Abstract
Aim: We aimed to explore the biomarkers for disease progression or the risk of nonsurvivors. Materials & methods: This study included 134 hospitalized patients with confirmed COVID-19 infection. The outcome of moderate versus severe versus critically ill patients and survivors versus nonsurvivors were compared. Results: An increase in the severity of COVID-19 pneumonia was positively associated with lower levels of platelets and albumin (all p < 0.05). In the critical group, the plasma levels of albumin continued to have a significant association for the risk of nonsurvivors (p < 0.05), even after adjusting for confounding factors. Conclusion: Albumin levels could be used as an independent predictor of the risk of nonsurvivors in critically ill patients with COVID-19.
Keywords: : albumin, biomarkers, COVID-19, critically ill patients, infection, pneumonia
A series of unexplained pneumonia cases (with a history of work or residence around the Huanan seafood wholesale market) were admitted to a hospital in Wuhan, Hubei province, China. Their clinical presentations were similar to viral pneumonia and some patients rapidly developed life-threatening acute respiratory diseases (ARDS) [1]. A novel coronavirus was then identified by sequencing the whole genome of the virus isolated from the patients and was named COVID-19 by the WHO [2,3]. To date, more than 80,000 confirmed cases have been identified in 34 provinces of China, more than 49,000 are from Wuhan city and the virus has been found in Japan, Thailand, South Korea, USA, etc [4,5].
Generally, the majority of COVID-19-positive patients are present with general symptoms of respiratory infection with a case fatality rate of 1.4–4% [3,6,7]. In some cases that develop severe or critical illness, death may be due to massive alveolar damage and progressive respiratory failure, with a higher mortality rate (38–60%) [8,9]. However, little is known regarding the clinical markers for the risk of nonsurvivors in patients with COVID-19.
The purpose of this study was to explore biomarkers for disease progression and the risk of nonsurvivors. We hope that our research will help clinicians identify patients with a high risk of nonsurvivors at an early stage.
Materials & methods
Study design & participants
In this retrospective study, we included discharged patients, including deaths, hospitalized with COVID-19 pneumonia in the Central Hospital of Wuhan from 1 January to 20 February 2020. COVID-19 was defined as a positive result on real time reverse transcriptase PCR and ‘ground-glass opacity’ on computed tomography (CT). This study was approved by the Ethics Commission of the Central Hospital of Wuhan. Written informed consent was waived by the Ethics Commission of the designated hospital under the criteria of emerging infectious diseases. The classification of diseases used is as described previously [10,11].
Participants’ characteristics & data collection
This study retrospectively analyzed the patients’ medical history, epidemiological data (including workplace), history of disease exposure, fever, cough, headache, diarrhea and chest pain, etc. The laboratory tests included liver function, kidney function, blood cell count, COVID-19 nucleic acid and tests for other respiratory viruses etc. Data regarding medical expenses, lung CT image, drugs prescribed and comorbidities were also analyzed [12].
Clinical outcomes
This study focused on discharged patients. The two patient subtypes included rehabilitation discharges and death cases.
Statistical analysis
All data were expressed as median interquartile range (IQR), or percentages (%). Categorical data were tested using Fisher’s exact test or x2 test. Normal distribution data were tested by independent t-test, while non-normal distribution data were tested by nonparametric Mann–Whitney U test. A binary logistic regression analysis was used to assess the independent predictors for the risk of nonsurvivors. To predict the risk of nonsurvivors, a receiver operating characteristic curve was plotted to determine the cut-off point for albumin. The data were analyzed using SPSS 20.0. A two-sided α score <0.05 was considered statistically significant.
Results
In this retrospective study, we included 134 discharged patients, including deaths. Patient demographics, characteristics, outcomes and medical expenses are summarized in Table 1. The median age of all the patients was 61.00 years, 69 (51.49%) of the patients were >60 years of age, 65 (48.51%) were <60 years of age and 75 (55.97%) of them were males. A total of 83.58, 96.27 and 100.00% had no history of smoking, drinking or a history of exposure to the Huanan seafood market, respectively. A total of 15 (11.19%) of the patients with COVID-19 were medical staff. Some patients had comorbidities including cardiovascular disease (44.03%), endocrine disorder (diabetes) (25.37%), digestive disorder (14.93%), respiratory disease (8.21%), neurological disease (17.16%) and solid tumor (9.70%). The median hospital stays and medical expenses for all the patients were 13.00 days and 24,093.38 yuan, respectively. Forty two (31.34%) patients died due to COVID-19 pneumonia.
Table 1. Demographics, characteristics, outcomes and medical expenses of patients with COVID-19.
| Characteristics | Patients | p-value | |||
|---|---|---|---|---|---|
| All (n = 134) | Moderate (n = 45) | Severe (n = 30) | Critical (n = 59) | ||
| Age, median (IQR), years | 61.00 (46.75–69.25) | 50.00 (31.00–63.00) | 59.50 (52.75–67.75) | 67.00 (56.00–75.00) | 0.000 |
| <60 (%) | 65 (48.51) | 31 (68.89) | 15 (50.00) | 19 (32.20) | 0.001 |
| ≧60 (%) | 69 (51.49) | 14 (31.11) | 15 (50.00) | 40 (67.80) | |
| Gender, (%) | |||||
| Females | 59 (44.03) | 21 (46.67) | 15 (50.00) | 23 (38.98) | 0.557 |
| Males | 75 (55.97) | 24 (53.33) | 15 (50.00) | 36 (61.02) | |
| Smoking, (%) | |||||
| Yes | 22 (16.42) | 8 (17.78) | 5 (16.67) | 9 (15.25) | 0.942 |
| No | 112 (83.58) | 37 (82.22) | 25 (83.33) | 50 (84.75) | |
| Drinking, (%) | |||||
| Yes | 5 (3.73) | 3 (6.67) | 1 (3.33) | 1 (1.69) | 0.412 |
| No | 129 (96.27) | 42 (93.33) | 29 (96.67) | 58 (98.31) | |
| Exposure to Huanan seafood market, (%) | |||||
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| No | 134 (100) | 45 (100.00) | 30 (100.00) | 59 (100.00) | |
| Occupation, (%) | |||||
| Medical staff | 15 (11.19) | 8 (17.78) | 5 (3.73) | 2 (3.39) | 0.039 |
| Nonmedical staff | 119 (88.81) | 37 (82.22) | 25 (83.33) | 57 (96.61) | |
| Chronic disease, (%) | |||||
| Cardiovascular disease | 59 (44.03) | 12 (40.00) | 14 (46.67) | 33 (55.93) | 0.014 |
| Hypertension | 44 (32.84) | 10 (33.33) | 11 (36.67) | 23 (38.98) | 0.173 |
| Endocrine disorder (diabetes) | 34 (25.37) | 6 (20.00) | 10 (33.33) | 18 (30.51) | 0.072 |
| Digestive disorder | 20 (14.93) | 5 (11.11) | 3 (9.99) | 12 (20.34) | 0.294 |
| Respiratory disease | 11 (8.21) | 2 (4.44) | 1 (3.33) | 8 (13.56) | 0.133 |
| Neurological disease | 23 (17.16) | 7 (15.56) | 5 (3.73) | 11 (18.64) | 0.915 |
| Solid tumor | 13 (9.70) | 5 (11.11) | 3 (9.99) | 5 (8.47) | 0.902 |
| Hospital stays, median (IQR), days | 13.00 (10.00–20.00) | 14.00 (11.00–20.00) | 15.50 (12.75–22.00) | 11.00 (7.00–17.00) | 0.009 |
| Clinical outcomes, (%) | |||||
| Rehabilitation discharge | 92 (68.66) | 45 (100.00) | 30 (100.00) | 17 (28.81) | 0.000 |
| Died | 42 (31.34) | 0 (0) | 0 (0) | 42 (71.19) | |
| Medical expenses, median (IQR), yuan (RMB) | 24,093.38 (10,217.39–44,511.04) | 13,588.28 (8825.39–30,426.43) | 24,169.73 (11,221.73–34,855.10) | 36,895.44 (12,726.83–61,336.93) | 0.000 |
According to the severity of COVID-19 pneumonia (Table 1), the increase in the median age, percentage of patients ≥60 years, percentage of patients with cardiovascular disease, and the median medical expenses occurred concomitantly with the severity of COVID-19 pneumonia (all p-values <0.05). However, the median hospital stay was the shortest in the critical group, as this may have been due to the quick disease progression and early patient death.
The clinical characteristics of the patients are summarized in Table 2. The most common symptoms were fever (84.33%), cough (76.87%), diarrhea (20.15%), muscle pain (19.40%), generally feeling sick and vomiting (11.19%), as well as 96.27% experiencing more than one symptom. Out of the 134 patients, 62 (46.27%) developed ARDS and 120 (89.55%) had bilateral pneumonia. Most were treated with oxygen (66.42%), antibiotics (97.76%), antiviral drugs (97.76%), hormones (66.42%) and immunoglobulin (59.70%). An increase in the percentage of patients with bilateral pneumonia and hormones used occurred concomitantly with an increase in the severity of COVID-19 pneumonia (all p < 0.05).
Table 2. Clinical characteristics and treatment strategies of patients with COVID-19.
| Characteristics | Patients | p-value | |||
|---|---|---|---|---|---|
| All (n = 134) | Moderate (n = 45) | Severe (n = 30) | Critical (n = 59) | ||
| Clinical symptoms, (%) | |||||
| Fever | 113 (84.33) | 36 (80.00) | 25 (83.33) | 52 (88.14) | 0.520 |
| Cough | 103 (76.87) | 32 (71.11) | 21 (70.00) | 50 (84.75) | 0.158 |
| Stuffy nose | 2 (1.49) | 0 (0) | 1 (3.33) | 1 (1.69) | 0.499 |
| Runny nose | 2 (1.49) | 0 (0) | 1 (3.33) | 1 (1.69) | 0.499 |
| Sneeze | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Sore throat | 5 (3.73) | 0 (0) | 1 (3.33) | 4 (6.78) | 0.194 |
| Chest pain | 9 (6.72) | 5 (11.11) | 1 (3.33) | 3 (5.08) | 0.335 |
| Diarrhea | 27 (20.15) | 6 (13.33) | 10 (33.33) | 11 (18.64) | 0.099 |
| Headache | 8 (5.97) | 3 (6.67) | 1 (3.33) | 4 (6.78) | 0.787 |
| Muscle pain | 26 (19.40) | 6 (13.33) | 8 (26.67) | 12 (20.34) | 0.349 |
| Feel sick and vomit | 15 (11.19) | 8 (17.78) | 2 (6.66) | 5 (8.47) | 0.221 |
| More than one symptom | 129 (96.27) | 41 (91.11) | 29 (96.67) | 59 (100.00) | 0.060 |
| Complications, (%) | |||||
| Acute kidney injury | 5 (3.73) | 0 (0) | 0 (0) | 5 (8.47) | 0.037 |
| Acute respiratory injury | 4 (2.99) | 0 (0) | 0 (0) | 4 (6.78) | 0.073 |
| Septic shock | 2 (1.49) | 0 (0) | 0 (0) | 2 (3.39) | 0.275 |
| Ventilator-associated pneumonia | 1 (0.75) | 0 (0) | 0 (0) | 1 (1.69) | 0.527 |
| Acute respiratory distress syndrome | 56 (41.79) | 0 (0) | 5 (16.67) | 51 (86.44) | 0.000 |
| Lung CT images, (%) | |||||
| Unilateral pneumonia | 13 (9.70) | 10 (22.22) | 2 (6.66) | 1 (1.69) | 0.002 |
| Bilateral pneumonia | 120 (89.55) | 35 (77.78) | 27 (90.00) | 58 (98.31) | 0.003 |
| Ground-glass turbidity | 117 (87.31) | 41 (91.11) | 27 (90.00) | 49 (83.05) | 0.417 |
| Treatment strategies, (%) | |||||
| Oxygen therapy | 89 (66.42) | 0 (0) | 30 (100.00) | 59 (100.00) | 0.000 |
| Extracorporeal membrane oxygenation | 1 (0.75) | 0 (0) | 0 (0) | 1 (1.69) | 0.527 |
| Antibiotics | 131 (97.76) | 44 (97.78) | 30 (100.00) | 57 (96.61) | 0.593 |
| Antiviral drugs | 131 (97.76) | 45 (100.00) | 30 (100.00) | 56 (94.92) | 0.142 |
| Antifungal drugs | 5 (3.73) | 0 (0) | 1 (3.33) | 4 (6.78) | 0.194 |
| Hormones | 89 (66.42) | 23 (51.11) | 18 (60.00) | 48 (81.36) | 0.004 |
| Immunoglobulin | 80 (59.70) | 25 (55.55) | 19 (63.33) | 36 (61.02) | 0.768 |
| Kidney replacement therapy | 1 (0.75) | 0 (0) | 0 (0) | 1 (1.69) | 0.527 |
| Invasive mechanical ventilation | 5 (3.73) | 0 (0) | 0 (0) | 5 (8.47) | 0.037 |
| Noninvasive mechanical ventilation | 41 (30.60) | 0 (0) | 0 (0) | 41 (69.49) | 0.000 |
CT: Computed tomography.
The laboratory results of the patients with COVID-19 are summarized in Table 3. An increase in the severity of COVID-19 pneumonia was associated with higher median values of leukocytes (p < 0.001), neutrophils (p < 0.001), D-dimer (p = 0.005), aspartate aminotransferase (p = 0.010), serum urea (p = 0.009), lactate dehydrogenase (p < 0.001), myoglobin (p = 0.026), glucose (p < 0.001), procalcitonin (p = 0.022) and C-reactive protein (p < 0.001), lower platelets count (p = 0.005), lower albumin (p < 0.001) and lower sodium (p = 0.03). The lymphocyte counts were lower in the critical group (p = 0.046). Few patients had co-infections with other viruses, bacteria, etc. The positivity rate of the first test for viral nucleic acid was 33.58%. Furthermore, with an increase in the severity of COVID-19 pneumonia, the positivity rate of the first test for viral nucleic acid increased accordingly (p = 0.010).
Table 3. Laboratory test results of patients with COVID-19.
| Characteristics | Patients | p-value | |||
|---|---|---|---|---|---|
| All (n = 134) | Moderate (n = 45) | Severe (n = 30) | Critical (n = 59) | ||
| Blood biochemical parameters, median (IQR) | |||||
| Leukocytes (3.5–9.5) 109/l | 5.42 (3.87–7.43) | 4.70 (3.34–6.07) | 4.78 (3.78–6.24) | 6.94 (4.49–12.54) | 0.000 |
| Neutrophils (1.8–6.3) 109/l | 3.74 (2.57–6.17) | 2.86 (1.83–4.13) | 3.54 (2.12–4.63) | 5.61 (3.32–10.92) | 0.000 |
| Lymphocytes (1.1–3.2) 109/l | 1.02 (0.64–1.35) | 1.15 (0.91–1.74) | 1.16 (0.93–1.34) | 0.74 (0.49–1.12) | 0.046 |
| Platelets (125–350) 109/l | 167.50 (130.75–216.00) | 193.50 (160.00–226.00) | 165.00 (141.25–228.25) | 147.50 (109.75–194.50) | 0.005 |
| Hemoglobin (130–175 g/l) | 126.00 (116.75–141.00) | 126.00 (121.00–139.25) | 126.50 (116.75–140.50) | 127.00 (110.75–142.25) | 0.938 |
| Monocytes (0.1–0.6) 109/l | 0.35 (0.25–0.47) | 0.36 (0.27–0.44) | 0.45 (0.29–0.53) | 0.28 (0.21–0.46) | 0.734 |
| Activated partial thromboplastin time (20–40) s | 29.20 (25.40–32.15) | 29.6 (26.05–31.50) | 26.40 (24.50–30.20) | 30.10 (26.50–34.00) | 0.099 |
| Fibrinogen (2–4) g/l | 3.06 (2.53–3.69) | 2.82 (2.28–3.25) | 3.13 (2.52–3.74) | 3.34 (2.67–3.91) | 0.128 |
| Prothrombin time (9–13) s | 11.80 (11.15–12.70) | 11.8 (11.15–12.45) | 11.40 (10.60–12.10) | 12.00 (11.40–13.15) | 0.027 |
| International normalized ratio (0.7–1.3) | 1.02 (0.96–1.10) | 1.02 (0.96–1.08) | 0.98 (0.92–1.05) | 1.04 (0.99–1.16) | 0.021 |
| D-dimer (0–1) μg/ml | 0.64 (0.31–1.46) | 0.34 (0.17–0.78) | 0.57 (0.33–0.88) | 1.15 (0.50–5.56) | 0.005 |
| Total cholesterol (<5.18) mmol/l | 3.58 (3.00–4.50) | 3.63 (3.01–4.17) | 3.84 (3.14–5.10) | 3.40 (2.93–3.94) | 0.433 |
| Triglyceride (<1.7) mmol/l | 1.06 (0.79–1.54) | 1.02 (0.73–1.51) | 1.11 (0.78–1.49) | 1.11 (0.86–1.73) | 0.343 |
| Total protein (65–85) g/l | 65.30 (62.70–71.20) | 64.80 (63.1–71.2) | 64.70 (61.23–71.30) | 66.15 (62.15–70.95) | 0.900 |
| Albumin (40–55) g/l | 38.10 (33.90–41.65) | 39.90 (38.30–42.90) | 37.00 (34.15–42.55) | 34.40 (31.38–39.15) | 0.000 |
| Alanine aminotransferase (9–50) U/l | 31.10 (13.10–33.20) | 21.10 (12.35–34.30) | 16.95 (13.03–28.90) | 23.70 (15.35–33.75) | 0.331 |
| Aspartate aminotransferase (15–40) U/l | 25.70 (18.40–36.80) | 20.30 (17.48–27.5) | 21.55 (16.65–34.00) | 31.60 (24.80–45.15) | 0.010 |
| Total bilirubin (2–20.4) μmol/l | 10.10 (7.10–15.40) | 8.35 (6.63–11.60) | 9.65 (7.70–15.43) | 13.00 (7.75–17.60) | 0.182 |
| Serum urea (1.7–8.3) mmol/l | 5.07 (3.70–6.71) | 4.46 (3.44–5.45) | 4.81 (3.47–6.60) | 6.13 (4.25–9.46) | 0.009 |
| Serum creatinine (57–111) μmol/l | 69.05 (56.25–84.23) | 63.15 (55.33–79.78) | 68.50 (53.85–80.35) | 73.10 (58.68–100.58) | 0.282 |
| Alkaline phosphatase (40–150) U/l | 50.50 (40.75–60.50) | 49.00 (41.00–58.00) | 50.00 (39.00–56.00) | 52.00 (40.75–67.50) | 0.570 |
| Sodium ion (137–147) mmol/l | 141.05 (137.93–143.28) | 142.75 (140.95–143.93) | 140.60 (138.70–142.55) | 139.90 (136.15–142.38) | 0.003 |
| Potassium ion (3.5–5.3) mmol/l | 4.14 (3.85–4.46) | 4.17 (3.98–4.43) | 4.19 (3.72–4.49) | 4.12 (3.80–4.49) | 0.999 |
| Calcium ion (2.2–2.7) mmol/l | 2.19 (2.03–2.35) | 2.21 (2.09–2.41) | 2.24 (2.05–2.49) | 2.13 (2.01–2.27) | 0.363 |
| pH value (7.35–7.45) | 7.45 (7.42–7.47) | 7.43 (7.39–7.47) | 7.46 (7.43–7.47) | 7.44 (7.42–7.46) | 0.265 |
| Partial pressure of carbon dioxide (35–45 mmHg) | 35.00 (31.00–39.00) | 36.00 (32.00–40.00) | 36.00 (31.00–38.00) | 34.00 (29.50–38.00) | 0.440 |
| Partial pressure of oxygen (80–100 mmHg) | 81.00 (58.00–105.00) | 100.00 (76.00–109.00) | 78.00 (62.00–105.00) | 66.00 (51.00–102.00) | 0.406 |
| Creatine kinase (38–174) U/l | 94.00 (57.00–161.00) | 81.00 (47.00–117.50) | 83.80 (56.50–151.50) | 125.00 (63.50–239.30) | 0.064 |
| Lactate dehydrogenase (80–285) U/l | 212.00 (164.00–317.00) | 168.00 (140.00–217.50) | 191.00 (163.00–236.00) | 296.00 (212.00–467.50) | 0.000 |
| Angiotensin-converting enzyme (12–68) U/l | 21.65 (17.03–24.88) | 22.70 (17.20–25.58) | 20.50 (16.50–23.00) | 21.00 (17.05–24.35) | 0.645 |
| Myoglobin (14.3–105.5) ng/ml | 36.30 (18.05–84.40) | 17.55 (12.83–35.28) | 36.30 (17.90–91.45) | 56.85 (32.85–174.10) | 0.026 |
| Troponin (<0.03) μg/l | 0.01 (0.00–0.03) | 0.003 (0.002–0.015) | 0.006 (0.002–0.030) | 0.02 (0.009–0.066) | 0.058 |
| Glucose (3.9–6.1) mmol/l | 5.90 (4.95–8.38) | 4.95 (4.36–6.39) | 5.48 (5.06–7.86) | 7.41 (5.78–11.03) | 0.000 |
| Procalcitonin (<0.04) ng/ml | 0.09 (0.05–0.25) | 0.05 (0.04–0.08) | 0.06 (0.05–0.11) | 0.22 (0.10–0.52) | 0.022 |
| Interleukin 6 (<7) pg/ml | 25.52 (9.64–57.32) | 7.70 (4.45–10.95) | 25.88 (2.22–31.82) | 26.88 (9.68–78.67) | 0.633 |
| Erythrocyte sedimentation rate (0–15) mm/h | 25.00 (15.25–52.00) | 25.00 (12.00–40.50) | 27.00 (16.00–62.50) | 27.00 (17.00–53.00) | 0.291 |
| Serum ferritin (21.8–274.7) ng/ml | 297.98 (190.98–876.80) | 210.20 (80.70–339.70) | 244.20 (190.99–876.80) | 818.92 (297.98–1339.86) | 0.483 |
| C-reactive protein (0–0.5) mg/dl | 2.69 (0.94–6.25) | 0.98 (0.35–3.28) | 2.47 (0.61–4.59) | 4.86 (2.36–10.60) | 0.000 |
| Co-infection (negative), (%) | |||||
| Other respiratory viruses | 2 (1.49) | 2 (4.44) | 0 (0) | 0 (0) | 0.134 |
| Bacteria | 8 (5.97) | 4 (8.88) | 1 (3.33) | 3 (5.08) | 0.566 |
| Fungus | 8 (5.97) | 2 (4.44) | 0 (0) | 6 (10.17) | 0.139 |
| Mycoplasma | 4 (2.99) | 1 (2.22) | 2 (6.66) | 1 (1.69) | 0.400 |
| Chlamydia | 3 (2.24) | 1 (2.22) | 2 (6.66) | 0 (0) | 0.133 |
| Viral nucleic acid (negative), (%) | |||||
| Test for the first time | |||||
| Negative | 89 (66.42) | 34 (75.56) | 24 (80.00) | 31 (52.54) | 0.010 |
| Positive | 45 (33.58) | 11 (24.44) | 6 (20.00) | 28 (47.46) | |
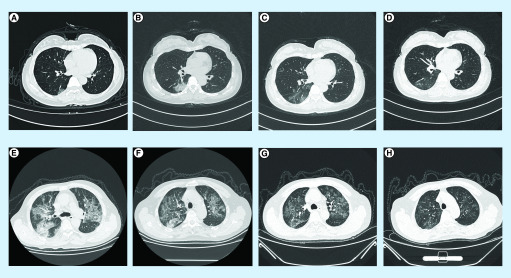
A 51-year-old female medical staff tested positive for COVID-19, possibly due to exposure in the hospital and she developed fever and cough that lasted for 3 days. Her chest CT (Figure 1) showed little ground glass opacities below the right lung (Figure 1A). On the 5th day of her admission (Figure 1B), the area of ground glass opacities below the right lung increased. On the 15th (Figure 1C) and 23rd (Figure 1D) days after treatment, the ground glass opacities in the right lung gradually reduced and the lung inflammation slowly resolved. A 66-year-old man infected with COVID-19 due to a meal, presented with fever, cough and shortness of breath for 6 days. His chest CT showed multiple, ground-glass turbidity (Figure 1E). On the 5th (Figure 1F), 9th (Figure 1G) and 20th (Figure 1H) day of admission after active treatment, the ground glass opacities in the lungs gradually reduced and the clinical symptoms improved.
Figure 1. Chest computed tomography images.
(A–D) A 51-year-old female medical staff. Initially of mild severity, her condition worsened after admission and she finally recovered slowly. (E–H) A 66-year-old man. Initially severe, the patient’s condition slowly improved.
The patients in the critical group were divided into group 1 (dead) and group 2 (surviving). The parameters affecting disease progression (p < 0.05) as in Tables 1– 3 were included in Table 4 to further explore whether disease progression was closely related to the risk of nonsurvivors. The clinical and laboratory indicators, closely related to the severity of patients with COVID-19 are summarized in Table 4. In group 1 and group 2, the median ages were 70.00 and 55.00 years, respectively (p < 0.001). More cases of cardiovascular disease (64.29 vs 35.29%; p = 0.042), ARDS (100.00 vs 52.94%; p < 0.001) and higher median values of D-dimer (1.67 vs 0.49; p = 0.017), lactate dehydrogenase (337.50 vs 258.00; p = 0.026) and glucose (7.85 vs 7.05; p = 0.031), along with lower counts of platelets (median, 134.00 vs 182.00; p = 0.028) and albumin (median, 33.45 vs 39.20; p < 0.001) were found in group 1, as compared with group 2.
Table 4. Clinical and laboratory indicators closely related to the severity of patients with COVID-19.
| Characteristics | Critical | p-value | ||
|---|---|---|---|---|
| All (n = 59) | Survivors (n = 17) | Nonsurvivors (n = 42) | ||
| Age, median (IQR), years | 67.00 (56.00–75.00) | 55.00 (43.50–61.50) | 70.00 (63.75–78.00) | 0.000 |
| <60 (%) | 19 (32.20) | 12 (70.59) | 9 (21.43) | 0.000 |
| ≧60 (%) | 40 (67.80) | 5 (29.411) | 33 (78.57) | |
| Chronic disease, (%) | ||||
| Cardiovascular disease | 33 (55.93) | 6 (35.29) | 27 (64.29) | 0.042 |
| Complications, (%) | ||||
| Acute kidney injury | 5 (8.48) | 0 (0) | 5 (11.90) | 0.308 |
| Acute respiratory distress syndrome | 51 (86.44) | 9 (52.94) | 42 (100.00) | 0.000 |
| Lung CT images, (%) | ||||
| Unilateral pneumonia | 1 (1.69) | 0 (0) | 1 (2.38) | 1.000 |
| Bilateral pneumonia | 58 (98.31) | 17 (100.00) | 41 (97.62) | 1.000 |
| Treatment strategies, (%) | ||||
| Oxygen therapy | 59 (100.00) | 17 (100.00) | 42 (100.00) | 1.000 |
| Hormones | 48 (81.36) | 12 (70.59) | 36 (85.71) | 0.293 |
| Invasive mechanical ventilation | 5 (8.47) | 2 (11.76) | 3 (7.14) | 0.620 |
| Noninvasive mechanical ventilation | 41 (69.49) | 14 (82.35) | 27 (64.29) | 0.222 |
| Blood biochemical parameters, Median (IQR) | ||||
| Leukocytes (3.5–9.5) 109/l | 6.94 (4.49–12.54) | 4.62 (3.63–11.43) | 7.11 (5.38–12.54) | 0.298 |
| Neutrophils (1.8–6.3) 109/l | 5.61 (3.32–10.92) | 3.37 (2.84–8.91) | 6.18 (3.61–11.27) | 0.240 |
| Lymphocytes (1.1–3.2) 109/l | 0.74 (0.49–1.12) | 0.95 (0.56–1.16) | 0.68 (0.46–1.04) | 0.801 |
| Platelets (125–350) 109/l | 147.50 (109.75–194.50) | 182.00 (119.50–268.50) | 134.00 (105.00–186.00) | 0.028 |
| Prothrombin time (9–13) s | 12.00 (11.38–13.08) | 11.80 (11.40–12.25) | 12.10 (11.35–13.30) | 0.514 |
| International normalized ratio (0.7–1.3) | 1.04 (0.98–1.16) | 1.03 (0.98–1.07) | 1.05 (0.98–1.18) | 0.505 |
| D-dimer (0–1) μg/ml | 1.06 (0.40–5.54) | 0.49 (0.18–0.93) | 1.67 (0.66–6.61) | 0.017 |
| Albumin (40–55) g/l | 34.40 (31.38–39.15) | 39.20 (34.25–43.20) | 33.45 (30.78–35.85) | 0.000 |
| Aspartate aminotransferase (15–40) U/l | 31.50 (24.58–45.03) | 29.60 (22.55–57.60) | 33.40 (25.68–45.28) | 0.333 |
| Serum urea (1.7–8.3) mmol/l | 6.04 (4.20–9.16) | 3.91 (2.90–6.90) | 6.55 (4.88–10.39) | 0.291 |
| Sodium ion (137–147) mmol/l | 139.90 (135.98–142.30) | 137.50 (132.90–141.85) | 139.90 (136.25–142.95) | 0.968 |
| Lactate dehydrogenase (80–285) U/l | 291.50 (211.25–458.75) | 258.00 (198.00–361.50) | 337.50 (213.75–509.50) | 0.026 |
| Myoglobin (14.3–105.5) ng/ml | 0.00 (0.00–53.88) | 0.00 (0.00–27.55) | 78.75 (43.25–378.93) | 0.051 |
| Glucose (3.9–6.1) mmol/l | 7.41 (5.78–11.03) | 7.05 (5.15–10.10) | 7.85 (6.30–13.10) | 0.031 |
| Procalcitonin (<0.04) ng/ml | 0.22 (0.10–0.52) | 0.17 (0.09–0.36) | 0.26 (0.10–0.57) | 0.057 |
| C-reactive protein (0–0.5) mg/dl | 3.91 (1.52–9.01) | 2.21 (1.02–6.28) | 6.11 (3.11–11.51) | 0.141 |
Taking nonsurvivors as the dependent variable, the significant risk factors (Table 4) were analyzed using a binary logistic regression. Even after adjusting for age, cardiovascular disease and ARDS, plasma platelets and albumin levels were still associated with the risk of nonsurvivors in the critical group (all p < 0.05; Table 5).
Table 5. Risk factors for nonsurvivors in the critical group by binary logistic regression analysis.
| Characteristics | Odds ratio | 95% CI for odds ratio | p-value | Odds ratio† | 95% CI for odds ratio† | p-value† |
|---|---|---|---|---|---|---|
| Age | 1.114 | 1.049–1.183 | 0.000 | / | / | / |
| Cardiovascular disease | 3.300 | 1.016–10.719 | 0.047 | / | / | / |
| Acute respiratory distress syndrome | 7.539E9 | 0.000 | 0.999 | / | / | / |
| Platelets | 0.998 | 0.978–0.997 | 0.012 | 0.980 | 0.963–0.996 | 0.018 |
| D-dimer | 1.112 | 0.951–1.301 | 0.185 | 1.718 | 0.411–7.183 | 0.458 |
| Albumin | 0.789 | 0.677–0.920 | 0.002 | 0.777 | 0.614–0.983 | 0.036 |
| Lactate dehydrogenase | 1.004 | 1.000–1.008 | 0.073 | 1.004 | 0.997–1.011 | 0.229 |
| Glucose | 1.176 | 0.977–1.417 | 0.087 | 1.102 | 0.855–1.419 | 0.453 |
Adjusted for age, cardiovascular disease and acute respiratory distress syndrome. Patients in the critical group were included in the analysis.
OR: Odds ratio.
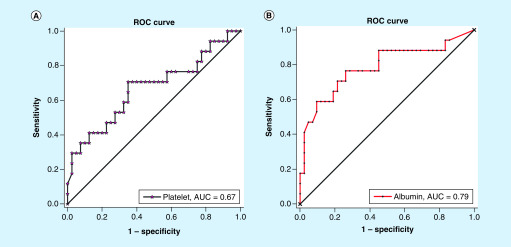
A receiver operating characteristic curve analysis was performed to verify the diagnostic accuracy of the platelets and albumin levels for the risk of nonsurvivors in the critical group. The area under the curve for platelets and albumin were 0.67 (95% CI: 0.50–0.83; p < 0.05) and 0.79 (95% CI: 0.64–0.93; p < 0.001), respectively. The optimal cut-off points for platelet and albumin were 155.00 109/l and 35.1 g/l, respectively. At this level, the Youden index was: 0.36 (platelet), 0.51 (albumin); sensitivity: 70.59% (platelet, 95% CI: 0.44–0.90), 76.47% (albumin, 95% CI: 0.50–0.93); specificity: 65.00% (platelet, 95% CI: 0.48–0.79), 73.81% (albumin, 95% CI: 0.58–0.85). The area under the curve for albumin was higher than that for platelets (Figure 2). Therefore, albumin may be a better predictive marker (cut-off point: 35.1 g/l) for the risk of nonsurvivors in the critical group.
Figure 2. Predictive factors for the risk of nonsurvivors in critical group.
The AUC for platelet (A) and albumin (B) were 0.67 (95% CI: 0.50–0.83; p < 0.05) and 0.79 (95% CI: 0.64–0.93; p < 0.001), respectively.
AUC: Area under the curve.
Discussion
In this study, we identified differences in clinical manifestations, laboratory tests, therapeutic interventions with severity and the risk of nonsurvivors in patients with confirmed COVID-19. We found that albumin may be an independent predictive marker (cut-off point: 35.1 g/l) for the risk of nonsurvivors in critically ill patients with confirmed COVID-19. Our findings highlight the clinical significance of focusing on the levels of albumin as a predictor of a high risk of nonsurvivors in critically ill patients with COVID-19.
Albumin is synthesized by the liver, which plays an important role in maintaining body nutrition and osmotic pressure [13]. A decrease in the levels of albumin is most likely due to liver damage and is likely to be caused by adverse drug reactions and systemic inflammation in critically ill patients with COVID-19. Although, several studies have reported that albumin may predict disease severity in patients with COVID-19 [14,15], we found that in critically ill patients, if the albumin level was less than 35.1 g/l, the risk of nonsurvivors is higher (sensitivity: 76.47%, 95% CI: 0.50–0.93; specificity: 73.81%, 95% CI: 0.58–0.85). However, as hypoproteinemia is defined as blood albumin <25 g/l [16], it will be too late to add albumin at this stage, as the risk of nonsurvivors would be greatly increased. Therefore, we recommend using drugs such as human albumin (that raise levels of albumin) to reduce risk of nonsurvivors, due to decreased levels of albumin (less than 35.1 g/l) in critically ill patients. This may also help clinicians identify patients with high risk of nonsurvivors at an early stage. Low levels of albumin also indicate that the patient’s nutritional status is poor and the body’s immunity is reduced. However, the host’s immune response against RNA viral infection is often weakened due to nutritional deficiencies, which may be overlooked during clinical diagnosis and treatment. Therefore, we recommend verifying the nutritional status of patients with COVID-19 before giving general treatments.
Similar to previous studies [8,10,11], the elderly and male patients with cardiovascular disease had higher morbidity and mortality. Fever and cough were the main symptoms. Elevated levels of neutrophils, D-dimer, lactate dehydrogenase, procalcitonin, C-reactive protein and decreased lymphocyte count were found. However, the elevation of D-dimer was not shown the independent risk factor of a nonsurvivor. In the critical group, there was one case with myocardial infarction, two cases with pulmonary thromboembolism and five cases with ischemic stroke in a group of nonsurvivors, however, there was only one case with ischemic stroke in the group of survivors; after analysis, there was no statistical difference between survivors and nonsurvivors in these three diseases. The proportion of patients without fever with COVID-19 was higher than that found in severe acute respiratory syndrome (SARS) coronavirus (1%) and Middle East respiratory syndrome (MERS) coronavirus infection (2%) [17]. Therefore, if surveillance teams focus on fever detection, patients with COVID-19 may be missed.
The case fatality rate (31.34%) of this study was similar to that by Prof Yu (28.4%), but significantly higher than that from other studies (1.4, 3.2, 11 and 15%) [1,3,8]. In addition, mortality rate in the critically ill was as high as 71.19%, probably because of the difference in sample sizes and case inclusion criteria. This study focused on discharged patients (including rehabilitation discharge and death cases) and no inpatients were included. The patients studied were infected with the first-generation of the virus, but the virus might be more virulent now.
The median hospital stay in the critical group was 1.5 days longer than that in the moderate group. Recently, studies have found that four patients with COVID-19 who met criteria for the discharge requirements had positive real time reverse transcriptase PCR results after 5–13 days in China. This suggests that at a proportion of the recovered patients may still be virus carriers [18]. Therefore, we recommend that recovering patients from the severe and critical groups should have to extend the duration of their hospital stay, so that they can get better rehabilitation services in the hospital.
In this study, the positivity rate for the first test for viral nucleic acid was 33.58%, however, similar to previous studies, the positivity rate increased to 78.36% after multiple tests [19]. These viral nucleic acid test results were inconsistent with the patient’s clinical symptoms and lung CT imaging. This may have been caused by irregular sampling, incorrect PCR amplification and interpretation as well as unstable kits. Therefore, rapidly optimizing the sampling site, ensuring quality of testing kits and standard operating procedure should be done urgently to provide the best infrastructure for the testing of COVID-19.
Previous studies have shown that SARS and Middle East respiratory syndrome coronavirus (MERS-CoV) infection increased the levels of serum proinflammatory cytokines [20,21]. In this study, we noted that higher median values of leukocytes, neutrophils and C-reactive protein were associated with the increased severity of COVID-19 pneumonia, which suggests that a cytokine storm is associated with disease severity. In this study, most patients were prescribed corticosteroids to reduce the inflammatory-induced lung injury. However, previous studies have reported that administering corticosteroids in patients with SARS and MERS did not affect mortality [22,23]. Therefore, we recommend low- and moderate-dose corticosteroids for critically ill COVID-19-positive patients. However, the efficacy and adverse reactions need to be further studied. Although immunoglobulins were used extensively for COVID-19, it should be noted that a third of all critically ill SARS patients developed venous thrombo-embolism including pulmonary embolism during the outbreak in Singapore in 2003 [24], due to an immunoglobulin-induced increase of viscosity in hypercoagulable states. Therefore, immunoglobulins should be used with caution in critically ill patients with significantly elevated D-dimer or decreased platelets to prevent nonsurvivors from pulmonary embolism.
Currently, there are no studies on the beneficial effects of antiviral drugs [10]. Arbidol, oseltamivir, lopinavir and ritonavir treatments are not effective. However, remdesivir is currently in Phase III clinical trials and its efficacy is unknown. At present, some traditional Chinese medicine decoctions were used clinically, as their main action is to improve lung function and enhance host immunity against COVID-19 infection [25].
There were some limitations in this study. First, some cases were incompletely recorded and laboratory testing indicators were not complete, which will cause some deviations. In addition, the sample size may not be large enough and some bias may have occurred. Also, we undoubtedly missed asymptomatic cases, so our study cohort may represent only the more serious COVID-19 cases. Finally, this is a single-center retrospective study, so these results need further confirmation with a multicentric study.
Conclusion
Albumin levels decreased with the progression of the disease and could be used as an independent predictor (cut-off point: 35.1 g/l) of the risk of nonsurvivors in critically ill patients with COVID-19. This may help clinicians to identify high risk of nonsurvivors among critically ill patients at an early stage.
Summary points.
Background
Exploring biomarkers closely related to death is of great significance for reducing the mortality of critically ill patients with COVID-19.
Materials & methods
Patients admitted to hospital due to COVID-19 pneumonia were collected and the clinical characteristics and clinical outcomes were analyzed.
Results
The severity of COVID-19 pneumonia was positively correlated with the decrease in levels of platelets and albumin (all p < 0.05). In the critical group, even after adjusting for age, cardiovascular disease and acute respiratory diseases, plasma levels of albumin were still significantly correlated with the risk of nonsurvivors (p < 0.05).
Conclusion
Levels of albumin could be used as an independent predictor of the risk of nonsurvivors in critically illness patients with COVID-19.
Author contributions
H Zhang and Y Cai had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. H Zhang and Y Cai also conceived and designed the experiments. J Li, ML Li, S Zheng, M Sun, Y Cai and H Zhang performed the experiments. J Li, M Li, X Li and A Deng analyzed the data. J Li wrote the paper. J Li, M Li, S Zheng and ML Li contributed equally.
Acknowledgments
We thank all health-care workers involved in the diagnosis and treatment of patients in Wuhan.
Financial & competing interests disclosure
This study was supported by the Health and Family Planning Commission of Wuhan City (WX18M02 and WX18C25). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study was approved by the Ethics Commission of the Central Hospital of Wuhan. Written informed consent was waived for retrospective study. The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Data availability
The data used to support the findings of this study are included within the article.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Chen N, Zhou M, Dong X. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223), 507–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maclaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA (2020) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Includes the basic characteristics of patients with new coronary pneumonia.
- 4.Holshue ML, Debolt C, Lindquist S. et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothe C, Schunk M, Sothmann P. et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z, Shi L, Wang Y. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223), 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng SQ, Peng HJ. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 9(2), 575 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu YH, Dong JH, An WM. et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J. Infect. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan JF, Yuan S, Kok KH. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395(10223), 514–523 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardi M, Angeli P, Claria J. et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The research is closely related to our theme.
- 14.Liu Y, Yang Y, Zhang C. et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 63(3), 364–374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Tao ZW, Lei W. et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. (Engl). (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet 386(9997), 995–1007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan L, Xu D, Ye G. et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology 200343 (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong CK, Lam CW, Wu AK. et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136(1), 95–103 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 104, 8–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 3(9), e343 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arabi YM, Mandourah Y, Al-Hameed F. et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 197(6), 757–767 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Lew TW, Kwek TK, Tai D. et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA 290(3), 374–380 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]