the canonical and non-canonical NF-κB pathways, only activation of the canonical pathway is impaired in diabetes/hyperglycemia; this suggests that the canonical NF-κB pathway and not the non-canonical pathway plays a key role in postischemic perfusion recovery. Thus, hyperglycemia in diabetes contributes to poorer experimental PAD outcomes by hyperphosphorylating PKCβ that results in impairment in ischemia-induced activation of the canonical NF-κB signaling pathway.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220920832 for Inhibition of protein kinase C beta phosphorylation activates nuclear factor-kappa B and improves postischemic recovery in type 1 diabetes by Satyanarayana Alleboina, Thomas Wong, Madhu V Singh and Ayotunde O Dokun in Experimental Biology and Medicine
Authors’ contributions
SA and TW performed experiments and analyzed results. MVS wrote the manuscript, researched data, and contributed to discussion, and AOD designed the studies and reviewed/edited the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Heart, Lung, and Blood Institute (R01 HL130399 to AOD).
ORCID iD
Madhu V Singh https://orcid.org/0000-0003-3048-4432
SUPPLEMENTAL MATERIAL
Supplemental material for this article is available online.
References
- 1.Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UKA, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382:1329–40 [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt W, Dormandy J, Nehler M, Harris K, Fowkes F. TASC II working group. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007; 33:S1–75 [DOI] [PubMed] [Google Scholar]
- 3.Tamarat R, Silvestre J-S, Huijberts M, Benessiano J, Ebrahimian TG, Duriez M, Wautier M-P, Wautier JL, Levy BI. Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice. PNAS 2003; 100:8555–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Dia Care 2016; 39:S244. [DOI] [PubMed] [Google Scholar]
- 5.Starkey JM, Haidacher SJ, LeJeune WS, Zhang X, Tieu BC, Choudhary S, Brasier AR, Denner LA, Tilton RG. Diabetes-induced activation of canonical and noncanonical nuclear factor-κB pathways in renal cortex. Diabetes 2006; 55:1252–9 [DOI] [PubMed] [Google Scholar]
- 6.Xi G, Shen X, Wai C, Vilas CK, Clemmons DR. Hyperglycemia stimulates p62/PKCζ interaction, which mediates NF-κB activation, increased Nox4 expression, and inflammatory cytokine activation in vascular smooth muscle. FASEB J 2015; 29:4772–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin AS., Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 1996; 14:649–83 [DOI] [PubMed] [Google Scholar]
- 8.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol 1994; 10:405–55 [DOI] [PubMed] [Google Scholar]
- 9.Scherer DC, Brockman JA, Chen Z, Maniatis T, Ballard DW. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci U S A 1995; 92:11259–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res 2011; 21:71–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridder DA, Schwaninger M. NF-κB signaling in cerebral ischemia. Neuroscience 2009; 158:995–1006 [DOI] [PubMed] [Google Scholar]
- 12.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol (Lond) 2008; 586:4055–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirziu D, Jaba IM, Yu P, Larrivée B, Coon BG, Cristofaro B, Zhuang ZW, Lanahan AA, Schwartz MA, Eichmann A, Simons M. Endothelial nuclear factor-κB-dependent regulation of arteriogenesis and branching. Circulation 2012; 126:2589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayo T, Koizumi A. Mapping of murine diabetogenic gene mody on chromosome 7 at D7Mit258 and its involvement in pancreatic islet and beta cell development during the perinatal period. J Clin Invest 1998; 101:2112–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997; 46:887–94 [DOI] [PubMed] [Google Scholar]
- 16.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science 1996; 272:728–31 [DOI] [PubMed] [Google Scholar]
- 17.McClung JM, McCord TJ, Keum S, Johnson S, Annex BH, Marchuk DA, Kontos CD. Skeletal muscle-specific genetic determinants contribute to the differential strain-dependent effects of hindlimb ischemia in mice. Am J Pathol 2012; 180:2156–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dokun AO, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, Marchuk DA, Annex BH. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation 2008; 117:1207–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L-P, Zhou J-P, Zhang J-X, Wang J-Y, Wang Z-Y, Pan M, Li L-F, Chen L, Li C-C, Wang K-K, Bai Y-P, Zhang G-G. MiR-15b-5p regulates collateral artery formation by targeting AKT3 (protein kinase B-3). Arterioscler Thromb Vasc Biol 2017; 37:957–68 [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Okeke E, Ayalew D, Wang D, Shahid L, Dokun AO. Modulation of miR29a improves impaired post-ischemic angiogenesis in hyperglycemia. Exp Biol Med (Maywood) 2017; 242:1432–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dokun AO, Chen L, Okutsu M, Farber CR, Hazarika S, Jones WS, Craig D, Marchuk DA, Lye RJ, Shah SH, Annex BH. ADAM12: a genetic modifier of preclinical peripheral arterial disease. Am J Physiol Heart Circ Physiol 2015; 309:H790–H803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dokun AO, Chen L, Lanjewar SS, Lye RJ, Annex BH. Glycaemic control improves perfusion recovery and VEGFR2 protein expression in diabetic mice following experimental PAD. Cardiovasc Res 2014; 101:364–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 2017; 18:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 2007; 7:803–15 [DOI] [PubMed] [Google Scholar]
- 25.Williams CR, Lu X, Sutliff RL, Hart CM. Rosiglitazone attenuates NF-κB-mediated Nox4 upregulation in hyperglycemia-activated endothelial cells. Am J Physiol, Cell Physiol 2012; 303:C213–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Kloting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt A-M, Stern DM, Haring H-U, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappa B. Diabetes 2001; 50:2792–808 [DOI] [PubMed] [Google Scholar]
- 27.Shao B, Bayraktutan U. Hyperglycaemia promotes cerebral barrier dysfunction through activation of protein kinase C-beta. Diabetes Obes Metab 2013; 15:993–9 [DOI] [PubMed] [Google Scholar]
- 28.Sims MW, Winter J, Brennan S, Norman RI, Ng GA, Squire IB, Rainbow RD. PKC-mediated toxicity of elevated glucose concentration on cardiomyocyte function. Am J Physiol Heart Circ Physiol 2014; 307:H587–97 [DOI] [PubMed] [Google Scholar]
- 29.Deissler HL, Lang GE. The protein kinase C inhibitor: ruboxistaurin. Dev Ophthalmol 2016; 55:295–301 [DOI] [PubMed] [Google Scholar]
- 30.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res 2007; 101:948–56 [DOI] [PubMed] [Google Scholar]
- 31.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol 2006; 47:921–9 [DOI] [PubMed] [Google Scholar]
- 32.Henke N, Schmidt-Ullrich R, Dechend R, Park J-K, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft Friedrich C, Scheidereit C, Muller Dominik N. Vascular endothelial cell-specific NF-κB suppression attenuates hypertension-induced renal damage. Circ Res 2007; 101:268–76 [DOI] [PubMed] [Google Scholar]
- 33.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-κB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 2009; 119:1284–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther 2017; 2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathan C, Ding A. Nonresolving inflammation. Cell 2010; 140:871–82 [DOI] [PubMed] [Google Scholar]
- 36.Ting AT, Bertrand M. More to life than NF-kappaB in TNFR1 signaling. Trends Immunol 2016; 37:535–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW, Lin-Shiau SY, Liu SH. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol 2005; 25:539–45 [DOI] [PubMed] [Google Scholar]
- 38.Shih VF, Tsui R, Caldwell A, Hoffmann A. A single NFkappaB system for both canonical and non-canonical signaling. Cell Res 2011; 21:86–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene DA, Lattimer SA, Sima AA. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med 1987; 316:599–606 [DOI] [PubMed] [Google Scholar]
- 40.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 1998; 47:859–66 [DOI] [PubMed] [Google Scholar]
- 41.Ciccarelli M, Vastolo V, Albano L, Lecce M, Cabaro S, Liotti A, Longo M, Oriente F, Russo GL, Macchia PE, Formisano P, Beguinot F, Ungaro P. Glucose-induced expression of the homeotic transcription factor Prep1 is associated with histone post-translational modifications in skeletal muscle. Diabetologia 2016; 59:176–86 [DOI] [PubMed] [Google Scholar]
- 42.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle 2006; 5:512–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao B, Bayraktutan U. Hyperglycaemia promotes human brain microvascular endothelial cell apoptosis via induction of protein kinase C-ßI and prooxidant enzyme NADPH oxidase. Redox Biol 2014; 2:694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem 1995; 270:28495–8 [DOI] [PubMed] [Google Scholar]
- 45.Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZY, Yamauchi T, Kuboki K, Meier M, Rhodes CJ, King GL. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest 1999; 103:185–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol 1995; 5:1394–403 [DOI] [PubMed] [Google Scholar]
- 47.Park KA, Byun HS, Won M, Yang K-J, Shin S, Piao L, Kim JM, Yoon W-H, Junn E, Park J, Seok JH, Hur GM. Sustained activation of protein kinase C downregulates nuclear factor-κB signaling by dissociation of IKK-γ and Hsp90 complex in human colonic epithelial cells. Carcinogenesis 2007; 28:71–80 [DOI] [PubMed] [Google Scholar]
- 48.Aiello LP, Clermont A, Arora V, Davis MD, Sheetz MJ, Bursell SE. Inhibition of PKC beta by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients. Invest Ophthalmol Vis Sci 2006; 47:86–92 [DOI] [PubMed] [Google Scholar]
- 49.Kelly DJ, Zhang Y, Hepper C, Gow RM, Jaworski K, Kemp BE, Wilkinson-Berka JL, Gilbert RE. Protein kinase C beta inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes 2003; 52:512–8 [DOI] [PubMed] [Google Scholar]
- 50.Joy SV, Scates AC, Bearelly S, Dar M, Taulien CA, Goebel JA, Cooney MJ. Ruboxistaurin, a protein kinase C beta inhibitor, as an emerging treatment for diabetes microvascular complications. Ann Pharmacother 2005; 39:1693–9 [DOI] [PubMed] [Google Scholar]
- 51.Tuttle KR, McGill JB, Bastyr EJ, 3rd, Poi KK, Shahri N, Anderson PW. Effect of ruboxistaurin on albuminuria and estimated GFR in people with diabetic peripheral neuropathy: results from a randomized trial. Am J Kidney Dis 2015; 65:634–6 [DOI] [PubMed] [Google Scholar]
- 52.Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW, Pkc-Drs P-D, Groups P-D. Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol 2007; 2:631–6 [DOI] [PubMed] [Google Scholar]
- 53.Sharp TE, 3rd, Kubo H, Berretta RM, Starosta T, Wallner M, Schena GJ, Hobby AR, Yu D, Trappanese DM, George JC, Molkentin JD, Houser SR. Protein kinase C inhibition with ruboxistaurin increases contractility and reduces heart size in a swine model of heart failure with reduced ejection fraction. JACC Basic Transl Sci 2017; 2:669–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220920832 for Inhibition of protein kinase C beta phosphorylation activates nuclear factor-kappa B and improves postischemic recovery in type 1 diabetes by Satyanarayana Alleboina, Thomas Wong, Madhu V Singh and Ayotunde O Dokun in Experimental Biology and Medicine