The coronavirus disease 2019 (COVID-19) pandemic has impacted congenital cardiac surgical programs with significant reduction in case load, implementation of patient triage strategies, and development of personal protective equipment (PPE) guidelines. This document summarizes current status and implications of COVID-19 in congenital cardiac surgery outlining recommendations from the Congenital Cardiac Anesthesia Society (CCAS;Figure 1).
Figure 1.
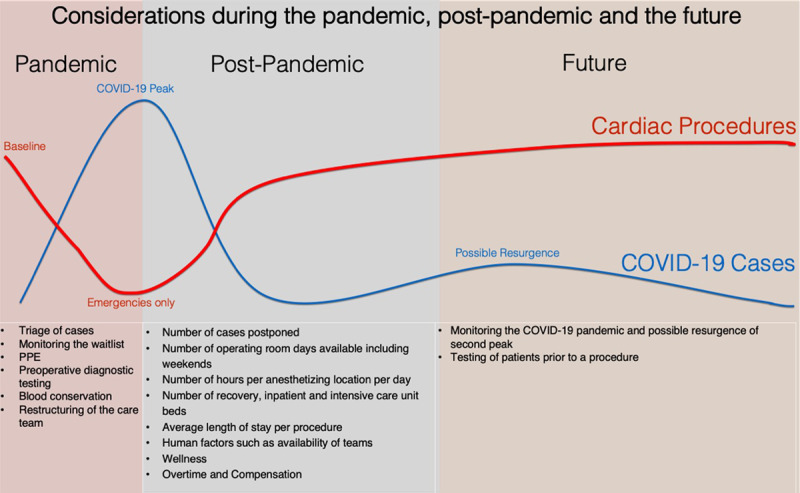
Considerations for Pediatric Heart Programs during COVID-19 pandemic, postpandemic, and the future. COVID-19 indicates coronavirus disease 2019; PPE, personal protective equipment.
REORGANIZATION OF HEART PROGRAM
Decrease in CaseLoadand Prioritization
In response to the rapid recognition of challenges faced by health care infrastructures related to the COVID-19 outbreak, a broad call to restrain “elective” surgical procedures was announced by the Center for Medicare and Medicaid Services (CMS) and the American College of Surgeons (ACS) in March 2020.1 Multidisciplinary committees should discuss cases prioritization based on ACS definition of emergent and urgent procedures, taking into consideration hospital capacity and local resources available during the COVID-19 pandemic (Table).2
Table.
Cardiac Lesions and Surgical Prioritization
| Elective Cases | Urgent Cases | Emergency Cases |
|---|---|---|
| No anticipated risk as a result of delaying the procedure more than 2 mo | Risk of patient deterioration or rapid disease progression if the procedure is delayed days to weeks | Delay more than 24–48 h is life threatening |
| Medically managed arrhythmias that is not lifethreatening awaiting an electrophysiologic study/ablation. Valvular regurgitation that is managed medically. Slowly progressive aortic stenosis scheduled for Ross operation. Pre-Fontan catheterization with adequate saturations (>75%) on room air. |
Transposition of the great arteries with intact ventricular septum (delay <1wk) and with ventricular septal defect (2–4wk). Hypoplastic left heart syndrome with restrictive atrial septum (Norwood procedure). Truncus arteriosus. Obstructive lesions that are stabilized with prostaglandins. Superior cavopulmonary anastomosis with decreasing saturations. Atrioventricular canal in a patient on “maximal” anticongestive therapy with failure to thrive and repeat hospitalization. Endocarditis with a hemodynamically stable patient. |
Obstructed total anomalous pulmonary venous return. Heart transplant. ECMO or VAD placement in hemodynamically unstable patient. PDA stents in an unstable patient on prostaglandin. AAOCA with recent hemodynamic instability. Stenotic RV-PA conduitwith severe ventricular dysfunction. Thrombosed shunt. Tamponade. Balloon atrial septostomy. |
Abbreviations: AAOCA, anomalous aortic origin of a coronary artery; ECMO, extracorporeal membrane oxygenation; PDA, patent ductus arteriosus; RV-PA, right ventricle to pulmonary artery; VAD, ventricular assist device.
Heart Transplantation
To date, most pediatric heart transplant programs in the United States continue to deem heart transplant as an urgent/emergent surgical procedure. The recommendation from the International Society of Heart and Lung Transplantation states that the patient should report no new-onset clinical symptoms consistent with COVID-19, no close contact with a confirmed case, and no travel within 14 days (https://ishlt.org/ishlt/media/documents/SARS-CoV-2_-Guidance-for-Cardiothoracic-Transplant-and-VAD-centers.pdf). In addition, the document recommends COVID-19 testing before transplant, assuming a rapid turn-around assay is available. Patients with positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection should not undergo transplantation. In regard to the processes that surround donor offers and procurement, the surgical recovery of organs by the local teams should be promoted whenever possible to minimize potential exposure and traveling across the country.
Monitoring the WaitList
Most congenital cardiac programs ceased scheduling elective procedures in anticipation of the surge in COVID-19–positive patients requiring hospitalization. This has resulted in a dynamic expanding procedural wait list which will have to be managed with consideration of the following:
-
Continuously reassessing and reprioritizing of all patients on list
-
a.
Implementing telehealth, virtual check-in, and/or remote monitoring
-
a.
-
Recognition that every patient’s clinical status and pending procedure is unique and a decision to further postpone must be multidisciplinary based on:
-
a.
Potential for significant deterioration which could result in harm such as therapy otherwise not indicated, prolonged hospital stay, or mortality
-
b.
Institutional abilities to schedule cases (physical plant, human resources)
-
c.
Time course of COVID-19 crisis
-
a.
Ongoing effective, timely communication with patients/families, referring physicians, and proceduralists
As restrictions lessen, a plan needs to be in place not only for caring for the remaining patients on the wait list but also for addition of new patients.
Resumption Of Activity
In the COVID-19 outbreak, defining the transition of pandemic to postpandemic remains unclear. The stringency and duration of strategies such as physical distancing and PPE use will impact the timing and magnitude of a potential “second wave” of infections which may prolong the pandemic phase. Formal documentation of herd immunity by testing individuals’ immune status may help define a stable SARS-CoV-2 resilient health care workforce, allowing faster return to higher surgical volumes. Prioritization based on medical needs across surgical specialties is important as many pediatric cardiac centers will share resources (eg, personnel) with other surgical specialties. Monitoring for clinical deterioration during recommended wait times mitigates harm, affords opportunities to inform future care plans, and provides a structured and just approach to return of surgical services.
Resuming Activityin Practice
In the recovery phase following the COVID-19 pandemic, delayed surgeries will need to be rescheduled. This will likely require overtime shifts and expanded utilization of currently available operating rooms and catheterization laboratories. In some institutions, additional operating rooms may be allocated to meet this demand, but that approach assumes that perioperative personnel can be mobilized. In many institutions, cardiac intensive care unit (CICU) and step-down cardiac floor capacities are a limiting factor in the number of cases that can be performed. Programs that have well-established “fast-track” protocols will be able to leverage that expertise to help relieve potential congestion in the CICU and step-down floor. In each program, serious consideration will have to be given to further delaying procedures associated with long CICU and hospital stays in favor of performing procedures with short stays. To put a finer point on it, a program may not be able to justify performing a procedure with an average CICU stay of 6–7 days in place of performing multiple cases with an average CICU stay of 1–2 days. “Fast-track” surgical procedures and diagnostic catheterizations could be performed on the weekend to reduce the inpatient volume during the week. Alternatively, these patients could be recovered in an extended recovery room environment allowing the CICU and step-down units to handle a larger caseload.
DIAGNOSTIC TESTING FOR SARS-COV-2
The purpose of testing is to facilitate diagnosis to guide supportive patient management and to implement measures to limit the spread of the virus. There are 2 broad categories of SARS-CoV-2 tests: those that detect the virus itself and those that test for the immune response of the host to the virus.
Tests for ViralRNA
The preferred testing method is the real-time reverse transcription-polymerase chain reaction (RT-PCR). Within 5 days of the onset of symptoms of COVID-19, patients have high viral loads in their upper and lower respiratory tracts.3 A nasopharyngeal or oropharyngeal swab may be used to collect a specimen. For patients with pneumonia, lower respiratory tract secretions may be collected from bronchoalveolar lavage fluid. Detection rates in each sample vary between patients and may change over the course of their illness.4 Testing patients for SARS-CoV-2 helps identify those who are infected, which is useful for individual patient management, as well as for implementing mitigation strategies to prevent spread in health care facilities and the community. It may also help with the appropriate choice of PPE for health care providers. Although these tests have become more widely available, the huge demand for them has created supply chain challenges. One of the current limitations regarding preoperative testing for SARS-CoV-2 comes from the variability in sensitivity and specificity of the different tests available.5 The rate of false negative for some of those tests can be as high as 30%. It is therefore extremely important to consider the sensitivity and positive predictive value of the test used in each institution before a decision to deescalade the level of PPE can be made. The vast majority of heart programs have started testing inpatients and outpatients scheduled for cardiac surgery and cardiac catheterization.
Antibody Tests
Antibody tests can identify individuals who have been exposed to SARS-CoV-2 and have developed immunity, which could protect them from future infections. Development of immunity may enable previously infected health care workers to return to work with relative safety.6 Congenital heart programs could consider deployment of these individuals to environments where the risk of infection is highest thereby protecting nonimmune members of the workforce from infection.
MINIMIZING THE IMPACT OF EXPOSURE TO COVID-POSITIVE INDIVIDUALS ON SMALL DEDICATED TEAMS
Cardiac anesthesia teams are composed of a small number of dedicated attendings and extended care providers. If a substantial number of cardiac anesthesia team members become ill or need to self-isolate due to exposure to a COVID-19–positive patient, colleague or family member, normal care delivery cannot be sustained. Segregating anesthesia providers and other cardiac specialty members into teams that are kept isolated from each other minimizes the risk of virus transmission. Attempting to operationalize team separation is challenging when faced with 24/7/365 coverage, simultaneous emergencies and illness (COVID-19 or otherwise), or exposure of one or more team members to a positive patient.
AIRWAY MANIPULATION
Aerosol-generating medical procedures (AGMP), such as endotracheal intubation and extubation, mask ventilation, tracheal suctioning, and inadvertent lung injury during positive pressure ventilation produce significant amounts of aerosolization and may be a significant source for the spread of SARS-CoV-2.7 Careful airway management of suspected or infected patients is therefore crucial. Adequate muscle relaxation and rapid sequence induction are recommended to avoid mask ventilation.8 Cuffed tubes should be utilized to minimize gas leaks. Selection of the nasal or oral route for intubation should be based on institutional experience and preference with consideration given to the priority of rapidly securing the airway. Once the trachea is intubated and the endotracheal tube secured, an inline suction device should be placed to avoid inadvertent contamination of the environment during tracheal suctioning. A high-efficiency particulate air (HEPA) filter should routinely be placed distal to the Y-connector of the circle system in addition to the filters normally placed on the inspiratory and expiratory limbs. Regardless of the site of extubation, appropriate PPE and room decontamination is essential.
PPE IN THE OPERATIVE ROOM OR THE CARDIAC CATHETERIZATION SUITE
In COVID-19–positive patients or suspected patients, PPE must be worn at all times. In asymptomatic patients who have not undergone COVID-19 testing, PPE is recommended during AGMP. Some institutions with well-established COVID-19 testing are relaxing the requirement for PPE during AGMP for patients who have tested negative in the preceding 24 hours. PPE should, at a minimum, include properly fitted N95 masks, eye and face shields, fluid impervious gowns, and gloves. When available, additional equipment such as positiveairwaypurifyingrespiratorsshould be considered to further reduce the risk of exposure. In all patients, except those tested to be negative for COVID-19, all nonessential personnel should be a minimum of 6 feet from the patient or ideally outside the room during AGMP. Only the minimum number of properly trained personnel should be in attendance during any AGMP. The use of video laryngoscopes is highly recommended both to assist in airway visualization and to maximize the distance from the patient to the caregiver’s face during the procedure. It is recommended that a “COVID” cart with all of the appropriate equipment be placed immediately outside the patient’s room to minimize time acquiring the necessary items. Finally, it is the responsibility of the provider to properly dispose of all contaminated materials into a double-sealed bag at the conclusion of the procedure while gowned in PPE to avoid accidental contamination of environmental staff.
To prevent contamination of anesthesia and perioperative personnel and workspace equipment, perioperative simulation should be promoted. Checklists should be created in collaboration with Surgical Quality and Safety teams.
CARING OF NEWBORN WITH CONGENITAL HEART DISEASE BORN OF A COVID-19–POSITIVE MOTHER
In retrospective analyses of neonates born to mothers with suspected or confirmed COVID-19, many newborns remained COVID-19 negative, but several exhibited respiratory distress and death havebeen reported.9,10 In 1 cohort, 3 newborns who were infected with SARS-CoV-2 had positive nasal and anal swabs at days 2 and 4 of life. All 3 experienced fever, lethargy, and pneumonia and were nasal swab negative by day 6 or 7 of life.10
The most likely route of transmission of SARS-CoV-2 frommother to newborn is via respiratory droplets during close contact. In the setting of acute maternal COVID-19 shortly before birth, maternal antibody production and fetal passive immunity may not have had time to develop, leaving the neonate relatively defenseless.11 There is currently no evidence of vertical transmission of SARS-CoV-2 from mother to fetus.
Multidisciplinary planning must occur ideally before and certainly after the birth of a child with CHD who is born of a suspected or confirmed COVID-19 mother. Severe COVID-19 symptoms in the mother may lead to fetal distress and preterm labor and delivery, which may gravely impact the newborn cardiopulmonary physiology.9 After delivery, the cord should be immediately clamped and the neonate isolated from the mother. If transport is required to the pediatric hospital after birth, full PPE is required throughout all phases. The newborn should be isolated and closely monitored for symptoms for 14 days. If emergent surgery is required, the operating room team must treat the newborn as SARS-CoV-2 infected and observe strict isolation procedures. In the middle ground are the neonates with lesions typically treated nonemergently within the first few days of life, such as the Norwood or arterial switch operations. The risks and benefits of proceeding with surgery versus waiting must be carefully weighed by a multidisciplinary team on a case by case basis.
CARDIOVASCULAR IMAGING
Echocardiography represents the primary imaging modality for diagnosis, management, and long-term surveillance of these patients. In response to the spread of COVID-19, the American Society of Echocardiography (ASE) has recently published a statement addressing protection of patients and echocardiography service providers to prevent viral transmission. Based on the recognition that children and those affected by CHD represent potential confounding elements in this pandemic, recommendations specific to the pediatric, fetal, and CHD populations have also been outlined in a supplement statement.12 These are based on acknowledging known differences between the pediatric and adult populations in terms of COVID-19 prevalence, symptomatology, and disease severity and other factors that impact imaging.13 Most echocardiography laboratories have adopted these ASE recommendations which CCAS supports. Key points of these continually updated guidelines suggest that echocardiographic studies be performed when they are expected to provide clinical patient benefit based on established appropriate use criteria with other examinations to be deferred/rescheduled.14 It is desirable to determine patient COVID-19 status whenever possible, to limit provider exposure during the examination, and to prevent equipment/reading room transmission.
Performance of transesophageal echocardiography (TEE) imaging is considered to increase the risk of SARS-CoV-2 spread secondary to both droplet and aerosol transmission. Currently, nonessential TEE examinations and those unlikely to impact clinical care have been or are being postponed or canceled. In many adults with CHD, these studies take place in sedated, nonintubated patients,a circumstance that could provoke aerosolization of a significant number of viral particles in infected patients. Consequently, alternative imaging modalities should be considered outside clinical settings that strongly merit the use of TEE such as during urgent or emergent cardiac surgery in neonates, children, and adults with CHD, and selected nonelective catheter-based interventions. Other situations require careful assessment of the benefit of the TEE study versus risks related to potential COVID-19 exposure and should also consider available PPE resources. Although epicardial echocardiography is considered a valuable adjunct to intraoperative imaging, TEE is known to overcome many of its limitations. Accordingly, during the COVID-19 pandemic, standardized TEE algorithms should be developed in conjunction with all stakeholders and influenced by local institutional policies, that consider study indications and COVID-19 status to guide procedural precautions and the use of PPE, as well as protocols for equipment handling, allowing for ongoing patient benefits and the safest application of the imaging modality.12,15
CARDIOPULMONARY BYPASS AND ANTICOAGULATION FOR COVID-19 PATIENTS
There are currently no reported cases of children with known COVID-19 undergoing cardiac surgery with cardiopulmonary bypass (CPB). There is no evidence that the anticoagulation strategy for a patient undergoing a procedure on CPB should be altered due to COVID-19, but the cardiac care team should be aware that the thrombosis risk is increased in this population.16 In the event CPB is utilized in a patient with COVID-19, scavenging of exhaust gas from the CPB machine is recommended. Currently, there are publications from China showing the COVID-19 virus in the blood.17 This would indicate it may be possible to transmit the virus to the exhaust ports of an oxygenator and out into the environment. However, plasma leakage into the hollow fiber oxygenator is the most likely way for the virus to be aerosolized from the CPB circuit. Fortunately, when oxygenators are used as recommended, plasma leakage is extremely uncommon. Scavenging systems are easy to apply and already utilized in many centers that routinely deliver inhaled anesthetic gases during CPB.
BLOOD SHORTAGE AND CONSERVATION
Although the reduction in elective cases has decreased blood product utilization, social isolation measures have resulted in a rapid decrease in overall blood donations. Daily communication with the blood center and key clinical teams is necessary to predict whether inventory is available to cover daily needs.
As cardiac anesthesiologists, it is our duty to implement perioperative blood conservation strategies. Blood conservation guidelines for neonates and children undergoing cardiac surgery have been published and provide a resource to help develop institutional protocols.18 Although not well studied in children with CHD, preoperative treatment of anemia and iron deficiency should be considered with either oral or intravenous iron. An erythropoietin stimulating agent may be considered in specific cases. Prophylactic administration of an antifibrinolytic should be used when appropriate. Efforts should also be made to minimize the resultant hemodilution of CPB, including reduction of the prime volume and the use of a nonblood prime in infants weighing >5 to 7 kg.19 Because units of red blood cells are aging, blood banks may not be able to provide fresh blood for neonatal cardiac patients. Due to the risk of hyperkalemia associated with older and large volume transfusions, physicians should be vigilant regarding the age of blood products received, the need for irradiation, and the use of washing techniques before transfusion. Nonallogeneic coagulation agents, like desmopressin, fibrinogen concentrate,20 or prothrombin thrombin complex concentrate, may be considered based on underlying coagulopathy. Multimodal center-specific transfusion algorithms (Figure 2) using a restrictive approach and coagulation assessment based on either standard coagulation tests or viscoelastic tests have been shown to be effective in reducing transfusion requirements.
Figure 2.
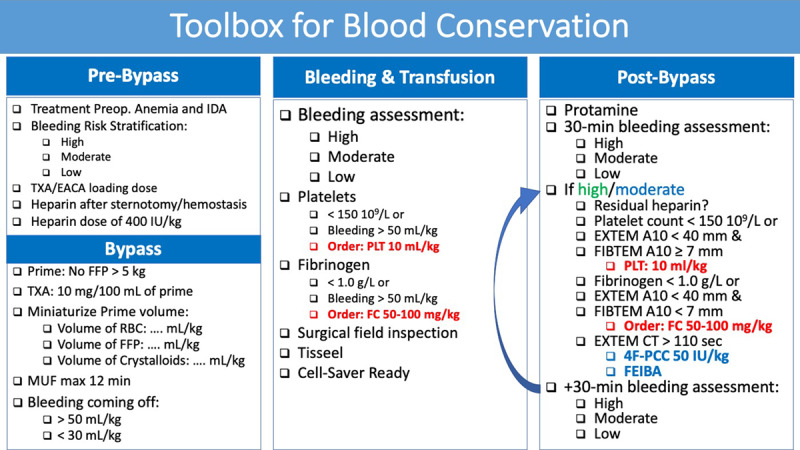
Toolbox for blood conservation that can be used as an example to design institutional toolbox. CT indicates clotting time; EACA, epsilon amino caproic acid; EXTEM, extrinsic test from thromboelastometry; FC, fibrinogen concentrate; FEIBA, factor eight inhibitor bypassing activity; FFP, fresh frozen plasma; FIBTEM, fibrinogen test from thromboelastometry; IDA, iron-deficiency anemia; MUF, modified ultrafiltration; PCC, prothrombin complex concentrate; PLT, platelets; RBC, red blood cell; TXA, tranexamic acid.
SUMMARY
The COVID-19 pandemic has impacted congenital cardiac surgical programs with significant reduction in case load, implementation of patient triage strategies, and development of PPE guidelines. Our opinion piece is based on current knowledge and experience. Due to the lack of robust evidence, strategies will be evolving, and recommendations will certainly change over the next months. It is important that congenital cardiac surgical programs collect data to understand the impact of the COVID-19 pandemic on the incidence of adverse events and resource utilization.
DISCLOSURES
Name: David Faraoni, MD, PhD.
Contribution: This author invited the coauthors, created the outline, wrote the manuscript, edited and formatted the draft.
Name: Lisa A. Caplan, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: James A. DiNardo, MD, FAAP.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: Nina A. Guzzetta, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: Wanda C. Miller-Hance, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: Gregory Latham, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: Mona Momeni, MD, PhD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: Susan C. Nicolson, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: James P. Spaeth, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: Katherine Taylor, BMed MMed, FANZCA.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: Mark Twite, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: David F. Vener, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: Luis Zabala, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
Name: Viviane G. Nasr, MD.
Contribution: This author helped write the manuscript, reviewed and approved the final version.
This manuscript was handled by: Jean-Francois Pittet, MD.
GLOSSARY
- AAOCA
- anomalous aortic origin of a coronary artery
- ACS
- American College of Surgeons
- AGMP
- aerosol-generating medical procedures
- ASE
- American Society of Echocardiography
- CCAS
- Congenital Cardiac Anesthesia Society
- CHD
- congenital heart disease
- CICU
- cardiac intensive care unit
- CMS
- Center for Medicare and Medicaid Services
- COVID-19
- coronavirus disease 2019
- CPB
- cardiopulmonary bypass
- CT
- clotting time
- EACA
- epsilon amino caproic acid
- ECMO
- extracorporeal membrane oxygenation
- EXTEM
- extrinsic test from thromboelastometry
- FC
- fibrinogen concentrate
- FEIBA
- factor eight inhibitor bypassing activity
- FFP
- fresh frozen plasma
- FIBTEM
- fibrinogen test from thromboelastometry
- HEPA
- high-efficiency particulate air
- IDA
- iron-deficiency anemia
- MUF
- modified ultrafiltration
- PCC
- prothrombin complex concentrate
- PDA
- patent ductus arteriosus
- PLT
- platelets
- PPE
- personal protective equipment
- RBC
- red blood cell
- RT-PCR
- reverse transcription-polymerase chain reaction
- RV-PA
- right ventricle to pulmonary artery
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- TEE
- transesophageal echocardiography
- TXA
- tranexamic acid
- VAD
- ventricular assist device
Funding: This study was solely supported by the Congenital Cardiac Anesthesia Society.
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
REFERENCES
- 1. Surgeons ACo: Guidelines for triage of Non-Emergent Surgical Procedure. Available at: https://www.facs.org/covid-19/clinical-guidance/triage. 2020. Accessed March 17, 2020.
- 2.Stephens EH, Dearani JA, Guleserian KJ, et al. COVID-19: crisis management in congenital heart surgery. Ann Thorac Surg. 2020. April 11 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang YW, Schmitz JE, Persing DH, Stratton CW. The laboratory diagnosis of COVID-19 infection: current issues and challenges. J Clin Microbiol. 26;58:e00512–e00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg Microbes Infect. 2020;9:747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melgaço JG, Azamor T, Ano Bom APD. Protective immunity after COVID-19 has been questioned: what can we do without SARS-CoV-2-IgG detection? Cell Immunol. 2020;353:104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matava CT, Kovatsis PG, Summers JL, et al. Pediatric airway management in COVID-19 patients - consensus guidelines from the Society for Pediatric Anesthesia’s Pediatric Difficult Intubation Collaborative and the Canadian Pediatric Anesthesia Society. Anesth Analg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 March 26 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D, Yang H, Cao Y, et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet .2020;149:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker PCA, Lewin MB, Donofrio MT, et al. Specific considerations for pediatric, fetal, and congenital heart disease patients and echocardiography service providers during the 2019 novel coronavirus outbreak: Council on Pediatric and Congenital Heart Disease Supplement to the Statement of the American Society of Echocardiography endorsed by the Society of Pediatric Echocardiography and the Fetal Heart Society. J Am Soc Echocardiogr. 2020 April 9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 14.Sachdeva R, Valente AM, Armstrong AK, et al. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 appropriate use criteria for multimodality imaging during the follow-up care of patients with congenital heart disease: a report of the American College of Cardiology Solution Set Oversight Committee and Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Pediatric Echocardiography. J Am Coll Cardiol. 2020;75:657–703. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Coll Cardiol. 2020 April 6 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 May 4 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraoni D, Meier J, New HV, Van der Linden PJ, Hunt BJ. Patient blood management for neonates and children undergoing cardiac surgery: 2019 NATA guidelines. J Cardiothorac Vasc Anesth. 2019;33:3249–3263. [DOI] [PubMed] [Google Scholar]
- 19.Dieu A, Rosal Martins M, Eeckhoudt S, et al. Fresh frozen plasma versus crystalloid priming of cardiopulmonary bypass circuit in pediatric surgery: a randomized clinical trial. Anesthesiology. 2020;132:95–106. [DOI] [PubMed] [Google Scholar]
- 20.Downey LA, Andrews J, Hedlin H, et al. Fibrinogen concentrate as an alternative to cryoprecipitate in a postcardiopulmonary transfusion algorithm in infants undergoing cardiac surgery: a prospective randomized controlled trial. Anesth Analg. 2020;130:740–751. [DOI] [PubMed] [Google Scholar]


