Abstract
Objective
To determine whether cytochrome P450 (CYP)2C19 haplotype associates with lansoprazole-associated adverse event frequency.
Study design
Respiratory adverse events from a clinical trial of lansoprazole in children with asthma were analyzed for associations with extensive or poor metabolizer (PM) phenotype based on CYP2C19 haplotypes. Carriers of CYP2C19*2, *3, *8, or *9 alleles were PMs; carriers of 2 wild-type alleles were extensive metabolizers (EMs). Plasma concentrations of lansoprazole were determined in PM and EM phenotypes.
Results
The frequency of upper respiratory infection among PMs (n = 45) was higher than that among EMs (n = 91), which in turn was higher than that in placebo subjects (n = 135; P = .0039). The frequency of sore throat (ST) was similarly distributed among EMs and PMs (P = .0015). The OR (95% CI) for upper respiratory infections in PMs was 2.46 (1.02–5.96) (P = .046); for EMs, the OR (95% CI) was 1.55 (0.86–2.79). The OR (95% CI) for ST in EMs and PMs was 2.94 (1.23–7.05, P = .016) vs 1.97 (1.09–3.55, P = .024), respectively. Mean ± SD plasma concentrations of lansoprazole were higher in PMs than in EMs: 207 ± 179 ng/mL vs 132 ± 141 ng/mL (P = .04).
Conclusions
Lansoprazole-associated upper respiratory infections and ST in children are related in part to CYP2C19 haplotype. Our data suggest that lansoprazole-associated adverse events in children may be mitigated by adjusting the conventional dose in PMs. Additional studies are required to replicate our findings.
Proton pump inhibitors (PPIs) are effective acid-suppressing drugs for the treatment of gastroesophageal reflux disease, laryngopharyngeal reflux, ulcers, and related disorders in both adults1 and children.2 PPIs are among the highest-selling drugs in the US and worldwide. Although they are considered safe, PPIs used on a long-term basis have been associated with several unexpected side effects, including bone fractures, reduced absorption of vitamin B12, reduced magnesium levels, hyperacid secretion, and gastric cancer.3,4 Additionally, long-term PPI use increases gastric pH from an acid to a less acid milieu, thus removing an effective barrier to ingested pathogens, which can result in bacterial overgrowth in gastric media.5–9 If ingested and aspirated, infected gastric media may lead to increased susceptibility to upper respiratory tract infections (URIs) including community-acquired pneumonia and bronchitis.10,11
The efficacy of PPIs to suppress gastric acid secretion is strongly correlated with plasma concentrations.12,13 The pharmacokinetics and pharmacodynamics of PPIs are influenced by cytochrome P450 (CYP)2C19 single nucleotide polymorphisms (SNPs).14–16 Several SNPs result in reduced efficiency of the CYP2C19 enzyme to clear PPIs, including lansoprazole.15 Alleles carrying these mutants are termed loss-of-function alleles and are designated CYP2C19*2 through CYP2C19*9.15 Individuals carrying one or more loss-of-function alleles are classified as either poor metabolizers (PMs) or intermediate metabolizers. Alleles carrying no loss-of-function SNPs are designated CYP2C19*1 (wild type [WT]), are associated with a more rapid clearance, and are responsible for the extensive metabolizer (EM) phenotype.15 CYP2C19*17 is a gain-of-function allele and is thought to contribute to the ultra rapid metabolizer phenotype.17,18
Recently, we completed a randomized placebo-controlled clinical trial of the PPI lansoprazole in children whose asthma symptoms were poorly controlled with inhaled corticosteroid treatment.19 We found that the addition of lansoprazole to existing asthma therapy improved neither symptoms nor lung function but was associated with a higher incidence of respiratory adverse events, including URIs, sore throat (ST), and bronchitis, compared with placebo. Given the propensity of PPIs to associate with respiratory infections and the relationship between PPI pharmacokinetics and genetic variability, we hypothesized that the respiratory adverse events associated with lansoprazole treatment in our clinical trial of lansoprazole treatment of children with poorly controlled asthma19 are associated with the CYP2C19 genotype.
Methods
Details of the clinical trial The Study of Acid-Reflux in Childhood Asthma have been previously published.19 A total of 306 children aged 6–17 years with poor asthma control while on inhaled corticosteroids were assigned to either matched placebo (n = 157) or lansoprazole (15 mg/d for children weighing <30 kg or 30 mg/d for those weighing ≥30 kg; n = 149) for 24 weeks. The primary outcome measure for The Study of Acid-Reflux in Childhood Asthma trial was the change in the Asthma Control Questionnaire score.20 A 0.5-point change in the Asthma Control Questionnaire score reflects a clinically important difference in asthma control. Secondary outcomes included the rate of acute episodes of poor asthma control, changes in spirometry, and exhaled nitric oxide.19 A targeted sample size of 300 participants provided 90% power to detect a −0.6-point change in the primary outcome. Additionally, research staff conducted structured interviews at 7 scheduled clinic visits during treatment using a questionnaire to determine the presence of the following: URI (cold), ST, strep throat, bronchitis, pneumonia, ear infection, and acute sinusitis (sinus infection). Only participants from whom parental permission and assent were obtained for storage and future use of genomic DNA samples were included in the present analysis. The study was approved by the institutional review board at each center.
DNA was isolated from saliva (Oragene•DNA DNA Self-Collection Kit [OG-100 Vial Format]; DNA Genotek; http://www.dnagenotek.com) or from blood collected at the randomization visit. Five CYP2C19 SNPs were genotyped: G681A (rs4244285; *2), G636A (rs4986893; *3), T358C (rs41291556; *8), G114A (rs17884712; *9), and –C806T (rs12248560; *17). Participants were classified as PMs if they carried ≥1 CYP2C19*2, *3, *8, or *9 alleles and as EMs if they carried 2 WT alleles. DNA was genotyped by using a LightTyper fluorescent assay with simple probe chemistry (Roche Applied Science, Indianapolis, Indiana) as previously described.21 Blood was drawn 2–3 hours after the final dose of lansoprazole or placebo before a meal in 119 individuals who volunteered for the genetic substudy. Plasma concentrations of lansoprazole were quantified by high-performance liquid chromatography as previously described.22 The limit of delectability was 10 ng/mL; the mean ± SD intra-assay precision values at 10 and 100 ng/mL were 5% ± 3% and 3% ± 1%, respectively.
Statistical Analyses
Adverse event frequencies were calculated for participants taking placebo and compared with those in participants taking lansoprazole and classified as either EMs or PMs. Because treatment assignment and metabolizer phenotype (PM vs EM) exist on the same causal pathway of increasing lansoprazole exposure, we used the Cochran-Armitage test for trend23 to determine the effect of lansoprazole exposure on event frequency. Logistic regression was performed to calculate the ORs for adverse events comparing lansoprazole vs placebo in patients with PM and EM separately. Analysis was performed with SAS version 9.2 (SAS Institute, Cary, North Carolina). One-tailed t test was used to test for differences in lansoprazole plasma concentrations between EMs and PMs.
Results
DNA was collected from 279 of 306 participants; 27 participants declined to participate in the genetic substudy. Adverse event data were not collected in 7 participants because they attended the randomization visit only. Data for adverse event frequencies and CYP2C19 genotype were available for 272 participants: 63% were male, 51% were black, 39% were white, and 1.8% were Asian. Eighty-eight percent and 93% of participants taking placebo and lansoprazole, respectively, visited the clinic at least 4 times; 66% and 67% of participants receiving placebo and lansoprazole treatment, respectively, completed all 7 visits. The call rate for the genotypes was >98%. Table I compares the allele frequencies for CYP2C19*2, *3, *8, *9, and *17 by race/ethnic group and are in agreement with published frequencies.24
Table I.
Allele frequencies of CYP2C19 SNPs by race/ethnic groups in children with poorly controlled asthma participating in placebo controlled clinical trial of lansoprazole
| CYP2C19 variant |
rs number | Whites (n = 107) | African Americans (n = 139) | Asians (n = 5) | Others (n = 28) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| Major allele | Minor allele | MAF | Major allele | Minor allele | MAF | Major allele | Minor allele | MAF | Major allele | Minor allele | MAF | ||
| *2 | rs4244285 | G | A | 0.118 | G | A | 0.1799 | G | A | 0.2 | G | A | 0.107 |
| *3 | rs4986893 | G | A | 0.005 | G | A | 0.0036 | G | A | 0.1 | G | A | 0 |
| *8 | rs41291556 | T | C | 0.0096 | T | C | 0 | T | C | 0 | T | C | 0 |
| *9 | rs17884712 | G | A | 0 | G | A | 0.018 | G | A | 0 | G | A | 0 |
| *17 | rs12248560 | C | T | 0.201 | C | T | 0.191 | C | T | 0.1 | C | T | 0.25 |
A, adenine; C, cytosine; G, guanine; MAF, minor allele frequency; T, thymine.
Table II compares the frequencies of respiratory adverse events in the individuals who participated in the genetic substudy and were assigned to the placebo and lansoprazole treatment arms. The relative risks (95% CI) of URI, ST, and bronchitis in participants taking lansoprazole compared with placebo were 1.30 (1.06–1.59, P = .012), 1.56 (1.20–2.03, P = .0007), and 5.11 (1.14–22.89, P = .0106). We did not subject data on participants with bronchitis or other adverse events to separate analyses owing to the small number of participants for each event.
Table II.
Comparison of adverse event frequencies in children with poorly controlled asthma assigned to either placebo or lansoprazole treatment arms and who volunteered for the genetic substudy
| Treatment group, No. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Adverse event | Placebo (n= 141) | Lansoprazole (n = 138) | Relative risk (95% CI) | P |
| URI | 71 (50.4%) | 90 (65.2%) | 1.30 (1.06–1.59) | .012 |
| ST | 51 (36.2%) | 78 (56.5%) | 1.56 (1.20–2.03) | .0007 |
| Group A Streptococcus | 12 (8.5%) | 6 (4.4%) | 0.51 (0.20–1.32) | .157 |
| Bronchitis | 2 (1.4%) | 10 (7.3%) | 5.11 (1.14–22.89) | .016 |
| Pneumonia | 5 (3.5%) | 5 (3.6%) | 1.02 (0.30–3.45) | .97 |
| Otitis media | 10 (7.1%) | 12 (8.7%) | 1.23 (0.55–2.74) | .62 |
| Acute sinusitis | 18 (12.8%) | 15 (10.9%) | 0.85 (0.45–1.62) | .63 |
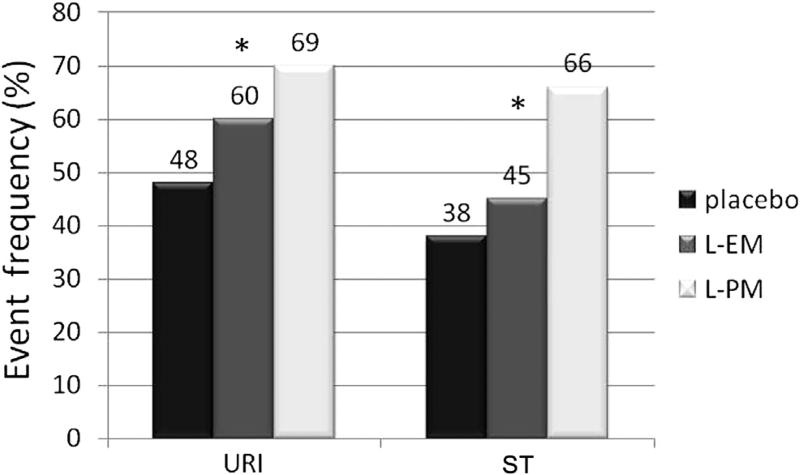
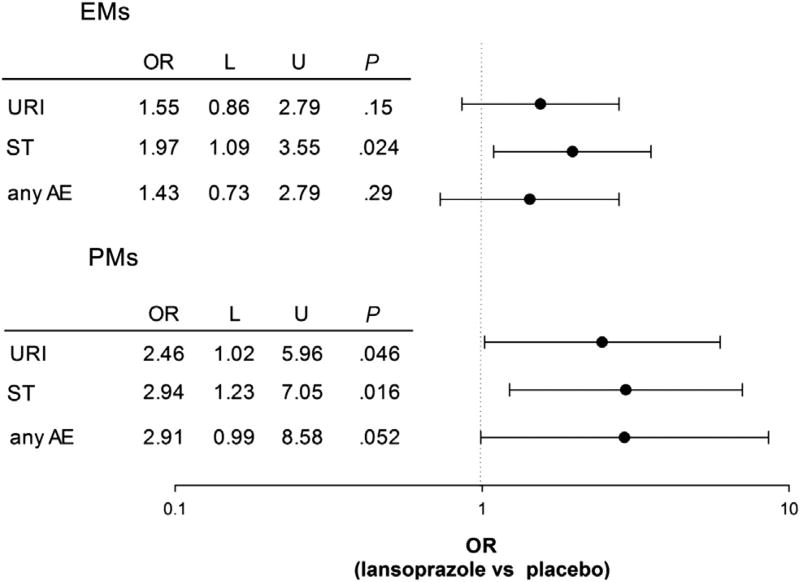
To address our hypothesis that lansoprazole-associated respiratory adverse events was related to CYP2C19 genotype, we compared the frequencies of URI and ST in PMs and EMs (Figure 1). The frequency of URI among participants classified as PMs was higher compared with EMs (31/45, 69%, vs 55/91, 60%), which in turn was higher than in placebo (65/136, 48%, P = .0039, Cochran-Armitage test for trend). The frequency of ST among participants classified as PMs was higher compared with EMs (30/45, 66%, vs 41/91, 45%), which in turn was higher than in placebo (51/136, 38%, Cochran-Armitage test for trend). To determine if the frequencies of URI and ST differed between PMs and EMs, we calculated ORs and 95% CIs (Figure 2). The OR for URI in PMs taking lansoprazole was significantly different from placebo (0.046); the OR (95% CI) for EMs was 1.55 (0.86–2.79) and was not significantly different from placebo. The ORs (95% CIs) for ST in both EMs and PMs were both significantly different from placebo (Figure 2). However, the OR for ST did not differ between PMs and EMs. Also shown in Figure 2, the OR (95% CI) for any adverse event (sum of all respiratory adverse events) trended higher for PMs than for EMs.
Figure 1.
Comparison of adverse event frequencies by metabolizer phenotype. Metabolizer phenotype classification was based on CYP2C19 haplotype. *P < .01 χ2 test for trend. L-EM, lansoprazole extensive metabolizer; L-PM, lansoprazole poor metabolizer.
Figure 2.
OR (95% CI) for associating URI, ST, and any adverse event (AE) with lansoprazole metabolizer phenotype. L, lower limit of 95% CI; U, upper limit of 95% CI.
To evaluate the influence of CYP2C19 genotype on the pharmacokinetics of lansoprazole, we compared plasma concentrations from samples drawn 2–3 hours after the final dose, which corresponds to the time at which maximal plasma concentrations are achieved following oral administration.13 Of the 119 participants who volunteered a blood sample for lansoprazole blood level determination, 63 were assigned placebo and 56 were assigned to receive 30 mg/d lansoprazole (none of the participants who were assigned to receive 15 mg/d lansoprazole volunteered a blood sample for lansoprazole plasma determination). Mean ± SD plasma concentrations of lansoprazole were higher in PMs (n = 23) compared with EMs (n = 33): 207 ± 179 vs 132 ± 141 ng/mL (P = .04).
Discussion
The acidic pH of the gastrointestinal tract represents a protective barrier from invasion by ingested pathogens.5,7 Chronic gastric acid suppression by PPI and histamine2 antagonist treatment can reduce the effectiveness of this protective barrier, alter the gastric flora of the stomach, and increase the risk of bacterial and viral colonization in the upper gastrointestinal tract in a dose-dependent manner.6,25 It has been proposed that reflux and aspiration of gastric contents containing colonized bacteria can lead to respiratory infections including pneumonia.9,10 Consistent with this sequence of events, several studies have reported that the long-term use of PPIs has been associated with community-acquired pneumonia.26–32 In the present study, we report that the frequencies of URI and ST were higher in children with poorly controlled asthma taking lansoprazole for 6 months compared with placebo. When stratified by metabolizer phenotype as determined by the genotype of loss-of-function CYP2C19 SNPs, PMs had higher frequencies of URI and ST compared with EMs and with those on placebo. This study suggests that lansoprazole-associated respiratory adverse events in children are related to CYP2C19 loss-of-function alleles.
The mechanism underlying the link between lansoprazole-associated respiratory side effects and CYP2C19 loss-of-function SNPs is consistent with a reduced clearance of and greater exposure to lansoprazole. Carriers of the CYP2C19 mutant variants G681A (rs4244285), G636A (rs4986893), T358C (rs41291556), and G114A (rs17884712) cause a loss of function for the enzyme CYP2C19, a reduced clearance of the substrate, and an increase in plasma concentrations compared with WT carriers following administration of equal doses. We postulate that the higher plasma concentrations of lansoprazole in PMs result in a greater suppression of acid secretion, a higher gastric pH, and a greater degree of dysbiosis compared with EMs. If refluxed and aspirated, infected gastric media would be expected to increase the risk of respiratory infections, especially in PMs.
Our findings are potentially important. If PMs are at greater risk of developing PPI-associated respiratory side effects compared with EMs, then it is possible that PMs, which comprise about one-third of the population in this country (proportion of individuals carrying 1 or 2 loss-of-function alleles), may be overdosed by conventional PPI treatment. It may be further reasoned that respiratory adverse events associated with chronic PPI use in children may be mitigated by adjusting the dose of the PPI downward in PM. Genotyping patients for CYP2C19 loss-of-function alleles prior to dosing or soon after initiating conventional PPI therapy followed by dosage adjustment is feasible in PMs. Commercial laboratories currently offer genetic testing for CYP2C19 loss-of-function alleles. Prescribing a PPI for an evidence-based indication and using genotype-guided dosing should reduce PPI-associated respiratory adverse events. The use of histamine2 receptor antagonists is probably not an alternative to PPI therapy as this class of drugs is also associated with respiratory adverse events. Additionally, the present study supports the recommendations of our earlier study that PPIs not be used to treat asthma and that there are safety concerns regarding long-term use in children.19
The link between chronic PPI use and the development of respiratory adverse events including community-acquired pneumonia has been questioned. A few population-based studies report no association between chronic PPI use and the development of community-acquired pneumonia,33–35 and the results of 3 meta-analyses failed to support an association between chronic PPI use and community-acquired pneumonia.11,36,37 It is possible that had patients been stratified by CYP2C19 metabolizer phenotype, an association between PPI use and pneumonia would have been observed in these studies. Additionally, other arguments against PPIs causing community-acquired pneumonia and other respiratory infections have been raised.38 First, PPI therapy reduces the risk of reflux and therefore reduces the risk of aspiration, which would diminish the risk of respiratory side effects. Second, the association between PPI use and pneumonia is driven by patients who had been prescribed PPI therapy during the past 30 days with no association in those taking PPIs chronically. This raises the issue of protopathic bias—that is, PPIs were being prescribed for symptoms of pneumonia before the diagnosis of pneumonia was made.38 Last, patients who were prescribed PPI therapy were sicker than those who were not prescribed PPI therapy and therefore more susceptible to developing pneumonia. These arguments do not apply to the present study, which emanated from a randomized, placebo-controlled clinical trial of lansoprazole in patients with poorly controlled asthma.19 Participants assigned to the lansoprazole arm were no sicker than those assigned to received placebo, and participants reporting respiratory adverse events were evenly distributed throughout the study.
In addition to our finding that lansoprazole-associated respiratory side effects are related to CYP2C19 loss-of-function alleles, we found that concentrations of lansoprazole were higher among PMs compared with EMs. These data are consistent with several pharmacokinetic studies demonstrating that the clearance of PPIs including lansoprazole is markedly reduced in individuals carrying one or more loss-of-function CYP2C19 alleles.14–16,18 We speculate that the higher prevalence of respiratory adverse events observed in PMs is due in part to a lower clearance of lansoprazole among carriers of the loss-of-function CYP2C19*2, *3, *8, and *9 alleles, which results in greater exposure to the drug, prolonged acid suppression, increased bacterial/viral colonization, and increased susceptibility to develop respiratory adverse events compared with EMs.
Our study has several limitations. Our genetic substudy was ancillary to a placebo-controlled, randomized clinical trial of lansoprazole, which was not powered as a genetic study. Consequently, we may not have had sufficient numbers to address the hypothesis that the frequency of PPI-associated respiratory adverse events is higher in PMs compared with EMs and that the findings in the present study may represent false positives. In a prospective trial, we would have collected more plasma concentration vs time data than the single sample collected in our substudy, which would have provided more definitive pharmacokinetic data. Central to our finding that PPI-associated respiratory adverse events are higher in PMs is that PMs have greater exposure to lansoprazole (higher area under the plasma concentration vs time curve) than EMs following administration of equal doses owing to a reduced clearance in carriers of the CYP2C19 loss-of-function alleles. Our study relied on participant recall and response to the side effect questionnaire to quantify respiratory adverse event frequency. Culture of gastric fluid7 or bronchoalveolar lavage9 for bacterial overgrowth is preferable to completion of questionnaires to evaluate the link between PPI use and respiratory infection. Another limitation of our study is that gastric pH was not measured in all participants prior to and after treatment. Central to our proposed mechanism of PPI-associated respiratory side effects is a higher gastric pH in PMs compared with EMs. Finally, we did not measure participant adherence to lansoprazole treatment. In a previous study, we quantified adherence by measuring plasma concentrations of montelukast and theophylline.39 Monitoring blood levels of lansoprazole to assess adherence would not have been feasible owing to once-daily dosing and to the drug’s very short half-life. These limitations notwithstanding, the results of our association study and blood level data taken together with the biological plausibility of the model of pH changes induced by PPIs with subsequent bacterial and viral overgrowth support a role for CYP2C19 variation in PPI-associated respiratory adverse events.
Glossary
- CYP
Cytochrome P450
- EM
Extensive metabolizer
- PM
Poor metabolizer
- PPI
Proton pump inhibitor
- SNP
Single nucleotide polymorphism
- ST
Sore throat
- URI
Upper respiratory tract infection
- WT
Wild type
Footnotes
The authors declare no conflicts of interest.
Registered with ClinicalTrials.gov: NCT00604851.
References
- 1.van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2006;3:CD002095. doi: 10.1002/14651858.CD002095.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Croom KF, Scott LJ. Lansoprazole: in the treatment of gastro-oesophageal reflux disease in children and adolescents. Drugs. 2005;65:2129–35. doi: 10.2165/00003495-200565150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Altman KW, Radosevich JA. Unexpected consequences of proton pump inhibitor use. Otolaryngol Head Neck Surg. 2009;141:564–6. doi: 10.1016/j.otohns.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Abraham NS. Proton pump inhibitors: potential adverse effects. Curr Opin Gastroenterol. 2012;28:615–20. doi: 10.1097/MOG.0b013e328358d5b9. [DOI] [PubMed] [Google Scholar]
- 5.Howden CW, Hunt RH. Relationship between gastric secretion and infection. Gut. 1987;28:96–107. doi: 10.1136/gut.28.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54–9. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theisen J, Nehra D, Citron D, Johansson J, Hagen JA, Crookes PF, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg. 2000;4:50–4. doi: 10.1016/s1091-255x(00)80032-3. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy DM. Current opinion in gastroenterology. Curr Opin Gastroenterol. 2010;26:624–31. doi: 10.1097/MOG.0b013e32833ea9d9. [DOI] [PubMed] [Google Scholar]
- 9.Rosen R, Johnston N, Hart K, Khatwa U, Katz E, Nurko S. Higher rate of bronchoalveolar lavage culture positivity in children with nonacid reflux and respiratory disorders. J Pediatr. 2011;159:504–6. doi: 10.1016/j.jpeds.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol. 2009;104(Suppl 2):S17–20. doi: 10.1038/ajg.2009.47. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther. 2010;31:1165–77. doi: 10.1111/j.1365-2036.2010.04284.x. [DOI] [PubMed] [Google Scholar]
- 12.Yacyshyn BR, Thomson AB. The clinical importance of proton pump inhibitor pharmacokinetics. Digestion. 2002;66:67–78. doi: 10.1159/000065588. [DOI] [PubMed] [Google Scholar]
- 13.Litalien C, Theoret Y, Faure C. Pharmacokinetics of proton pump inhibitors in children. Clin Pharmacokinet. 2005;44:441–66. doi: 10.2165/00003088-200544050-00001. [DOI] [PubMed] [Google Scholar]
- 14.Furuta T, Shirai N, Sugimoto M, Ohashi K, Ishizaki T. Pharmacogenomics of proton pump inhibitors. Pharmacogenomics. 2004;5:181–202. doi: 10.1517/phgs.5.2.181.27483. [DOI] [PubMed] [Google Scholar]
- 15.Furuta T, Shirai N, Sugimoto M, Nakamura A, Hishida A, Ishizaki T. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet. 2005;20:153–67. doi: 10.2133/dmpk.20.153. [DOI] [PubMed] [Google Scholar]
- 16.Hagymasi K, Mullner K, Herszenyi L, Tulassay Z. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics. 2011;12:873–88. doi: 10.2217/pgs.11.4. [DOI] [PubMed] [Google Scholar]
- 17.Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79:103–13. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Kearns GL, Leeder JS, Gaedigk A. Impact of the CYP2C19*17 allele on the pharmacokinetics of omeprazole and pantoprazole in children: evidence for a differential effect. Drug Metab Dispos. 2010;38:894–7. doi: 10.1124/dmd.109.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, Castro M, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 2012;307:373–81. doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. 2010;36:1410–6. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 21.Lima JJ, Mohapatra S, Feng H, Lockey R, Jena PK, Castro M, et al. A polymorphism in the NPPA gene associates with asthma. Clin Exp Allergy. 2008;31:1117–23. doi: 10.1111/j.1365-2222.2008.02955.x. [DOI] [PubMed] [Google Scholar]
- 22.Karol MD, Granneman GR, Alexander K. Determination of lansoprazole and five metabolites in plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1995;668:182–6. doi: 10.1016/0378-4347(95)00068-t. [DOI] [PubMed] [Google Scholar]
- 23.Wikipedia. [Accessed on April 17, 2013];Cochran–Armitage test for trend. Available at http://en.wikipedia.org/wiki/Cochran-Armitage_test_for_trend.
- 24.Saito M, Ozawa S. Genetic polymorphisms and haplotypes of major drug metabolizing enzymes in East Asians and their comparison with other ethnic populations. Current Pharmacogenomics. 2007;5:78–127. [Google Scholar]
- 25.Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23:3–10. doi: 10.1111/j.1365-2036.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- 26.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955–60. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 27.Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117:e817–20. doi: 10.1542/peds.2005-1655. [DOI] [PubMed] [Google Scholar]
- 28.Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med. 2007;167:950–5. doi: 10.1001/archinte.167.9.950. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez LA, Ruigomez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology. 2009;20:800–6. doi: 10.1097/EDE.0b013e3181b5f27d. [DOI] [PubMed] [Google Scholar]
- 30.Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2009;18:269–75. doi: 10.1002/pds.1715. [DOI] [PubMed] [Google Scholar]
- 31.Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med. 2010;123:47–53. doi: 10.1016/j.amjmed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Hermos JA, Young MM, Fonda JR, Gagnon DR, Fiore LD, Lawler EV. Risk of community-acquired pneumonia in veteran patients to whom proton pump inhibitors were dispensed. Clin Infect Dis. 2012;54:33–42. doi: 10.1093/cid/cir767. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149:391–8. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 34.Gau JT, Acharya U, Khan S, Heh V, Mody L, Kao TC. Pharmacotherapy and the risk for community-acquired pneumonia. BMC Geriatr. 2010;6:45–53. doi: 10.1186/1471-2318-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dublin S, Walker RL, Jackson ML, Nelson JC, Weiss NS, Jackson LA. Use of proton pump inhibitors and H2 blockers and risk of pneumonia in older adults: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2010;19:792–802. doi: 10.1002/pds.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sultan N, Nazareno J, Gregor J. Association between proton pump inhibitors and respiratory infections: a systematic review and meta-analysis of clinical trials. Can J Gastroenterol. 2008;22:761–6. doi: 10.1155/2008/821385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estborn L, Joelson S. Occurrence of community-acquired respiratory tract infection in patients receiving esomeprazole: retrospective analysis of adverse events in 31 clinical trials. Drug Saf. 2008;31:627–36. doi: 10.2165/00002018-200831070-00008. [DOI] [PubMed] [Google Scholar]
- 38.Moayyedi P, Leontiadis GI. The risks of PPI therapy. Nat Rev Gastroenterol Hepatol. 2012;9:132–9. doi: 10.1038/nrgastro.2011.272. [DOI] [PubMed] [Google Scholar]
- 39.Irvin CG, Kaminsky DA, Anthonisen NR, Castro M, Hanania NA, Holbrook JT, et al. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med. 2007;175:235–42. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]