An infectious disease with a new viral pathogen has garnered the world's attention over the past 3 months in ways that no other human infection has to date. Coronavirus disease 2019, also known as COVID-19, is accelerating around the world in pandemic proportions. This infectious disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 [SARS CoV 2 (1)], which was first detected in China and has now spread to over more than 150 countries. On March 17, the WHO announced that the number of confirmed cases outside of China had exceeded those inside of China (2), and counts on March 25, showed 413,467 confirmed cases and 18,433 associated deaths, globally (3). The latest global count updates can be found at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/.
SARS-CoV-2 is a positive-sense, single-stranded RNA virus belonging to the genus Betacoronavirus, and phylogenetically related (88%–89% similarity) to the two bat-derived SARS-like coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21. Coronaviruses are capable of rapid mutation and recombination leading to novel coronaviruses that have differing transmission profiles, as was witnessed in the 2002 epidemic of Severe Acute Respiratory Syndrome coronavirus (SARS-CoV), and the Middle East Respiratory Syndrome coronavirus (MERS-CoV) in 2012. Genetic analysis suggests that SARS-CoV-2 is a novel coronavirus (4) that enabled its initial transmission from an animal source, possibly bats to humans, either directly or via dropping-contaminated materials, in a local seafood and wild animal market in Wuhan, Hubei Province, China (5,6), and now is being transmitted in exponentially rising numbers between humans. The virus enters the human host cell via angiotensin-converting enzyme-2 receptors that are expressed on the outer membrane of tissue-specific cells found in the heart, lungs, small intestine, kidneys, testes, as well as other organs to a lesser degree.
Early in the epidemic, individuals who had contact with travelers to China or other geographical hot-spots were deemed at risk. With evolution of the epidemic into a pandemic, community contacts, with no obvious exposure, now account for the majority of the viral spread. Unlike SARS-CoV and MERS-CoV, however, the human-to-human transmission of SARS-CoV-2 is far more efficient. The reproduction number (R0) of an infection is the expected number of cases resulting directly from 1 case, in a population where all individuals are susceptible to the infection. Whereas SARS-CoV and MERS-CoV had R0 overall values of 0.3 to 2.9 and 0.5 to 3.5, respectively, the R0 for SARS-CoV-2 is currently estimated at 2.7 (7,8). The mean incubation period for COVID-19 is 5 to 6 days, ranging from 2.1 to 11.1 days (2.5--97.5th percentile) (9), with both symptomatic and asymptomatic human-to-human transmission now causing local, regional, and global spread of the virus. Despite the efficient spread, the case-fatality rate, which could change as the total number of infected cases accumulates, is currently at or less than 4.3% (3), with different rates based on age, sex, geographical region (10) and other yet to be identified factors. Regardless, despite a much more efficient infection transmission rate, the SARS-CoV-2 fatality rate overall is much lower than either SARS (10%, 8098 cases and 774 deaths) or MERS-CoV (34%, 2494 cases and 858 deaths) (11).
Clinical presentation: Although adults tend to present stereotypically with acute respiratory symptoms, the presentation of children appears more variable. Adults are suspected as having COVID-19 when they have an acute respiratory infection with fever, cough and/or shortness of breath, and no other etiology that fully explains the clinical presentation (WHO, February 27, 2020, and the European Centre for Disease Prevention and Control [ECDC] case definition). Additionally, there is growing experience that anosmia, hyposmia, and dysgeusia are associated with COVID-19, and may be present before any other symptoms of infection present (12). In contrast, the symptoms in children are sometimes indistinguishable from those associated with common respiratory infections. One case series of 34 children from the Province of Shenzhen, China described the clinical manifestations of the previously well children, with 65% having common respiratory symptoms (fever [50%] and cough [38%]), 26% only mild disease, and 9% as asymptomatic (13). In a series of 20 children from the Province of Zhejiang, China, they described the presenting symptoms of low-to-moderate or no fever, rhinitis, cough, fatigue, headache, and diarrhea, and only in the more severe cases were dyspnea, cyanosis, and poor feeding observed (14). Although gastrointestinal symptoms are considered rare with coronaviral infections, prior studies of other coronaviruses (15), as well as the experience with SARS-CoV-2 suggest that vomiting and diarrhea may be among the presenting symptoms in adults and in up to 10% of children (16,17). In 204 adults (mean age 54.9 years) from China with COVID-19, 48.5% experienced 1 or more digestive symptoms, including: lack of appetite (40.1%), diarrhea (14%), vomiting (4%), and abdominal pain (2%). More importantly, 3.4% of the patients with COVID-19 had no respiratory symptoms but did experience digestive symptoms (18). Despite mild or no symptoms, there is evidence that transmission of the infection is still possible by these infected individuals (19).
The impact of COVID-19 on 2143 children (<18 years of age), reported to the Chinese Center for Disease Control and Prevention between January 16 and February 8, 2020, was evaluated, and published in Pediatrics in March 2020 (20). Two-thirds of the children (1412, 65.9%) were suspected to have COVID-19, and 731 (34.1%) were laboratory-confirmed to have SARS-CoV-2. Boys and girls were affected similarly, with 4% of children remaining asymptomatic, 51% having mild illness, and 39% moderate illness. Only 6% of the reported children had severe or critical illness, compared with 18.5% of reported adults, and just 1 14-year-old boy died, which is a remarkably low fatality rate. Infants, however, were found to have higher rates of serious illness than older children. About 11% of infants had severe or critical illness compared with 7% of children ages 1 to 5 years, 4% of those 6 to 10 years of age, 4% of those 11 to 15 years, and 3% of those 16 years and older. It is important to note that this study was limited by a short window of study duration and a high percentage of severe and critical illness without laboratory confirmation of SARS-CoV-2, leaving open the possibility of other respiratory infections confounding the results being characterized (eg, influenza, RSV).
Radiological and laboratory findings early in the course of COVID-19 disease have been described as stereotypically including ground glass opacities of the lung, lymphopenia, and elevated C-reactive protein (CRP). In 1 study of 108 adults in China with confirmed SARS-CoV-2 positivity by reverse transcription-polymerase chain reaction testing and stereotypical symptoms, the laboratory results showed normal white blood cell count (WBC) in 90%, normal or reduced lymphocyte count in 60%, but elevated CRP in 99% of patients (21). Computed tomography (CT) of the lungs revealed ground glass opacity in 60% and vascular thickening in 80%, with a typically patchy (86%) and peripheral (90%) distribution involving anywhere from 1 (35%) to 4 to 5 (43%) lung lobes. Pregnant women may exhibit a normal temperature, leukocytosis, and lymphopenia more commonly than nonpregnant adults (22). Additionally, in the same study, the lung findings by CT were more commonly mixed or complete consolidation in the pregnant women (22). The ability to differentiate between mild and severe COVID-19 was analyzed in 43 adult patients in China (23). The highest specificity (93.3%) and sensitivity (96.4%) for the early prediction of severity in COVID-19 patients was with IL-6 and D-Dimer tandem testing.
In series of 4 (22) and 5 (24) children, on the other hand, the pulmonary involvement with COVID-19 was mild or moderate, with only focal patchy ground glass opacity or consolidation.
Risk of exposure and severe illness: The immediate risk of exposure to this virus is changing rapidly, and as the outbreak expands geographically, that risk is increasing. At present, optimal studies in population-based epidemiology are lacking as second and third generation testing is not yet available (ie, sensitivity and specificity >90%) nor is population-based testing. Individuals living in or having visited places where community spread of SARS-CoV-2 is reported are at elevated risk of exposure, and this increases with contact with known persons infected with SARS-CoV-2. Likewise, healthcare workers caring for patients with COVID-19 are at elevated risk of infection. Endoscopists are at particular risk given the recently identified exposure of the endoscopist's face to biological material during the procedure (25), and substantiated by the observation that droplets aerosolized from SARS-CoV patients in 2003 reached individuals located more than 6 feet away (26). The presence of SARS-CoV-2 in colonic biopsy specimens and stool also suggests that the risk is not limited to upper endoscopy (16,27). Additionally, the presence of SARS-CoV-2 in stool suggests the possibility of fecal-oral spread in addition to aerosolized droplet spread, and underscores the importance of frequent hand-washing. Furthermore, it is now recognized that spread may occur not only via direct contact with aerosolized virus (emitted via cough or sneeze), but that the virus survives on surfaces. In particular, van Doremalen et al) (28) conducted a recent study of the stability of SARS-CoV-2 when aerosolized and deposited on different surfaces (plastic, stainless steel, copper, and cardboard) (28). SARS-CoV-2 remains stable in aerosols for greater than 3 hours, and is more stable on plastic and stainless steel than on copper or cardboard. Although the investigators found that SARS-CoV-2 remains detectable for up to 72 hours on plastic and stainless steel, it is still detectable on copper up to 4 hours, and on cardboard up to 24 hours.
Early information from China, and from subsequently highly-impacted communities, show that subsets of our populations are at higher risk of severe disease. Those at increased risk of severe disease include: older adults, with risk increasing by age (especially those >60 years), infants under 12 months of age, individuals with serious chronic medical conditions, such as those with cancer, end-stage renal disease on dialysis, diabetes, poorly controlled hypertension, coronary artery disease, heart failure, or pre-existing chronic lung disease. In contrast, however, adults and children on immunomodulating drugs, or immunosuppressed for other reasons, do not seem to be more prone to acquiring the SARS-CoV-2 infection or developing a more severe disease form (29).
Treatment: Currently no antiviral drugs are licensed by the US Food and Drug Administration (FDA) or European Medicines Agency (EMA) to treat patients with COVID-19. In the United States, the National Institutes of Health (NIH) and collaborators at many university research centers are working on the development of candidate vaccines and therapeutics for COVID-19. Similar rapid developments are reported in other countries, such as in Germany and in Switzerland.
Remdesivir is an investigational antiviral drug that was reported to have in vitro activity against SARS-CoV-2 (30) and successful compassionate use is anecdotally reported (31). In China, multiple clinical trials of investigational therapeutics have been implemented, including 2 phase 3, randomized, double-blind, placebo-controlled multicenter clinical trials of remdesivir (32). In the United States, a NIH-adaptive, randomized, double-blind, placebo-controlled, clinical trial of investigational therapeutics for hospitalized COVID-19 patients was approved by the FDA; the first investigational therapeutic to be studied is remdesivir. Other treatment trials for severely affected COVID-19 patients in the United States are also available (see Fig. 1); for information on specific clinical trials underway for treatment of patients with COVID-19, see clinicaltrials.gov, and www.chictr.org.
FIGURE 1.
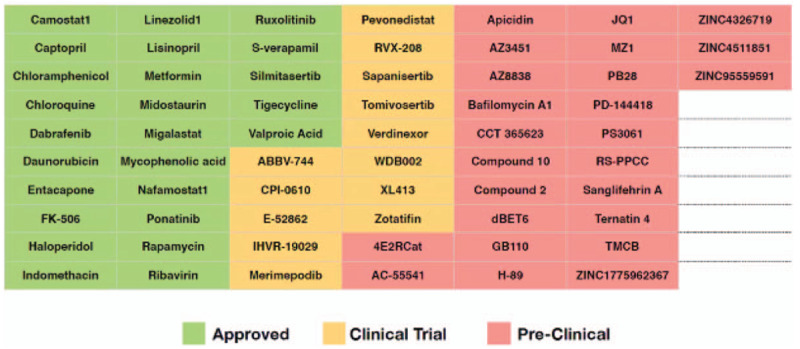
Existing drugs targeting severe acute respiratory syndrome coronavirus 2 human host factors. Data from (33).
Recent publications have brought attention to the possible benefit of chloroquine, a broadly used antimalarial drug, for treatment of patients infected by the novel coronavirus (SARS-CoV-2). In particular, in vitro data suggest that chloroquine inhibits SARS CoV-2 replication (30), and clinical trials of chloroquine in China in patients infected by SARS-CoV-2 have suggested promise (34). These reports will represent the first successful treatment of an acute viral disease in humans with chloroquine, however, validation is clearly needed (35). On the basis of reports from China indicating that hydroxychloroquine, in combination with azithromycin, may shorten the duration of infection, Gautret et al (36) prospectively studied 30 confirmed COVID-19 patients in France, treating each with either hydroxychloroquine, hydroxychloroquine in combination with azithromycin, or standard of care with neither. Six of the subjects were asymptomatic, 22 with symptoms of the upper respiratory tract (ie, sneezing, headaches, and sore throats), and 8 with lower respiratory tract symptoms (coughing). Hydroxychloroquine was found more effective than no treatment in these small cohorts, and when combined with azithromycin it was even more effective. More multicenter well-powered studies are, however, needed, utilizing patients at different stages of COVID-19 disease in order to see if hydroxychloroquine with or without azithromycin are truly effective and in what patient populations. Data from a small randomized trial conducted by the Shanghai Public Health Clinical Center, of 30 subjects randomized to receive 400 mg of hydroxychloroquine per day for 5 days on top of standard supportive care versus just standard supportive care found no difference in the groups, and hence no added benefit of hydroxychloroquine treatment (37). Furthermore, one must take into account that both hydroxychloroquine and azithromycin have the potential to increase QT and, the combination appears in Micromedex under the category of major interaction.
At present, no clinical trial evidence supports the use of vitamin C for COVID-19. On the basis of anecdotal observations that patients with severe SARS-CoV-2-associated disease and sepsis often have reduced vitamin C levels, however, randomized controlled trials are underway to evaluate if this antioxidant could play a role in the treatment of critically ill COVID-19 patients. One such trial currently underway is administering 1500 mg of vitamin C intravenously to critically ill patients with COVID-19, with the same massive dose repeated 3 to 4 times each day. Each dose is over 16 times the daily recommended dietary allowance of vitamin C, which is 90 mg for adult men and 75 mg for adult women.
Biologics: For the treatment of severe COVID-associated lung disease, studies in adults are considering “biologic” compounds targeting blockage of either the interleukin (IL)-1 or IL-6 pathways. When COVID-19 infects the respiratory tract, it can cause mild or highly acute respiratory distress syndrome with consequent release of pro-inflammatory cytokines, including IL-1β and IL-6. COVID-19 binds to the Toll Like Receptor (TLR) causing the release of pro-IL-1β, which is cleaved by caspase-1, followed by inflammasome activation and production of active mature IL-1β, which mediates lung inflammation, fever, and fibrosis (see Fig. 2). Suppression of the pro-inflammatory IL-1 family members and IL-6 has a therapeutic effect in many inflammatory diseases, including viral infections. Cytokine IL-37, for instance, has the ability to suppress innate and acquired immune response and the capacity to inhibit inflammation via the IL-18Rα receptor, acting on mTOR, thereby increasing adenosine monophosphate kinase. IL-37 inhibits class II histocompatibility complex molecules and inflammation by suppressing MYD88 and subsequently IL-1β, IL-6, TNF, and CCL2. The suppression of IL-1β by IL-37 in the inflammatory state induced by coronavirus-19 could be a potential route for therapeutic molecules, and merits further study. Another inhibitory cytokine is IL-38, the most recently recognized cytokine of the IL-1 family members, produced by several immune cells including B cells and macrophages. IL-38 is also a suppressor cytokine that inhibits IL-1β and other pro-inflammatory IL-family members. IL-38 is a potential therapeutic cytokine, which inhibits inflammation in viral infections including that caused by coronavirus-19, providing yet another novel relevant therapeutic strategy (39).
FIGURE 2.
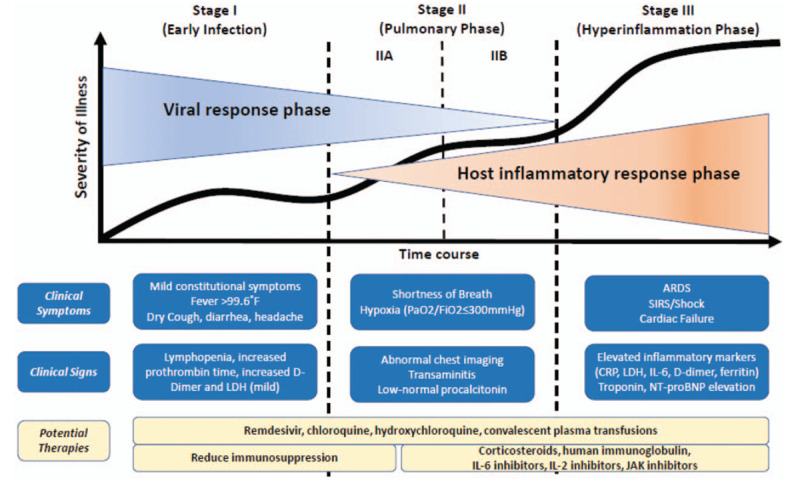
Classification of COVID-19 Disease States and Potential Therapeutic Targets. Data from (38). The figure shows 3 escalating phases of disease progression with COVID-19, with associated signs, symptoms, and potential phase-specific therapies. ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; IL = interleukin; JAK = janus kinase; LDH = lactate dehydrogenase; SIRS = systemic inflammatory response syndrome.
The FDA is expediting the use of a blood plasma treatment for seriously ill patients with COVID-19. Called plasma-derived therapy, or convalescent plasma, this treatment involves injecting the plasma, rich with anti-COVID antibodies, from individuals who have recovered from the disease, into the affected patient. Similarly, plasmapheresis or plasma exchange, which has been successfully employed in some acute auto-immune neurological diseases (eg, Guillain-Barre Syndrome), is also being evaluated. Results are still too preliminary to recommend these approaches in the management of COVID-19 disease at present (40,41).
Vaccination: Phase 1 clinical trials of potential COVID-19 vaccines have begun in Seattle, Washington, United States (US, partnership between US National Institute of Allergy and Infectious Diseases, NIAID, and Moderna, a biotechnology company based in Cambridge, Massachusetts) (42), and China (CanSino Biologics with Beijing Institute of Biotechnology). In addition, groups in Germany, the United Kingdom, and the US have vaccines in the preclinical stages of development.
Recommendations for gastroenterology practices: Routine gastroenterology practice poses increased risk of exposure and potential viral transmission during ambulatory interaction, especially during endoscopic procedures (27,43). Current guidelines recommend postponing elective nonurgent endoscopic procedures until the SARS-CoV-2 pandemic has subsided in the local area to limit un-necessary exposure and spread [on March 14, 2020, the US Surgeon General advised hospitals to postpone all elective surgeries (6)]. In contrast, the only endoscopic procedures that should be conducted are those in which the life of the patient is threatened (eg, significant acute bleeding), function of the organ may be at risk (eg, liver biopsy for the diagnosis of autoimmune hepatitis), or the delay of the procedure could significantly change long-term prognosis (eg, suspected cancer). The diagnosis of celiac disease, for instance, can, in many cases, especially in asymptomatic patients, be postponed for a few months without significant impact on prognosis, while the benefit to family and caregiver in lowered risk of COVID-exposure is clear. Consideration should be, however, given to the risk imposed on the patient, family (arriving to the hospital) and the caregiver (exposure to positive patients and family) compared with the risk of false positive or negative diagnosis. When biopsy is needed and not done for celiac disease because of the COVID-19 pandemic, patients should be advised keep gluten in the diet, if possible, until a diagnosis is made. Similarly, an effort to delay endoscopies because of abdominal pain, heartburn, diarrhea, and other GI manifestations should be exercised, where the delay will not significantly impact long-term prognosis. In contrast, when a disease like inflammatory bowel disease is suspected and delay in diagnosis would be dangerous for the patient, and treatment is reliant on endoscopic evaluation, exposure risks of endoscopy may be warranted.
The use of telemedicine is now a critical tool for the pediatric gastroenterologists and their patients, whether in the academic setting or private practice. As the COVID-19 pandemic continues to spread across the United States, increasing numbers of practices and hospitals are turning to telehealth to safely care for patients (44,45). Recently, Medicare announced it would be expanding reimbursement for office, hospital, and other telehealth visits in the United States to help slow the spread of the novel coronavirus. Before this expansion, Medicare only provided limited telehealth payments, particularly targeted at those patients living in rural areas. Very recently, the American Academy of Pediatrics has provided valuable information to facilitate pediatric practitioners to successfully employ telehealth in the care of their patients. The recently published AAP guideline, entitled Telehealth Payer Policy in Response to COVID-19 (https://downloads.aap.org/DOPA/Telehealth_2_rev.pdf and https://www.aap.org/en-us/professional-resources/practice-transformation/telehealth/Pages/compendium.aspx), which outlines policy changes aiming to alleviate barriers to telehealth care, along with a webinar on telehealth and guidance on structuring your practice during the pandemic are tools that can be employed in both the academic and private practice pediatric gastroenterologist office to facilitate ongoing quality care of their patients.
Through telehealth, patients can stay home and have a virtual visit with their regular physician or an on-demand physician, instead of presenting at a clinic and potentially exposing themselves or others to infection. Although society is trying to “flatten the curve” and reduce the incidence of new SARS-CoV-2 infections, individuals need to not go to the ER unless really sick, avoid public transportation (eg, buses, trains), and take refuge at home, physically distancing from others as much as possible. Further, if the SARS-CoV-2 pandemic in the United States reaches the scale of that in other hard-hit countries like China and Italy, then physicians will need to determine how to manage caring for patients with coronavirus, in addition to all their other patients. Pediatric gastroenterologists care for a number of digestive conditions of childhood that require routine and regular follow-up. Using telemedicine or telehealth, pediatric GI providers can continue to provide care for their patients, safely and without increasing risk for either the healthcare staff and/or the patient and their family.
Recommendations are as follows:
-
1.
Classification of procedures into nonurgent/postpone and urgent/perform may be useful.
-
2.
Prescreen all patients for high-risk exposure or symptoms:
-
a.
Patients should be asked about history of fever or respiratory symptoms, family members or close contacts with COVID-19 suggestive symptoms, and any contact with a confirmed case of COVID-19.
-
b.
Any patient or visitor with fever or respiratory symptoms must be given a surgical mask to wear.
-
a.
-
3.
Make sure appropriate personal protective equipment (PPE) is available and worn by all members of the endoscopy team, and know how to don and doff PPE appropriately (https://www.cdc.gov/hai/pdfs/ppe/ppe-sequence.pdf).
-
4.
Check body temperature of the patient and their attendant upon entrance to the endoscopy unit or clinic building.
-
5.
Conservation of PPE is critical
-
a.
Only essential personnel should be present in cases.
-
b.
Consider extended use or reuse of surgical masks and eye protection in accordance with hospital policies.
-
a.
-
6.
For COVID-19 positive patients, or those under investigation, isolation precautions should be taken with procedures performed in negative pressure rooms.
-
7.
Consider phone follow-up at 7 and 14 days to patients exposed to COVID-19, to ask about new diagnosis, or development of symptoms, suggestive of COVID-19.
-
8.
Centers should strategically assign available personnel.
-
a.
It is important to minimizing concomitant exposure of those with similar or unique skill sets.
-
b.
Nonphysician practitioners and fellows that cannot participate in cases may be helpful screening and triaging patients, or performing virtual visits.
-
a.
-
9.
For office visits, consider offering visits remotely, via telemedicine platforms or telephone, in order to decrease the office density of patients and decrease the potential for exposure, while providing needed care.
-
10.
Address staff needs and institute policies that protect our workforce.
-
11.
Patients on immunosuppressive drugs should continue taking their medications as we know that the risk of undertreated disease outweighs the chance of contracting coronavirus (29). These patients should also follow CDC guidelines for at-risk groups by avoiding crowds and limiting travel.
The summary of what we know is as follow:
-
1.
Cough, fever, and fatigue are the most common symptoms in adults.
-
2.
The incidence of GI symptoms including nausea and/or diarrhea are unknown but reports vary from <5% to almost 50% or greater. Further, there are some reports of isolated diarrhea preceding cough and fever.
-
3.
The virus may be present in GI secretions, important for the pediatric gastroenterologist to be aware of, and, viral RNA is detectable in stool. Gastrointestinal infection and potential fecal-oral transmission must be considered for this pathogen.
-
4.
Asymptomatic spread can occur during the prodromal phase (the mean incubation period is ∼5 days; range of 0–14 days), with viral shedding at its greatest when symptoms begin.
-
5.
Abnormal liver biochemistries are observed in 20% to 30% of persons infected by COVID-19.
-
6.
Lymphocyte counts drop in adults with COVID-19 infection, but is less common in children. Increased total white count is a poor prognostic sign.
-
7.
Adults over the age of 60 years, infants, and individuals with severe chronic health conditions, such as cardiovascular disease, lung disease, diabetes, or decompensated cirrhosis, are at higher risk of developing more severe illness if infected with SARS-CoV-2.
-
8.
Best protection against community viral transmission include:
-
a.
Social distancing of at least 6 feet (eg, avoidance of crowds)
-
b.
Cough etiquette (ie, into your elbow)
-
c.
Wash hands thoroughly with soap (at least 20 seconds under running water)
-
d.
Do not touch your face
-
a.
-
9.
Personal protective equipment in the healthcare environment (27):
-
a.
In a low-risk situation:
-
i.
Surgical mask
-
ii.
Goggles or eye shield
-
iii.
Gown
-
iv.
Gloves
-
i.
-
b.
In a high-risk situation or with a known COVID-positive patient:
-
i.
N95 or FFFP2-3 respirator
-
ii.
Goggles
-
iii.
Water-resistant gown
-
iv.
Gloves
-
v.
All evaluations in a negative pressure room
-
i.
-
a.
Recent updated guidelines are as follows:
-
1.
Appropriate PPE for healthcare personnel depending on the COVID risk: https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe.html
-
2.
Cleaning and disinfecting rooms or areas visited by individuals with suspected or confirmed COVID-19: https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/cleaning-disinfection.html
-
3.
For the conduct of clinical trials during the COVID-19 pandemic:
-
4.
Additional resources and guidelines are as follows:
-
a.
JOINT GI SOCIETY MESSAGE: COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers: https://www.asge.org/home/joint-gi-society-message-covid-19.
-
b.
American Academy of Pediatrics (AAP) updates on COVID-19 with guidelines for pediatric providers and parents: https://www.healthychildren.org/English/health-issues/conditions/chest-lungs/Pages/2019-Novel-Coronavirus.aspx
-
c.
The AAP also has regular updates for the Redbook on line: https://redbook.solutions.aap.org/selfserve/sspage.aspx?selfservecontentid=rbo_outbreaks_page_3.
-
d.
The Crohn's Colitis Foundation has regularly updated guidelines for providers that care for patients with inflammatory bowel disease (IBD, Crohn disease, ulcerative colitis): Resources for IBD Healthcare Professionals: 2019 Novel Coronavirus (COVID-19); https://www.crohnscolitisfoundation.org/coronavirus/professional-resources
-
e.
Lastly an invaluable resource is the Centers for Disease Control and Prevention (CDC), updated daily: https://www.cdc.gov/coronavirus/2019-ncov/index.html
-
a.
In conclusion, the authors would like the pediatric gastroenterology, hepatology, and nutrition world-wide community to know that we are in this together. United, with evidence-based information shared throughout our professional societies and then employed by our members, the curve can be flattened, new incident SARS-CoV-2 infections reduced, and the care for our patients with or without COVID-19 can continue. Research is a major pillar for maintaining optimal medical care and moving towards a better future for our children. These are, however, challenging times for medical research. It is our hope that the effects of the coronavirus pandemic will be short-lived relative to the long-term benefits of the clinical trials being conducted, ultimately for the benefit of children we care for with chronic and debilitating digestive, liver, pancreatic and nutritional disease. Additionally, we of the pediatric gastroenterology community need to share our collective experiences (eg, SECURE-IBD, www.covidibd.org) dealing with this infection, as well as sharing and employing guidelines (eg, WHO, CDC, AAP) based on science for what we do every day. As a global Pediatric Gastroenterology, Hepatology and Nutrition community—we can overcome this pandemic safely—and thereby get back to our lives, and our families, a little older, wiser and with the knowledge that we did not do it alone!
Footnotes
This article has been developed as a Journal CME Activity by NASPGHAN Visit http://www.naspghan.org/content/59/en/Continuing-Medical-Education-CME to view instructions, documentation, and the complete necessary steps to receive CME credit for reading this article.
The authors report no conflicts of interest.
REFERENCES
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020; 5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cyranoski D. When will the coronavirus outbreak peak? Nature 2020. [Google Scholar]
- 3. World Health Organization. Coronavirus disease (COVID-19) outbreak, Situation report 57. 2020. [Google Scholar]
- 4.Chen L, Liu W, Zhang Q, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect 2020; 9:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 2020; 55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 2020; 395:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19, an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travelers from Wuhan, China, 20-28 January 2020. Euro Surveill 2020; 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Mahase E. Coronavirus: COVID-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. Br Med J 2020; 368:m641. [DOI] [PubMed] [Google Scholar]
- 12. Hopkins C, Kumar N. Lost sense of smell as marker of COVID-19 infection. The Royal College of Surgeons of England: British Rhinological Society; 2020. [Google Scholar]
- 13.Wang XF, Yuan J, Zheng YJ, et al. Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen. Zhonghua Er Ke Za Zhi 2020; 58:E008. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZM, Fu JF, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esper F, Ou Z, Huang YT. Human coronaviruses are uncommon in patients with gastrointestinal illness. J Clin Virol 2010; 48:131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020; [Epub ahead of print]. [Google Scholar]
- 21.Han R, Huang L, Jiang H, et al. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. Am J Roentgenol 2020. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Liu F, Li J, et al. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Cui H, Li K, et al. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston ER, Habib-Bein N, Dueker JM, et al. Risk of bacterial exposure to the endoscopists face during endoscopy. Gastrointest Endosc 2019; 89:818–824. [DOI] [PubMed] [Google Scholar]
- 26.Wong TW, Lee CK, Tam W, et al. Outbreak Study Group. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis 2004; 10:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Repici A, Maselli R, Colombo M, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med: Massachusetts Medical Society; 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl 2020; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus nCoV) in vitro. Cell Res 2020; 30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holshue ML, DeBolt C, Lindquist S, et al. Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko W-C, Rolain J-M, Lee N-Y, et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents 2020; 105933 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioSci Trends 2020; 14:72–73. [DOI] [PubMed] [Google Scholar]
- 35.Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res 2020; 177:104762 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gautret P, Lagiera J-C, Parolaa P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID - 19 results of an open - label non - randomized clinical trial. Int J Antimicrob 2020; 105949 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haelle T. Hydroxychloroquine use for COVID-19 coronavirus shows no benefit in first small—but limited—controlled trial. Forbes 2020. [Google Scholar]
- 38.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transpl 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 2020; 34: [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Xiong J, Bao L, et al. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 2020; 20:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care 2020; 24:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coronavirus latest: most infections and deaths are now outside, China. Nature 2020. [Google Scholar]
- 43. Yafei Z, Xiaodan Z, Hongling W, et al. Suggestions of Infection Prevention and Control in Digestive Endoscopy During Current 2019-nCoV Pneumonia Outbreak in Wuhan, Hubei Province, China. World Endo Org 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenhalgh T, Wherton J, Shaw S, et al. Video consultations for covid-19. BMJ 2020; 368:m998. [DOI] [PubMed] [Google Scholar]
- 45.Reeves JJ, Hollandsworth HM, Torriani FJ, et al. Rapid response to COVID-19: health informatics support for outbreak management in an academic health system. J Am Med Inform Assoc 2020; pii: ocaa037 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


