Abstract
In malaria, the evidence concerning the nucleotide-binding, oligomerization domain (NOD) 2 (NOD2) receptor is fragmented and the stimuli that might activate NOD2 are not well characterized. We investigated the role of NOD2 in vitro in the response of macrophages to Plasmodium falciparum products. Immortalized or primary bone marrow derived macrophages from wild type C57Bl/6 mice, or knockout mice for NOD2 or its adaptor proteins, were either primed with interferon gamma or left untreated, and stimulated with parasite products. Both lysates of infected erythrocytes or hemozoin induced higher levels of nitric oxide in primed than in unprimed wild type macrophages. When stimulated with hemozoin, primed macrophages knockout for NOD2, or for its adaptor proteins, produced significantly lower nitric oxide levels compared to wild type cells. Differently from hemozoin, the use of β-hematin (synthetic hemozoin) as stimulus showed that NOD2 is dispensable. Furthermore, the production of inflammatory cytokines by wild type cells treated with hemozoin was not dependent on NOD2. These data indicate that parasite components present in the hemozoin, differently from β-hematin, induce the production of nitric oxide through the activation of NOD2, whereas the production of inflammatory cytokines, like TNF-α or MIP-2 (CXCL2), seems to be NOD2 independent.
Keywords: Plasmodium falciparum, natural hemozoin, synthetic hemozoin (β-hematin), Nitric oxide, Cytokines, NOD2
1. Introduction
Malaria remains one of the world’s most important infectious diseases. In 2013, the WHO estimated that 207 million individuals were infected with malaria and that 627,000 people died, mostly children [1]. There are five species of Plasmodium that infect humans: P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. However, the most deadly species is P. falciparum (Pf), which is responsible for the most severe forms of the disease. Severe Pf infections are characterized by high parasitemia, sequestration of infected red blood cells (iRBC) in the deep vasculature and excessive host inflammatory responses. The life cycle of Pf comprises two main stages: an asymptomatic liver phase and the blood phase which causes the typical symptoms of malaria. During the blood stage, the parasites undergo a series of developmental changes that typically last 48 hours. At the end of the cycle, the late stage parasitized erythrocytes, called schizonts, burst and release merozoites, which infect new red blood cells and maintain the disease in the host. At schizogony, parasite products and toxins are released into the blood stream and interact with different host tissues and cells, triggering a strong inflammatory response, and thus contributing to the paroxysm of malaria [2]. Increasing evidence suggests that parasite products activate the host innate immune system through defined pattern recognition receptors (PRRs). For example, the protein anchor glycosylphosphatidylinositol (GPI) has been associated mainly with the activation of toll-like receptor (TLR)-2 and, to a lesser extent, of TLR4 [3]. Another toxin which has also been shown to interact with PRRs is hemozoin (HZ) which can be isolated from in vitro cultures (natural HZ, nHZ) or obtained by chemical synthesis (β-hematin, βH, from hemin) [4]. This brownish crystal is formed inside the digestive food vacuole of the parasite as a detoxification product of hemoglobin [5]. However, while HZ is non-toxic for the parasite, its presence in host tissues has been associated with both activation, and inhibition of the host inflammatory responses [6–8]. Both events can be differentially involved in the pathogenesis of the severe forms of malaria, such as anemia and cerebral malaria [9, 10]. The production of inflammatory mediators, such as macrophage inflammatory proteins, MIP-1 alpha, MIP-1 beta, nitric oxide (NO) and matrix metalloproteinase (MMP)-9, has been demonstrated upon interaction of nHZ with host immune cells [10, 11], whereas a deleterious effects of nHZ on bone marrow macrophage has been correlated with the dyserythropoiesis observed in severe malaria anemia [9].
It has been previously reported that parasite products associated with it, such as Pf DNA, are able to activate innate immune receptors [12]. We have shown that plasmodial DNA activates the endolysosomal TLR-9 within the phagolysosome [13]. Similar reports have shown that the lysates of merozoites contain a DNA-protein complex which can activate TLR9 in the endosome [14]. It has also been proposed that serum components, such as fibrinogen, bind to nHZ, thus activating TLR4 [15]. Moreover, nHZ is able to generate potent peroxidation and hydroxylation products from polyunsaturated fatty acids by non-enzymatic haem catalysis [8].
A very important role of nHZ appears to be the activation of a NLR (Nucleotide-binding Oligomerization Domain (NOD)-like receptor family member, the NOD-like Receptor containing Pyrin domain 3 (NLRP3), an essential element of the inflammasome complex. nHZ has properties that are similar to other crystals like silica and triggers the NLRP3 in primed cells [16, 17]. In addition, following the phagocytosis of nHZ or iRBC, nHZ is capable of destabilizing the phagolysosome, thus allowing ingested DNA and other parasite products access to the cytosol, where nucleic acid receptors, including AIM2 (Absent in melanoma 2), are activated [13]. Other members of the NLRs family, the NOD1 and NOD2 proteins, can sense peptidoglycans (PGNs) of bacterial cell walls, and have been associated with chronic inflammatory disorders related to bacterial infections such as Crohn’s disease [18], and Blau syndrome [19], but also to viral [20], as well as to protozoal infections, such as Toxoplasma [21]. Sharma et al. reported that CARD15/NOD2, the gene that encodes the NOD2 protein, was up-regulated in malaria patients [22]. Using the murine model of P. berghei ANKA, NOD1 and NOD2 have been shown to modulate the inflammatory responses although they seem not to influence parasitemia or survival, but only the extent of the inflammation [23]. NOD1 and NOD2 activation seems thus to be involved in the pathogenesis of the disease more than outcome. However, which parasite proteins or parasite-derived products trigger this activity during the blood stage of the infection, is presently not clear.
It has been reported that muramyl dipeptide (MDP) synergizes with TLR ligands to activate NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) and MAPK (mitogen-activated protein kinases) [24], and after priming with mouse interferon gamma (IFN-γ) induces macrophages to release NO through a mechanism that involves NOD2 activation [25]. Interestingly, earlier reports indicated that nHZ and IFN-γ increases NO production in macrophages through MAPK and NFκB dependent pathways [26], but the exact upstream mechanisms leading to these activities have not been elucidated, yet. On the basis of these observations, we asked the question of whether malarial nHZ could contribute to the inflammation in malaria via the activation of NOD2. Our study focused on the production of NO by IFN-γ primed macrophages treated with nHZ or synthetic βH or lysates of iRBC. Moreover, the involvement of RIP2 and CARD9, two adaptor proteins of NOD2, was also investigated. Finally, the production of cytokines and chemokines related to NOD2 activation by macrophages stimulated with nHZ was evaluated. The availability of immortalized bone marrow derived macrophages from C57BL/6 mice (BMDMs) and from knockout (KO) mice represented an important tool to discriminate NOD2 related effects.
2. Materials and Methods
2.1. Reagents
DMEM high glucose, fetal bovine serum (FBS, EU-Superior Quality), RPMI 1640, HEPES, L-glutamine, and bioluminiscent MycoAlert® kit were obtained from Euroclone. Albumax II (lipid-rich bovine serum albumin), sodium bicarbonate, gentamicin, zymosan, and Alexa Fluor® 488 goat anti-rabbit antibody were obtained from Life Technologies. Hypoxanthine, glucose, ciprofloxacin, mouse recombinant IFN-γ, hemin porcine, Giemsa’s solution (methylene blue, eosin, azure B), E-Toxate test (Limulus amebocyte lysate), Griess reagents (sulphanilamide, napthtylenthylenediamine dihydrochloride, phosphoric acid [H3PO4]), formalin solution 10%, and fluoroshield with DAPI were purchased from Sigma-Aldrich. MDP was obtained from Vinci-Biochem. Ultra-pure lipopolysaccharide (LPS) and apigenin (4’,5,7-trihydroxyflavone) were obtained from Alexis Biochemicals. Cytokine Array Panel A was purchased from R&D Systems. NOD2 polyclonal antibody was obtained from Cayman Chemicals. Goat Serum (Normal) was purchased from Dako. Chemiluminescent detection reagents were obtained from Bio-Rad. Blood from healthy donors was obtained from AVIS Comunale Milano.
Porcine hemin (98%) for βH preparation was purchased from Fluka® and used without purification. All other chemical reagents used in the synthesis of βH were purchased from Sigma Aldrich® (Vorna Valley, South Africa). Double distilled deionised Millipore® Direct-Q water was used to prepare all aqueous solutions. The pH was determined using a Crison MicropH 2000 pH meter.
2.2. Cell culture
Immortalized BMDMs from C57Bl/6 mice wild type (WT), NOD2-KO, RIP2-KO, or CARD9-KO were generated as described elsewhere [13, 17] in the laboratories of Drs. Douglas Golenbock and Kate Fitzgerald (University of Massachusetts, Worcester, USA). Cells were maintained in DMEM, supplemented with 10% (v/v) FBS, 25 mM HEPES, 4 mM L-glutamine, and 10 μg/ml of ciprofloxacin. For qRT-PCR experiment, primary BMDMs-WT and -NOD2-KO cells were cultured at 37°C in 5% CO2 in DMEM containing L-glutamine (CellGro, Herndon, VA) with 10% (v/v) FBS (HyClone Laboratories; Logan, UT), and 20% L929 supernatants to generate BMDMs.
2.3. Parasite culture
Pf strain 3D7 was maintained in vitro as previously described with slight modifications [27], in ARh+ red blood cells at 1–2% hematocrit in media containing RPMI 1640, supplemented with 0.75% (w/v) Albumax II, 25 mM HEPES, 27 mM sodium bicarbonate, 2 mM L-glutamine, 0.37 mM hypoxanthine, 11 mM glucose, and 0.087 mM gentamicin. Parasites were grown under a standard gas mixture of 1% O2, 5% CO2, and 94% N2 and maintained at 37°C. Parasitemia was assessed by Giemsa staining, and cultures were diluted regularly with fresh erythrocytes and warmed media several times per week. The culture was checked routinely for Mycoplasma contamination through bioluminescence kit detection.
2.4. Malarial HZ and parasite preparation
2.4.1. nHZ preparation
nHZ was prepared following the protocol described by Parroche and colleagues with some modifications [12]. The malarial pigment was isolated using an LS column (MACS Miltenyi Biotec, USA) from the malaria cultures when the parasitemia reached >5% at the stage of young trophozoites (rings). The culture hematocrit was diluted to 0.5%−1% using frozen supernatants collected when the malaria cultures were split during continuous in vitro growth, in order to retain the pigment released during schizogony. The column was equilibrated with PBS, and the cultures were passed through. The retained nHZ was thoroughly washed, and eluted in 4 ml PBS. The crystals were then pelleted, concentrated, and frozen in PBS at −20°C. Preparations of nHZ from different stocks were thawed, pooled together, and finely dispersed with a 1 ml syringe. Finally, an aliquot was diluted in 1M NaOH to determine the heme content by spectrophotometry (λ=405 nm). The concentration of heme was calculated by comparison to a standard curve of hemin that was analyzed the same way. The doses of HZ used to stimulate PBMC were calculated based on the iron content of trophozoites reported previously [28]. Knowing that a trophozoite contains about 519 fg of hemozoin heme and assuming a hematocrit of 42%, approximately 50 μM nHZ would be released in vivo in patients with at 1% parasitemia. This is the highest dose of nHZ used in this study. The preparations were checked for endotoxin contamination by the Limulus amebocyte lysate test (E-toxate kit, sensitivity of 0.05–0.1 EU per ml); all preparations were endotoxin free.
2.4.2. Lysate preparations of infected red blood cells
Whole infected erythrocytes (Wh-iRBCs) were prepared from Pf in vitro cultures with 5% parasitemia (late stage trophozoites). The culture was diluted to 0.5%−1% hematocrit with warm media and passed twice through an LS column to completely deplete the nHZ present free in the medium. A LD column (MACS Miltenyi Biotec, USA) was subsequently used to separate infected erythrocytes containing late trophozoites or schizonts. After elution from the column, parasites were centrifuged at 1500 rpm for 5 minutes; the supernatant discarded and fresh warm malaria culture media was added. Parasite enrichment was checked by Giemsa staining and 90–95% parasitemia was routinely obtained. The number of Wh-iRBCs per ml was counted with a Neubauer chamber. An aliquot of this preparation was used to prepare the lysates of infected erythrocytes (Ly-iRBCs). Briefly, the aliquot was frozen and thawed several times, and placed in different tubes in order to get the final ratios of 1:1, 3:1, 4:1 or 6:1 of iRBCs with respect to macrophages. Thus, the number of iRBCs per well was 1×105, 3×105, 4×105, or 6×105 according to the experiments performed. The ratios used in these experiments were chosen based on clinical and experimental data published previously [29] and fall within the range that could be observed in malaria patients. In fact, ratios of iRBC : monocytes from 1:1 to 6:1 could be easily reached in vivo, in plasma of malaria patients with 0.1% parasitemia.
2.4.3. Production of synthetic hemozoin, beta-hematin-
βH was prepared at a pentanol-aqueous interface according to a method, adapted from earlier publications [30, 31]. Citrate buffer solutions (20 mM, 50 ml × 6, pH 4.80) were prepared from citric acid and a NaOH slurry. Pentanol (15 ml × 6) and the buffer solutions were then transferred to several Schott-Duran crystallisation dishes of 9 cm internal diameter. The two immiscible liquids formed a distinct interface and were allowed to incubate at 37°C in a thermostated water-bath for 60 min. Hemin solid was first dissolved in 0.1 M NaOH by sonication and then mixed with acetone and MeOH (1: 9 v/v) in a 6:4 v/v ratio to make the 2 mg/ml heme stock solution. Fe(III)PPIX or heme stock solution (1.5 ml) was then gently syringed on top of the pentanol layer in each dish. The higher density heme solution settled at the interface between the pentanol and aqueous buffer solutions almost immediately following addition. The crystallisation reaction was allowed to proceed at 37°C for 6 h. The reactions were quenched with water and the contents of the crystallisation dish were filtered by vacuum filtration using Whatman® filter disks (0.22 μm). The resulting crystals were washed with 1 ml of a solution of 5% pyridine, 40% acetone, 20% HEPES buffer (0.02 M) and water (v/v) to bind and solubilise unreacted heme by formation of a previously reported bis-pyridyl complex. The remaining dark brown solid was dried over 48 h in a desiccator over P4O10. ATR-FT-IR (attenuated total reflection) infrared spectroscopy was used to confirm the formation of βH on a Bruker Platinum ATR mounted on a Bruker Tensor 27 FT-IR Spectrometer. Confirmation of diagnostic βH stretching frequencies of the Fe (III) centre of one heme molecule coordinated C-O of the carboxylate moiety of an adjacent heme molecule at 1207 and 1660 cm−1 respectively were confirmed.
2.5. Nitric oxide (NO) determination
2.5.1. Stimulation of cells
Immortalized mouse BMDMs-WT, -NOD2-KO, -RIP2-KO, or -CARD9-KO cells were seeded overnight at 37°C in 5% CO2 in 96-well plates placing 1×105 cells per well in 100 μl. On the next day, where indicated, macrophages were pre-treated for 1 h with the iNOS (inducible Nitric Oxide synthase) inhibitor apigenin, a natural flavonoid. Cells were primed with IFN-γ (12.5 U/ml, final concentration) for 2 hours and then left untreated (medium) or stimulated with nHZ, βH, parasite preparations (Wh-iRBCs, Ly-iRBCs) or their controls (Wh-uRBCs or Ly-uRBCs), MDP, or LPS, resulting in a final volume of 200 μl, and incubated for 24 or 48 h.
2.5.2. Nitric oxide (NO) Assay
At the end of incubation, the production of NO was evaluated through the Griess reagents (1% [w/v] sulphanilamide, 0.1% [w/v] napthtylenthylene diamine dihydrochloride, and 2.5% [v/v] H3PO4). One hundred microliters of fresh supernatant were mixed with an equal volume of Griess reagent. After 10 minutes incubation at room temperature, the nitrite accumulated was measured in a spectrophotometer (Synergy 4 microplate reader, Biotek, Germany) at 540 nm. The levels of NO were determined using a nitrite (NaNO2) standard curve.
2.6. Cytokine production
2.6.1. Stimulation of mouse cells and cytokine array experiments
To measure cytokine production we used the Proteome Profiler Mouse Cytokine Array Panel A Array Kit (R&D Systems Inc.), which consists of individual nitrocellulose membranes each containing 40 capture antibodies spotted in duplicates and simultaneously profiles the relative levels of multiple cytokines in the samples. Briefly, immortalized BMDMs-WT were seeded overnight at 37°C in 5% CO2 in 6-well plates (1×106 cells/well) in a final volume of 2 ml per well. Then, cells were primed with IFN-γ (12.5 U/ml) for 2 hours, then left untreated (medium alone) or treated with nHZ, and incubated for further 24 h. Then, cell supernatants were collected, centrifuged, and then mixed with a cocktail of biotinylated detection antibodies, and subjected to the cytokine array. The streptavidin-HRP and chemiluminescent detection reagents were added sequentially, and the secreted cytokines were detected. The films were scanned (Camag Reprostar 3) and the spot area was determined using densitometric analysis software.
2.7. Quantitative Real Time-PCR experiment
Primary BMDM from WT and NOD2-KO mice were stimulated for 2 or 4 h with 50 μM nHZ and 5 μg/mL MDP (InvivoGen; San Diego, CA). The RNA from WT and NOD2-KO cells were extracted with RNeasy kit (Qiagen; Valencia, CA) according to the manufacturer’s instructions. The cDNA was synthesized with iScript cDNA Synthesis Kit (BioRad; Hercules, CA), and quantitative RT-PCR analysis was performed with the iQ SYBR Green Supermix (Biorad; Hercules, CA) on a CFX96 Real-Time PCR detection System (BioRad; Hercules, CA) using primers designed from PrimerBank [32]. The specificity of amplification was assessed for each sample by melting curve analysis. Relative quantification was performed using standard curve analysis and the expression data were presented as a mean of normalized expression to the Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) level using qGene [33] of three biological replications.
Primers used in the qRT-PCR experiments:
| Primer name | Sequence (5’-> 3’) | PrimerBank ID |
|---|---|---|
| TNFα Fwd | CCCTCACACTCAGATCATCTTCT | 7305585a1 |
| TNFα Rev | GCTACGACGTGGGCTACAG | |
| CXCL10 Fwd | CCAAGTGCTGCCGTCATTTTC | 10946576a1 |
| CXCL10 Rev | GGCTCGCAGGGATGATTTCAA | |
| CXCL2 Fwd | CCAACCACCAGGCTACAGG | 6677885a1 |
| CXCL2 Rev | GCGTCACACTCAAGCTCTG | |
| CXCL1 Fwd | CTGGGATTCACCTCAAGAACATC | 6680109a1 |
| CXCL1 Rev | CAGGGTCAAGGCAAGCCTC | |
| CCL3 Fwd | TTCTCTGTACCATGACACTCTGC | 6755432a1 |
| CCL3 Rev | CGTGGAATCTTCCGGCTGTAG | |
| CCL4 Fwd | TTCCTGCTGTTTCTCTTACACCT | 7305459a1 |
| CCL4 Rev | CTGTCTGCCTCTTTTGGTCAG | |
| A20 Fwd | GAACAGCGATCAGGCCAGG | 31543880a1 |
| A20 Rev | GGACAGTTGGGTGTCTCACATT | |
| IL1RA Fwd | GCTCATTGCTGGGTACTTACAA | 13624317a1 |
| IL1RA Rev | CCAGACTTGGCACAAGACAGG | |
| GAPDH Fwd | AGGTCGGTGTGAACGGATTTG | 6679937a1 |
| GAPDH Rev | TGTAGACCATGTAGTTGAGGTCA |
2.8. Immunofluorescence for NOD2
2.8.1. Cell stimulation and Immunostaining procedure
Immortalized BMDMs were plated at a concentration of 2.5×104 cells per well on sterile 12 millimeter glass coverslips previously coated with poly-L-lysine. Cells were pre-treated with IFN-γ for 2 h, stimulated with medium, nHZ (30 μM), or MDP (2.5 μg/ml), and incubated for 6 h. Upon incubation, supernatants were discarded, and cells were washed with PBS and fixed in buffered formalin solution (Sigma) for 15 minutes. Cells were washed and treated with methanol for 10 minutes, washed again and treated with 0.1% bovine serum albumin (BSA) in PBS for 5 minutes while gently shaking. Then, NOD2 polyclonal antibody (diluted 1:400 in PBS containing 1% BSA and 3% goat serum) was added, incubated an additional hour at room temperature, and cells were washed. Finally, Alexa Fluor® 488 goat anti-rabbit antibody (diluted 1:1000 in PBS plus 1% BSA and 3% goat serum) was added and incubated for 30 min in the dark. At the end of the incubation, the cells were washed and the supernatants removed. Finally, a drop of Fluoroshield with DAPI was placed on a glass slide. Coverslips containing cells were placed over the mounting medium and examined by fluorescence microscopy (Nikon Eclipse Ti Series). Images were captured by using a digital camera (Nikon Digital Sight) and by keeping constant microscope settings (laser power, exposure times). Captured images were subsequently processed using the Nis-Elements D3.2 software.
2.9. Statistical analyses
Differences between groups were analyzed for statistical significance by using Student’s t-test. P<0.01 or P<0.05 were considered as statistically significant.
3. Results
3.1. Infected erythrocyte lysates or nHZ induce iNOS dependent release of NO in macrophages primed with IFN-γ
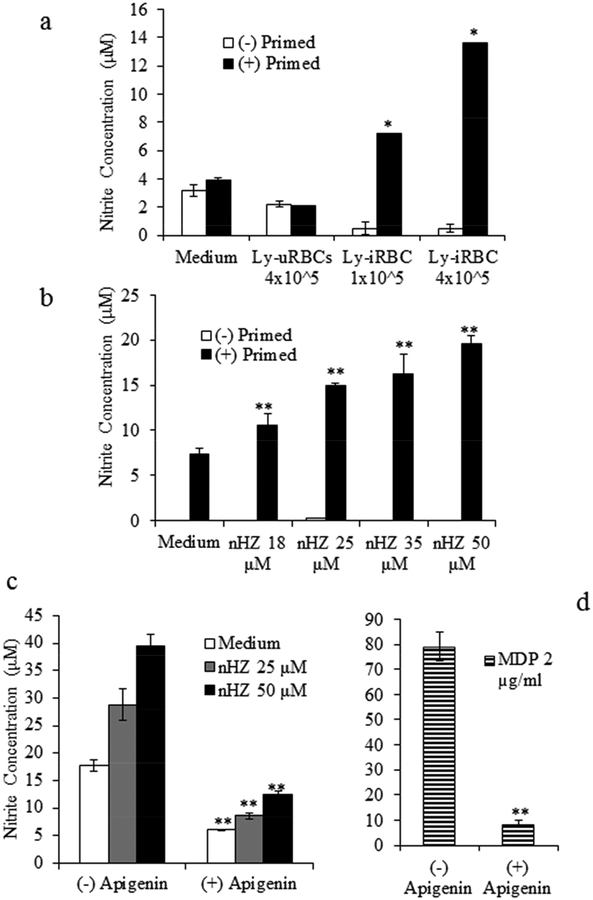
The production of NO upon stimulation of IFN-γ-primed immortalized BMDMs-WT with Ly-iRBCs or nHZ was investigated. The doses of lysates corresponded to 1×105 or 4×105 iRBCs per well. The ratio of macrophages to non-infected erythrocytes was 1:4, and the ratios of macrophages to infected RBC was 1:1 and 1:4, respectively. After 24 h of stimulation with Ly-iRBCs, a dose-dependent production of NO was observed only in the IFN-γ-primed BMDMs-WT, but not in the absence of IFN-γ-priming (Fig. 1a). The production of NO by IFN-γ-primed BMDMs was 3x or 7x higher using 1×105 or 4×105 Ly-iRBCs, respectively, compared to the medium controls, or macrophages stimulated with the lysate of uninfected RBCs (Ly-uRBCs) (Fig.1a). Subsequently, BMDMs-WT cells were treated with different doses of nHZ in the presence or absence of IFN-γ-priming. In the absence of IFN-γ, BMDMs-WT did not produce any NO upon incubation with nHZ (Fig. 1b). In contrast, when macrophages were primed with IFN-γ, the stimulation with nHZ resulted in a dose-dependent release of NO into the culture supernatant. A three-fold increase over the control at the highest nHZ concentration (50 μM) was seen (Fig. 1b). In the presence of apigenin, an iNOS inhibitor, the levels of NO were significantly reduced compared to control cells treated with nHZ alone (Fig. 1c). This observation was consistent at all of the nHZ concentrations tested. In the same manner, apigenin decreased the levels of NO induced by stimulation with MDP (Fig. 1d), indicating that iNOS is involved in the induction of NO by nHZ.
Fig. 1. Lysates of infected erythrocytes or natural hemozoin induced release of NO in macrophages primed with IFN-γ, and was dependent on iNOS.
Immortalized BMDMs were pre-treated with medium alone, (−) primed (white bars), or with 12.5 U/ml IFN-γ, (+) primed (black bars). After 1–2 h, macrophages were stimulated with: (a) Medium, Ly-uRBCs (4×105 per well), or Ly-iRBCs (1×105 or 4×105 per well) for 24h and, (b) Medium or nHZ at different heme concentrations (18, 25, 35, or 50 μM). To test the involvement of iNOS, macrophages were incubated for 1 h with medium alone, (−) apigenin, or in the presence of the iNOS inhibitor, (+) apigenin, prior to priming, and then stimulated with: medium, or nHZ (25 or 50 μM) (c), or MDP (2 μg/ml) (d) alone or in the presence of apigenin (40 μM). The production of nitrite, as an indication of NO levels, was assessed by Griess reagents in the culture supernatants. Results are reported as a mean of triplicate determinations ± S.D. (error bars). The graph is representative of one experiment out of four independent tests. Significant differences *, p < 0.05 or **, p < 0.01 are indicated.
3.2. The release of nitric oxide induced by lysates of P. falciparum infected erythrocytes or nHZ, but no synthetic hemozoin, in IFN-γ primed macrophages is NOD2 dependent
We next stimulated IFN-γ-primed macrophages from WT and NOD2-KO mice with Ly-iRBCs for 48 h. A dose-dependent production of NO was observed in the WT cells, whereas the release of NO in controls (medium and Ly-uRBCs) was negligible (Fig. 2a). Remarkably, NO production elicited by Ly-iRBCs was significantly lower in IFN-γ-primed macrophages from the NOD2-KO mice (Fig. 2a). Similarly, the response to Wh-iRBC was also dependent on NOD2 (data not shown). As expected, the release of NO induced by MDP in the NOD2-KO cells was significantly reduced compared to WT, while the amount of NO induced by LPS was equivalent in both WT and NOD2-KO BMDMs (Fig. 2b). The stimulation of WT macrophages by nHZ induced a dose-dependent release of NO which was completely abolished in the NOD2-KO macrophages (Fig. 2c). Different results were obtained when βH was used to stimulate WT and NOD2-KO BMDMs. βH is a crystalline material made of Fe(III) protoporphyrin IX which lacks the parasite-derived components usually associated with nHZ (Pf DNA, RNA, proteins, or lipids). As depicted in Fig. 2d, when WT BMDMs were stimulated with synthetic βH, the amount of NO released into the supernatants was lower (approximately half) compared with that induced by nHZ at equivalent heme content indicating that βH is less potent than nHZ in stimulating WT BMDMs. However, the levels of NO elicited in the NOD2-KO BMDMs were not significantly different from WT macrophages, suggesting that differently from nHZ, the stimulation by βH is NOD2 independent. Taken together, these results indicate that NO production induced by Ly-iRBCs or nHZ in IFN-γ-primed macrophages involves NOD2 expression, and that components of nHZ other than the crystal itself are involved in the activation of NOD2.
Fig. 2. Lysates of infected erythrocytes and natural hemozoin induced NO production in primed macrophages in a NOD2 dependent manner.
Immortalized BMDMs-WT or -NOD2-KO were pre-treated with 12.5 U/ml IFN-γ for 1–2 h, and then stimulated with: (a) Medium, Ly-uRBCs (6×105 per well), or Ly-iRBCs (1×105, 3×105, or 6×105 per well); (b) Medium, MDP (1 or 2 μg/ml), or LPS (25 or 50 ng/ml); (c) Medium or nHZ (17.8, 25, 35, or 50 μM); or (d) Medium, nHZ (15 or 30 μM), or βH (15 or 30 μM). Forty-eight hours later, production of nitrite was assessed by Griess reagents in the culture supernatants. Results are reported as a mean of triplicate determinations ± S.D. (error bars). Significant differences (*, p < 0.05 or **, p < 0.01) are indicated.
In order to determine if NOD2 protein expression was enhanced upon stimulation with nHZ, immunostaining using indirect immunofluorescence and antibody against NOD2 was performed. The results show a clear NOD2-specific fluorescent signal in both MDP (NOD2 ligand) and nHZ treated cells, compared to non-specific background fluorescence present in cells treated with medium only, thus confirming induction of NOD2 receptor protein expression by nHZ (Fig. 3).
Fig. 3. Natural hemozoin qualitatively enhances expression of NOD2.
An indirect immunofluorescence staining was applied in immortalized BMDMs primed with IFN-γ for 1–2 h and treated for 24 h with Medium, MDP (2.5 μg/ml), or nHZ (30 μM). Cells were probed with NOD2 polyclonal antibody and Alexa Fluor® 488 goat anti-rabbit antibody (FITC) (see methodology section). The figures are one representative experiment out of three independent tests.
3.3. Expression of the NOD2 adaptors, RIP2 and CARD9, is obligatory for NO production induced by nHZ
RIP2 (CARD3) and CARD9 are two adaptors that are activated through CARD domains upon ligand recognition and activation of NOD2. We found that NO production in IFN-γ-primed immortalized BMDMs-RIP2-KO or CARD9-KO upon nHZ stimulation was completely abolished compared to BMDMs-WT at all the concentrations tested (Fig. 4a). Similar results were seen when preparations of Wh-iRBCs or Ly-iRBCs were used as stimuli (data not shown) or when the production of NO was elicited by MDP (Fig. 4b). On the other hand, when BMDMs-RIP2-KO or CARD9-KO cells were stimulated with LPS, a robust response was seen. These data suggest that RIP2 and CARD9 are involved in the signaling triggered by nHZ for the secretion of NO in IFN-γ-primed macrophages.
Fig. 4. NO production in primed macrophages stimulated with natural hemozoin is dependent on two NOD2 adaptors, RIP2 and CARD9.
Immortalized BMDMs-WT, -RIP2-KO, or -CARD9-KO, were pre-treated with 12.5 U/ml IFN-γ for 1–2 h, and then stimulated with: (a) Medium, or nHZ (10, 25, and 50 μM); and (b), MDP (2 μg/ml) or LPS (25 ng/ml). Forty-eight hours later, production of nitrite was assessed by Griess reagents in the culture supernatants. Results are reported as a mean of triplicate determinations ± S.D. (error bars). The graphs are representative of one experiment out of three independent experiments. Significant differences (* p < 0.05 or ** p < 0.01) are indicated.
3.4. The inflammatory response by hemozoin stimulated macrophages is NOD2 independent
As shown in Fig. 5, the stimulatory activity of nHZ (25 μM) was confirmed measuring the production of cytokines and chemokines by immortalized BMDMs-WT cells, in IFN-γ-primed or unprimed cells. The results are expressed as a ratio between the levels of cytokines produced by nHZ-stimulated cells compared to untreated cells. Among the cytokines analyzed, TNF-α and MIP-2 (CXCL2) showed the highest stimulatory index, especially in unprimed compared to primed cells. The stimulation of RANTES (CCL5), MIP-1β (CCL4), IL1-Ra (IL-1F3) and IP-10 (CXCL10) was lower than that of TNF or MIP2, but still higher in unprimed than primed cells, suggesting a negative modulatory activity of IFN-γ for the secretion of these markers. On the contrary, the stimulation of MIP-1α and JE appeared slightly increased in primed vs unprimed cells. Knowing that some of these cytokines are related to NOD2 activation [34], we investigated the role of NOD2 by qRT-PCR. The experiments were done using primary, unprimed, BMDMs-WT or BMDMs from NOD-2 KO mice stimulated with nHZ or MDP for 2 or 4 hours (Fig. 6). The data confirmed the observations made with the protein array shown in Fig. 5. We observed a significant up-regulation of mRNA of TNF-α, A20 (TNFAIP3), IL1-RA, CXCL1 (KC), CXCL2 (MIP-2), CCL3 (MIP-1α), CCL4 (MIP-1β), and CXCL10 (IP-10) in both primary BMDMs-WT and NOD2-KO cells. The differences between these two groups did not reach statistical significance, implying that NOD2 is not involved in the stimulation of cytokine production by nHZ. On the contrary, MDP induced the mRNA expression of TNF-α, CXCL1, CXCL2 and CXCL10 in a NOD2 dependent way, as expected.
Fig. 5. Natural hemozoin stimulated differentially in primed or unprimed cells with IFN-γ.
Immortalized BMDMs-WT were primed or not with 12.5 U/ml IFN-γ for 2 h, and then stimulated with medium alone or with nHZ (25 μM) for 24 h. Cytokine and chemokine production were detected in duplicates in the supernatants through an array for cytokines (R&D). Values represent the fold increase of each protein detected in the sample stimulated with nHZ respect to their non-stimulated control (medium alone). Ratios around 1 were considered as a basal production of cytokines by macrophages. The values are representative of one out of two independent experiments.
Fig. 6. Natural hemozoin up-regulated cytokine mRNA levels in macrophages in a NOD2 independent manner.
Primary BMDMs-WT, or -NOD2-KO were stimulated for 2 and 4 hours with 50 μM nHZ and 5 μg/ml MDP. The mRNA levels for (a) TNF-α, (b) A20 (TNFAIP3), (c) IL1RA, (d) CXCL1 (KC), (e) CXCL2 (MIP-2), (f) CCL3 (MIP1-a), (g) CCL4 (MIP-1b), and (h) CXCL10 (IP-10) were detected by real-time quantitative PCR and normalized to GAPDH level of expression. Data are expressed as means ± SEM and are representative of three experiments. Significance is represented relative to the medium of WT and NOD2-KO as follows: *0.05>p>0.01, **0.01>p>0.001, ***0.001>p>0.0001, ****p<0.0001.
4. Discussion
Malaria is the protozoan disease that has the greatest historical impact upon human beings. Plasmodium parasites have a complex life cycle, which may account for the correspondingly intricate immune responses. Immunity to malaria is present, but typically, it takes years to develop, it requires constant re-exposure to be maintained and does not last long upon leaving an endemic area. In this view, it is not surprising that attempts at developing a vaccine have only been partially successful. Although most cases of malaria develop a mild disease, the failure to properly treat it can result in the rapid development of life-threatening or even fatal complications, especially in children below 5 years of age or pregnant women [1]. Therefore, a better understanding of the innate and adaptive immune responses to malaria may help in developing new tools and new prophylactic interventions.
The data presented here shed light on the mechanism used by macrophages to release NO when triggered by a parasite-derived molecule such as nHZ. Nitric oxide is an important mediator of resistance to parasitic infection and, along with cytokines and chemokines, it is part of the inflammatory response induced by malaria parasites or their products [35]. NO has been shown to influence malaria pathogenesis and outcome in different ways and controversial data have been published. Several reports claim a beneficial effect of NO in controlling parasitemia in uncomplicated malaria cases [36], but elevated NO levels might cause oxidant damage to red blood cells that leads to anemia in severe malaria [37]. Alternatively, it has been claimed that NO has anti-inflammatory effects able to prevent cerebral malaria and there is epidemiological evidence that NO plasma levels are decreased in cerebral malaria [38]. More recently, the data on the genetic polymorphisms of the NOS2 promoter and malaria severity confirm the complexity of the in vivo situation and may help explaining the controversy of the data [39]. HZ has been reported to induce iNOS transcripts and NO in blood mononuclear cells of children with severe malaria anemia [7]. The exact signaling events that lead to NO production by HZ have not been clarified, yet.
Data in the literature suggest that the innate immune responses to malaria are driven either by molecules present on the surface of whole iRBCs or by their toxic products released into the circulation after schizont rupture. These products can trigger PRRs, such as the membrane-bound TLR2, TLR4 and TLR9 [3, 12, 15], or members of the intracellular NLR family such as NLRP3 [13, 16]. The cognate receptor, NOD2, and its adaptor CARD9, have been shown to be involved in the cytokine response which characterizes malaria infections in the murine model of P. berghei ANKA, although no direct effects on peripheral parasitemia or survival were seen [23, 40], indicating that these proteins are involved in the pathogenesis, but they are not critical for the outcome of the disease. In the other hand, CAR15 (NOD2), the gene that encodes NOD2, has been shown to be up-regulated during febrile malaria in human patients [22]. In our model, NOD2 expression seemed to be unnecessary for the production of cytokines or chemokines, after stimulation of IFN-γ primed macrophages with nHZ. This is different from the data reported in the P. berghei ANKA model [23], described above. The reasons for these discrepancies are not known, but it is always difficult to reconcile in vivo and in vitro data, especially when different parasites species are used. We tried to overcome the limitation of the in vitro system by adjusting the doses of nHZ and of the lysate of iRBC, as well as the ratio of macrophages to iRBCs to be as close as possible to the situation occurring in vivo in malaria patients. If we consider that one trophozoite contains approximately 47 fg of iron per cell [28], which means about 519 fg of hemozoin heme per trophozoites, and assuming a hematocrit of 42%, a parasitemia of 1% would be sufficient to release in vivo the same amount of hemozoin used in this study (50 μM at the highest dose). The ratios of macrophages to infected RBCs were chosen based on data published elsewhere [24] and are in the range of what could be observed in vivo, since with a parasitemia of 0.1%, ratios from 1:1 to 1:6 iRBC to monocytes could be easily obtained in the blood. Therefore, our in vitro conditions can be considered representative of certain in vivo situations. However, it is likely that at the site of parasite sequestration, the local concentration of iRBC may be much higher and in a different relationship with the number of monocytes.
At variance with what was observed with the inflammatory cytokines, the production of NO by IFN-γ primed macrophages follows a different pathway since its stimulation by nHZ clearly involved NOD2, as well as RIP2 or CARD9 activation, two adaptor proteins downstream from NOD2 [41] that signal through NFkB and MAPK, respectively. The results were different when primed macrophages were stimulated with βH, the synthetic crystals made of Fe(III) protoporphyrin IX. In the latter case, the production of NO was lower than that induced by nHZ, but it was not dependent on NOD2. This observation raises the possibility that other components present in the preparation of nHZ (such as DNA, proteins or lipids), but not the Fe(III)PPIX structure, interact directly or indirectly with NOD2 for NO production. At present, the exact contribution of contaminating DNA, proteins or lipids on the activation of NOD2 dependent NO production, has not been investigated. Based on the previous data of our group, it appears that after phagocytosis of nHZ by macrophages, the DNA of Pf which is bound to nHZ, dissociates from the crystals and promotes a destabilization of the phagolysosome allowing the content to access the cytosol, where DNA receptors become activated [13]. It is plausible that a similar process could take place in the case of NOD2 stimulation by nHZ.
Our findings tend to exclude the contribution of proteins since preliminary data using nHZ treated with proteases did not modify the results (data not shown). The involvement of lipids co-purified with nHZ cannot be completely ruled out at present. It is known that nHZ heme is able to generate large amounts of biologically potent peroxidation and hydroxylation products from polyunsaturated fatty acids (PUFA) by non-enzymatic heme catalysis [8] and the biological activity of nHZ has been related to the lipids associated with the heme moiety [42]. We have previously shown that natural, but not lipid-free, hemozoin enhances the production of MMPs in human microvascular endothelial cells [43]. Hydroxy-PUFA (HETEs, HODEs) and the terminal aldehyde 4-hydroxynonenal (4-HNE) are responsible for several nHZ effects on phagocytes like inhibition of erythropoiesis, dendritic cells maturation, or up-regulation of several cytokines and chemokines [8, 42]. Additional studies are in progress to clarify the role of lipids in the production of NO.
Taken together these results are consistent with current data that inflammation during malaria occurs through TLR signalling [3, 44] and confirm that nHZ is an immunologically active parasite product through which the NOD2 pathway can trigger NO production. βH is also immunologically active, as shown earlier. It activates NO production in a NOD2 independent way and it has been shown to stimulate the NOD-like Receptor containing Pyrin domain 3 (NLRP3) inflammasome, resulting in the production of IL-1β and IL-18 [16, 45].
Evidence on the literature show that NOD1 and NOD2 are involved principally in the pathogenesis of malaria disease, more than termination. A similar conclusion is reported for some bacteria infections. In Legionella pneumophila infections, the NOD1/NOD2 signaling is not critical for host resistance, but both NOD1 and NOD2 participate in the recognition of these bacteria in vivo [46].
For bacteria as Coxiella burnetii and Brucella abortus, NOD1 and NOD2 play no role in restricting bacterial replication in murine models of infection [47, 48] although they seems to regulate the production of cytokines. In general, the roles of NOD1 and NOD2 in the induction and resolution of inflammatory processes are largely unknown and albeit NOD2 seems to have a marginal role for the outcome of malaria, knowing its involvement in the production of NO, may allow the use of new pharmacological interventions where modulation of NO production is required.
Acknowledgments
This work was supported by an NIH grant: NIH-NIAID R01AI079293 (DTG, KF, RG, YC, BR), and PRIN 2013.
We thank Paola Misiano for helpful discussion and review of the manuscript. We thank Paola Verducci and Tiziana Bianchi from AVIS Comunale Milano for coordinate blood supply for parasite culture.
The UCT group would like to acknowledge Colyn Bourhill for his contributions as a vacation student and the National Research Foundation (NRF) and Medical Research Council of South Africa for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO WHO. World malaria report; 2013. [Google Scholar]
- 2.White NJ, Pukrittayakamee S, Hien TT, et al. Malaria. Lancet 2014;383:723–735. [DOI] [PubMed] [Google Scholar]
- 3.Krishnegowda G, Hajjar AM, Zhu J, et al. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem 2005;280:8606–8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagola S, Stephens PW, Bohle DS, et al. The structure of malaria pigment beta-haematin. Nature 2000;404:307–310. [DOI] [PubMed] [Google Scholar]
- 5.Egan TJ. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J Inorg Biochem 2008;102:1288–1299. [DOI] [PubMed] [Google Scholar]
- 6.Polimeni M, Valente E, Aldieri E, et al. Haemozoin induces early cytokine-mediated lysozyme release from human monocytes through p38 MAPK- and NF-kappaB-dependent mechanisms. PLoS One 2012;7:e39497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller CC, Kremsner PG, Hittner JB, et al. Elevated nitric oxide production in children with malarial anemia: hemozoin-induced nitric oxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect Immun 2004;72:4868–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarzer E, Kuhn H, Valente E, et al. Malaria-parasitized erythrocytes and hemozoin nonenzymatically generate large amounts of hydroxy fatty acids that inhibit monocyte functions. Blood 2003;101:722–728. [DOI] [PubMed] [Google Scholar]
- 9.Skorokhod OA, Caione L, Marrocco T, et al. Inhibition of erythropoiesis in malaria anemia: role of hemozoin and hemozoin-generated 4-hydroxynonenal. Blood 2010;116:4328–4337. [DOI] [PubMed] [Google Scholar]
- 10.Prato M, D’Alessandro S, Van den Steen PE, et al. Natural haemozoin modulates matrix metalloproteinases and induces morphological changes in human microvascular endothelium. Cellular Microbiology 2011;13:1275–1285. [DOI] [PubMed] [Google Scholar]
- 11.Perkins DJ, Were T, Davenport GC, et al. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci 2011;7:1427–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A 2007;104:1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalantari P, Deoliveira RB, Chan J, et al. Dual Engagement of the NLRP3 and AIM2 Inflammasomes by Plasmodium-Derived Hemozoin and DNA during Malaria. Cell Rep 2014;6:196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Gowda NM, Kumar S, et al. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J Immunol 2010;184:4338–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrera V, Skorokhod OA, Baci D, et al. Host fibrinogen stably bound to hemozoin rapidly activates monocytes via TLR-4 and CD11b/CD18-integrin: a new paradigm of hemozoin action. Blood 2011;117:5674–5682. [DOI] [PubMed] [Google Scholar]
- 16.Shio MT, Tiemi Shio M, Eisenbarth SC, et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog 2009;5:e1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008;9:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 19.Rosé CD, Doyle TM, McIlvain-Simpson G, et al. Blau syndrome mutation of CARD15/NOD2 in sporadic early onset granulomatous arthritis. J Rheumatol 2005;32:373–375. [PubMed] [Google Scholar]
- 20.Sabbah A, Chang TH, Harnack R, et al. Activation of innate immune antiviral responses by Nod2. Nat Immunol 2009;10:1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw MH, Reimer T, Sánchez-Valdepeñas C, et al. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol 2009;10:1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S, DeOliveira RB, Kalantari P, et al. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity 2011;35:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finney CA, Lu Z, LeBourhis L, et al. Disruption of Nod-like receptors alters inflammatory response to infection but does not confer protection in experimental cerebral malaria. Am J Trop Med Hyg 2009;80:718–722. [PubMed] [Google Scholar]
- 24.Scott MJ, Chen C, Sun Q, et al. Hepatocytes express functional NOD1 and NOD2 receptors: a role for NOD1 in hepatocyte CC and CXC chemokine production. J Hepatol 2010;53:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tötemeyer S, Sheppard M, Lloyd A, et al. IFN-gamma enhances production of nitric oxide from macrophages via a mechanism that depends on nucleotide oligomerization domain-2. J Immunol 2006;176:4804–4810. [DOI] [PubMed] [Google Scholar]
- 26.Jaramillo M, Gowda DC, Radzioch D, et al. Hemozoin increases IFN-gamma-inducible macrophage nitric oxide generation through extracellular signal-regulated kinase- and NF-kappa B-dependent pathways. J Immunol 2003;171:4243–4253. [DOI] [PubMed] [Google Scholar]
- 27.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976;193:673–675. [DOI] [PubMed] [Google Scholar]
- 28.Egan TJ. Physico-chemical aspects of hemozoin (malaria pigment) structure and formation. J Inorg Biochem 2002;91:19–26. [DOI] [PubMed] [Google Scholar]
- 29.Artavanis-Tsakonas K, Eleme K, McQueen KL, et al. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol 2003;171:5396–5405. [DOI] [PubMed] [Google Scholar]
- 30.Egan TJ, Chen JY, de Villiers KA, et al. Haemozoin (beta-haematin) biomineralization occurs by self-assembly near the lipid/water interface. FEBS Lett 2006;580:5105–5110. [DOI] [PubMed] [Google Scholar]
- 31.Ambele MA, Sewell BT, Cummings FR, et al. Synthetic Hemozoin (β-Hematin) Crystals Nucleate at the Surface of Neutral Lipid Droplets that Control Their Sizes. Cryst Growth Des 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Spandidos A, Wang H, et al. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res 2012;40:D1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon P Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 2003;19:1439–1440. [DOI] [PubMed] [Google Scholar]
- 34.Carneiro LA, Magalhaes JG, Tattoli I, et al. Nod-like proteins in inflammation and disease. J Pathol 2008;214:136–148. [DOI] [PubMed] [Google Scholar]
- 35.Riley EM, Wahl S, Perkins DJ, et al. Regulating immunity to malaria. Parasite Immunol 2006;28:35–49. [DOI] [PubMed] [Google Scholar]
- 36.Perkins DJ, Kremsner PG, Schmid D, et al. Blood mononuclear cell nitric oxide production and plasma cytokine levels in healthy gabonese children with prior mild or severe malaria. Infect Immun 1999;67:4977–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobolewski P, Gramaglia I, Frangos J, et al. Nitric oxide bioavailability in malaria. Trends Parasitol 2005;21:415–422. [DOI] [PubMed] [Google Scholar]
- 38.Hobbs MR, Udhayakumar V, Levesque MC, et al. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet 2002;360:1468–1475. [DOI] [PubMed] [Google Scholar]
- 39.Trovoada MeJ, Martins M, Ben Mansour R, et al. NOS2 Variants Reveal a Dual Genetic Control of Nitric Oxide Levels, Susceptibility to Plasmodium Infection, and Cerebral Malaria. Infect Immun 2014;82:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hafalla JC, Burgold J, Dorhoi A, et al. Experimental cerebral malaria develops independently of caspase recruitment domain-containing protein 9 signaling. Infect Immun 2012;80:1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi K, Inohara N, Hernandez LD, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 2002;416:194–199. [DOI] [PubMed] [Google Scholar]
- 42.Prato M, Gallo V, Arese P. Higher production of tumor necrosis factor alpha in hemozoin-fed human adherent monocytes is dependent on lipidic component of malarial pigment: new evidences on cytokine regulation in Plasmodium falciparum malaria. Asian Pac J Trop Med 2010;3:85–89. [Google Scholar]
- 43.Prato M, Gallo V, Giribaldi G, et al. Role of the NF-κB transcription pathway in the haemozoin- and 15-HETE-mediated activation of matrix metalloproteinase-9 in human adherent monocytes. Cell Microbiol 2010;12:1780–1791. [DOI] [PubMed] [Google Scholar]
- 44.Gowda DC. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol 2007;23:596–604. [DOI] [PubMed] [Google Scholar]
- 45.Dostert C, Guarda G, Romero JF, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One 2009;4:e6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreira LO, Zamboni DS. NOD1 and NOD2 Signaling in Infection and Inflammation. Front Immunol 2012;3:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benoit M, Bechah Y, Capo C, et al. Role of the cytoplasmic pattern recognition receptor Nod2 in Coxiella burnetii infection. Clin Microbiol Infect 2009;15 Suppl 2:154–155. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira FS, Carvalho NB, Zamboni DS, et al. Nucleotide-binding oligomerization domain-1 and −2 play no role in controlling Brucella abortus infection in mice. Clin Dev Immunol 2012;2012:861426. [DOI] [PMC free article] [PubMed] [Google Scholar]