The asymmetric unit of the title compound contains two independent molecules, consisting of perimidine and phenol units, which are linked through an N—H⋯O hydrogen bond. Intramolecular O—H⋯N hydrogen bonds are observed in both independent molecules.
Keywords: crystal structure, perimidine, phenol, Hirshfeld surface
Abstract
The asymmetric unit of the title compound, C17H14N2O, contains two independent molecules each consisting of perimidine and phenol units. The tricyclic perimidine units contain naphthalene ring systems and non-planar C4N2 rings adopting envelope conformations with the C atoms of the NCN groups hinged by 44.11 (7) and 48.50 (6)° with respect to the best planes of the other five atoms. Intramolecular O—H⋯N hydrogen bonds may help to consolidate the molecular conformations. The two independent molecules are linked through an N—H⋯O hydrogen bond. The Hirshfeld surface analysis of the crystal structure indicates that the most important contributions for the crystal packing are from H⋯H (52.9%) and H⋯C/C⋯H (39.5%) interactions. Hydrogen bonding and van der Waals interactions are the dominant interactions in the crystal packing. Density functional theory (DFT) optimized structures at the B3LYP/ 6–311 G(d,p) level are compared with the experimentally determined molecular structure in the solid state. The HOMO–LUMO behaviour was elucidated to determine the energy gap.
Chemical context
1-H Perimidines are defined as peri-naphtho-fused pyrimidines (Varsha et al., 2010 ▸). They were first discovered in 1874 (De Aguiar, 1874 ▸) and are characterized either by a binding deficit or an excess of π binding (Woodgate et al., 1987 ▸). They are used as intermediates in dyes, dyeing and polymerization systems (Watanab et al., 1977 ▸) and have been recognized as new carbene ligands (Bazinet et al., 2003 ▸), attracting great interest (Bu et al., 2001 ▸; Starshikoy et al., 1973 ▸). 1-H Perimidines also exhibit important biological activities (Zhou et al., 2019 ▸), having the potential to act as anti-inflammatory agents (Zhang et al., 2017 ▸) and inhibitors of enzymes (Alam et al., 2016 ▸) and to have applications in fluorescence (Giani et al., 2016 ▸), catalysis (Behbahani et al., 2017 ▸), corrosion inhibition (He et al., 2018 ▸) and in coordination chemistry (Booysen et al., 2016 ▸; Mahapatra et al., 2015 ▸).
Perimidines are obtained by the condensation of 1,8-diaminonaphthalene with various carbonyl groups. As a continuation of our research into the development of new perimidine derivatives with potential pharmacological applications, we have studied the reaction of the condensation of salicylaldehyde and 1,8- diaminonaphthalene in ether under agitation at room temperature to give the title compound in good yield. The title compound was obtained for the first time and characterized by single-crystal X-ray diffraction techniques as well as by Hirshfeld surface analysis. The results of the calculations by density functional theory (DFT), carried out at the B3LYP/6-311G (d,p) level, are compared with the experimentally determined molecular structure in the solid state.
Structural commentary
The asymmetric unit of the title compound, I, contains two crystallographically independent molecules each consisting of perimidine and phenol units, where the tricyclic perimidine units contain naphthalene ring systems and non-planar C4N2 rings (Fig. 1 ▸). A puckering analysis of the non-planar six-membered C4N2, B (N1A/N2A/C1A/C9A–C11A) and B′ (N1A/N2A/C1A/C9B–C11B) rings gave the parameters q 2 = 0.9280 (12) Å, q 3 = 0.1829 (12) Å, Q T = 0.9459 (13) Å, θ2 = 75.85 (15)° and φ 2= 134.47 (18)° for B and q 2 = 0.5320 (11) Å, q 3 = 0.3791 (11) Å, Q T = 0.6533 (14) Å, θ2 = 54.33 (12)° and φ 2= −5.47 (13)° for B′; both rings adopt envelope conformations, where atoms C1A and C1B are at the flap positions and at distances of 0.6044 (12) and −0.6590 (13) Å, respectively, from the best planes through the other five atoms. The C4N2 rings may alternatively be described as being hinged about the N⋯N vectors with the N1A/C1A/N2A and N1B/C1B/N2B planes being inclined by 44.11 (7) and 48.50 (6)°, respectively, to the best planes through the other five atoms (N1A/N2A/C9A–C11A) and (N1B/N2B/C9B–C11B). Rings A (C2A–C7A), C (C10A–C15A), D (C9A/C10A/C15A–C18A) and A′ (C2B–C7B), C′ (C10B–C15B), D′ (C9B/C10B/C15B–C18B) are oriented at dihedral angles of A/C = 76.78 (4), A/D = 78.49 (4), C/D = 2.09 (3)° and A′/C′ = 88.43 (3), A′/D′ = 88.31 (3), C′/D′ = 3.26 (4)°. Intramolecular O—H⋯N hydrogen bonds (Table 1 ▸) may be effective in consolidating the conformations of the two independent molecules.
Figure 1.
The asymmetric unit of the title compound with the atom-numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1A—H1OA⋯N1A | 0.86 (2) | 2.66 (2) | 3.1072 (16) | 113.8 (17) |
| O1A—H1OA⋯N2A | 0.86 (2) | 2.03 (2) | 2.7763 (16) | 144.6 (19) |
| O1B—H1OB⋯N1B | 0.84 (2) | 2.20 (3) | 2.8835 (16) | 138 (2) |
| O1B—H1OB⋯N2B | 0.84 (2) | 2.47 (2) | 3.0196 (16) | 123 (2) |
| N1B—H1NB⋯O1A | 0.865 (17) | 2.331 (17) | 3.1608 (18) | 160.8 (14) |
Supramolecular features
In the crystal, the two molecules in the asymmetric unit are linked through an N—H⋯O hydrogen bond (Table 1 ▸, Fig. 1 ▸).
Hirshfeld surface analysis
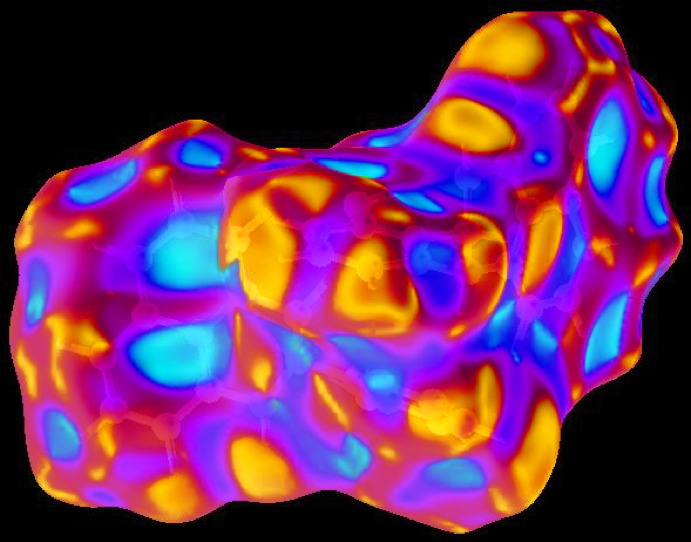
In order to visualize the intermolecular interactions in the crystal of the title compound, a Hirshfeld surface (HS) analysis (Hirshfeld, 1977 ▸; Spackman & Jayatilaka, 2009 ▸) was carried out by using Crystal Explorer 17.5 (Turner et al., 2017 ▸). In the HS plotted over d norm (Fig. 2 ▸), the white surface indicates contacts with distances equal to the sum of van der Waals radii, and the red and blue colours indicate distances shorter (in close contact) or longer (distinct contact) than the van der Waals radii, respectively (Venkatesan et al., 2016 ▸). The bright-red spots indicate their roles as the respective donors and/or acceptors.
Figure 2.
View of the three-dimensional Hirshfeld surface of the title compound plotted over d norm in the range −0.1813 to 1.6330 a.u.
The shape-index of the HS is a tool to visualize the π–π stacking by the presence of adjacent red and blue triangles; if there are no adjacent red and/or blue triangles, then there are no π–π interactions. Fig. 3 ▸ clearly suggests that there are no π–π interactions in I. The overall two-dimensional fingerprint plot (McKinnon et al., 2007 ▸) is shown in Fig. 4 ▸ a, and those delineated into H⋯H, H⋯C/C⋯H, H⋯O/O⋯H, H⋯N/N⋯H and C⋯C contacts are illustrated in Fig. 4 ▸ b–f, respectively, together with their relative contributions to the Hirshfeld surface. The most important interaction is H⋯H, contributing 52.9% to the overall crystal packing, which is reflected in Fig. 4 ▸ b as widely scattered points of high density due to the large hydrogen content of the molecule, with the tip at d e = d i = 1.10 Å. The pair of characteristic wings in the fingerprint plot delineated into H⋯C/C⋯H contacts, Fig. 4 ▸ c, (39.5% contribution to the HS) have the tips at d e + d i = 2.50 Å. The scattered points in the pair of spikes in the fingerprint plot delineated into H⋯O/O⋯H (Fig. 4 ▸ d, 5.7% contribution) have a symmetrical distribution with the tips at d e + d i = 2.49 Å. The H⋯N/N⋯H contacts (Fig. 4 ▸ e, 1.3% contribution) have a distribution of points with the tips at d e + d i = 2.72 Å. Finally, the C⋯C interactions (0.5% contribution to the overall crystal packing) are reflected in Fig. 4 ▸ f as low density wings with the tips at d e + d i = 3.60 Å.
Figure 3.
Hirshfeld surface of the title compound plotted over shape-index.
Figure 4.
The full two-dimensional fingerprint plots for the title compound, showing (a) all interactions, and delineated into (b) H⋯H, (c) H⋯C/C⋯H, (d) H⋯O/O⋯H, (e) H⋯N/N⋯H and (f) O⋯C/C⋯O interactions. The d i and d e values are the closest internal and external distances (in Å) from given points on the Hirshfeld surface contacts.
The Hirshfeld surface representations with the function d norm plotted onto the surface are shown for the H⋯H, H⋯C/C⋯H and H⋯O/O⋯H interactions in Fig. 5 ▸ a–c, respectively.
Figure 5.
The Hirshfeld surface representations with the function d norm plotted onto the surface for (a) H⋯H, (b) H⋯C/C⋯H and (c) H⋯O/O⋯H interactions.
The Hirshfeld surface analysis confirms the importance of H-atom contacts in establishing the packing. The large number of H⋯H and H⋯C/C⋯H interactions suggest that van der Waals interactions and hydrogen bonding play the major roles in the crystal packing (Hathwar et al., 2015 ▸).
DFT calculations
The optimized structure of the title compound, I, in the gas phase was generated theoretically via density functional theory (DFT) using standard B3LYP functional and 6–311 G(d,p) basis-set calculations (Becke, 1993 ▸) as implemented in GAUSSIAN 09 (Frisch et al., 2009 ▸). The theoretical and experimental results were in good agreement (Table 2 ▸). The highest-occupied molecular orbital (HOMO), acting as an electron donor, and the lowest-unoccupied molecular orbital (LUMO), acting as an electron acceptor, are very important parameters for quantum chemistry. When the energy gap is small, the molecule is highly polarizable and has high chemical reactivity. The DFT calculations provide some important information on the reactivity and site selectivity of the molecular framework. E HOMO and E LUMO, which clarify the inevitable charge-exchange collaboration inside the studied material, electronegativity (χ), hardness (η), potential (μ), electrophilicity (ω) and softness (σ) are recorded in Table 3 ▸. The significance of η and σ is for the evaluation of both the reactivity and stability. The electron transition from the HOMO to the LUMO energy level is shown in Fig. 6 ▸. The HOMO and LUMO are localized in the plane extending from the whole 2-(2,3-dihydro-1H-perimidin-2-yl)phenol ring. The energy band gap [ΔE = E LUMO - E HOMO] of the molecule is 1.4933 eV, the frontier molecular orbital energies E HOMO and E LUMO being −3.2606 and −1.7673 eV, respectively.
Table 2. Comparison of selected X-ray and DFT geometrical parameters (Å, °).
| Bonds/angles | X-ray | B3LYP/6–311G(d,p) |
|---|---|---|
| C1A—N1A | 1.4597 (17) | 1.40941 |
| C1A—N2A | 1.4646 (19) | 1.35557 |
| C1A—C2A | 1.5079 (17) | 1.43731 |
| C1A—H1A | 0.9800 | 1.03211 |
| N1A—C9A | 1.3944 (17) | 1.42420 |
| N1A—H1N1 | 0.873 (19) | 1.00630 |
| O1A—C3A | 1.3693 (18) | 1.40953 |
| O1A—H1OA | 0.86 (2) | 0.97032 |
| C2A—C7A | 1.388 (2) | 1.42763 |
| C2A—C3A | 1.3923 (19) | 1.42630 |
| N2A—C11A | 1.4081 (17) | 1.36897 |
| N1A—C1A—N2A | 106.61 (11) | 115.07 |
| N1A—C1A—C2A | 110.09 (11) | 125.03 |
| N2A—C1A—C2A | 109.23 (11) | 109.89 |
| N1A—C1A—H1A | 110.3 | 110.17 |
| N2A—C1A—H1A | 110.3 | 110.03 |
| C2A—C1A—H1A | 110.3 | 110.08 |
| C9A—N1A—C1A | 117.08 (11) | 117.82 |
| C9A—N1A—H1N1 | 115.0 (12) | 114.98 |
| C3A—O1A—H1OA | 106.1 (14) | 107.84 |
Table 3. Calculated energies.
| Molecular Energy (a.u.) (eV) | Compound I |
|---|---|
| Total Energy TE (eV) | −22880.3725 |
| E HOMO (eV) | −3.2606 |
| E LUMO (eV) | −1.7673 |
| Gap, ΔE (eV) | 1.4933 |
| Dipole moment, μ (Debye) | 3.3491 |
| Ionization potential, I (eV) | 3.2606 |
| Electron affinity, A | 1.7673 |
| Electronegativity, χ | 2.5139 |
| Hardness, η | 0.7466 |
| Electrophilicity index, ω | 4.2322 |
| Softness, σ | 1.3393 |
| Fraction of electron transferred, ΔN | 3.0042 |
Figure 6.
The energy band gap of the title compound.
Database survey
Similar perimidine derivatives have also been reported in which the groups at position 2 are almost coplanar with the perimidic nucleus. Examples related to the title compound, I, are II (Ghorbani, 2012 ▸), III (Fun et al., 2011 ▸), IV (Maloney et al., 2013 ▸), V (Cucciolito et al., 2013 ▸) and VI (Manimekalai et al., 2014 ▸), where III and V are most closely related while II, IV and VI are more distant relatives.
Synthesis and crystallization
0.35 mol (1.48 g) of 1,8-diaminonaphthalene and 18.8 mmol (2 ml) of salicylaldehyde were introduced into a 250 ml flask and 30 ml of ether were added thereto. The mixture was stirred magnetically for 3 days. The grey precipitate that formed was recovered by filtration, washed with ether, rinsed with ethanol and dried under Büchner. The resulting brownish powder was recrystallized several times from ethanol to obtain colourless 2-(2,3-dihydro-1H-perimidin-2-yl)phenol product (R f = 0.70 in hexane/ethyl acetate (1:0.5), yield: 97% A significant quantity of the colourless monocrystalline product was obtained by the slow evaporation of the solvent after 15 days.
Refinement
Crystal data, data collection and structure refinement details are summarized in are summarized in Table 4 ▸. The H atoms of OH and NH groups were located in difference-Fourier maps and refined freely. The C-bound H atoms were positioned geometrically, with C—H = 0.93 Å (for aromatic H atoms) and 0.98 Å (for methine H atom) and constrained to ride on their parent atoms, with U iso(H) = 1.2U eq(C).
Table 4. Experimental details.
| Crystal data | |
| Chemical formula | C17H14N2O |
| M r | 262.30 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 293 |
| a, b, c (Å) | 9.0710 (4), 12.0526 (7), 24.6120 (11) |
| β (°) | 95.999 (4) |
| V (Å3) | 2676.1 (2) |
| Z | 8 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.08 |
| Crystal size (mm) | 0.60 × 0.35 × 0.05 |
| Data collection | |
| Diffractometer | Rigaku XtaLAB PRO |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2018 ▸) |
| T min, T max | 0.212, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 29344, 6395, 4554 |
| R int | 0.042 |
| (sin θ/λ)max (Å−1) | 0.690 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.046, 0.120, 1.03 |
| No. of reflections | 6395 |
| No. of parameters | 379 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.19, −0.21 |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989020005939/lh5957sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020005939/lh5957Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989020005939/lh5957Isup3.cdx
Supporting information file. DOI: 10.1107/S2056989020005939/lh5957Isup4.cml
CCDC reference: 1976884
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
Professor Nahossé Ziao is thanked for allowing the synthesis to be undertaken in the Laboratory of Thermodynamics and Physical Chemistry of the Environment (LTPCM), University Nangui, Abrogoua, Côte d’Ivoire.
supplementary crystallographic information
Crystal data
| C17H14N2O | F(000) = 1104 |
| Mr = 262.30 | Dx = 1.302 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.0710 (4) Å | Cell parameters from 8291 reflections |
| b = 12.0526 (7) Å | θ = 2.8–29.0° |
| c = 24.6120 (11) Å | µ = 0.08 mm−1 |
| β = 95.999 (4)° | T = 293 K |
| V = 2676.1 (2) Å3 | Plate, colourless |
| Z = 8 | 0.60 × 0.35 × 0.05 mm |
Data collection
| Rigaku XtaLAB PRO diffractometer | 6395 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, Rigaku micromax 003 | 4554 reflections with I > 2σ(I) |
| Rigaku Integrated Confocal MaxFlux double bounce multi-layer mirror optics monochromator | Rint = 0.042 |
| Detector resolution: 5.811 pixels mm-1 | θmax = 29.4°, θmin = 2.7° |
| ω scans | h = −12→11 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2018) | k = −15→16 |
| Tmin = 0.212, Tmax = 1.000 | l = −33→32 |
| 29344 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: other |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.046 | Hydrogen site location: mixed |
| wR(F2) = 0.120 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0558P)2 + 0.390P] where P = (Fo2 + 2Fc2)/3 |
| 6395 reflections | (Δ/σ)max = 0.001 |
| 379 parameters | Δρmax = 0.19 e Å−3 |
| 0 restraints | Δρmin = −0.21 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1A | 0.56889 (14) | 0.53907 (11) | 0.62210 (5) | 0.0396 (3) | |
| H1A | 0.638920 | 0.526271 | 0.595013 | 0.047* | |
| N1A | 0.41985 (13) | 0.50532 (11) | 0.60023 (5) | 0.0437 (3) | |
| H1N1 | 0.4106 (19) | 0.4334 (16) | 0.5970 (7) | 0.066* | |
| O1A | 0.43505 (13) | 0.56864 (10) | 0.72308 (4) | 0.0584 (3) | |
| H1OA | 0.438 (2) | 0.6117 (18) | 0.6953 (9) | 0.088* | |
| C2A | 0.61653 (14) | 0.47595 (12) | 0.67387 (5) | 0.0405 (3) | |
| N2A | 0.56102 (14) | 0.65756 (10) | 0.63467 (4) | 0.0435 (3) | |
| H1NA | 0.646 (2) | 0.6821 (15) | 0.6495 (7) | 0.065* | |
| O1B | 0.04282 (12) | 0.30406 (13) | 0.63319 (5) | 0.0708 (4) | |
| H1OB | 0.043 (3) | 0.318 (2) | 0.6666 (10) | 0.106* | |
| N1B | 0.20034 (13) | 0.37845 (10) | 0.73446 (4) | 0.0411 (3) | |
| H1NB | 0.2567 (17) | 0.4294 (14) | 0.7230 (6) | 0.049* | |
| C1B | 0.25725 (13) | 0.26647 (11) | 0.72676 (5) | 0.0375 (3) | |
| H1B | 0.347428 | 0.254276 | 0.751615 | 0.045* | |
| C3A | 0.54903 (15) | 0.49467 (12) | 0.72131 (5) | 0.0443 (3) | |
| C2B | 0.28946 (14) | 0.24906 (11) | 0.66876 (5) | 0.0369 (3) | |
| N2B | 0.14081 (13) | 0.19020 (11) | 0.74018 (5) | 0.0419 (3) | |
| H2NB | 0.1541 (17) | 0.1222 (14) | 0.7310 (6) | 0.050* | |
| C4A | 0.59490 (18) | 0.43710 (15) | 0.76890 (6) | 0.0582 (4) | |
| H4A | 0.550760 | 0.450973 | 0.800663 | 0.070* | |
| C3B | 0.17999 (14) | 0.26533 (12) | 0.62532 (5) | 0.0433 (3) | |
| C5A | 0.70642 (19) | 0.35908 (16) | 0.76890 (7) | 0.0661 (5) | |
| H5A | 0.736838 | 0.320194 | 0.800742 | 0.079* | |
| C4B | 0.20960 (17) | 0.24421 (13) | 0.57226 (6) | 0.0503 (4) | |
| H4B | 0.135102 | 0.252304 | 0.543578 | 0.060* | |
| C6A | 0.77248 (17) | 0.33852 (16) | 0.72245 (8) | 0.0645 (5) | |
| H6A | 0.846957 | 0.285454 | 0.722639 | 0.077* | |
| C5B | 0.34887 (19) | 0.21132 (14) | 0.56196 (6) | 0.0568 (4) | |
| H5B | 0.368794 | 0.198052 | 0.526223 | 0.068* | |
| C7A | 0.72813 (15) | 0.39693 (14) | 0.67520 (6) | 0.0519 (4) | |
| H7A | 0.773844 | 0.383022 | 0.643784 | 0.062* | |
| C6B | 0.45901 (18) | 0.19793 (14) | 0.60424 (7) | 0.0582 (4) | |
| H6B | 0.553804 | 0.177175 | 0.597066 | 0.070* | |
| C9A | 0.35196 (14) | 0.56636 (11) | 0.55642 (5) | 0.0378 (3) | |
| C7B | 0.42863 (15) | 0.21533 (12) | 0.65727 (6) | 0.0456 (3) | |
| H7B | 0.502848 | 0.204217 | 0.685787 | 0.055* | |
| C10A | 0.38759 (13) | 0.68071 (11) | 0.55413 (5) | 0.0348 (3) | |
| C9B | 0.15927 (15) | 0.40010 (13) | 0.78695 (5) | 0.0444 (3) | |
| C11A | 0.49532 (14) | 0.72763 (11) | 0.59321 (5) | 0.0379 (3) | |
| C10B | 0.10259 (14) | 0.30998 (13) | 0.81524 (5) | 0.0455 (3) | |
| C12A | 0.52565 (18) | 0.83872 (12) | 0.59224 (6) | 0.0494 (4) | |
| H12A | 0.597660 | 0.868995 | 0.617626 | 0.059* | |
| C11B | 0.09143 (14) | 0.20261 (12) | 0.79161 (5) | 0.0422 (3) | |
| C13A | 0.44833 (19) | 0.90655 (13) | 0.55310 (6) | 0.0559 (4) | |
| H13A | 0.468574 | 0.982195 | 0.553059 | 0.067* | |
| C12B | 0.02694 (16) | 0.11725 (16) | 0.81762 (6) | 0.0578 (4) | |
| H12B | 0.018478 | 0.047355 | 0.801591 | 0.069* | |
| C14A | 0.34396 (17) | 0.86426 (13) | 0.51502 (6) | 0.0509 (4) | |
| H14A | 0.293631 | 0.911237 | 0.489454 | 0.061* | |
| C13B | −0.0259 (2) | 0.1357 (2) | 0.86819 (8) | 0.0763 (6) | |
| H13B | −0.069782 | 0.077655 | 0.885520 | 0.092* | |
| C15A | 0.31142 (14) | 0.75013 (12) | 0.51393 (5) | 0.0407 (3) | |
| C14B | −0.0142 (2) | 0.2368 (2) | 0.89238 (8) | 0.0829 (7) | |
| H14B | −0.048187 | 0.246358 | 0.926437 | 0.099* | |
| C16A | 0.20329 (16) | 0.70134 (14) | 0.47590 (5) | 0.0508 (4) | |
| H16A | 0.152944 | 0.744820 | 0.448781 | 0.061* | |
| C15B | 0.04892 (18) | 0.32860 (18) | 0.86688 (6) | 0.0644 (5) | |
| C17A | 0.17231 (16) | 0.59168 (15) | 0.47860 (6) | 0.0552 (4) | |
| H17A | 0.100450 | 0.561244 | 0.453204 | 0.066* | |
| C16B | 0.0568 (2) | 0.4373 (2) | 0.88808 (8) | 0.0871 (7) | |
| H16B | 0.023178 | 0.451434 | 0.921832 | 0.105* | |
| C18A | 0.24558 (16) | 0.52286 (13) | 0.51861 (6) | 0.0505 (4) | |
| H18A | 0.222179 | 0.447803 | 0.519553 | 0.061* | |
| C18B | 0.1656 (2) | 0.50467 (16) | 0.80913 (7) | 0.0639 (4) | |
| H18B | 0.204313 | 0.563464 | 0.790689 | 0.077* | |
| C17B | 0.1125 (3) | 0.5215 (2) | 0.86015 (8) | 0.0844 (6) | |
| H17B | 0.115746 | 0.592467 | 0.875106 | 0.101* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1A | 0.0369 (6) | 0.0440 (8) | 0.0368 (6) | −0.0004 (6) | −0.0008 (5) | 0.0035 (5) |
| N1A | 0.0475 (6) | 0.0373 (6) | 0.0431 (6) | −0.0065 (5) | −0.0108 (5) | 0.0045 (5) |
| O1A | 0.0697 (7) | 0.0588 (7) | 0.0487 (6) | 0.0109 (6) | 0.0153 (5) | 0.0095 (5) |
| C2A | 0.0347 (6) | 0.0438 (8) | 0.0409 (7) | −0.0056 (6) | −0.0055 (5) | 0.0066 (6) |
| N2A | 0.0470 (6) | 0.0416 (7) | 0.0385 (6) | −0.0103 (5) | −0.0113 (5) | 0.0042 (5) |
| O1B | 0.0442 (6) | 0.1251 (12) | 0.0413 (6) | 0.0251 (6) | −0.0033 (5) | −0.0002 (6) |
| N1B | 0.0451 (6) | 0.0392 (7) | 0.0390 (6) | −0.0011 (5) | 0.0046 (5) | −0.0005 (5) |
| C1B | 0.0320 (6) | 0.0436 (8) | 0.0360 (6) | 0.0035 (5) | −0.0009 (5) | 0.0022 (5) |
| C3A | 0.0443 (7) | 0.0458 (8) | 0.0414 (7) | −0.0062 (6) | −0.0017 (6) | 0.0052 (6) |
| C2B | 0.0372 (6) | 0.0352 (7) | 0.0383 (6) | −0.0002 (5) | 0.0039 (5) | 0.0013 (5) |
| N2B | 0.0443 (6) | 0.0397 (7) | 0.0424 (6) | −0.0002 (5) | 0.0079 (5) | 0.0026 (5) |
| C4A | 0.0635 (10) | 0.0667 (11) | 0.0422 (8) | −0.0147 (8) | −0.0045 (7) | 0.0124 (7) |
| C3B | 0.0396 (7) | 0.0510 (9) | 0.0392 (7) | 0.0017 (6) | 0.0038 (5) | 0.0024 (6) |
| C5A | 0.0567 (9) | 0.0738 (12) | 0.0624 (10) | −0.0109 (9) | −0.0196 (8) | 0.0315 (9) |
| C4B | 0.0583 (9) | 0.0533 (9) | 0.0386 (7) | −0.0017 (7) | 0.0010 (6) | −0.0004 (6) |
| C6A | 0.0413 (8) | 0.0676 (11) | 0.0809 (12) | 0.0057 (8) | −0.0115 (8) | 0.0250 (9) |
| C5B | 0.0715 (10) | 0.0573 (10) | 0.0441 (8) | 0.0027 (8) | 0.0179 (7) | −0.0076 (7) |
| C7A | 0.0364 (7) | 0.0584 (10) | 0.0594 (9) | 0.0023 (7) | −0.0020 (6) | 0.0109 (7) |
| C6B | 0.0520 (9) | 0.0632 (11) | 0.0622 (9) | 0.0089 (8) | 0.0195 (7) | −0.0082 (8) |
| C9A | 0.0369 (6) | 0.0431 (8) | 0.0325 (6) | −0.0025 (6) | −0.0001 (5) | 0.0012 (5) |
| C7B | 0.0396 (7) | 0.0461 (8) | 0.0511 (8) | 0.0034 (6) | 0.0045 (6) | −0.0031 (6) |
| C10A | 0.0343 (6) | 0.0415 (7) | 0.0288 (6) | 0.0002 (5) | 0.0048 (5) | 0.0022 (5) |
| C9B | 0.0421 (7) | 0.0532 (9) | 0.0364 (7) | 0.0082 (6) | −0.0033 (5) | −0.0045 (6) |
| C11A | 0.0416 (7) | 0.0405 (8) | 0.0316 (6) | −0.0032 (6) | 0.0032 (5) | 0.0015 (5) |
| C10B | 0.0359 (7) | 0.0647 (10) | 0.0349 (6) | 0.0115 (6) | −0.0008 (5) | 0.0031 (6) |
| C12A | 0.0647 (9) | 0.0424 (8) | 0.0401 (7) | −0.0106 (7) | 0.0007 (6) | −0.0014 (6) |
| C11B | 0.0313 (6) | 0.0564 (9) | 0.0381 (7) | 0.0073 (6) | −0.0007 (5) | 0.0114 (6) |
| C13A | 0.0798 (11) | 0.0378 (8) | 0.0506 (8) | −0.0022 (8) | 0.0099 (8) | 0.0058 (6) |
| C12B | 0.0459 (8) | 0.0708 (11) | 0.0566 (9) | 0.0013 (8) | 0.0049 (7) | 0.0234 (8) |
| C14A | 0.0613 (9) | 0.0492 (9) | 0.0427 (8) | 0.0103 (7) | 0.0082 (7) | 0.0139 (6) |
| C13B | 0.0614 (11) | 0.1090 (18) | 0.0607 (11) | 0.0030 (11) | 0.0162 (8) | 0.0329 (11) |
| C15A | 0.0390 (7) | 0.0512 (9) | 0.0327 (6) | 0.0044 (6) | 0.0072 (5) | 0.0074 (6) |
| C14B | 0.0694 (12) | 0.137 (2) | 0.0460 (9) | 0.0154 (13) | 0.0225 (8) | 0.0196 (12) |
| C16A | 0.0443 (7) | 0.0714 (11) | 0.0352 (7) | 0.0037 (7) | −0.0030 (6) | 0.0124 (7) |
| C15B | 0.0549 (9) | 0.0997 (15) | 0.0388 (8) | 0.0167 (9) | 0.0057 (7) | −0.0008 (8) |
| C17A | 0.0482 (8) | 0.0757 (12) | 0.0383 (7) | −0.0105 (8) | −0.0119 (6) | 0.0019 (7) |
| C16B | 0.0949 (15) | 0.122 (2) | 0.0457 (9) | 0.0253 (14) | 0.0137 (9) | −0.0216 (11) |
| C18A | 0.0539 (8) | 0.0520 (9) | 0.0429 (7) | −0.0127 (7) | −0.0078 (6) | −0.0002 (6) |
| C18B | 0.0766 (11) | 0.0603 (11) | 0.0528 (9) | 0.0090 (9) | −0.0019 (8) | −0.0138 (8) |
| C17B | 0.1031 (16) | 0.0873 (16) | 0.0611 (11) | 0.0239 (13) | 0.0011 (11) | −0.0309 (11) |
Geometric parameters (Å, º)
| C1A—N1A | 1.4597 (17) | C6B—C7B | 1.378 (2) |
| C1A—N2A | 1.4646 (19) | C6B—H6B | 0.9300 |
| C1A—C2A | 1.5079 (17) | C9A—C18A | 1.3728 (18) |
| C1A—H1A | 0.9800 | C9A—C10A | 1.4181 (19) |
| N1A—C9A | 1.3944 (17) | C7B—H7B | 0.9300 |
| N1A—H1N1 | 0.873 (19) | C10A—C11A | 1.4154 (17) |
| O1A—C3A | 1.3693 (18) | C10A—C15A | 1.4189 (18) |
| O1A—H1OA | 0.86 (2) | C9B—C18B | 1.372 (2) |
| C2A—C7A | 1.388 (2) | C9B—C10B | 1.416 (2) |
| C2A—C3A | 1.3923 (19) | C11A—C12A | 1.368 (2) |
| N2A—C11A | 1.4081 (17) | C10B—C11B | 1.418 (2) |
| N2A—H1NA | 0.870 (19) | C10B—C15B | 1.426 (2) |
| O1B—C3B | 1.3616 (17) | C12A—C13A | 1.395 (2) |
| O1B—H1OB | 0.84 (2) | C12A—H12A | 0.9300 |
| N1B—C9B | 1.4059 (17) | C11B—C12B | 1.374 (2) |
| N1B—C1B | 1.4644 (18) | C13A—C14A | 1.360 (2) |
| N1B—H1NB | 0.865 (17) | C13A—H13A | 0.9300 |
| C1B—N2B | 1.4638 (17) | C12B—C13B | 1.397 (3) |
| C1B—C2B | 1.5013 (17) | C12B—H12B | 0.9300 |
| C1B—H1B | 0.9800 | C14A—C15A | 1.406 (2) |
| C3A—C4A | 1.387 (2) | C14A—H14A | 0.9300 |
| C2B—C7B | 1.3833 (18) | C13B—C14B | 1.356 (3) |
| C2B—C3B | 1.3949 (18) | C13B—H13B | 0.9300 |
| N2B—C11B | 1.3942 (17) | C15A—C16A | 1.412 (2) |
| N2B—H2NB | 0.862 (17) | C14B—C15B | 1.421 (3) |
| C4A—C5A | 1.381 (3) | C14B—H14B | 0.9300 |
| C4A—H4A | 0.9300 | C16A—C17A | 1.354 (2) |
| C3B—C4B | 1.3840 (19) | C16A—H16A | 0.9300 |
| C5A—C6A | 1.368 (3) | C15B—C16B | 1.410 (3) |
| C5A—H5A | 0.9300 | C17A—C18A | 1.401 (2) |
| C4B—C5B | 1.373 (2) | C17A—H17A | 0.9300 |
| C4B—H4B | 0.9300 | C16B—C17B | 1.353 (3) |
| C6A—C7A | 1.383 (2) | C16B—H16B | 0.9300 |
| C6A—H6A | 0.9300 | C18A—H18A | 0.9300 |
| C5B—C6B | 1.375 (2) | C18B—C17B | 1.406 (3) |
| C5B—H5B | 0.9300 | C18B—H18B | 0.9300 |
| C7A—H7A | 0.9300 | C17B—H17B | 0.9300 |
| N1A—C1A—N2A | 106.61 (11) | N1A—C9A—C10A | 117.35 (11) |
| N1A—C1A—C2A | 110.09 (11) | C6B—C7B—C2B | 121.03 (13) |
| N2A—C1A—C2A | 109.23 (11) | C6B—C7B—H7B | 119.5 |
| N1A—C1A—H1A | 110.3 | C2B—C7B—H7B | 119.5 |
| N2A—C1A—H1A | 110.3 | C11A—C10A—C9A | 120.35 (11) |
| C2A—C1A—H1A | 110.3 | C11A—C10A—C15A | 119.31 (12) |
| C9A—N1A—C1A | 117.08 (11) | C9A—C10A—C15A | 120.30 (11) |
| C9A—N1A—H1N1 | 115.0 (12) | C18B—C9B—N1B | 122.14 (15) |
| C1A—N1A—H1N1 | 112.7 (12) | C18B—C9B—C10B | 120.75 (14) |
| C3A—O1A—H1OA | 106.1 (14) | N1B—C9B—C10B | 117.02 (13) |
| C7A—C2A—C3A | 118.36 (13) | C12A—C11A—N2A | 121.94 (12) |
| C7A—C2A—C1A | 120.67 (13) | C12A—C11A—C10A | 120.34 (12) |
| C3A—C2A—C1A | 120.97 (12) | N2A—C11A—C10A | 117.56 (12) |
| C11A—N2A—C1A | 117.26 (10) | C9B—C10B—C11B | 120.84 (12) |
| C11A—N2A—H1NA | 112.9 (12) | C9B—C10B—C15B | 119.55 (15) |
| C1A—N2A—H1NA | 111.1 (12) | C11B—C10B—C15B | 119.53 (15) |
| C3B—O1B—H1OB | 107.7 (17) | C11A—C12A—C13A | 119.88 (14) |
| C9B—N1B—C1B | 114.90 (11) | C11A—C12A—H12A | 120.1 |
| C9B—N1B—H1NB | 113.0 (10) | C13A—C12A—H12A | 120.1 |
| C1B—N1B—H1NB | 112.5 (10) | C12B—C11B—N2B | 122.37 (15) |
| N2B—C1B—N1B | 106.09 (10) | C12B—C11B—C10B | 120.51 (14) |
| N2B—C1B—C2B | 110.09 (11) | N2B—C11B—C10B | 117.02 (12) |
| N1B—C1B—C2B | 110.99 (10) | C14A—C13A—C12A | 121.31 (14) |
| N2B—C1B—H1B | 109.9 | C14A—C13A—H13A | 119.3 |
| N1B—C1B—H1B | 109.9 | C12A—C13A—H13A | 119.3 |
| C2B—C1B—H1B | 109.9 | C11B—C12B—C13B | 119.89 (19) |
| O1A—C3A—C4A | 117.37 (13) | C11B—C12B—H12B | 120.1 |
| O1A—C3A—C2A | 122.12 (12) | C13B—C12B—H12B | 120.1 |
| C4A—C3A—C2A | 120.50 (14) | C13A—C14A—C15A | 120.62 (13) |
| C7B—C2B—C3B | 118.45 (12) | C13A—C14A—H14A | 119.7 |
| C7B—C2B—C1B | 120.52 (11) | C15A—C14A—H14A | 119.7 |
| C3B—C2B—C1B | 121.02 (11) | C14B—C13B—C12B | 121.03 (18) |
| C11B—N2B—C1B | 116.45 (11) | C14B—C13B—H13B | 119.5 |
| C11B—N2B—H2NB | 114.1 (10) | C12B—C13B—H13B | 119.5 |
| C1B—N2B—H2NB | 114.4 (10) | C14A—C15A—C16A | 123.37 (13) |
| C5A—C4A—C3A | 119.71 (16) | C14A—C15A—C10A | 118.52 (12) |
| C5A—C4A—H4A | 120.1 | C16A—C15A—C10A | 118.08 (13) |
| C3A—C4A—H4A | 120.1 | C13B—C14B—C15B | 121.50 (16) |
| O1B—C3B—C4B | 117.87 (12) | C13B—C14B—H14B | 119.3 |
| O1B—C3B—C2B | 121.81 (12) | C15B—C14B—H14B | 119.3 |
| C4B—C3B—C2B | 120.30 (12) | C17A—C16A—C15A | 120.50 (13) |
| C6A—C5A—C4A | 120.55 (14) | C17A—C16A—H16A | 119.8 |
| C6A—C5A—H5A | 119.7 | C15A—C16A—H16A | 119.8 |
| C4A—C5A—H5A | 119.7 | C16B—C15B—C14B | 124.57 (18) |
| C5B—C4B—C3B | 120.04 (14) | C16B—C15B—C10B | 117.87 (18) |
| C5B—C4B—H4B | 120.0 | C14B—C15B—C10B | 117.52 (18) |
| C3B—C4B—H4B | 120.0 | C16A—C17A—C18A | 121.78 (13) |
| C5A—C6A—C7A | 119.76 (16) | C16A—C17A—H17A | 119.1 |
| C5A—C6A—H6A | 120.1 | C18A—C17A—H17A | 119.1 |
| C7A—C6A—H6A | 120.1 | C17B—C16B—C15B | 121.10 (17) |
| C4B—C5B—C6B | 120.27 (13) | C17B—C16B—H16B | 119.4 |
| C4B—C5B—H5B | 119.9 | C15B—C16B—H16B | 119.4 |
| C6B—C5B—H5B | 119.9 | C9A—C18A—C17A | 119.91 (14) |
| C6A—C7A—C2A | 121.10 (15) | C9A—C18A—H18A | 120.0 |
| C6A—C7A—H7A | 119.4 | C17A—C18A—H18A | 120.0 |
| C2A—C7A—H7A | 119.4 | C9B—C18B—C17B | 118.95 (19) |
| C5B—C6B—C7B | 119.85 (14) | C9B—C18B—H18B | 120.5 |
| C5B—C6B—H6B | 120.1 | C17B—C18B—H18B | 120.5 |
| C7B—C6B—H6B | 120.1 | C16B—C17B—C18B | 121.76 (19) |
| C18A—C9A—N1A | 123.03 (13) | C16B—C17B—H17B | 119.1 |
| C18A—C9A—C10A | 119.42 (12) | C18B—C17B—H17B | 119.1 |
| N2A—C1A—N1A—C9A | 53.40 (15) | C1A—N2A—C11A—C10A | 27.40 (17) |
| C2A—C1A—N1A—C9A | 171.78 (12) | C9A—C10A—C11A—C12A | −177.51 (12) |
| N1A—C1A—C2A—C7A | 110.84 (15) | C15A—C10A—C11A—C12A | 0.02 (18) |
| N2A—C1A—C2A—C7A | −132.41 (14) | C9A—C10A—C11A—N2A | −1.84 (18) |
| N1A—C1A—C2A—C3A | −68.59 (16) | C15A—C10A—C11A—N2A | 175.69 (11) |
| N2A—C1A—C2A—C3A | 48.16 (16) | C18B—C9B—C10B—C11B | 177.89 (14) |
| N1A—C1A—N2A—C11A | −50.96 (15) | N1B—C9B—C10B—C11B | 1.32 (18) |
| C2A—C1A—N2A—C11A | −169.89 (11) | C18B—C9B—C10B—C15B | 1.2 (2) |
| C9B—N1B—C1B—N2B | 57.04 (13) | N1B—C9B—C10B—C15B | −175.39 (12) |
| C9B—N1B—C1B—C2B | 176.61 (11) | N2A—C11A—C12A—C13A | −174.43 (13) |
| C7A—C2A—C3A—O1A | −177.96 (13) | C10A—C11A—C12A—C13A | 1.0 (2) |
| C1A—C2A—C3A—O1A | 1.5 (2) | C1B—N2B—C11B—C12B | −155.68 (12) |
| C7A—C2A—C3A—C4A | 1.3 (2) | C1B—N2B—C11B—C10B | 27.86 (16) |
| C1A—C2A—C3A—C4A | −179.23 (13) | C9B—C10B—C11B—C12B | −175.50 (12) |
| N2B—C1B—C2B—C7B | −118.22 (14) | C15B—C10B—C11B—C12B | 1.21 (19) |
| N1B—C1B—C2B—C7B | 124.63 (13) | C9B—C10B—C11B—N2B | 1.03 (18) |
| N2B—C1B—C2B—C3B | 61.14 (16) | C15B—C10B—C11B—N2B | 177.74 (12) |
| N1B—C1B—C2B—C3B | −56.01 (16) | C11A—C12A—C13A—C14A | −0.9 (2) |
| N1B—C1B—N2B—C11B | −55.21 (14) | N2B—C11B—C12B—C13B | −177.43 (13) |
| C2B—C1B—N2B—C11B | −175.37 (11) | C10B—C11B—C12B—C13B | −1.1 (2) |
| O1A—C3A—C4A—C5A | 178.05 (14) | C12A—C13A—C14A—C15A | −0.3 (2) |
| C2A—C3A—C4A—C5A | −1.3 (2) | C11B—C12B—C13B—C14B | −0.3 (3) |
| C7B—C2B—C3B—O1B | −176.49 (14) | C13A—C14A—C15A—C16A | 179.26 (14) |
| C1B—C2B—C3B—O1B | 4.1 (2) | C13A—C14A—C15A—C10A | 1.3 (2) |
| C7B—C2B—C3B—C4B | 2.2 (2) | C11A—C10A—C15A—C14A | −1.20 (18) |
| C1B—C2B—C3B—C4B | −177.14 (13) | C9A—C10A—C15A—C14A | 176.34 (12) |
| C3A—C4A—C5A—C6A | 0.3 (3) | C11A—C10A—C15A—C16A | −179.24 (11) |
| O1B—C3B—C4B—C5B | 176.17 (15) | C9A—C10A—C15A—C16A | −1.70 (18) |
| C2B—C3B—C4B—C5B | −2.6 (2) | C12B—C13B—C14B—C15B | 1.5 (3) |
| C4A—C5A—C6A—C7A | 0.5 (3) | C14A—C15A—C16A—C17A | −176.90 (14) |
| C3B—C4B—C5B—C6B | 0.8 (2) | C10A—C15A—C16A—C17A | 1.0 (2) |
| C5A—C6A—C7A—C2A | −0.4 (3) | C13B—C14B—C15B—C16B | 176.13 (19) |
| C3A—C2A—C7A—C6A | −0.5 (2) | C13B—C14B—C15B—C10B | −1.3 (3) |
| C1A—C2A—C7A—C6A | −179.92 (14) | C9B—C10B—C15B—C16B | −0.9 (2) |
| C4B—C5B—C6B—C7B | 1.4 (3) | C11B—C10B—C15B—C16B | −177.64 (15) |
| C1A—N1A—C9A—C18A | 153.25 (13) | C9B—C10B—C15B—C14B | 176.72 (14) |
| C1A—N1A—C9A—C10A | −31.88 (17) | C11B—C10B—C15B—C14B | 0.0 (2) |
| C5B—C6B—C7B—C2B | −1.7 (2) | C15A—C16A—C17A—C18A | −0.2 (2) |
| C3B—C2B—C7B—C6B | −0.1 (2) | C14B—C15B—C16B—C17B | −176.93 (19) |
| C1B—C2B—C7B—C6B | 179.29 (14) | C10B—C15B—C16B—C17B | 0.5 (3) |
| C18A—C9A—C10A—C11A | 179.05 (12) | N1A—C9A—C18A—C17A | 174.10 (13) |
| N1A—C9A—C10A—C11A | 3.99 (17) | C10A—C9A—C18A—C17A | −0.7 (2) |
| C18A—C9A—C10A—C15A | 1.53 (19) | C16A—C17A—C18A—C9A | 0.0 (2) |
| N1A—C9A—C10A—C15A | −173.53 (11) | N1B—C9B—C18B—C17B | 175.37 (15) |
| C1B—N1B—C9B—C18B | 151.26 (14) | C10B—C9B—C18B—C17B | −1.0 (2) |
| C1B—N1B—C9B—C10B | −32.21 (16) | C15B—C16B—C17B—C18B | −0.4 (3) |
| C1A—N2A—C11A—C12A | −157.00 (13) | C9B—C18B—C17B—C16B | 0.6 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1A—H1OA···N1A | 0.86 (2) | 2.66 (2) | 3.1072 (16) | 113.8 (17) |
| O1A—H1OA···N2A | 0.86 (2) | 2.03 (2) | 2.7763 (16) | 144.6 (19) |
| O1B—H1OB···N1B | 0.84 (2) | 2.20 (3) | 2.8835 (16) | 138 (2) |
| O1B—H1OB···N2B | 0.84 (2) | 2.47 (2) | 3.0196 (16) | 123 (2) |
| N1B—H1NB···O1A | 0.865 (17) | 2.331 (17) | 3.1608 (18) | 160.8 (14) |
Funding Statement
This work was funded by Hacettepe University Scientific Research Project Unit grant 013 D04 602 004 to T. Hökelek.
References
- Alam, M. & Lee, D.-U. (2016). Comput. Biol. Chem. 64, 185–201. [DOI] [PubMed]
- Bazinet, P., Yap, G. P. A. & Richeson, D. S. (2003). J. Am. Chem. Soc. 125, 13314–13315. [DOI] [PubMed]
- Becke, A. D. (1993). J. Chem. Phys. 98, 5648–5652.
- Behbahani, F. K. & Golchin, F. M. (2017). J. Taibah Univ. Sci. 11, 85–89.
- Booysen, I. N., Ebinumoliseh, I., Sithebe, S., Akerman, M. P. & Xulu, B. (2016). Polyhedron, 117, 755–760.
- Bu, X., Deady, L. W., Finlay, G. J., Baguley, B. C. & Denny, W. A. (2001). J. Med. Chem. 44, 2004–2014. [DOI] [PubMed]
- Cucciolito, M. E., Panunzi, B., Ruffo, F. & Tuzi, A. (2013). Acta Cryst. E69, o1133–o1134. [DOI] [PMC free article] [PubMed]
- De Aguiar, A. (1874). Ber. Dtsch. Chem. Ges. 7, 309–319.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J. A. Jr, Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J. & Fox, D. J. (2009). GAUSSIAN09. Gaussian Inc., Wallingford, CT, US
- Fun, H.-K., Chanawanno, K. & Chantrapromma, S. (2011). Acta Cryst. E67, o715–o716. [DOI] [PMC free article] [PubMed]
- Ghorbani, M. H. (2012). Acta Cryst. E68, o2605. [DOI] [PMC free article] [PubMed]
- Giani, A. M., Lamperti, M., Maspero, A., Cimino, A., Negri, R., Giovenzana, G. B., Palmisano, G. & Nardo, L. (2016). J. Lumin. 179, 384–392.
- Hathwar, V. R., Sist, M., Jørgensen, M. R. V., Mamakhel, A. H., Wang, X., Hoffmann, C. M., Sugimoto, K., Overgaard, J. & Iversen, B. B. (2015). IUCrJ, 2, 563–574. [DOI] [PMC free article] [PubMed]
- He, X., Mao, J., Ma, Q. & Tang, Y. (2018). J. Mol. Liq. 269, 260–268.
- Hirshfeld, H. L. (1977). Theor. Chim. Acta, 44, 129–138.
- Mahapatra, A. K., Maji, R., Maiti, K., Manna, S. K., Mondal, S., Das Mukhopadhyay, C., Goswami, S., Sarkar, D., Mondal, T. K., Quah, C. K. & Fun, H. K. (2015). Sens. Actuators B Chem. 207, 878–886.
- Maloney, S., Slawin, A. M. Z. & Woollins, J. D. (2013). Acta Cryst. E69, o246. [DOI] [PMC free article] [PubMed]
- Manimekalai, A., Vijayalakshmi, N. & Selvanayagam, S. (2014). Acta Cryst. E70, o959. [DOI] [PMC free article] [PubMed]
- McKinnon, J. J., Jayatilaka, D. & Spackman, M. A. (2007). Chem. Commun. pp. 3814–3816. [DOI] [PubMed]
- Rigaku OD (2018). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spackman, M. A. & Jayatilaka, D. (2009). CrystEngComm, 11, 19–32.
- Starshikoy, N. M. & Pozharskii, F. T. (1973). Chem. Heterocycl. Compd. 9, 922–924.
- Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D. & Spackman, M. A. (2017). CrystalExplorer17. The University of Western Australia.
- Varsha, G., Arun, V., Robinson, P. P., Sebastian, M., Varghese, D., Leeju, P., Jayachandran, V. P. & Yusuff, K. M. M. (2010). Tetrahedron Lett. 51, 2174–2177.
- Venkatesan, P., Thamotharan, S., Ilangovan, A., Liang, H. & Sundius, T. (2016). Spectrochim. Acta A Mol. Biomol. Spectrosc. 153, 625–636. [DOI] [PubMed]
- Watanab, K. & Hareda, H. (1977). Chem. Abstr. 8499.
- Woodgate, P. D., Herbert, J. M. & Denny, W. A. (1987). Heterocycles, 26, 1029–1036.
- Zhang, H. G., Wang, X. Z., Cao, Q., Gong, G. H. & Quan, Z. S. (2017). Bioorg. Med. Chem. Lett. 27, 4409–4414. [DOI] [PubMed]
- Zhou, D. C., Lu, Y. T., Mai, Y. W., Zhang, C., Xia, J., Yao, P. F., Wang, H. G., Huang, S. L. & Huang, Z. S. (2019). Bioorg. Chem. 91, 103131. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989020005939/lh5957sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020005939/lh5957Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989020005939/lh5957Isup3.cdx
Supporting information file. DOI: 10.1107/S2056989020005939/lh5957Isup4.cml
CCDC reference: 1976884
Additional supporting information: crystallographic information; 3D view; checkCIF report