Abstract
Corona Virus Disease 2019 (COVID-19) is caused by the novel coronavirus SARS-CoV-2. Emerging genetic and clinical evidence suggests similarities between COVID-19 patients and those with severe acute respiratory syndrome and Middle East respiratory syndrome. Hematological changes such as lymphopenia and thrombocytopenia are not rare in COVID-19 patients, and a smaller population of these patients had leukopenia. Thrombocytopenia was detected in 5–41.7% of the patients with COVID-19. Analyzing the dynamic decrease in platelet counts may be useful in the prognosis of patients with COVID-19. However, the mechanisms underlying the development of thrombocytopenia remain to be elucidated. This review summarizes the hematological changes in patients infected with SARS-CoV-2 and possible underlying mechanisms of thrombocytopenia development.
Keywords: COVID-19, SARS-CoV-2, Thrombocytopenia
Highlights
-
•
Early diagnosis and treatment of COVID-19 patients can reduce mortality.
-
•
There is no laboratory test index to predict disease progression and prognosis.
-
•
Progressive decline in platelets may be a prognostic factor for COVID-19 patients.
-
•
Monitoring platelet count may be an effective index for COVID-19 progression.
1. Introduction
Patients with pneumonia (due to unknown etiologies) were identified in early December 2019 in Wuhan, Hubei, China. The causative agent of this disease was identified as a novel strain of the coronavirus (CoV) named SARS-CoV-2 and the disease was named Corona Virus Disease 2019 (COVID-19) by the World Health Organization. The symptoms of COVID-19 are complex; fever and cough were the most common symptoms. Hematological changes such as lymphopenia, thrombocytopenia, and coagulation disorder in these patients are not rare [1,2,3]. Patients with COVID-19 share similar hematological changes (especially lymphopenia and thrombocytopenia) as those in patients with severe acute respiratory syndrome coronavirus (SARS) and Middle East respiratory syndrome (MERS) [4,5]. However, the mechanism(s) involved in the induction of these changes are poorly understood. This review summarizes the hematological changes in patients infected with CoV and possible mechanisms of thrombocytopenia in patients with COVID-19.
2. Coronavirus types and their receptors
Six types of human CoVs have been identified till date: HCoV-NL63 and HCoV-229E are Alphacoronaviruses and HCoV-OC43, HCoVHKU1, SARS-CoV, and Middle East respiratory syndrome coronavirus (MERS-CoV) are Betacoronaviruses (Table I ) [[6], [7], [8], [9], [10]]. SARS-CoV-2 is the seventh member of the RNA-containing enveloped CoV family. SARS-CoV-2 and SARS-CoV reside on different branches of the phylogenetic tree, but the genome of SARS-CoV-2 shares more than 85% homology with that of SARS-CoV [7]. HCoV-229E, OC43, NL63, and HKU1 cause mild respiratory diseases. The last two decades have seen fatal infections caused by SARS-CoV and MERS-CoV [8].
Table I.
| CoV type | Genus | Receptors |
|---|---|---|
| HCoV-229E | α-Coronavirus | hAPN (CD13) |
| HCoV-OC43 | β-Coronavirus | HLA class I |
| HCoV-NL63 | α-Coronavirus | ACE2 |
| HCoV-HKU1 | β-Coronavirus | ? |
| SARS-CoV | β-Coronavirus | ACE2 |
| MERS-CoV | β-Coronavirus | DPP4 (CD26) |
| SARS-CoV-2 | β-Coronavirus | ACE2 |
CoVs use cell surface receptors to enter host cells [9]. SARS-CoV primarily binds to the angiotensin-converting enzyme 2 (ACE2) [10], whereas MERS-CoV interacts with dipeptidyl peptidase 4 (DPP4; also known as CD26; Table I). Similar to SARS-CoV, COVID-19 develops upon binding of SARS-CoV-2 viral particles to ACE2, but not to other CoV receptors, such as aminopeptidase N and DPP4 [7]. SARS-CoV has similar antigenic characteristics as human HCoV-229E [11,12]. HCoV-229E enters monocytes and macrophages via CD13 and induces cell apoptosis [13]. In addition, Betacoronaviruses can utilize CEACAMla (CD66a) as receptors [4,14].
3. Clinical manifestations and treatment of COVID-19
Patients with COVID-19 can be divided into four categories based on their clinical manifestations: light, common, severe, and critical. Guan et al. performed a retrospective study (n = 1099) demonstrated that COVID-19 is associated with a wide range of symptoms [1]. Fever (87.9%) and cough (67.7%) were the most common symptoms, whereas diarrhea (3.7%) and vomiting (5.0%) were rare [1]. Among the cohorts analyzed, some SASR-CoV-2 infected individuals were asymptomatic, thereby making the diagnosis and treatment even more challenging. The most common complication in symptomatic patients was pneumonia (79.1%) followed by acute respiratory distress syndrome (3.37%) and shock (1.00%) [1]. The treatment for COVID-19 includes providing oxygen, mechanical ventilation, intravenous antibiotics, and antiviral drugs [1,2,3]. Use of antibiotics in the early stages of disease has no effect and steroid hormones have not been reported to be effective. Administering antibiotics to patients with potential bacterial or fungal infection helps in preventing infection and reducing complications and mortality [1,2,3]. Patients with severe COVID-19 infection and symptoms are subjected to mechanical ventilation or extracorporeal membrane oxygenation; however, some severe and critical patients do not respond well to this therapeutic regimen [1,2,3].
4. Hematological changes in patients with CoV infection
SARS patients commonly manifest lymphopenia, thrombocytopenia, and leukopenia. During the onset of SARS, patients exhibit a reduction in peripheral CD4+ and CD8+ T lymphocytes [15]. A retrospective cohort study comprising 16 MERS-CoV-infected patients showed that 31% and 40% of the patients developed thrombocytopenia on day 1 and 21, respectively [5]. Similarly, a retrospective study performed on patients with COVID-19 (n = 1099) showed 82.1% and 36.2% of patients with lymphopenia and thrombocytopenia on admission, respectively, and 33.7% of patients with leukopenia [1]. Different studies have reported varying rates of thrombocytopenia in COVID-19 [1,2,[16], [17], [18], [19]]. This could be attributed to the different number of patients and proportion of severe patients (Table II ). Severe patients exhibited prominent abnormalities as compared to non-severe patients. The postmortem biopsy of a patient who died from severe COVID-19 revealed a drastic reduction in the number of peripheral hyperactivated CD4+ and CD8+ T cells [20]. Patients with severe disease and fatal outcomes present with a decreased lymphocyte/white blood cell ratio compared to the non-severe patients [21]. The platelet-to-lymphocyte ratio is an inflammatory marker that reflects the extent of systemic inflammation and cytokine storm [21,22]. Qu et al. showed that, among 30 hospitalized patients with COVID-19, those presenting with increased platelet counts during the development of disease had poor prognoses. Severe patients undergoing treatment showed an increase followed by a decrease in platelet content [[21], [22], [23]]. Thus, dynamic changes in platelet counts, neutrophil/lymphocyte ratio, and platelet-to-lymphocyte ratio may have prognostic value in determining disease severity [[21], [22], [23]]. However, the underlying mechanisms remain to be understood.
Table II.
| Author [reference] (patient cohort) | Thrombocytopenia (%): all patients | Thrombocytopenia (%): non-severe patients | Thrombocytopenia (%): severe patients | Platelet count (109/L): all patients | Platelet count (109/L): non-severe patients | Platelet count (109/L): severe patients |
|---|---|---|---|---|---|---|
| Huang et al. (n = 41) [2,18] | 5 | 8 | 4 | 164.5 | 196 | 140 |
| Chen et al. (n = 99) [18,19] | 12 | NA | NA | 213.5 | NA | NA |
| Guan et al. (n = 1099) [1,18] | 36.2 | 31.6 | 57.7 | 168 | 172 | 137.5 |
| Liu et al. (n = 12) [16,18] | 41.7 | 66.7 | 16.7 | 160.3 | 186.2 | 139.5 |
| Zhou F et al. (n = 137) [17,18] | 7 | 1 | 20 | 206 | 220 | 165.5 |
NA represents (not applicable).
5. Thrombocytopenia and coagulation disorders in severe and critical COVID-19 patients
Thrombocytopenia was detected in 5–41.7% in COVID-19 patients. Severe and/or critical patients had decreased platelet counts [24,25] as well as coagulation disorders. However, there is no data on the role of thrombocytopenia in increasing the risk of bleeding in COVID-19 patients. A meta-analysis of 1779 COVID-19 patients showed that thrombocytopenia was associated with a threefold-enhanced risk of severe COVID-19 and a lower platelet count correlated with mortality [18]. A similar retrospective study performed on patients with COVID-19 (n = 383) showed a threefold-correlation between thrombocytopenia (at hospital admission) and mortality as compared to patients without thrombocytopenia [26]. Thus, platelet count is an independent risk factor for in-hospital mortality. Increments of 50 × 109/L platelets are associated with 40% decreases in mortality [26]. Thus, platelet count may serve as a simple and inexpensive biomarker for disease severity and risk of mortality of patients in the intensive care unit [18,26]. This study (n = 383) showed that the incidence of thrombocytopenia in patients with comorbidities (chronic obstructive pulmonary disease, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, and chronic kidney disease) was between 2.9% and 26.5%. Thrombocytopenia prevalence was highest in patients with hypertension (26.5%) [26]. However, this data remains indicative because of the lack of data on thrombocytopenia in patients without comorbidities. Thrombocytopenia is usually detected during the diagnosis of COVID-19. The correlation between the rate of decrease in patient platelet counts and disease progression remains to be investigated. This will help to understand the mechanisms of thrombocytopenia in COVID-19 patients.
Thrombocytopenia is a common consequence of infectious diseases. The most common pathogens that induce thrombocytopenia are viruses (28%) and bacteria (28%) followed by fungi (15%) [27]. The incidence of thrombocytopenia in hospitalized patients with viral influenza or community-acquired pneumonia is less than 5% [28,29]. In contrast, thrombocytopenia can be detected in ~50% of patients with septic shock [30]. A minority of patients with cytomegalovirus infection (2.7%–18.3%) exhibit thrombocytopenia [[31], [32], [33]], whereas mild thrombocytopenia is common in patients with Epstein-Barr-mediated infectious mononucleosis (25%–50%) and occurs in uncomplicated cases during the acute phase of the disease [34,35]. During the 2002 SARS outbreak, 20%–55% of patients had thrombocytopenia that was identified as a risk factor for mortality [4]. Similarly, thrombocytopenia was found in 24.3–46.6% of MERS patients (Table III ) [36].
Table III.
Incidence of thrombocytopenia in infectious diseases.
| Disease | Thrombocytopenia (%) |
|---|---|
| Influenza [28] | <5% |
| CAP [29] | <5% |
| Infectious mononucleosis [[34], [35]] | 25–50% |
| Cytomegalovirus infection [[31], [32], [33]] | 2.7–18.3% |
| SARS [4] | 20–55% |
| MERS [36] | 24.3–46.6% |
| COVID-19 [18] | 5–41.7% |
CAP: community-acquired pneumonia; SARS: severe acute respiratory syndrome coronavirus; MERS: Middle East respiratory syndrome.
During the progression of COVID-19, ~50% of the patients show increased levels of D-dimer and all the patients who expired showed an increase in D-dimer content. D-dimers were higher in patients with severe disease as compared to that in patients with mild disease, suggesting increased risk of thrombosis in severe patients [24,25]. Severe or critical patients manifest with significant coagulation disorders. Coagulation disorders and disseminated intravascular coagulation (DIC) are important causes of death in patients with severe disease. Multiple organ dysfunction induced by diffuse microvascular damage is one of the main causes of death in COVID-19 patients [24,25].
Therefore, timely monitoring of patient platelet counts, dynamic blood coagulation function, and DIC will improve treatment and prognosis of patients with COVID-19. Moreover, evaluating the lymphocyte count dynamics and inflammatory index, including LDH, CRP, and IL-6 may also help determine the prognosis and initiate therapy to improve disease outcomes [23].
6. Possible mechanisms of thrombocytopenia caused by CoV infection
6.1. Attack on hematopoietic stem cells (HSCs)
HSCs differentiate into megakaryocyte-erythrocyte progenitors that develop into mature erythrocytes and megakaryocytes (MKs). MKs differentiate, mature, and generate platelets in the bone marrow (BM). One MK produces 10–20 proplatelets, and ultimately releasing approximately 1000–3000 platelets in its lifetime [[37], [38], [39]].
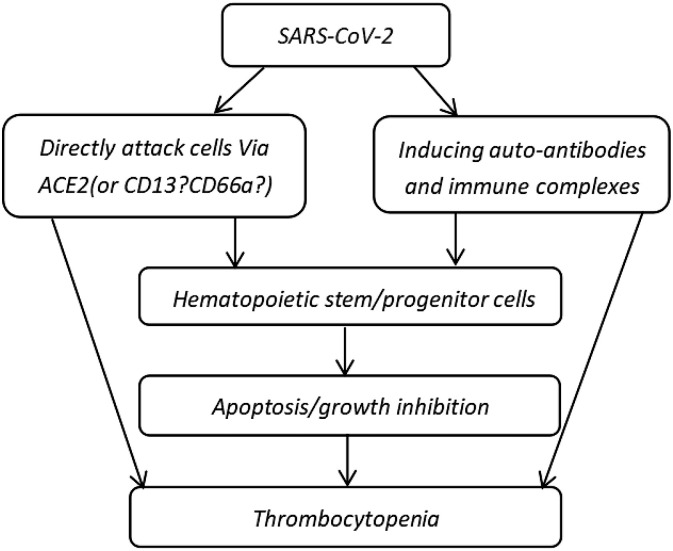
SARS-CoV-2 and SARS-CoV use the same receptor, ACE2, to invade host cells and tissues. The ACE2 surface receptor is expressed in hematopoietic and lymphoid tissues [40]. Thus, SARS-CoV-2 and SARS-CoV binds to ACE2 on the surface of hematopoietic tissues and organs. CD13 is present on the surface of human BM CD34+ stem cells, platelets, MKs, and MK cell lines [4,41]. EACAMla (CD66a) is an adhesion molecule expressed on the surface of BM CD34+ cells, MK cell line M-07e, and platelets. SARS-CoV infects a small population of human MK progenitor cells and CD34+ cells [42]. Thus, CD13 and CD66a are potential receptors for the internalization of SARS-CoV-2 into CD34+ cells and MK cell lines followed by rapid viral replication and apoptosis (Fig. 1 ).
Fig. 1.
Hypothesis of the mechanisms employed by SARS-CoV-2 in attacking hematopoietic stem/progenitor cells [40,41,42,45,46].
Interactions between viruses and host cells result in the production of specific antibodies [43,44]. Autoimmune antibodies triggered by viral infection lead to specific cell death. Thus, SARS-CoV-2 infection may induce the production of autoantibodies and CD34+ cell-induced cell death. Thrombocytopenic patients infected with HIV-1 possess antibodies against platelet proteins that cross-react with the HIV-1 gp160/120 antigens and elevate the level of circulating immune complexes [45,46]. Platelets coated with antibodies or immune complexes are recognized by cells of the reticuloendothelial system and are targeted for destruction. Hematopoietic and other blood cells expressing similar antigens may also sustain immune injury when coated with anti-platelet antibodies and immune complexes (Fig. 1) [4]. Taken together, antibodies and/or immune complexes attack hematopoietic cells and suppress hematopoiesis, thereby reducing the production of platelets. Thus, SARS-CoV-2 may directly attack HSCs or hematopoietic progenitor cells. CoV particles may simultaneously damage the circulatory system by inducing the production of autoantibodies and immune complexes and reducing platelet production, thereby resulting in thrombocytopenia (Fig. 1).
7. MK differentiation and maturation
7.1. Abnormal BM microenvironment and decreased production of thrombopoietin (TPO)
TPO is the most potent stimulus for megakaryopoiesis either alone or in combination with other cytokines. TPO is synthesized and released by a variety of cells, including BM stromal cells and liver cells. Liver cells are the primary source for serum TPO [38,39]. Liver cells also express ACE2 on their cell surface. Thus, SARS-CoV-2 may bind to these ACE2 molecules and induce liver damage, thereby hampering TPO production, and eventually inhibiting the differentiation and maturation of MKs.
A dysfunctional local renin–angiotensin system results in abnormalities in the BM microenvironment [47,48]. SARS-CoV particles and inflammatory cytokines, such as IL-1β and TNFα, enhance ACE2 shedding from the cell surface. This reduces the functionality of ACE2, thereby resulting in the malfunction of the renin-angiotensin system, and increased inflammation [44]. Elevated levels of chemokines, inflammatory, growth, and anti-inflammatory factors may affect the hematopoietic microenvironment. Further, imbalances in the BM microenvironment may affect the function of endothelial and mesenchymal stem cells, thereby decreasing TPO production and MK differentiation and maturation, thereby resulting in thrombocytopenia.
7.2. Cellular immunity and cytokine storm
In addition to TPO, multiple cytokines, including IL-3, -6, -9, -1, and stem cell factor, stimulate MK production [49]. In contrast, Tumor growth factor-β (TGF-β), platelet factor 4, and interferon-α (IFN-α) inhibits MK production [50].
Patients with SARS show increased levels of TGF-β. Plasma from patients with active SARS showed an inhibitory effect on the formation of MK-colony forming units, which could be neutralized using anti-TGF-β antibodies, suggesting that virus-induced TGF-β-mediated cytokine storm inhibited megakaryocytopoiesis, thereby resulting in thrombocytopenia [42,51].
IFN-α is an important antiviral cytokine produced by virus-infected cells that inhibit MK production [43]. IFN-α suppresses the expression of transcription factors that regulate megakaryopoiesis. IFN-α directly inhibits TPO-mediated MK growth by inducing the suppressor of cytokine signaling 1 in vitro. Ultrastructural studies have shown that IFN-α inhibits MK maturation [52,53]. Collectively, these observations indicate that IFN-α positively influences thrombocytopenia.
7.3. Lung damage
In addition to the BM, MKs and hematopoietic progenitors have been detected in the blood vessels outside the lung tissue. During thrombocytopenia or a reduction in BM-derived HSCs, hematopoietic progenitors migrate from the lung to the BM, regenerate different blood cells, and supplement the number of platelets. MKs outside pulmonary vessels are smaller than those inside; further, and MKs inside the pulmonary vessels alone release platelets [54].
SARS-CoV-2 infection is associated with ACE2-induced lung injury. This can be attributed to multiple mechanisms. When the virus attacks the lungs, viral replication ensues in the invaded cells that results in the apoptosis of epithelial and endothelial cells and vascular leakage, thereby triggering the release of high concentrations of pro-inflammatory cytokines and chemokines [44]. The lungs of patients with SARS exhibit diffuse alveolar damage with pulmonary congestion, edema, formation of hyaline membrane, and fibrosis [4,11]. Majority of the hospitalized COVID-19 patients have pneumonia [1,2,20]. Chest computed tomography scans reveal ground-glass opacity and bilateral patchy shadowing in patients with COVID-19; however, these patients do not exhibit pulmonary fibrosis as prominently as patients with SARS [55].
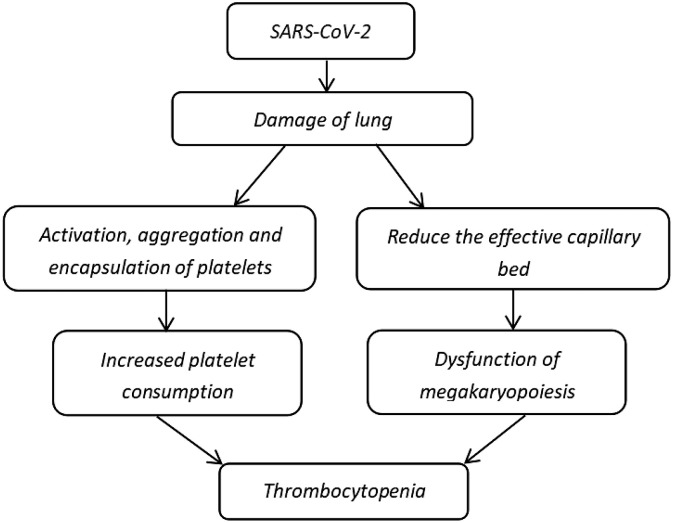
Extensive alveolar damage reduces the availability of effective capillary beds in the lung and affects MK fragmentation and production of platelets for pulmonary microcirculation, thereby leading to thrombocytopenia (Fig. 2 ) [56,57].
Fig. 2.
Possible mechanisms of thrombocytopenia caused by lung damage [54,56,57].
7.4. Increased platelet consumption and drug-induced thrombocytopenia
The attack and replication of viruses trigger apoptosis, vascular leakage, and release of pro-inflammatory cytokines and chemokines in the lungs [44]. This damage to the lung tissue and pulmonary endothelial cells results in the activation, aggregation, and encapsulation of platelets in the lung and increases platelet consumption or thrombogenesis that leads to thrombocytopenia.
The treatment regimen for COVID-19 includes antiviral drugs, such as ribavirin and fluoroquinolones, that inhibit hematopoietic function [58,59]; however, they also cause liver damage and affect the production of TPO, thus indirectly causing thrombocytopenia.
8. Discussion
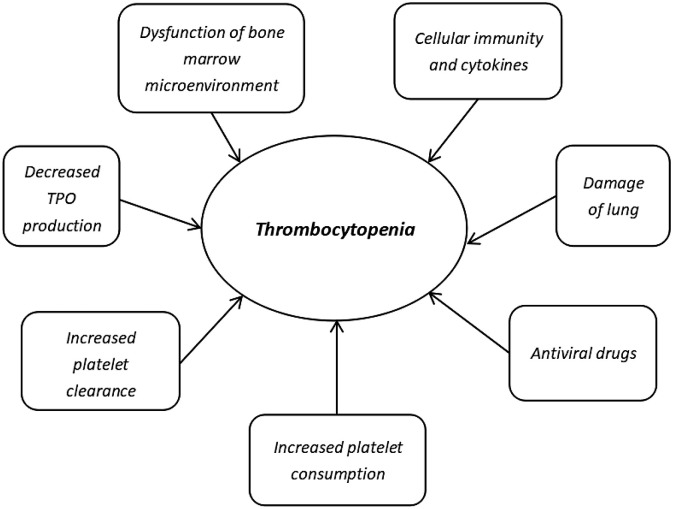
Thrombocytopenia is one of the most common symptoms in patients with COVID-19. The pathophysiological processes include direct attack of hematopoietic stem/progenitor cells and damage to the lungs by autoantibodies and immune complexes by coronavirus. Decreased TPO production, increased platelet clearance and platelet consumption, dysfunctional BM microenvironment, lung damage, and antiviral drugs may lead to the development of thrombocytopenic in patients with COVID-19 (Fig. 3 ). These complex processes can be attributed to DIC and multiple organ dysfunction syndrome that lead to the death of severe patients.
Fig. 3.
Possible indirect mechanisms involved in the development of thrombocytopenia [[42], [43], [44],[56], [57], [58], [59]].
Most patients with mild to moderate disease have favorable outcomes upon treatment, while severe or critical patients have poor outcomes combined with a high mortality rate. Early diagnosis and treatment of severe patients with COVID-19 can greatly reduce mortality. However, there is no laboratory test index to predict disease progression and prognosis. Combined with the aforementioned mechanisms, a progressive decline in platelet counts may be a prognostic factor for cytokine storm, myelosuppression, and platelet destruction induced by an uncontrolled immune response in patients with COVID-19. Therefore, monitoring platelet count may serve as a simple and effective index for disease progression. Further studies on larger patient cohorts are warranted to confirm this hypothesis. The possible mechanisms discussed here have following implications: First, targeted treatment can be designed based on the extent of thrombocytopenia. Glycyrrhizic acid and MSCs have immunoregulatory and anti-inflammatory effects [60,61] that help reduce the probability of cytokine storms. Moreover, glycyrrhizic acid has antiviral effects, and MSCs promote tissue repair and maintain homeostasis in BM microenvironments [62,63]. Second, damage to hematopoietic stem/progenitor cells can be treated using Astragalus polysaccharide to promote the recovery of hematopoietic function [64]. Combining Astragalus polysaccharides with rhTPO or TPORA may serve as a promising regimen to treat hematopoietic cell damage and reduce TPO production.
Funding source
This work was supported by the Natural Science Foundation of Guangdong, Education and Research of Guangdong Province (2017A030313767 [to J.Y.Y.]) and National Natural Science Foundation of China (81770116 [to M.Y.]).
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing and the language editor Yasmin.
Contributor Information
Jieyu Ye, Email: jieyu.ye@gmail.com.
Mo Yang, Email: yangm1091@126.com.
References
- 1.Guan W.J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang M. Hematological findings in SARS patients and possible mechanisms (review) Int. J. Mol. Med. 2004;14(2):311–315. [PubMed] [Google Scholar]
- 5.Al-Tawfiq J.A. Hematologic, hepatic, and renal function changes in hospitalized patients with Middle East respiratory syndrome coronavirus. Int. J. Lab. Hematol. 2017;39(3):272–278. doi: 10.1111/ijlh.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Q. Inferring the hosts of coronavirus using dual statistical models based on nucleotide composition. Sci. Rep. 2015;5 doi: 10.1038/srep17155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu A. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Z. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):E59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayawardena N. Virus–receptor interactions: Structural insights for oncolytic virus development. Oncolytic Virother. 2019;8:39–56. doi: 10.2147/OV.S218494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge X.Y. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiris J.S. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C.J. Identification of a novel protein 3a from severe acute respiratory syndrome coronavirus. FEBS Lett. 2004;565(1-3):111–116. doi: 10.1016/j.febslet.2004.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomura R. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004;78(16):8701–8708. doi: 10.1128/JVI.78.16.8701-8708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999;9(2):67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 15.Wong R.S. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326(7403):1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu R., Ling Y., Zhang Y.H., Wei L.Y., Chen X., Li X.M. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasparyan A.Y., Ayvazyan L., Mukanova U., Yessirkepov M., Kitas G.D. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann. Lab. Med. 2019;39:345–357. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020 doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang N. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mei H., Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19. Zhonghua Xue Ye Xue Za Zhi. 2020;41(0):E002. doi: 10.3760/cma.j.issn.0253-2727.2020.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Sun W., Guo Y., Chen L., Zhang L., Zhao S. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets. 2020;16:1–7. doi: 10.1080/09537104.2020.1754383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J.H., Hou W., Jin Q., Yang Y.D., Yang Y.H. Clinical characteristics and treatment of thrombocytopenia patients with severe infection. Zhonghua Yi Yuan Gan Ran Xue Za Zhi. 2019;29(11):1638–1642. doi: 10.11816/cn.ni.2019-181270. [DOI] [Google Scholar]
- 28.Leung C.H., Tseng H.K., Wang W.S., Chiang H.T., Wu A.Y., Liu C.P. Clinical characteristics of children and adults hospitalized for influenza virus infection. J. Microbiol. Immunol. Infect. 2014;47(6):518–525. doi: 10.1016/j.jmii.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Prina E., Ferrer M., Ranzani O.T., Polverino E., Cilloniz C., Moreno E. Thrombocytosis is a marker of poor outcome in community-acquired pneumonia. Chest. 2013;143(3):767–775. doi: 10.1378/chest.12-1235. [DOI] [PubMed] [Google Scholar]
- 30.Levi M., Opal S.M. Coagulation abnormalities in critically ill patients. Crit. Care. 2006;10(4):222. doi: 10.1186/cc4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin M.J., Kim Y., Choi E.M., Shim Y.J., Kim H.S., Suh J.K. Clinical characteristics and treatment courses for cytomegalovirus-associated thrombocytopenia in immunocompetent children after neonatal period. Blood Res. 2018;53(2):110–116. doi: 10.5045/br.2018.53.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celikel E., Tezer H., Kanik-Yuksek S., Gulhan B., Ozkaya-Parlakay A., Yarali N. Evaluation of 98 immunocompetent children with cytomegalovirus infection: importance of neurodevelopmental follow-up. Eur. J. Pediatr. 2015;174(8):1101–1107. doi: 10.1007/s00431-015-2513-9. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui W.A., Al S.I., Jha A. Early clinical manifestations and laboratory findings before and after treatment of cytomegalovirus infection in kidney transplant patients. Saudi J. Kidney Dis. Transpl. 2017;28(4):774–781. [PubMed] [Google Scholar]
- 34.RL Carter. Platelet levels in infectious mononucleosis. Blood. 1965;25:817–821. [PubMed] [Google Scholar]
- 35.Walter R.B., Hong T.C., Bachli E.B. Life-threatening thrombocytopenia associated with acute Epstein-Barr virus infection in an older adult. Ann. Hematol. 2002;81(11):672–675. doi: 10.1007/s00277-002-0557-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhou M., Qi J., Li X., Zhang Z., Yao Y., Wu D. The proportion of patients with thrombocytopenia in three human-susceptible coronavirus infections: a systematic review and meta-analysis. Br. J. Haematol. 2020;189(3):438–441. doi: 10.1111/bjh.16655. [DOI] [PubMed] [Google Scholar]
- 37.Drissen R. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat. Immunol. 2016;17(6):666–676. doi: 10.1038/ni.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111(3):981–986. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karagiannis P., Eto K. Manipulating megakaryocytes to manufacture platelets ex vivo. J. Thromb. Haemost. 2015;13:S47–S53. doi: 10.1111/jth.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan C. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection. medRxiv. 2020 doi: 10.1101/2020.02.12.20022418. 2020.02.12.20022418. [DOI] [Google Scholar]
- 41.Jeffers S.A. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. U. S. A. 2004;101(44):15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang M. Thrombopoietin levels increased in patients with severe acute respiratory syndrome. Thromb. Res. 2008;122(4):473–477. doi: 10.1016/j.thromres.2007.12.021. doi:10.1016/j.thromres.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020 doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 44.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scaradavou A. HIV-related thrombocytopenia. Blood Rev. 2002;16(1):73–76. doi: 10.1054/blre.2001.0188. [DOI] [PubMed] [Google Scholar]
- 46.Nardi M. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell. 2001;106(5):551–561. doi: 10.1016/s0092-8674(01)00477-9. [DOI] [PubMed] [Google Scholar]
- 47.Strawn W.B. Renin-angiotensin system expression in rat bone marrow haematopoietic and stromal cells. Br. J. Haematol. 2004;126(1):120–126. doi: 10.1111/j.1365-2141.2004.04998.x. [DOI] [PubMed] [Google Scholar]
- 48.Haznedaroglu I.C., Malkan U.Y. Local bone marrow renin-angiotensin system in the genesis of leukemia and other malignancies. Eur. Rev. Med. Pharmacol. Sci. 2016;20(19):4089–4111. [PubMed] [Google Scholar]
- 49.Sugimoto N., Eto K. Platelet production from induced pluripotent stem cells. J. Thromb. Haemost. 2017;15(9):1717–1727. doi: 10.1111/jth.13736. [DOI] [PubMed] [Google Scholar]
- 50.Behrens K., Alexander W.S. Cytokine control of megakaryopoiesis. Growth Factors. 2018;36(3-4):89–103. doi: 10.1080/08977194.2018.1498487. [DOI] [PubMed] [Google Scholar]
- 51.Yang M., Ng M.H., Li C.K. Thrombocytopenia in patients with severe acute respiratory syndrome (review) Hematology. 2005;10(2):101–105. doi: 10.1080/10245330400026170. [DOI] [PubMed] [Google Scholar]
- 52.Yamane A. Interferon-alpha 2b-induced thrombocytopenia is caused by inhibition of platelet production but not proliferation and endomitosis in human megakaryocytes. Blood. 2008;112(3):542–550. doi: 10.1182/blood-2007-12-125906. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q., Fox N.E., Kaushansky K. Interferon-alpha directly inhibits thrombopoietin-induced megakaryocyte proliferation and differentiation. Zhonghua Xue Ye Xue Za Zhi. 2001;22(6):296–299. [PubMed] [Google Scholar]
- 54.Lefrançais E. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi H. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30086-4. S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niinikoski J. Effect of oxygen-induced lung damage on tissue oxygen supply. Acta Chir. Scand. 1973;139(7):591–595. [PubMed] [Google Scholar]
- 57.Martin J.F., Slater D.N., Trowbridge E.A. Abnormal intrapulmonary platelet production: a possible cause of vascular and lung disease. Lancet. 1983;1(8328):793–796. doi: 10.1016/s0140-6736(83)91851-2. [DOI] [PubMed] [Google Scholar]
- 58.Peyrin-Biroulet L. Interaction of ribavirin with azathioprine metabolism potentially induces myelosuppression. Aliment. Pharmacol. Ther. 2008;28(8):984–993. doi: 10.1111/j.1365-2036.2008.03812.x. [DOI] [PubMed] [Google Scholar]
- 59.De Silva E., Kim H. Drug-induced thrombocytopenia: focus on platelet apoptosis. Chem. Biol. Interact. 2018;284:1–11. doi: 10.1016/j.cbi.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 60.Akutagawa K., Fujita T., Ouhara K., Takemura T., Tari M., Kajiya M. Glycyrrhizic acid suppresses inflammation and reduces the increased glucose levels induced by the combination of Porphyromonas gulae and ligature placement in diabetic model mice. Int. Immunopharmacol. 2019;68:30–38. doi: 10.1016/j.intimp.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 61.Chen S., Cui G., Peng C., Lavin M.F., Sun X., Zhang E. Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res Ther. 2018;9(1) doi: 10.1186/s13287-018-0846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun Z.G., Zhao T.T., Lu N., Yang Y.A., Zhu H.L. Research progress of glycyrrhizic acid on antiviral activity. Mini-Rev. Med. Chem. 2019;19(10):826–832. doi: 10.2174/1389557519666190119111125. [DOI] [PubMed] [Google Scholar]
- 63.Xu C., Fu F., Li X., Zhang S. Mesenchymal stem cells maintain the microenvironment of central nervous system by regulating the polarization of macrophages/microglia after traumatic brain injury. Int. J. Neurosci. 2017;127(12):1124–1135. doi: 10.1080/00207454.2017.1325884. [DOI] [PubMed] [Google Scholar]
- 64.Li Q., Xing W., Gong X., Wang Y., Sun H. Astragalus polysaccharide promotes proliferation and osteogenic differentiation of bone mesenchymal stem cells by down-regulation of microRNA-152. Biomed. Pharmacother. 2019;115:108927. doi: 10.1016/j.biopha.2019.108927. [DOI] [PubMed] [Google Scholar]