To the Editor,
In the current coronavirus 2019 (COVID-19) pandemic, tocilizumab has gained widespread attention as a therapeutic option for the treatment of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) infection [1,2]. Tocilizumab is an interleukin-6 receptor (IL-6R) antagonist previously used for various rheumatological diseases, and now applied to treat cytokine release syndrome in COVID-19. IL-6R antagonists, however, may lead to higher susceptibility for opportunistic infections and increase the risk for pneumonia, urinary tract infections and cellulitis [3].
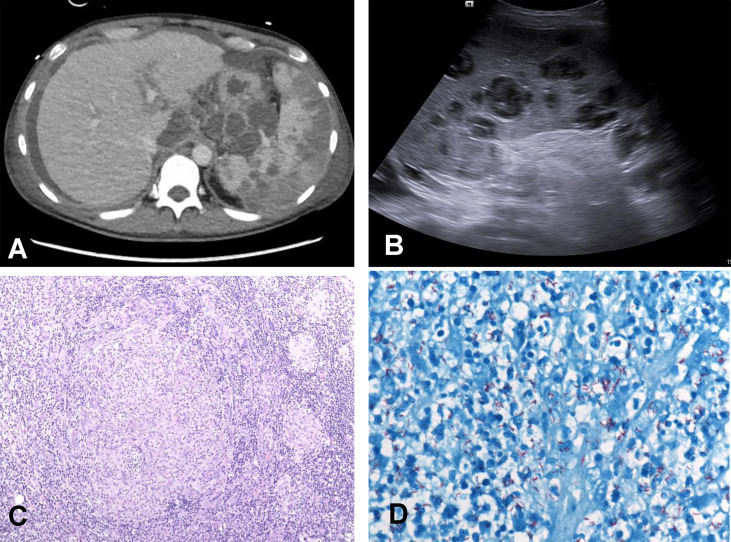
Here, we report the case of a 46-year-old woman who presented with epigastric pain to our emergency department. Two weeks before admission, she had received her first treatment with tocilizumab for systemic sclerosis and systemic lupus erythematosus overlap syndrome with predominant polyarthritis. On examination, painful and enlarged cervical, inguinal and abdominal lymph nodes were found. Laboratory analyses revealed leucocytosis of 18.7 G/L and elevated C-reactive protein of 40.3 mg/L (normal range <5 mg/L). Sonography and computed tomography showed a massive retroperitoneal lymph node bulk with central necrotic transformation (Fig. 1 A), ascites and splenomegaly with multiple abscesses (Fig. 1B). No pleural effusions or pulmonary infiltrates were present at first. Despite broad antibiotic treatment, the patient rapidly deteriorated and was admitted to the intensive care unit on day 10. She developed multi-organ failure (SOFA score 8 points), necessitating fluid resuscitation, noradrenaline (norepinephrine) therapy and invasive mechanical ventilation. Because of the critical condition of the patient, several investigations were performed simultaneously. Initial tests including blood, urine, bronchial lavage and ascites cultures, as well as repeated interferon-gamma release assays, human immunodeficiency virus, galactomannan and β-d-glucan were negative. Ziehl–Neelsen staining of bronchial lavage and ascites were negative. Microscopic differential blood count was unremarkable, but adenosine deaminase in plasma was elevated (46 U/L, normal range <15 U/L). One enlarged inguinal lymph node was removed because aggressive lymphoma was suspected in the differential diagnosis. Histopathological work-up revealed no signs of malignant cells, but granulomas surrounded by epithelioid cells (Fig. 1 C, 10 × magnification). Serological tests for Bartonella henselae and quintana, Toxoplasma gondii, Leptospira, Francisella tularensis and Yersinia were negative. However, Ziehl–Neelsen staining showed acid-fast rods (Fig. 1 D, 60 × magnification), and mycobacterial PCR detected high concentrations of Mycobacterium tuberculosis DNA complexes in the explanted inguinal lymph node. On 16S pan-bacterial PCR analyses no other bacterial species were found. Therefore, the diagnosis of sepsis due to disseminated tuberculosis was made, and treatment was switched to tuberculostatic chemotherapy with isoniazid, rifampicin, pyrazinamide and ethambutol on day 15. The patient slowly recovered with targeted chemotherapy and was discharged from the intensive care unit fully oriented on day 23. Five to 8 weeks later, cultures from multiple locations, including specimens from the inguinal lymph node and bronchial lavage, showed growth of Mycobacterium tuberculosis, which were pan-sensible on antibiotic susceptibility testing. Finally, an obtained third-party history from the patient's sister provided the information of possible tuberculosis during childhood, which was not recalled by the patient herself. She had recently not been abroad and had no known contact to an infected person. Before treatment with tocilizumab was started, interferon-gamma assay tests were negative. Nevertheless, we suppose that reactivation of latent tuberculosis took place in our patient, as new-onset infection is highly unlikely in a low-prevalence area such as Austria. Thus, this case also points to the fact that interferon-gamma release assays may be false negative both before treatment with tocilizumab, and during disseminated tuberculosis [4].
Fig. 1.
(A) Computed tomography scan of the abdomen showing a retroperitoneal lymph node bulk with necrotic transformation, and splenomegaly with multiple abscesses. (B) Abdominal sonography showing multiple splenic abscesses. (C) Hematoxylin and eosin (HE) staining of the explanted inguinal lymph node revealing granulomas surrounded by epitheloid cells. (D) Ziehl-Neelsen staining of the lymph node with numerous acid-fast rods.
In summary, fulminant sepsis due to M. tuberculosis infection may be triggered not only by tumour necrosis factor alpha inhibitors [5], but also by IL-6R antagonists such as tocilizumab. High clinical suspicion for reactivation of latent tuberculosis should be maintained even when interferon-gamma assays are negative before and during IL-6R antagonist therapy.
Transparency declaration
Conflict of interest: The authors declare that they have no competing interests. Funding: none. Ethics approval and consent to participate: The Institutional Review Board of the Medical University of Graz approved this clinical study (EK-Nr.: 31-273 ex 18/19). Consent for publication: Written informed consent was obtained from the patient for publication.
Authors contributions
ACR, GH and PE treated the patient on ICU, FRV performed the histopathological staining. ACR and PE analysed the data and wrote the manuscript. FRV and JH critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript and agreed to be accountable for all aspects related to accuracy and integrity of the work.
Editor: E. Bottieau
References
- 1.Wu R., Wang L., Kuo H.D., Shannar A., Peter R., Chou P.J., et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winthrop K.L., Mariette X., Silva J.T., Benamu E., Calabrese L.H., Dumusc A., et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors) Clin Microbiol Infect. 2018;24:S21–S40. doi: 10.1016/j.cmi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Yamasue M., Komiya K., Usagawa Y., Umeki K., Nureki S.I., Ando M., et al. Factors associated with false negative interferon-gamma release assay results in patients with tuberculosis: a systematic review with meta-analysis. Sci Rep. 2020;10:1607. doi: 10.1038/s41598-020-58459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai J.W., Zhang S., Ruan Q.L., Yu Y.Q., Zhang B.Y., Liu Q.H., et al. The risk of tuberculosis in patients with rheumatoid arthritis treated with tumor necrosis factor-alpha antagonist: a metaanalysis of both randomized controlled trials and registry/cohort studies. J Rheumatol. 2015;42:2229–2237. doi: 10.3899/jrheum.150057. [DOI] [PubMed] [Google Scholar]