Abstract
Background
The clinical characteristics and outcome of COVID-19 in children are different from those in adults. We aimed to describe the characteristics of infants under 1 year of age (excluding newborns) with COVID-19.
Methods
We retrospectively retrieved data of 36 infants with SARS-CoV-2 infection in Wuhan Children’s Hospital from January 26 to March 22, 2020. Clinical features, chest imaging findings, laboratory tests results, treatments and clinical outcomes were analyzed.
Results
The mean age of the infected infants was 6.43 months, with a range of 2–12 months. 61.11% of the patients were males and 38.89% females. 86.11% of the infants were infected due to family clustering. Cough (77.78%) and fever (47.22%) were the most common clinical manifestations. Chest CT scan revealed 61.11% bilateral pneumonia and 36.11% unilateral pneumonia. 47.22% of the infants developed complications. Increased leucocytes, neutrophils, lymphocytes, and thrombocytes were observed in 11.11, 8.33, 36.11 and 44.44% of infants, respectively. Decreased leucocytes, neutrophils, thrombocyte and hemoglobin were observed in 8.33, 19.44, 2.78 and 36.11% of infants, respectively. Increased C-reactive protein, procalcitonin, lactate dehydrogenase, alanine aminotransferase, creatine kinase and D-dimer were observed in 19.44, 67.74, 47.22, 19.44, 22.22 and 20.69% of infants, respectively. Only one infant had a high level of creatinine. Co-infections with other respiratory pathogens were observed in 62.86% of infants. CD3 (20.69%), CD4 (68.97%), CD19 (31.03%) and Th/Ts (44.83%) were elevated; CD8 (6.9%) and CD16+CD56 (48.28%) was reduced. IL-4 (7.69%), IL-6 (19.23%), IL-10 (50%), TNF-α (11.54%) and IFN-γ (19.23%) were elevated. Up to March 22, 97.22% of infants recovered, while a critical ill infant died. When the infant’s condition deteriorates rapidly, lymphocytopenia was discovered. Meanwhile, C-reactive protein, D-dimer, alanine aminotransferase, creatine kinase, creatinine, IL-6 and IL-10 increased significantly.
Conclusions
In the cohort, we discovered that lymphocytosis, elevated CD4 and IL-10, and co-infections were common in infants with COVID-19, which were different from adults with COVID-19. Most infants with COVID-19 have mild clinical symptoms and good prognosis.
Keywords: COVID-19, Coronavirus infant infection, SARS-CoV-2
Introduction
An epidemic of coronavirus disease 2019 (COVID-19), which was caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1, 2], had affected more than 1,914,916 individuals worldwide in 4 months, and led to 123,010 deaths [3]. According to the report from the Chinese Center for Disease Control and Prevention, most affected patients were 30–79 years of age, few were under 19 years and over 80 years [4]. The total case-fatality rate was 2.3%, and it was much higher in patients aged over 70 years. Most affected individuals were classified as mild, 19% were severe or critical ill. SARS-CoV-2 infection can cause systematic involvements including respiratory system (presenting as fever, cough, dyspnea, rhinobyon, rhinorrhea, and pneumonia), digestive system abnormalities (diarrhea, constipation, nausea and vomiting), fatigue, myalgia and chill [5–7]. Multiple complications might occur, and life-threatening conditions such as respiratory failure, septic shock, and multiple organ dysfunction or failure might occur in critically ill patients [8]. SARS-CoV-2 can affect different groups of individuals with varying severity.
Several studies have reported the clinical characteristics of pediatric patients with COVID-19; children may be more likely to experience a milder form of the disease than adult patients [9–12]. The reasons of children showing milder clinical symptoms and signs and a better prognosis than adults are still unclear. Several hypotheses have been proposed by the researchers. It’s clear that the immune systems of children and adults are different with respect to their composition and functional responsiveness, especially infants with some maternal antibodies during the first months of life [13].
Infants are a special group who are dependent on the care of family members and are easily affected by family clustering. To our knowledge, no study has been done to comprehensively investigate the clinical features of infant patients under 1 year of age with COVID-19. We aimed to investigate the clinical and laboratory characteristics of infant patients under 1 year of age (excluding newborns) with COVID-19.
Methods
Patients
We included 36 patients under 1 year (excluding newborns) treated in Wuhan Children’s Hospital, the only designated hospital for treating young COVID-19 patients under 16 years of age in Wuhan, from January 26 to March 22, 2020. Two infants had been reported in our previous report [14]. All patients in this cohort were confirmed with SARS-CoV-2 infection by positive results of virus nucleic acid test in nasopharyngeal swab specimens in clinical laboratory of Wuhan Children’s Hospital.
Clinical classifications of the COVID-19 patients were classified according to “Interim Guidance for Diagnosis and Treatment of Coronavirus Disease 2019 (the 7th edition)” released by National Health Commission [15]. Patients were defined with common type of COVID-19 when meeting the following criteria: with fever, respiratory symptom and pneumonia signs on chest imaging. Patients were defined as severe cases when meeting with any of the following criteria: (1) increased respiratory rate: ≥50 times/min; (2) oxygen saturation <92% under a resting state; (3) assisted breathing (moans, nasal flaring, and three concave sign), cyanosis, intermittent apnea; (4) lethargy, convulsion; (5) poor feeding, bad appetite, and even dehydration. Critical ill patients were defined with any of the following criteria: (1) respiratory failure which requires mechanical ventilation; (2) septic shock; (3) accompanied by other organ failure that needs intensive care unit treatment.
Data collection
Demographics and baseline characteristics (included age, sex, underlying diseases, exposure history, onset dates, confirmed dates and clinical staging), clinical characteristics (included signs and symptoms, lung auscultation, chest imaging examination and complications), laboratory tests, treatments and clinical outcomes of each patient were obtained from the electronical medical record system of Wuhan Children’s Hospital. Latent period, days from illness onset to diagnosis confirmation and duration of illness were calculated based on the definite date that can be collected; information of some patients was not available.
Laboratory tests were conducted at admission, including SARS-CoV-2 RT-PCR assay for nasopharyngeal swab specimens, blood routine examination, serum biochemistry, lymphocyte subsets assays, cytokine assays and other respiratory pathogens. Blood routine examination: leucocytes, neutrophils, lymphocytes, thrombocyte, hemoglobin. Serum biochemistry: C-reactive protein, procalcitonin, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, creatine kinase, creatinine and D-dimer. Lymphocyte subsets assays: CD3, CD4, CD8, CD19, CD16+CD56 and Th/Ts. Cytokine assays: IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ. Other respiratory pathogens such as influenza A virus (H1N1, H3N2, H7N9), influenza B virus, cytomegalovirus (CMV), Epstein–Barr (EB) virus, rat mycoplasma pneumoniae (MP), respiratory syncytial virus, parainfluenza virus, and adenovirus were tested.
Continuous laboratory tests result of severe and critical ill infants were collected.
Results
Demographics and baseline characteristics
Thirty-six infants with COVID-19 were analyzed in our report, with an average age of 6.43 months (2-12 months) (Table 1). 61.11% of them were males and 38.89% females. 25% of infants had underlying diseases, including eczema (5.56%), lacrimal sac dredge (2.78%), erythema multiforme (2.78%), pneumonia (Influenza A and mycoplasma infection) (2.78%), hypothyroidism (2.78%), atrial septal defect (2.78%), cleft palate (2.78%), traumatic intracranial hemorrhage (2.78%). Thirty-one infants (86.11%) were infected due to family clustering, and five (13.89%) had no clear history of exposure. The mean latent period was 6 days (1–22 days), and it was longer than 10 days in five infants. The mean time from illness onset to discharge was 16.83 days (7–36 days). Thirty-three (91.67%) infants were classified as common type, two as severe and critical ill type (5.56%, one patient in each type), and one asymptomatic infection (2.78%).
Table 1.
Demographics and baseline characteristics of 36 infants with SARS-CoV-2 infection
| Variables | Values |
|---|---|
| Age (mon), mean (range) | 6.43 (2–12) |
| Sex, n (%) | |
| Male | 22 (61.11%) |
| Female | 14 (38.89%) |
| Underlying diseases, n (%) | |
| Eczema | 2 (5.56%) |
| Lacrimal sac dredge | 1 (2.78%) |
| Erythema multiforme | 1 (2.78%) |
| Pneumonia (Influenza A, mycoplasma) | 1 (2.78%) |
| Hypothyroidism | 1 (2.78%) |
| Atrial septal defect | 1 (2.78%) |
| Cleft palate | 1 (2.78%) |
| Traumatic intracranial hemorrhage | 1 (2.78%) |
| Exposure history, n (%) | |
| Family clusters | 31 (86.11%) |
| No clear exposure history | 5 (13.89%) |
| Latent period (d), mean (range) | 6 (1–22) |
| Time from illness onset to discharged (d), mean (range) | 16.83 (7–36) |
| Clinical classifications, n (%) | |
| Asymptomatic carrier | 1 (2.78%) |
| Common | 33 (91.67%) |
| Severe | 1 (2.78%) |
| Critical ill | 1 (2.78%) |
Clinical manifestations and chest CT scan
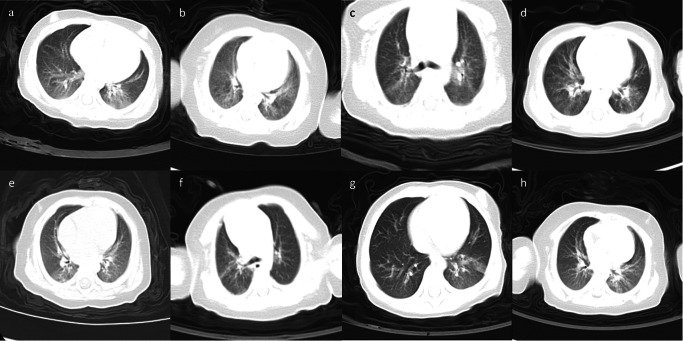
Cough (77.78%) and fever (47.22%) were the most common clinical manifestations, followed by diarrhea (25%), expectoration (22.22%), nasal congestion/discharge (16.67%), nausea/vomiting (11.11%), polypnea (5.56%) and constipation (2.78%). Abnormality of lung auscultation (rales) were found in 6 infants (16.67%). Chest CT scan showed bilateral pneumonia in 61.11% of the patients, and unilateral pneumonia in 36.11%. Imaging findings included thin scattered patches, multiple patchy or scattered ground glass opacity, increased short strip or patchy density, patchy blurry shadows and cord-like dense shadows (Fig. 1).
Fig. 1.
Chest CT images of eight infants with COVID-19. Bilateral pneumonia (a–d): a blurred shadow of the left and right inferior lungs; b the brightness of bilateral lungs is uneven, with thin scattered patches; c multiple patchy ground glass opacity can be seen in bilateral lungs; d scattered ground glass opacity of both lungs. Unilateral pneumonia (e–h): e patchy blurry shadows and cord-like dense shadows of the upper and lower lobe of right lung; f patchy ground glass opacity in the posterior segment of the right upper lobe and the posterior base of the lower lobe, local cord-like dense shadows, thickening of the right interlobular fissure; g patchy ground glass opacity and increased short strip density of the left lower lung; h increased patchy density of the left lung
Seventeen infants (47.22%) developed complications during the course of the disease; the more common were myocardial damage (19.44%), abnormal liver function (13.89%) and hypogammaglobulinemia (11.11%), while the less common were respiratory alkalosis (2.78%), pulmonary atelectasis (2.78%) and intussusception, toxic encephalopathy, status epilepticus, disseminated intravascular coagulation (DIC), septic shock and multiple organ dysfunction syndrome (MODS) (2.78%).
Laboratory results
Blood routine and blood biochemistry
Based on laboratory test results at admission, increased leucocytes, neutrophils, lymphocytes, and thrombocyte were observed in 11.11, 8.33, 36.11 and 44.44% of infants, respectively (Table 2). Decreased leucocytes, neutrophils, thrombocyte and hemoglobin were observed in 8.33, 19.44, 2.78 and 36.11% of infants, respectively. The inflammatory markers C-reactive protein and procalcitonin were elevated in 19.44 and 67.74% of infants, respectively. 47.22% of patients had elevated lactate dehydrogenase level. Twenty-seven infants (75%) had elevated aspartate aminotransferase, and seven (19.44%) had elevated alanine aminotransferase. Elevated total bilirubin and creatine kinase was observed in 2.78 and 22.22% of infants, respectively. Only one infant (2.85%) who died in our cohort had a high level of creatinine. Elevated D-dimer level was observed in 20.69% of infants. 62.86% of infants were co-infected with CMV, EB or MP.
Table 2.
Laboratory results of 36 infants with SARS-CoV-2 infection
| Laboratory tests (reference values) | Patients (n) | Diagnosis (n, %) | ||
|---|---|---|---|---|
| Decreased | Normal | Increased | ||
| Leucocytes count (5.5–12 × 109/L) | 36 | 3 (8.33%) | 29 (80.56%) | 4 (11.11%) |
| Neutrophils count (1.08–5.9 × 109/L) | 36 | 7 (19.44%) | 26 (72.23%) | 3 (8.33%) |
| Lymphocytes count (1.15–6 × 109/L) | 36 | – | 23 (63.89%) | 13 (36.11%) |
| Thrombocyte count (100–378 × 1012/L) | 36 | 1 (2.78%) | 19 (52.78%) | 16 (44.44%) |
| Hemoglobin (110–140 g/L) | 36 | 13 (36.11%) | 23 (63.89%) | – |
| C-reactive protein (0–3 mg/L) | 36 | – | 29 (80.56%) | 7 (19.44%) |
| Procalcitonin (0–0.05 ng/mL) | 31 | – | 10 (32.26%) | 21 (67.74%) |
| Lactate dehydrogenase (120–300 U/L) | 36 | – | 19 (52.78%) | 17 (47.22%) |
| Aspartate aminotransferase (10–40 U/L) | 36 | – | 9 (25%) | 27 (75%) |
| Alanine aminotransferase (9–60 U/L) | 36 | – | 29 (80.56%) | 7 (19.44%) |
| Total bilirubin (2–19 μmol/L) | 36 | 1 (2.78%) | 34 (94.44%) | 1 (2.78%) |
| Creatine kinase (20–250 U/L) | 36 | – | 28 (77.78%) | 8 (22.22%) |
| Creatinine (18–35 μmol/L) | 35 | 7 (20%) | 27 (77.14%) | 1 (2.85%) |
| D-dimer (0–0.55 mg/L FEU) | 29 | – | 23 (79.31%) | 6 (20.69%) |
| Lymphocyte subsets | ||||
| CD3+ T% (38.56–70.06%) | 29 | – | 23 (79.31%) | 6 (20.69%) |
| CD4+ T% (14.21–36.99%) | 29 | – | 9 (31.03%) | 20 (68.97%) |
| CD8+ T% (13.24–38.53%) | 29 | 2 (6.9%) | 27 (93.1%) | – |
| CD19+ B% (10.86–28.03%) | 29 | 2 (6.9%) | 18 (62.07%) | 9 (31.03%) |
| CD16+ CD56+ % (7.92–33.99%) | 29 | 14 (48.28%) | 15 (51.72%) | – |
| Th/Ts (0.96–2.05) | 29 | 1 (3.45%) | 15 (51.72%) | 13 (44.83%) |
| Cytokine assays | ||||
| IL-2 (0–11.4 pg/mL) | 26 | – | 26 (100%) | – |
| IL-4 (0–12.9 pg/mL) | 26 | – | 24 (92.31%) | 2 (7.69%) |
| IL-6 (0–20.9 pg/mL) | 26 | – | 21 (80.77%) | 5 (19.23%) |
| IL-10 (0–5.9 pg/mL) | 26 | – | 13 (50%) | 13 (50%) |
| TNF-α (0–5.9 pg/mL) | 26 | – | 23 (88.46%) | 3 (11.54%) |
| IFN-γ (0–17.3 pg/mL) | 26 | – | 21 (80.77%) | 5 (19.23%) |
IL interleukin, TNF tumor necrosis factor, IFN interferon, U unit. Decreased means the value lower than the reference values; increased means the value higher than the reference values
Continuous laboratory tests were carried out during the disease course of critical ill infant who died in the cohort, the results varied widely. On admission, the complete blood count results were basically normal. With the rapid deterioration of the condition, increased neutrophils and decreased lymphocytes were discovered. Meanwhile, sharp increases were found in C-reactive protein (202 mg/L), D-dimer (41.49 mg/L FEU), alanine aminotransferase (375 U/L), creatine kinase (20,702 U/L) and creatinine (260 μmol/L).
Lymphocyte subsets and cytokine assays
T lymphocyte subsets that tested at admission are shown in Table 2. The percentages of CD3+ and CD4+ T cells were elevated in 20.69 and 68.97% of the patients, respectively; the percentage of CD8+ T cell was reduced in 6.9% of the patients. CD19+ B cell was elevated in 31.03% and reduced in 6.9%. The percentage of CD16+CD56+ NK lymphocyte was reduced in 48.28%. Th/Ts was elevated in 44.83% and reduced in 3.45%. Elevated level of IL-4 (7.69%), IL-6 (19.23%), IL-10 (50%), TNF-α (11.54%) and IFN-γ (19.23%) were observed in our cohort (Table 2).
At admission, IL-6 (117.88 pg/ml) and IL-10 (17.42 pg/ml) of the death infant were elevated. With the rapid deterioration of the condition, the level of IL-6 and IL-10 reach up to 3868.86 and 326.93 pg/ml, respectively.
Treatment modalities and outcomes
97.22% of the infants received antiviral agents (interferon, virazole) which were administered mainly by inhalation. 63.89% of patients were treated with traditional Chinese medicine (including herbal medicine, acupuncture and massage) (Table 3). According to the syndrome differentiation of traditional Chinese medicine, different syndrome types are given corresponding prescriptions and medicines according to the syndrome. One dose of Chinese herbal medicine was taken twice daily. According to the microbiological tests, 41.67% of patients were given antibiotics (cefmetazole, azithromycin). Only three infants received oxygen therapy (8.33%). Symptomatic treatments were carried out in patients with complications, such as myocardial damage (calcium dibutyryl cyclic adenosine monophosphate, fructose sodium diphosphate), liver function damage (atomolam, glucuronolactone), intussusception (enterostomy), toxic encephalopathy, status epilepticus, DIC, septic shock and MODS (hemopurification, transfusions). Up to March 22, 35 (97.22%) infants recovered and were discharged home, while one infant died of respiratory circulatory failure.
Table 3.
Clinical treatments and outcomes of 36 infants with SARS-CoV-2 infection
| Items | Value, n (%) |
|---|---|
| Treatments | |
| Oxygen therapy | 3 (8.33%) |
| Antibiotic treatment (cefmetazole, zithromycin) | 15 (41.67%) |
| Antiviral treatment (interferon, virazole) | 35 (97.22%) |
| Traditional Chinese medicine | 23 (63.89%) |
| Outcomes | |
| Discharged | 35 (97.22%) |
| Death | 1 (2.78%) |
Discussion
SARS-CoV-2 affects all populations, while infected children commonly have milder clinical manifestations than adults [9, 13]. Limited cases aged under 1 year with COVID-19 have been reported [16–18]. Most reports are case descriptions, and no systematic clinical and serological features of infants with COVID-19 have been reported. This study systematically investigated the clinical and laboratory characteristics of infant patients under 1 year of age (excluding newborns) with COVID-19.
In the current cohort, 61.11% of infants was male. 91.67% of the infants were classified as common type. The proportion was higher than that of adult patients and older children [4, 7, 19]. Cough (77.78%) and fever (47.22%) were the most common clinical manifestations. No subjective descriptions of symptoms such as fatigue, myalgia, headache and dizziness were recorded because this series of patients aged under 1 year cannot speak yet.
SARS-CoV-2 infection may cause pulmonary infiltration of lymphocytes and/or cell damage through apoptosis or pyroptosis [20]. Lymphopenia is common in adult patients, and the severity of lymphocytopenia had been identified as potential risk factors of poor prognosis [4, 21]. However, no lymphopenia was observed in laboratory test results at admission in our cohort, conversely 36.11% of infant patients had lymphocytosis, 68.97% had increased level of CD4 cells in the infants. In the death case, lymphopenia was observed when the disease deteriorates rapidly. The results suggest that no serious T cell functional impairments occur in infants with mild SARS-CoV-2 infection, and less damage of infection has been caused to the immune system than adults that result in better clinical prognosis.
Severe pneumonia caused by severe acute respiratory syndrome CoV (SARS-CoV) and Middle East respiratory syndrome CoV (MERS-CoV) is often associated with massive inflammatory cell infiltration and elevated pro-inflammatory cytokine/chemokine responses resulting in acute lung injury, and acute respiratory distress syndrome (ARDS)[22]. In this series of infants with COVID-19, proinflammatory cytokines including IL-6, TNF-α and IFN-γ were increased. High IL-6 level had been identified as potential risk factors of poor prognosis in COVID-19 [15, 23, 24]. Serum level of IL-6 (117.88 pg/mL) at admission in the death infant with COVID-19 was significantly higher than normal level. When the disease deteriorates rapidly, the level of IL-6 up to 3868.86 pg/mL. IL-10 has a positive impact on the production of proinflammatory cytokines, which is associated with persistence in some other viral infections, such as hepatitis C virus (HCV) and hepatitis B virus (HBV) [25]. SARS patients with severe disease had very low levels of IL-10 [22, 26], increased level of IL-10 was only found in the convalescent SARS patients [27]. Different from SARS-CoV infection, IL-10 increased in half of the infants with COVID-19 in the cohort. In most infants, the increasing amplitude of IL-10 was not significant. In the death case, the level of IL-10 up to 326.93 pg/mL when the disease deteriorates rapidly. The reason for why IL-10 was so different between SARS and COVID-19 is still unclear, further research is needed to characterize the immune response to coronavirus infection.
The infant who died in our cohort had intussusception and fever on admission. Her condition deteriorated rapidly with severe infections, febrile convulsion, along with a large number of laboratory abnormalities. Significantly elevated indicators, such as alanine aminotransferase, creatine kinase and creatinine, suggested MODS. Many laboratory indicators have been confirmed to effectively assess disease severity and predict outcome in patients with COVID-19. Liu et al. [23] and Chen et al [28] demonstrated that COVID-19 patient with high level of C-reactive protein (>41.8 mg/L) and D-dimer (>1 μg/mL) were more likely to have severe complications and poor prognosis. In out cohort, C-reactive protein and D-dimer level of the infant increased to as high as of 202 mg/L and 41.49 μg/mL, respectively. The infant developed a series of complications and died of respiratory circulatory failure. This was consistent with their previous studies. At an early stage, significantly high level of C-reactive and D-dimer remind us the poor prognosis of infant with COVID-19.
There are no specific drugs to SARS-CoV-2 infection and the vaccine is still under study. Interferon inhalation and traditional Chinese medicine were the main therapeutic modalities for the patients. 62.86% of the infants were found to have co-infection with different types of other pathogens. Co-infections were common in pediatric patients, which is different from adults infected with SARS-CoV-2 [29]. 47.2% of infants developed complications including myocardial damage, abnormal liver function and hypogammaglobulinemia. Symptomatic treatment should be given for co-infections and complications.
Though most infected children have mild or no symptoms, clinicians should be vigilant in treating pediatric patients with SARS-CoV-2 infection. Infants may have high viral load even with mild symptoms [30] and being infectious. Actually, we are facing more challenges when managing infants. Almost 80% of the infants in this series were infected due to family member clustering infection. Therefore, we should highlight family clustering and prevent children from infection due to family clustering. Once infection detected, isolation and treatment should be undertaken immediately.
In conclusion, most infants with COVID-19 have mild clinical symptoms and good prognosis. Elevated leukocyte, CD4 and IL-10, co-infections with different types of pathogens were common in infants with COVID-19, which were different from adults with COVID-19. The level of C-reactive protein, D-dimer and IL-6 was significantly high in the infant who died with COVID-19.
Acknowledgements
We would like to thank the parents and children for participating in the study. We thank the doctors and nursing staff of Wuhan Children’s Hospital for their detailed assessments and dedicated care of these infant patients.
Author contributions
Dan Sun and Xue Chen contributed equally to this work. Dan Sun, Xue Chen, Xiao-Xia Lu, Han Xiao and Fu-Rong Zhang collected and analyzed data and drafted the manuscript. Hui Li and Zhi-Sheng Liu designed the project and revised the manuscript. All other authors helped in the clinical management of the patients, and have read and approved the final manuscript.
Funding
None.
Compliance with ethical standards
Ethical approval
This study was approved by the Ethics Committee of Wuhan Children’s Hospital. Informed consent was obtained from parents or guardians of patients.
Conflict of interest
All the authors declared no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hui Li, Email: tianyirabbit@163.com.
Zhi-Sheng Liu, Email: liuzsc@126.com.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Coronavirus disease (COVID-2019) situation reports. April 15, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200415-sitrep-86-covid-19.pdf?sfvrsn=c615ea20_6. Accessed 10 Apr 2020.
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020 doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients co-infected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng F, Liao C, Fan QH, Chen HB, Zhao XG, Xie ZG, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020 doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Q, Chen YC, Chen CL, Chiu CH. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;19:670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji LN, Chao S, Wang YJ, Li XJ, Mu XD, Lin MG, et al. Clinical features of pediatric patients with COVID-19: a report of two family cluster cases. World J Pediatr. 2020 doi: 10.1007/s12519-020-00356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/s1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020 doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodin P. Why is COVID-19 so mild in children? Acta Paediatr. 2020 doi: 10.1111/apa.15271. [DOI] [PubMed] [Google Scholar]
- 14.Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. 2020 doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Commission NH (2020) Diagnosis and treatment of pneumonia caused by 2019-nCoV (trial version 7). http://www.gov.cn/zhengce/zhengceku/2020-03/04/5486705/files/ae61004f930d47598711a0d4cbf874a9.pdf. Accessed 3 Apr 2020.
- 16.Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, et al. A 55-day-old female infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong H, Wang Y, Chung HT, Chen CJ. Clinical characteristics of novel coronavirus disease 2019 (COVID-19) in newborns, infants and children. Pediatr Neonatol. 2020 doi: 10.1016/j.pedneo.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Song Z, Xiao K. High-resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J Comput Assist Tomogr. 2020 doi: 10.1097/rct.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 22.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mubarak A, Alturaiki W, Hemida MG. Middle East respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J Immunol Res. 2019;2019:6491738. doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasui F, Kai C, Kitabatake M, Inoue S, Yoneda M, Yokochi S, et al. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181:6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020 doi: 10.1172/jci137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020 doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kam KQ, Yung CF, Cui L, Tzer Pin Lin R, Mak TM, Maiwald M, et al. A well infant with coronavirus disease 2019 with high viral load. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]