Abstract
Precise control of plant stem cell proliferation is necessary for the continuous and reproducible development of plant organs1,2. The peptide ligand CLAVATA3 (CLV3) and its receptor CLV1 maintain stem cell homeostasis within a deeply conserved negative feedback circuit1,2. In Arabidopsis, CLV1 paralogs also contribute to homeostasis, by compensating for the loss of CLV1 through transcriptional upregulation3. Here we show that compensation4,5 operates in diverse lineages for both ligands and receptors, but while the core CLV signaling module is conserved, compensation mechanisms have diversified. Transcriptional compensation between ligand paralogs operates in tomato, facilitated by an ancient gene duplication that impacted the domestication of fruit size. In contrast, we found little evidence for transcriptional compensation between ligands in Arabidopsis and maize, and receptor compensation differs between tomato and Arabidopsis. Our findings show that compensation among ligand and receptor paralogs is critical for stem cell homeostasis, but that diverse genetic mechanisms buffer conserved developmental programs.
Keywords: Robustness, Buffering, Redundancy, Compensation, Stem Cells, Meristem, Tomato, Arabidopsis, Maize, CRISPR/Cas9
Editorial Summary
A study of a stem cell receptor-ligand signaling module across tomato, maize and Arabidopsis identifies different genetic mechanisms of compensation that contribute to homeostasis.
Plant development is driven by the replenishment of stem cells in growing apices known as meristems. In shoot meristems the receptor kinase CLV1 and its ligand CLV3 function in a negative feedback circuit that dampens stem cell proliferation by regulating expression of the stem cell promoting transcription factor WUSCHEL (WUS)1,2. This core CLV signaling module is deeply conserved. Mutations in orthologs in the distantly related plants Arabidopsis, maize, rice, and tomato all cause similar stem cell over-proliferation, resulting in meristem enlargement and excess organs1,2. Mutations that partially disrupt CLV signaling have been important in domestication, making the CLV-module an attractive crop improvement target1,6. However, both the CLV3-EMBRYO SURROUNDING REGION (CLE) ligands and their receptors are part of large gene families7, suggesting that CLV signaling and the phenotypic consequences arising from its perturbation could be influenced by widespread redundancy and compensation.
In Arabidopsis, stem cell homeostasis is mediated both through CLV-WUS negative feedback, and through genetic buffering by CLV1 paralogs3. The severity of the clv1 phenotype is buffered by the paralogous BARELY ANY MERISTEM (BAM) receptors through an “active compensation” mechanism3,4. In active compensation, genes change their behavior to compensate for genetic or environmental perturbation, like the loss of a paralog. In contrast, in passive compensation, paralogs do not change their behavior under perturbation, and are closer to being truly redundant4. Passive compensation between paralogs is often assumed, but active compensation between paralogous genes is widespread in yeast8,9. In the case of the Arabidopsis BAMs, their expression levels increase, and their expression domains change when CLV1 is compromised, compensating actively for CLV1 loss3. It is unclear whether there is similar active compensation between CLE ligands, or whether compensation mechanisms are as conserved as the core CLV-module4,7.
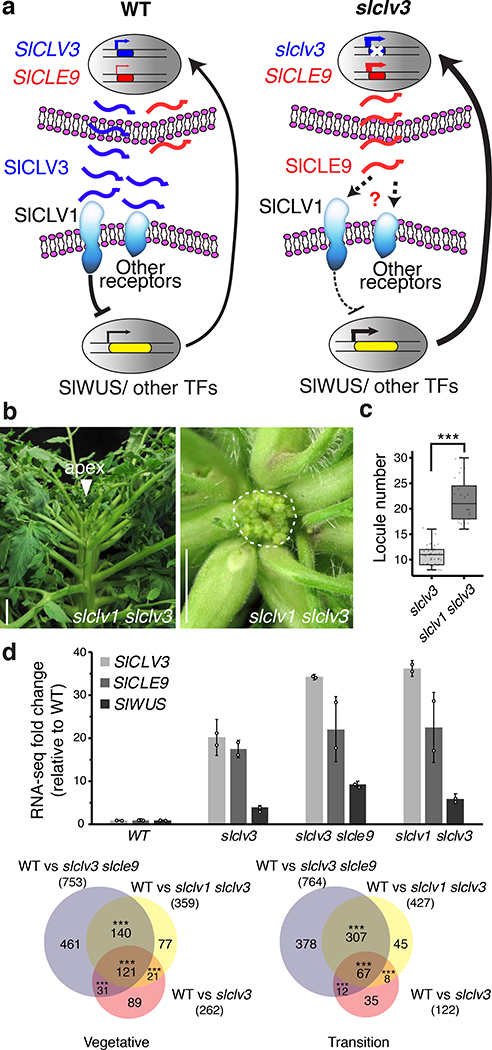
Our previous work suggested that there is active compensation between the tomato CLV3 ortholog, SlCLV3, and another CLE, SlCLE9. The stem-cell repressive activity of SlCLV3 requires arabinosylation of the mature dodecapeptide6. SlCLV3 expression increases 15-fold in arabinosyltransferase enzyme mutants, consistent with loss of stem cell homeostasis due to disrupted negative feedback. Interestingly, SlCLE9 expression also increases substantially6. SlCLE9 is the closest paralog of SlCLV3, and thus might be functionally similar to SlCLV3 (Fig. 1a)7. Therefore, SlCLE9 represented a good candidate for an active SlCLV3 compensator in tomato.
Fig. 1. Buffering of stem cell homeostasis in tomato depends on transcriptional compensation from SlCLE9.
a, Clustering of CLE proteins from Brassicaceae and Solanaceae. b, Wild type (WT) and slclv3 tomato inflorescences. Red arrowheads, branches. White arrowheads in insets, locule number. c, qRT-PCR in vegetative meristems of SlCLV3 and SlCLE9, normalized to SlUBIQUITIN (SlUBI). Mean ± SEM; two biological with three technical replicates (n = 30 meristem per replicate). d, Representative inflorescence of slcle9. Red arrowhead, branch. e, Quantification and distribution of locule number in WT, slclv3 and slcle9 (n = 105, 43 and 166). f, Side and top-down view of slclv3 slcle9. White arrowhead, Apex; white dotted circle, meristem. g, Primary meristems from WT, slclv3, slcle9, and slclv3 slcle9. h, Quantification of meristem width and height from WT single and higher-order mutants (n= 5, 17, 5, 12 and 7). Boxplots, 25th–75th percentile; whiskers, full data range; center line, median. One-way anova and Tukey test; letters represent significance groups at p < 0.05 in e,h. Scale bars, 1 cm in b, d, f; 100 μm in g.
To dissect the relationship between SlCLE9 and SlCLV3, we first phenotyped slclv3 homozygous null mutants generated by CRISPR/Cas9, and quantified expression of both genes in meristems. As expected, slclv3 mutants developed enlarged meristems, fasciated stems, and increased floral organ and fruit locule number (Fig. 1b)6. Notably, both SlCLV3 and SlCLE9 were upregulated more than 40-fold in slclv3 meristems, well beyond the meristem size increase (Fig. 1c). This suggested SlCLE9 repressed stem cell proliferation alongside SlCLV3. However, slcle9 null mutants resembled wild type plants, with a subtle effect on locule number (Fig. 1d, e, Supplementary Fig. 1a, Supplementary Table 1). We therefore generated slclv3 slcle9 double mutant plants, and they were dramatically more fasciated than slclv3 mutants, with thicker stems, more leaves, and a remarkably enlarged primary shoot meristem (Fig. 1f–h, Supplementary Table 2). Side shoots showed similar phenotypes, and developed severely fasciated flowers and fruits with twice as many locules as slclv3 mutants (Supplementary Fig. 1b–d). Notably, a third SlCLE homolog (SlCLE3) was upregulated threefold in slclv3 mutants (Supplementary Fig. 1c). However, null mutations in SlCLE3 did not further enhance slclv3 slcle9 double mutants, nor increase locule number in the slcle9 background (Supplementary Fig. 1, Supplementary Table 1). Thus, even though SlCLE3 is upregulated in slclv3 mutants, SlCLE3 shows no evidence of compensation. Thus, loss of SlCLV3 triggers an active compensation mechanism4, where upregulation of SlCLE9 buffers stem cell homeostasis in tomato.
The discovery of active CLE compensation in tomato prompted us to ask if similar mechanisms existed in other plants. Notably, CLV receptor and ligand mutant phenotypes in Arabidopsis suggested CLE compensation. Arabidopsis clv1 bam1/2/3 quadruple mutants, in which all CLV3 receptor function is lost, exhibit extreme meristem over-proliferation, well beyond clv3 mutants3. This phenotypic similarity to tomato slclv3 slcle9 double mutants suggested additional CLE genes could buffer stem cell homeostasis in Arabidopsis3,10. However, unlike tomato, there are no close CLV3 paralogs in Arabidopsis (Fig. 1a). Therefore, to identify putative CLE compensators, we selected 18 meristem-expressed CLE genes (Supplementary Fig. 2a, Supplementary Table 3, Methods), and measured their expression in wild type and clv3 inflorescence apices (Fig 2a). As in tomato, CLV3 expression rose dramatically (>100-fold) in clv3 mutants (Fig. 2a). However, none of the other CLE homologs increased more than two-fold. We confirmed these findings using transcriptome data, which identified no other upregulated CLEs (Supplementary Fig. 2b, Supplementary Table 4, Methods). Therefore, if any Arabidopsis CLEs buffer against clv3 disruption it is primarily through a passive compensation mechanism4, involving one or more CLE genes with little change in their expression.
Fig. 2. Arabidopsis stem cell homeostasis is controlled by multiple redundant CLEs.
a, qRT-PCR of CLV3 and 18 additional CLEs from inflorescence apices (dashed circles in pictures) of WT and clv3 mutants. Normalized to AtUBI. Mean ± SEM; two biological with three technical replicates (n = 40 per replicate). b and c, Representative stems and quantification of stem width from WT, clv3 and cle dodeca (n = 20, 20 and 20). d and e, Confocal micrographs of vegetative and inflorescence meristems and meristem size quantification from WT, clv3 and cle dodeca (n = 26, 30 and 29). f and g, Top-down view of inflorescences and flower numbers from WT, clv3, cle dodeca (n = 47, 104 and 173). h, quantification of locule number from WT, clv3, cle dodeca and clv1 bam1/2/3 (n = 149, 199, 199 and 26). Boxplots, 25th–75th percentile; whiskers, full data range; center line, median. One-way anova and Tukey test; letters represent significance groups at p < 0.05 in c, e, g and h. Scale bars, 300 μm in a; 1 cm in b; 50 μm and 100μm in d; 1 cm in f.
To test for passive CLE compensation in Arabidopsis, we took a multiplex CRISPR/Cas9 approach. None of the existing cle null mutants are fasciated or have increased locule number11,12, CLV3 has no close paralogs, and our transcriptomics yielded no clear compensator candidates (Fig. 1a, Fig. 2a, Supplementary Fig. 2b, Supplementary Table 4). This lack of clear candidates makes dissecting passive CLE compensation gene-by-gene challenging, requiring many mutant combinations, further complicated by linked CLE genes. We therefore used multiplex CRISPR/Cas9 to simultaneously mutate 11 Arabidopsis CLEs with known repressive activity in peptide assays13, in a clv3 mutant background. Notably, homozygous clv3 cle multi-gene mutants (hereafter referred to as cle dodeca, see Methods), in which 9 mutations disrupt the CLE peptide (Supplementary Fig. 2c), showed dramatic enhancement of stem fasciation, meristem size, and flower production over clv3 single mutants (Fig. 2b–g, Supplementary Table 2). However, this enhancement was not nearly as extreme as in clv1 bam1/2/3 quadruple receptor mutants, and locule number was only subtly affected. This suggests additional CLEs, beyond those targeted here, buffer stem cell homeostasis (Fig. 2h, Supplementary Fig 3a–b, Supplementary Table 1,2). Thus, unlike in tomato where a single CLE paralog compensates through active upregulation, many CLEs work together to compensate passively for the loss of CLV3 in Arabidopsis4.
We next examined the relative contributions of CLV1 versus BAM receptors to compensation in both Arabidopsis and tomato. We found that in quadruple clv1 bam1/2/3 receptor mutants’ locule number and vegetative meristem size were both considerably increased relative to both clv1 clv3 and clv3 bam1/2/3 quadruple mutants (Supplementary Fig. 3). This demonstrates that in Arabidopsis passive CLE compensation is mediated by shared CLV1 and BAM receptor function. In contrast, as tomato SlCLE9 and SlCLV3 are close paralogs, we hypothesized that active compensation might rely on SlCLV1 (Fig. 3a). We tested this by generating slclv1 slclv3 double mutants, where fasciation was dramatically enhanced, approaching the severity of slclv3 slcle9 mutants. This contrasts with Arabidopsis, where clv1 does not enhance clv3 (Fig. 3b, c, Supplementary Fig. 1d, 4b, d; Supplementary Table 1)14. We also generated slclv1 slcle9 double mutants, and found that slcle9 did not enhance slclv1 (Supplementary Fig. 4d, Supplementary Table 1). We then analyzed the transcriptome profiles from vegetative and transition meristems of wild type, slclv1 and slclv3 single mutants, and slclv1 slclv3 and slclv3 slcle9 double mutants, showing that SlCLV3, SlCLE9 and SlWUS were all upregulated to similar levels in both double mutants (Fig. 3d, Supplementary Table 5). These double mutants shared a significant overlap of differentially expressed genes (87.6% in transition meristems) compared to slclv3 and each other (Fig. 3e). Additionally, CRISPR/Cas9-generated null mutations in SlCLV2 (encoding the ortholog of the co-receptor CLV2) did not enhance slclv3, similar to Arabidopsis clv2 clv3 mutants (Supplementary Fig. 4)15. These analyses show that active SlCLE9 compensation in tomato acts primarily through SlCLV1, whereas passive CLE compensation in Arabidopsis requires multiple receptor paralogs.
Fig. 3. SlCLE9 compensation acts primarily through the receptor kinase SlCLV1.
a, A proposed model showing buffering of stem cell homeostasis by SlCLE9 acts primarily through SlCLV1 when SlCLV3 is compromised, achieving partial suppression of SlWUS through negative feedback. b, Side and top-down view of slclv1 slclv3 showing enlarged meristem flanked by multiple fasciated floral buds (dashed circle). c, Quantification of locule number from slclv3 (n = 43) and slclv1 slclv3 (n = 24). d, Fold change in expression of SlCLV3, SlCLE9 and SlWUS relative to wild-type RNA-seq from slclv1, slclv3 and slclv1 slclv3 and slcv3 slcle9 mutants (mean ± SD). Data are from 2 biological replicates (4-fold change, 1 cpm cutoff, FDR < 0.10). e, Venn diagrams of RNA-seq data of differentially-expressed genes (DEGs) comparing the indicated mutant genotypes for vegetative and transition stages of meristem maturation. Boxplots, 25th–75th percentile; whiskers, full data range; center line, median. ***P = 5×10−22, two-tailed, two-sample t-test in c. ***P < 0.001 of 1,000 simulations from random sampling in e. Scale bars, 2 cm in b.
Since CLE compensation is active in tomato and passive in Arabidopsis, we next asked if there were similar differences in CLV receptor signaling. In Arabidopsis, the BAM receptors are upregulated when CLV1 is compromised in an active compensation mechanism3. However, transcriptome profiling in tomato showed that none of the four BAM (SlBAM) homologs16 were dramatically upregulated in slclv1 or slclv3 slcle9 meristems, suggesting the lack of an active receptor compensation mechanism (Supplementary Fig. 4c). Nonetheless, we tested for active compensation genetically by disrupting the only SlBAM that was upregulated more than 1.5-fold in mutant meristems, SlBAM4 (Supplementary Fig 4c). We could not distinguish slbam4 single mutants from wild type, and locule number in slclv1 slbam4 double mutants was the same as in slclv1. Similarly, slbam1 single mutants and slbam1 slbam4 double mutants had wild type locule numbers, and locule number in slclv1 slbam1 slbam4 triple mutants remained indistinguishable from slclv1 (Supplementary Fig. 4, Supplementary Table 1). This contrasts with Arabidopsis, where locule number in clv1 mutants is enhanced step-wise by the bam mutants3,17. However, our results indicate some receptor compensation, as all available receptor mutant combinations show weaker fasciation than slclv3 slcle9 mutants (Supplementary Fig. 4d, Supplementary Table 1). Thus, additional receptors, potentially including SlBAMs, likely contribute to SlCLV3 and SlCLE9 signaling beyond SlCLV1, however their relative contributions appear to be different from the active compensation observed in Arabidopsis.
The elevated expression of SlCLE9 in slclv3 mutant meristems nearly matches SlCLV3 levels in wild type, however compensation only partially masks slclv3 stem cell homeostasis defects, suggesting that expression differences, barring possible differences in expression domains, are not responsible for the limited efficiency of SlCLE9 compensation (Supplementary Table 5). Peptide sequence differences likely limit compensation efficiency; four amino acid substitutions distinguish SlCLE9 and SlCLV3 dodecapeptides and synthetic SlCLE9 peptides are less potent than SlCLV36. To test this genetically, we expressed the SlCLE9 dodecapeptide in the context of the SlCLV3 gene. Whereas slclv3 mutants were nearly fully rescued when transformed with a genomic construct containing the SlCLV3 coding region with native upstream and downstream regulatory sequences (gSlCLV3SlCLV3), fasciation could not be complemented by replacing the SlCLV3 dodecapeptide with that of SlCLE9 (gSlCLV3SlCLE9) (Fig. 4a,b). Thus, active compensation efficiency is dampened by weaker activity of SlCLE9 peptide.
Fig. 4. Buffering impacted tomato domestication alongside a dynamic evolution of active compensation in flowering plants.
a, Representative inflorescences from slclv3 T0 plants gSlCLV3SlCLV3 and gSlCLV3SlCLE9. b, Quantification of locules from gSlCLV3SlCLV3 (n = 38) and gSlCLV3SlCLE9 (n = 28). Blue and red dashed lines, mean values WT and slclv3. c, qRT-PCR of SlCLE9 from reproductive meristems of WT and fas. Mean ± SEM; two biological and three technical replicates (n = 30 per replicate). d, Quantification and distribution of locule number in fas (n = 59) and fas slcle9 (n = 59). e, Synteny analysis of SlCLV3 and SlCLE9 chromosomal blocks. Red triangles, SlCLE9-like fragments with partial dodecapeptides. f, Clustering analysis of cluster 1D7 containing SlCLE9-like sequences. g, Gene models, gRNAs target sites (red arrows), primers (black arrows), and ZmCLE7 and ZmFCP1 CRISPR/Cas9 alleles. h, Representative ears and ear primordia from WT, Zmfcp1, Zmcle7 and Zmfcp1 Zmcle7. j, Fold-change of ZmCLE7, ZmFCP1, ZmWUS1 and ZmWUS2 relative to wild-type (blue line) in RNA-seq from Zmcle7 (mean ± SD; two biological replicates, 2-fold change, 5 cpm cutoff, FDR < 0.10). k and l, Representative micrographs and quantification of inflorescence transition meristem size from WT, Zmfcp1, Zmcle7 and Zmfcp1 Zmcle7 (n = 7, 15, 15 and 7). Boxplots, 25th–75th percentile; whiskers, full data range; center line, median. ***P = 1×10−21, **P = 0.005; two-tailed, two-sample t-test in b, d. One-way anova and Tukey test; letters, significance groups at p < 0.05 in l. Scale bars, 1 cm in a, h; 500 μm in j and 100 μm in k.
The tomato domestication mutation fasciated (fas) disrupts the promoter of SlCLV3, reducing expression and promoting a moderate increase in locule number6. We hypothesized that SlCLE9 compensation might mitigate the severity of this weaker natural slclv3 allele. Supporting this, SlCLE9 expression increased four-fold in fas meristems and still compensated, as locule number was higher in fas slcle9 double mutants compared to fas alone (Fig. 4c,d). However, this enhanced fasciation did not reach the severity of slclv3 single mutants, indicating the SlCLE9 compensation mechanism may scale with SlCLV3 dosage. Notably, fas was a major contributor to increasing fruit size during domestication6, and our results suggest the impact of fas on locule number might have been too extreme, were it not for active compensation by SlCLE9.
To investigate the origin of SlCLE9 compensation, we traced the syntenic blocks containing SlCLV3 and SlCLE9 through eudicot evolution. Both blocks were found in the order Solanales, throughout the Solanaceae family and extending to the Convolvulaceae, as represented by Ipomoea trifida, the progenitor of sweet potato (Fig. 4e). Outside the Solanales, only CLV3-like genes were found in syntenic blocks, indicating that SlCLE9 and SlCLV3 originated from a Solanales-specific duplication event, although we cannot exclude the possibility of two independent duplications, specific to the Solanaceae and Ipomoea18,19. Interestingly, following the emergence of SlCLE9, synteny within the SlCLV3 block degraded faster than within the SlCLE9 block20, leaving SlCLE9 as a clearer CLV3 syntenic ortholog than SlCLV3 (Fig. 4e). Once emerged, SlCLE9-like genes underwent a dynamic history of retention, duplication and loss, marked by two SlCLE9-like fragments in pepper, and independent losses of SlCLE9 from potato and eggplant (Fig. 4e, Supplementary Table 6). A broader CLE clustering supported the orthology of Solanales CLV3-like genes, and showed that the pepper SlCLE9-like sequences did not fall into any subcluster, supporting their identity as pseudogenes (Fig. 4f). Thus, SlCLE9-like genes likely emerged more than 30 million years ago, prior to Solanaceae diversification. Critically, active compensation mediated by SlCLE9 could only have arisen after SlCLE9 emerged, and is specific to tomato and, potentially, its relatives in the Solanales.
Broader CLE clustering showed that the grasses, which are monocots separated from eudicots by ~150 million years21, typically harbor two closely related CLV3-like genes in their genomes, represented by the rice stem cell regulators FON2 and FCP1 (Fig. 4f)22. These paralogs originated from a monocot-specific duplication event, independent from the duplication leading to SlCLV3 and SlCLE9 (Fig. 4e,f) 7. This mirroring between grasses and tomato led us to ask whether CLV3-like duplication in monocots also led to the evolution of active compensation. We mutated the maize orthologs of FON2 (ZmCLE7) and FCP1 (ZmFCP1) (Fig. 4g). Zmfcp1 mutants are fasciated23, but this phenotype was suppressed when introgressed into the standard B73 genotype (Fig 1e, Fig. 4h,i). In contrast, Zmcle7 null mutants had strongly fasciated ears (Fig. 4f–i). Notably, expression profiling of Zmcle7 inflorescence meristems showed that only ZmCLE7 and ZmFCP1 were significantly upregulated among more than 50 maize CLEs (Fig. 4j, Supplementary Table 7). Significantly, ear fasciation was enhanced in double mutants, suggesting ZmFCP1 partially compensates for Zmcle7. However, unlike slcle9 and slclv3 in tomato, inflorescence transition meristems from Zmfcp1 and Zmcle7 single mutants were each increased in size compared to WT, and double mutants were additive. Thus, although maize shows molecular hallmarks of active compensation, with another ZmCLE upregulated in Zmcle7 mutants, our comparisons of the single and double mutants suggest a passive mechanism, in which these CLE paralogs could have redundant, but unequal, roles in stem cell homeostasis (Fig, 4h–l)23.
We have discovered the independent evolution of CLE compensation in the monocots and the eudicots, driven by independent gene duplication events. Given the distant relationships between Arabidopsis, maize, and tomato, the genetic buffering of stem cell homeostasis uncovered in these species may reflect a formative feature of meristem biology. Interestingly, compensation is only partial, with compensators being less potent than the primary gene, paralleling principles of paralog compensation in yeast8. Partial compensators, like tomato SlCLE9, the Arabidopsis BAMs, and maize ZmFCP1 could function both to buffer stem cell homeostasis and provide developmental flexibility24, and could participate in meristem size changes that occur during developmental transitions25. They could also have as yet undiscovered primary roles in other contexts, as is likely for the BAMs26, maintaining subsidiary roles in shoot meristems. Similarly, paralogous gene pairs in yeast are rarely truly redundant27,28. While the core CLV-module is deeply conserved, our work shows that CLV compensation mechanisms, which shaped at least one domestication event, are diverse. This genetic complexity of CLV-module compensation, which could contribute to the tolerance of CLV-WUS feedback to changes in CLV3 expression29, identifies an underappreciated barrier to modification of these genes for crop improvement1,30. Our work provides a roadmap to dissect the genetic complexity underlying CLV compensation in other plants, and also compensation mechanisms involving other gene families in different developmental programs, which will be important for intelligent manipulation of plant development to enhance crop productivity.
Online Methods
Plant materials and growth conditions
Seeds of tomato cultivar M82 and derived CRISPR mutants used for plant phenotyping were directly sown and germinated in soil on 96-cell plastic flats and grown as previously31. Arabidopsis plants were grown under continuous light conditions at 25°C. The mutant clv1–101, clv3–9, bam1–4, bam2–4, and bam3–2 alleles in the isogenic Col-0 background used in this study are from a previous report and were genotyped as therein4. Double and higher order mutants not generated using CRISPR were created by standard crossing and appropriate genotypes were selected using gene specific mutant genotyping primers. Maize CRISPR/Cas9-derived mutants and wild-type segregants were sown directly on soil and grown under standard long-day greenhouse conditions (16-h light/8-h dark photoperiod) or in the field.
Plant phenotyping
Tomato meristem imaging and size measurement were performed as previously described7,32. Briefly, hand-dissected tomato meristems at late vegetative and transition meristem (11 and 13 days after germination) were captured on a Nikon SMZ1500 microscope. Arabidopsis inflorescences apices images were also captured similarly. To quantify meristem width in Arabidopsis, 5-week-old inflorescence meristems from Col-0 (WT), clv3–9 (clv3), and cle dodeca were removed, hand dissected, and fixed in FAA (2% formaldehyde, 5% acetic acid, 60% ethanol) overnight at 4°C. Tissue was dehydrated in an ethanol series (70%, 80%, 95%, 100%) for 30 min each at room temp and cleared in methyl salicylate overnight. Meristems were mounted in methyl salicylate in a glass bottom petri dish (MatTek #P35G-1.5–10-C) and imaged on an inverted Zeiss LSM 710 confocal microscope. Signal corresponds to structural autofluorescence following excitation with a 488nm Argon laser and emission detected in two broad windows (green: 504–597nm, red: 629–731nm). Images were edited and processed using ImageJ (v.2.0.0-rc-68/1.52e) where gamma was adjusted (0.5) in order to ensure complete delineation of the L1 layer of the inflorescence meristems (intensity data were not used for any downstream analysis). Measurements were made in ImageJ spanning the width of the meristem where the first primordia were visible on each side. As clv1 bam1/2/3 quadruple mutants rarely make a main inflorescence, vegetative meristems from seedlings were compared across genotypes. Seedling meristem perimeter, width, and height in Arabidopsis, were analyzed in 10 day old seedlings grown on ½ MS media plates lacking sucrose for Col-0 (WT), clv3–9 (clv3), clv1–101 (clv1), cle dodeca, clv1–101 clv3–9 (clv1 clv3), bam1/2/3, clv-1–101 bam1/2/3 (clv1 bam1/2/3), and clv3–9 bam123 (clv3 bam1/2/3). Plants were hand dissected to expose meristems and fixed in FAA and mounted as described above for the inflorescence meristems. The shoot apical meristems were imaged with an inverted Zeiss LSM 710 confocal microscope following the same procedure as for the inflorescence meristems. SAM measurements were made in Image J. The perimeter measurement spanned the entirety of the meristem excluding primordia. The width was measured spanning the width of the meristem where the first primordia were visible on each side while the height was measured spanning from the outer top portion of the meristem to the bottom portion where the meristem and primordia differentiate. (N>10 for each genotype imaged). For Arabidopsis locule number quantifications the primary inflorescence of independent individuals per genotype were analyzed and mature, opened flowers were counted. Specific N values differ, see figures for numbers. For clv1 bam1/2/3 mutant plants the main inflorescence rarely bolts and flowers were counted from lateral shoots as necessary. To quantify Arabidopsis flower production, all mature flowers (fully open and with perianth organs abscised), were counted on the main inflorescence stems by eye. Arabidopsis stem fasciation measurements were taken with a digital SPI caliper (model 15–719-8) 70 millimeters up from the rosette of 25 day old plants. For quantification and imaging of maize meristems and ears, the following genotypes were obtained from a segregating F2 population from a cross between Zmcle7 and Zmfcp1 CRISPR/Cas9-generated mutants: B73 (WT), Zmcle7 Zmfcp1/+ (Zmcle7), Zmcle7/+ Zmfcp1 (Zmfcp1) and Zmcle7 Zmfcp1. Apices were dissected and imaged directly in the scanning electron microscope (Hitachi) or fixed and cleared for measuring, as described for Arabidopsis.
CRISPR/Cas9 constructs for generating tomato, Arabidopsis and maize CLE mutants
To generate CRISPR/Cas9 mutants in tomato, a binary plasmid was built containing a functional Cas9 driven by a constitutive promoter (CaMV 35S) and two guide RNAs (gRNA) each driven by the Arabidopsis U6 (AtU6) promoter using Golden Gate cloning31,33,34. The final binary vectors were introduced into the M82 tomato line by Agrobacterium tumefaciens–mediated transformation as previously described33,35. First-generation (T0) transgenic plants were transplanted in soil and grown under greenhouse conditions. Genotyping of CRISPR/Cas9-generated mutations was performed as previously described31. Stable non-transgenic, homozygous plants were used for phenotyping and crosses. The CRISPR/Cas9 construct for producing the Arabidopsis cle dodeca mutant was built using the pCUT vector system36. Twelve 20 bp gRNA target sites were selected upstream of the dodecapeptide coding region in the genomic sequence of each target CLE gene. Three separate gRNA arrays gene were synthesized by GeneArts (Thermofisher) as groups of 4 AtU6::gRNA tandem constructs36, which were cloned together by restriction enzyme digestion into the recipient GeneArts pMA plasmid to generate a single vector hosting 12 gRNA units. The 12-stacked gRNA unit was then cloned into the pCUT4 binary vector as described in36. A separate set of 12-stacked gRNA units were cloned into the pCUT6 binary vector. clv3–9 plants were transformed with the pCUT4 CRISPR binary construct by floral dipping and T1 transgenic seed derived was selected on B5 media lacking sucrose and containing 100mg/L hygromycin. T1 plants were screened for editing efficiency by sequencing of CLE gene PCR products from leaf DNA. Plants were scored as having efficient editing by confirmation of overlapping sequencing traces originating at −3 position from the PAM site. As no obvious phenotypes were observed in T1 plants and no homozygous mutants in the T1 were expected36, T2 seed was sown on selective B5 media, DNA was collected from T2 plants, each targeted CLE gene was amplified via PCR, and products were directly sequenced via Sanger sequencing. We noted that some gRNAs from the pCUT4 set did not appear to work and a heterozygous pCUT4 T2 line was transformed with the pCUT6 gRNA set to target the remaining CLE genes and potentially obtain larger deletion mutations. From T2 of this transformation, higher order CLE mutant combinations were identified that contained lower order homozygous fixed alleles and from those plants the next generation was screened on hygromycin-containing B5 media, and basta, to identify heterozygous Cas9 transgenic plant lines. A select line was propagated to the T3 generation and subjected to additional rounds of sequencing. As no obvious phenotypes arose in the T3, this process was continued until homozygous mutants were selected for all CLE genes by the T6 generation. Plants lacking Cas9 were confirmed by PCR and screening on both basta and hygromycin plates. CLE genes from Cas9-free mutants were re-sequenced in independent plants to assure fixed mutations, and seed was propagated from a single fixed mutant plant. To generate CRISPR/Cas9 mutants in maize genes ZmFCP1 and ZmCLE7, a binary plasmid was built containing a monocot-optimized Cas9 driven by maize UBI (ZmUBI1) promoter with two gRNAs under and introduced into the maize genotype Hi-II by A. tumefaciens-mediated tissue culture transformation37. Maize calli were genotyped by PCR at the target sites of both genes and those carrying mutant alleles were selected for plant regeneration. T0 plants carrying mutant alleles were then backcrossed two to three times to B73 to segregate away the transgene.
RNA extraction and quantitative RT-PCR
Quantitative RT-PCR (qRT-PCR) for both tomato and Arabidopsis was performed as previously described32. Briefly, total RNA from vegetative meristems of tomato plants and dissected shoot apices from inflorescences of Arabidopsis was extracted with the PicoPure RNA Extraction kit and the RNeasy mini kit (Qiagen), respectively. One microgram of total RNA was treated with DNase I and used for cDNA synthesis with a SuperScript III reverse-transcriptase kit (Invitrogen). qPCR was performed using gene-specific primers (Supplementary Table 8) in the iQ SYBR Green SuperMix (Bio-Rad) reaction system on the CFX96 Real-Time system (Bio-Rad), following the manufacturer’s instructions.
Meristem transcriptome profiling
Total RNA from tomato vegetative and transition meristems was extracted using the PicoPure RNA Extraction kit (Arcturus) from 20–40 meristems per replicate for each genotype, yielding 200–1000ng RNA. RNA sequencing libraries were prepared using the Kapa mRNA Hyper prep kit (Roche). The quality of each RNA sequencing library was tested using a Bioanalyzer 2100 (Agilent). Paired-end 75-base sequencing was performed on the Illumina Hiseq sequencing platform. Two biological replicates were used for all library constructions7,32. For maize RNAseq, inflorescence meristems (∼0.5 mm) from a segregating population were dissected from growing ears (2–7 mm length). Total RNA was extracted using Direct-zol RNA kit (Zymo Research) and sequenced by Illumina NextSeq 500 platform at the Cold Spring Harbor Laboratory Genome Center (Woodbury, NY). Reads for the wild-type tomato M82 and slclv mutants were trimmed by quality using Trimmomatic v0.32 (parameters: ILLUMINACLIP:TruSeq3-PE-2.fa:2:40:15:1:FALSE LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:50)38 and aligned to the reference genome sequence of tomato (SL3.00)39 using Tophat2 v2.1.1 (parameters:–b2-very-sensitive –read-mismatches 2 –read-edit-dist 2 –min-anchor 8 –splice-mismatches 0 –min-intron-length 50 –max-intron-length 50000 –max-multihits 20)40. Alignments were sorted with samtools41 and gene expression quantified as unique read pairs aligned to reference annotated gene features (ITAG3.2) using HTSeq-count v0.6.08 (parameters:–format = bam –order = name –stranded = no –type = exon –idattr = Parent)42. Maize RNA-seq data was trimmed with Trimmomatic v0.36 (parameters: ILLUMINACLIP:./TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:20 MINLEN:50)38 and aligned to reference genome (B73 RefGen v3)43 using Tophat2 v2.1.1 (parameters: –b2-sensitive –read-mismatches 2 –read-edit-dist 2 –min-anchor 8 –splice-mismatches 0 –min-intron-length 50 –max-intron-length 50000 –max-multihits 20)40. Aligned reads were then sorted with samtools41 and gene expression quantified as unique read pairs aligned to reference annotated gene features in the maize (B73 AGPv3.22) using HTSeq-count v0.6.08 (parameters:–format = bam –order = name –stranded = no –type = exon –idattr = Parent)42. All statistical analyses of gene expression were conducted in R44. Significant differential expression between meristem stages in wild-type M82 and maize inflorescence ear tips was identified with edgeR45 using 4-fold change, average 1 CPM, and FDR ≤ 0.10 cutoffs or 2-fold change, average 5 CPM, and FDR ≤0.10, respectively46.
Quantification and statistical analysis
For tomato and locule number quantifications, at least 3 primary or secondary inflorescences from ≥ 3 individuals per genotype were analyzed. For tomato and maize meristem measurements at least 5 independent plants were analyzed per genotype, and 10–15 independent plants used for Arabidopsis fixation and inflorescence meristem imaging. For Arabidopsis seedling meristem measurements 11 to 16 independent seedlings were analyzed per genotype. For Arabidopsis carpel number quantifications, N>140 per genotype were counted, with the exception of clv1 bam1/2/3 mutants which have reduced flower production owing to extreme stem overgrowth (N= 26). For qRT-PCR experiments, two biological and three technical replicates were analyzed per experiment. Statistical analysis was performed using two-tailed, two-samples Student’s t-test and one-way anova with Tukey test (alpha = 0.05). Raw data and specific number of plants (N), meristems, flowers or fruits (n) is shown in Supplementary Tables 1 and 2. RNAseq differential expression analysis for tomato and maize is shown in Supplementary Tables 5 and 7. All raw data are provided in Supplementary Tables 1–8.
Transgenic complementation of SlCLV3 and SlCLE9
The genomic DNA sequences of SlCLV3 (gCLV3gCLV3: 3,261 bp in total with 1,995 bp upstream and 666 bp downstream). To mutate SlCLV3 dodecapeptide into SlCLE9 within gCLV3gCLV3 (gCLV3gSlCLE9) PCR products were amplified from pDONOR221-gCLV3gCLV3 and a vector containing the genomic region of SlCLE9 (pDONOR221-gCLE9) with overlapping primers (Supplementary Table 8) by using KOD Xtreme™ Hot Start DNA Polymerase (Merck). The resulting PCR products were digested with DpnI (NEB) and transformed into DH5a competent cells. Sanger sequencing confirmed pDONOR221-gCLV3gCLV3, gCLV3gSlCLE9 and colonies were recombined into binary vector pGWB40147 for transgenic complementation.
CLE clustering
We constructed Hidden Markov Models (HMM) particular to each CLE cluster defined by Goad et al., 20177. We generated HMMs that included all angiosperm CLE sequences in a particular cluster, as well as Brassicaceae, Solanaceae and monocot-specific HMMs. We searched Brassicaceae, Poaceae and Solanaceae genomes with these HMMs and used the retrieved sequences (E<0.001) in downstream clustering analyses. For the clustering analysis focused on CLV3 and SlCLE9, we included only those CLE pro-peptide sequences in cluster 1D, as well as those sequences identified in our synteny analysis (Supplementary Table 6). We submitted either all full CLE pre-propeptide sequences from Brassicaceae and Solanaceae, or only cluster 1D CLE pre-propeptide sequences, to an all-by-all BLAST using the MPI Bioinformatics Toolkit48, and the results were visualized by clustering using CLANS ver 1.049. The resulting clusters were named according to the conventions set by Goad et al., 20177. Based on the clusters formed in the above analysis, the full pre-propeptide translations of CLE genes from clusters 1D1 and 1D2 were chosen for further analysis.
SlCLV3, SlCLE9 synteny analysis
To find genomic regions orthologous to the tomato SlCLE9/SlCLV3 regions in each target species, the peptide sequences of each of the 4 genes flanking SlCLE9 and SlCLV3 were used to run CoGe BLAST50 on the target species’ genome using the tblastn search algorithm. Groundcherry genomic fragments were obtained from Lemmon et al., 201851. For each search, the genomic regions that contained the three best matches were compared to SlCLE9 and SlCLV3 regions using CoGe GEvo50 at a scale of 160,000 bp centered on the matched gene, using the (B)LastZ: Large Regions algorithm with a score threshold of 3,000. If two or more genes in this region aligned to genes in the tomato SlCLE9 or SlCLV3 regions, it was considered syntenic.
CLE peptide collection from syntenic regions
For each syntenic region match, GEvo alignment parameters were adjusted to detect CLEs, which are often missed by the default parameters. Two strategies were employed: First, the (B)LastZ: Large Regions algorithm with a reduced score threshold of 2,000, and second, the BlastN: Small Regions algorithm with a mismatch penalty reduced to −1. If either of these strategies found an alignment to SlCLE9 or SlCLV3 in the syntenic region, that portion of sequence was extracted and aligned to both SlCLE9 and SlCLV3 using MAFFT ver. 7.31352 using the L-INS-i algorithm and BLOSUM45 scoring matrix. From those individual alignments, we attempted to extract a CLE peptide translation, over the full pre-propeptide if possible, or just the dodeca region if alignment quality was poor. The CaCLE9 pseudogene was identified by aligning the pepper genomic region syntenic to the SlCLE9 region using MAFFT ver. 7.31352 with a lowered gap offset value of 0.001. This aligned SlCLE9 to an unannotated region of the pepper genome at chromosome 6 starting at position 9321808. In this alignment, the sequences that underlie the SlCLE9 exons share ~80% nucleotide identity, but the pepper sequence has stop codons in all three reading frames, and a portion of what aligns to the SlCLE9 dodecapeptide is deleted. Further analysis with Eukaryotic GeneMark.hmm version 3.4753 accurately predicted the three SlCLE9 exons but predicted no exons in the orthologous pepper region. To verify genome assembly integrity at this locus, the region was amplified from pepper genomic DNA (Supplementary Table 8), and Sanger sequenced, which had no discrepancies to the assembly sequence. All efforts to find a similar feature in the potato genome failed, which suggests that the entire CLE9 coding region is absent in potato.
Data software and availability
Raw data for all quantifications is included as Supplementary Tables. All RNAseq data from tomato are available in NCBI. Tomato SRA project and BioProject accession are SRP161864 and PRJNA491365, respectively. Maize SRA projects and Bioproject accessions are SRR7970748, SRR7970747, SRR7970749, SRR7970750 and PRJNA494874, respectively. RNA-seq data from Arabidopsis was obtained from Klepikova et al., 201554 and Mandel et al., 201655.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary Material
Acknowledgements
We thank C. Brooks, A. Krainer, and J. Dalrymple for technical support, P. Keen for assistance with tomato transformation, T. Mulligan, S. Vermylen, and S. Qiao from CSHL, and staff from Cornell University’s Long Island Horticultural Research and Extension Center in Riverhead, New York, for assistance with plant care, H. Shinohara and Y. Matsubayashi from Nagoya University for tomato peptide binding assays. Initial work from Z.L.N. was supported by funds from Virginia Polytechnic Institute and State University. This research was supported by a PEW Latin American Fellowship (29661) to D.R.-L., a NIGMS-MIRA award from National Institutes of Health under award number R35GM119614–01 to Z.L.N., an Agriculture and Food Research Initiative competitive grant # 2016–67013-24572 of the USDA National Institute of Food and Agriculture and the Next-Generation BioGreen 21 Program SSAC; project PJ01322602 from the Rural Development Administration, Republic of Korea to D.J., an Agriculture and Food Research Initiative competitive grant # 2015–67013-22823 of the USDA National Institute of Food and Agriculture to Z.B.L., and the National Science Foundation Plant Genome Research Program (IOS-1732253) to J.V.E. and Z.B.L and (IOS-1546837) to D.J., M.E.B., Z.L.N, and Z.B.L.
References
- 1.Somssich M, Je BI, Simon R & Jackson D CLAVATA-WUSCHEL signaling in the shoot meristem. Development 143, 3238–3248 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Soyars CL, James SR & Nimchuk ZL Ready, aim, shoot: stem cell regulation of the shoot apical meristem. Curr. Opin. Plant Biol. 29, 163–168 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Nimchuk ZL, Zhou Y, Tarr PT, Peterson BA & Meyerowitz EM Plant stem cell maintenance by transcriptional cross-regulation of related receptor kinases. Development 142, 1043–1049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diss G, Ascencio D, DeLuna A & Landry CR Molecular mechanisms of paralogous compensation and the robustness of cellular networks. J. Exp. Zoolog. B Mol. Dev. Evol. 322, 488–499 (2014). [DOI] [PubMed] [Google Scholar]
- 5.El-Brolosy MA & Stainier DYR Genetic compensation: A phenomenon in search of mechanisms. PLOS Genet. 13, e1006780 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu C et al. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 47, 784–792 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Goad DM, Zhu C & Kellogg EA Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytol. 216, 605–616 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Kafri R, Bar-Even A & Pilpel Y Transcription control reprogramming in genetic backup circuits. Nat. Genet. 37, 295–299 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Kafri R, Springer M & Pilpel Y Genetic Redundancy: New Tricks for Old Genes. Cell 136, 389–392 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Nimchuk ZL CLAVATA1 controls distinct signaling outputs that buffer shoot stem cell proliferation through a two-step transcriptional compensation loop. PLoS Genet. 13, e1006681 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi YL et al. A Collection of Mutants for CLE-Peptide-Encoding Genes in Arabidopsis Generated by CRISPR/Cas9-Mediated Gene Targeting. Plant Cell Physiol. 58, 1848–1856 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Gregory EF, Dao TQ, Alexander MA, Miller MJ & Fletcher JC The signaling peptide-encoding genes CLE16, CLE17 and CLE27 are dispensable for Arabidopsis shoot apical meristem activity. PloS One 13, e0202595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betsuyaku S, Sawa S & Yamada M The Function of the CLE Peptides in Plant Development and Plant-Microbe Interactions. Arab. Book 9, e0149 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark SE, Running MP & Meyerowitz EM CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121, 2057–2067 (1995). [Google Scholar]
- 15.Kayes JM & Clark SE CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125, 3843–3851 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Li H et al. Evolution of the leucine‐rich repeat receptor‐like protein kinase gene family: Ancestral copy number and functional divergence of BAM1 and BAM2 in Brassicaceae. J. Syst. Evol. 54, 204–218 (2016). [Google Scholar]
- 17.Deyoung BJ & Clark SE BAM receptors regulate stem cell specification and organ development through complex interactions with CLAVATA signaling. Genetics 180, 895–904 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bombarely A et al. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nat. Plants 2, 16074 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Hoshino A et al. Genome sequence and analysis of the Japanese morning glory Ipomoea nil. Nat. Commun. 7, 13295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeling M et al. Fractionation mutagenesis and similar consequences of mechanisms removing dispensable or less-expressed DNA in plants. Curr. Opin. Plant Biol. 15, 131–139 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Stecher G, Suleski M & Hedges SB TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 34, 1812–1819 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Suzaki T, Yoshida A & Hirano H-Y Functional diversification of CLAVATA3-related CLE proteins in meristem maintenance in rice. Plant Cell 20, 2049–2058 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Je BI et al. Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat. Genet. 48, 785–791 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Abley K, Locke JCW & Leyser HMO Developmental mechanisms underlying variable, invariant and plastic phenotypes. Ann. Bot. 117, 733–748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernier G The Control of Floral Evocation and Morphogenesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 175–219 (1988). [Google Scholar]
- 26.Depuydt S et al. Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor-like kinase BAM3. Proc. Natl. Acad. Sci. U. S. A. 110, 7074–7079 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kafri R, Levy M & Pilpel Y The regulatory utilization of genetic redundancy through responsive backup circuits. Proc. Natl. Acad. Sci. U. S. A. 103, 11653–11658 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soria PS, McGary KL & Rokas A Functional divergence for every paralog. Mol. Biol. Evol. 31, 984–992 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Müller R, Borghi L, Kwiatkowska D, Laufs P & Simon R Dynamic and compensatory responses of Arabidopsis shoot and floral meristems to CLV3 signaling. Plant Cell 18, 1188–1198 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher JC The CLV-WUS Stem Cell Signaling Pathway: A Roadmap to Crop Yield Optimization. Plants 7, 87 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Leal D, Lemmon ZH, Man J, Bartlett ME & Lippman ZB Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 171, 470–480.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Park SJ, Jiang K, Schatz MC & Lippman ZB Rate of meristem maturation determines inflorescence architecture in tomato. Proc. Natl. Acad. Sci. U. S. A. 109, 639–644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks C, Nekrasov V, Lippman ZB & Van Eck J Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR-associated9 system. Plant Physiol. 166, 1292–1297 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner S, Engler C, Weber E, Gruetzner R & Marillonnet S Fast track assembly of multigene constructs using Golden Gate cloning and the MoClo system. Bioeng. Bugs 3, 38–43 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Gupta S & Van Eck J Modification of plant regeneration medium decreases the time for recovery of Solanum lycopersicum cultivar M82 stable transgenic lines. Plant Cell Tissue Organ Cult. 127, 417–423 (2016). [Google Scholar]
- 36.Peterson BA et al. Genome-Wide Assessment of Efficiency and Specificity in CRISPR/Cas9 Mediated Multiple Site Targeting in Arabidopsis. PloS One 11, e0162169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Char SN et al. An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol. J. 15, 257–268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolger AM, Lohse M & Usadel B Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485, 635–641 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim D et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anders S, Pyl PT & Huber W HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnable PS et al. The B73 maize genome: complexity, diversity, and dynamics. Science 326, 1112–1115 (2009). [DOI] [PubMed] [Google Scholar]
- 44.RTeam DC (2015). R Core Team (2015). R: A language and environment for statistical computing. (R Found. Stat. Comput; Vienna, Austria.). [Google Scholar]
- 45.Robinson MD, McCarthy DJ & Smyth GK edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemmon ZH et al. The evolution of inflorescence diversity in the nightshades and heterochrony during meristem maturation. Genome Res. 26, 1676–1686 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawa T et al. Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71, 2095–2100 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann L et al. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at its Core. J. Mol. Biol. 430, 2237–2243 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Frickey T & Lupas A CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics 20, 3702–3704 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Lyons E et al. Finding and comparing syntenic regions among Arabidopsis and the outgroups papaya, poplar, and grape: CoGe with rosids. Plant Physiol. 148, 1772–1781 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemmon ZH et al. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 4, 766–770 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Katoh K & Standley DM MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Besemer J & Borodovsky M GeneMark: web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Res. 33, W451–454 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klepikova AV, Logacheva MD, Dmitriev SE & Penin AA RNA-seq analysis of an apical meristem time series reveals a critical point in Arabidopsis thaliana flower initiation. BMC Genomics 16, 466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mandel T et al. Differential regulation of meristem size, morphology and organization by the ERECTA, CLAVATA and class III HD-ZIP pathways. Development 143, 1612–1622 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.