Abstract
Background
Meningioma is the most common primary intracranial tumor. It is usually slow growing and benign, and surgery is the main treatment modality. There are limited data on return to work following meningioma surgery. The objective of this study was to determine the patterns of sick-leave rate prior to surgery, and up to 2 years after, in patients compared to matched controls.
Methods
Data on patients ages 18 to 60 years with histologically verified intracranial meningioma between 2009 and 2015 were identified in the Swedish Brain Tumor Registry (SBTR) and linked to 3 national registries after 5 matched controls were assigned to each patient.
Results
We analyzed 956 patients and 4765 controls. One year prior to surgery, 79% of meningioma patients and 86% of controls were working (P < .001). The proportion of patients at work 2 years after surgery was 57%, in contrast to 84% of controls (P < .001). Statistically significant negative predictors for return to work in patients 2 years after surgery were high (vs low) tumor grade, previous history of depression, amount of sick leave in the year preceding surgery, and surgically acquired neurological deficits.
Conclusion
There is a considerable risk for long term sick leave 2 years after meningioma surgery. Neurological impairment following surgery was a modifiable risk factor increasing the risk for long-term sick leave. More effective treatment of depression may facilitate return to work in this patient group.
Keywords: meningioma, neurosurgery, quality of life
Meningiomas are the most common primary intracranial tumors.1,2 According to recent guidelines, surgical removal is indicated in cases with radiological growth, presence of clinical symptoms, or if a nonbenign tumor (ie, World Health Organization [WHO] grade II or III) is suspected.3 Surgical treatment has in case series been shown to increase survival4 and quality of life,5 and more extensive resection is related to lower recurrence rate.6 Thus, complete tumor removal is recommended in situations where it can be performed safely without excessive morbidity or mortality. However, because complications and neurological deterioration related to tumor resection are not trivial,2,7,8 careful risk-benefit considerations are essential for optimal management.
There is a positive correlation between employment and quality of life for patients with brain disorders.9,10 In a recent study, 1 out of 3 patients with meningioma were unable to return to work (RTW) after surgery,11 whereas 2 small, older studies reported that 17% to 19% were unable to return to their previous employment or premorbid level of activity.12,13 These studies were limited by small sample sizes and by their cross-sectional design, not allowing a closer analysis of temporal patterns. Thus, large-scale information about dynamics in perioperative working capacity is lacking, and importantly no studies have used adequately matched controls to compare with the normal patterns of sick leave. Further, to our knowledge, there are no established predictors for postoperative working capacity for meningioma patients.
The primary aim of this study was to determine the rate and patterns of sick leave prior to surgery and up to 2 years after in patients with intracranial meningiomas in Sweden operated on between 2009 and 2015.
Materials and Methods
This study is reported according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.14 We used data from multiple nationwide Swedish registries, linked through the unique personal identification numbers allocated to Swedish citizens, to perform a population-based matched cohort study. The specific registries are described in detail below. Definitions of important variables are presented in Supplementary Table 1.
The Swedish Brain Tumor Registry
The Swedish Brain Tumor Registry (SBTR) is a regionally based nationwide registry of adult (age ≥ 18 years) patients diagnosed with primary brain tumors carrying information on patient tumor and treatment characteristics. For a selection of relevant clinical variables registered, see Supplementary Table 2. The SBTR covers data from 1999 and onward in histopathologically verified tumors. The Swedish health-care system is divided into 6 different regions that each contains 1 neurosurgical clinic providing care to patients with tumors of the CNS. There are no other institutions providing brain tumor surgery in Sweden. The level of registration coverage in the SBTR from the different regions has varied somewhat over time. Registration rate is defined as the percentage of diagnoses in the SBTR that corresponds to diagnoses reported to the compulsory National Cancer Registry. In our study, a minimum registration rate of 80% was required to be included in the analysis in any given year for each region to provide representative population-based data. For this reason, from the “southern Sweden” region, we used data from 2012 to 2013 only. For the remaining 5 regions, data inclusion covered the entire time period from 2009 to 2015. Further details of the SBTR and the definition of clinical variables in meningioma patients are described in our earlier work.15
Statistics Sweden
Statistics Sweden (www.scb.se) is a government agency responsible for coordinating the official statistics in Sweden. We used the registry to identify controls that were matched for year of birth, sex, municipality of residence, and educational level. Each case was individually matched with 5 controls. All controls were unique, that is, belonging to 1 case only and not used for additional similar cases. From Statistics Sweden we also extracted data on educational level and income for the patients and controls included in the analyses.
Swedish Social Insurance Agency
The Social Insurance Agency is the Swedish government agency administrating social insurance that provides financial security in the event of illness. This agency is also responsible for holding official statistics on sick leave, including temporary compensation, compensation for longer-lasting illness (> 10 days), and compensation for permanent incapacity to work. The information provided was time periods for compensation, type of compensation, and extent of compensation (25%, 50%, 75%, or 100%). The data from Swedish Social Insurance Agency were accessed November 30, 2018.
The National Board of Health and Welfare
The National Board of Health and Welfare is a government agency with a wide range of responsibilities within the fields of social and health services, such as providing and developing statistics on health and medical care in different registries. From the National Patient Registry (NPR) we received data on inpatient and outpatient visits, including dates and diagnostic and procedural codes, for the period 2007 to 2016. Since 2001 this registry has been subject to mandatory reporting both from private and public hospitals but does not include primary health care. The NPR thus contains information about all contacts with specialist health care with diagnoses coded according to the 10th revision of the International Classification of Diseases (ICD-10). The ICD-10 codes were used to classify comorbidity according to the Elixhauser comorbidity index.16,17 The underreporting of contacts in NPR has been estimated to be less than 1% according to the National Board of Health and Welfare (www.socialstyrelsen.se). The National Prescription Registry (NPrR) was established July 1, 2005, and has since been subject to mandatory registration of drug prescriptions. From the NPrR we received information concerning drugs prescribed according to the Anatomical Therapeutic Chemical classification system, and date of dispensing, during the period 2007 to 2017. In this study, we extracted information on any antiepileptic (N03A) and antidepressant (N06A) drug prescription for patients and controls. The registries under the National Board of Health and Welfare were accessed March 16, 2018.
Final Patient Selection
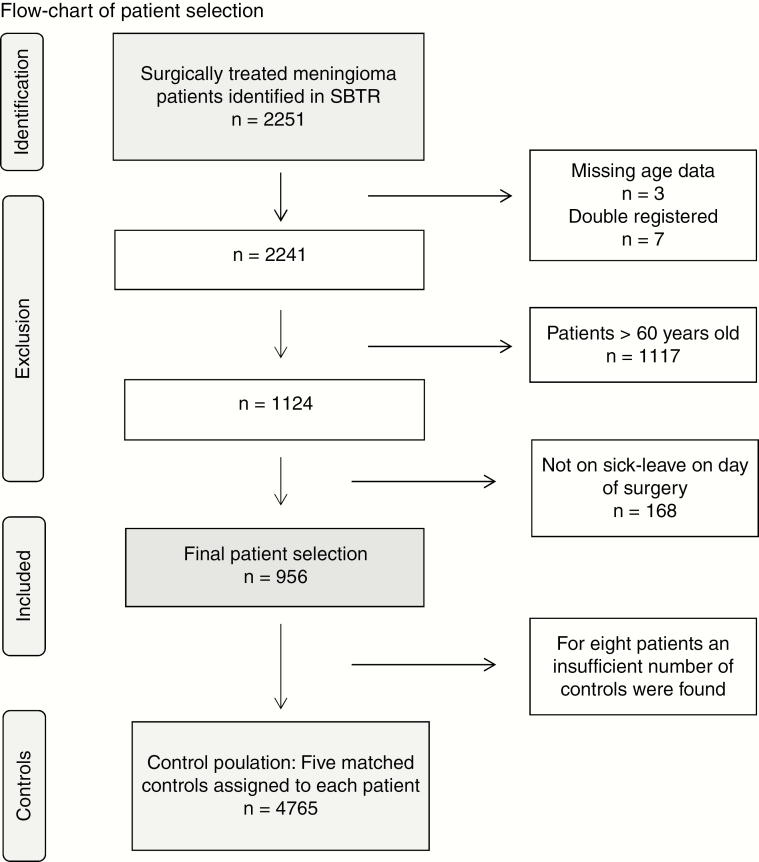
The patient selection process is outlined in Fig. 1. From the SBTR we identified 2251 adult (age ≥ 18 years) patients with a first-time histological diagnosis of intracranial meningioma according to the 2007 WHO classification of brain tumors,18 with an index date between January 1, 2009, and July 31, 2015. Patients with radiological suspected meningioma without histological diagnosis were not included in the present study. Because return to work was a major focus, we included patients between ages 18 and 60 years (n = 1124), as done by others.19 All patients who were not on sick leave at the index date were excluded (n = 168) because this indicated that these patients were not involved in activity related to work during the studied time period. Included patients (n = 956) were assigned 5 matched controls each (n = 4765). For 8 cases the number of controls was incomplete.
Fig. 1.
Patient Selection SBTR indicates Swedish Brain Tumor Registry.
Statistics
Data from the registries were imported into corresponding tables in a mySQL database. Sick-leave compensation (degree and time period) from 2 sources was combined for each individual using Python. Other data derivations were conducted using mySQL. R was used for statistical analyses. The index date, and date of diagnosis, were defined as the date of surgery. For each day from 1 year (365 days) prior to 2 years (730 days) after index date the rates of sick-leave compensation, both partial and full, and rates of mortality were computed and displayed in stacked graphs for cases and controls separately. From this computation we also derived rates of RTW and net days absent.
Continuous variables were summarized using the median, first, and third quartiles and compared between cases and controls using the Mann-Whitney U test. Categorical variables were summarized using counts and proportions and compared between cases and controls using the Fisher exact test.
Univariable logistic regression and multivariable logistic regression were used to examine predictors of RTW and independent predictors of RTW, respectively. RTW at 2 years was defined as any work-related activity (partial or complete) at 2 years postoperatively. Covariates were chosen based on presumed relevance. As demographic variables we included age and sex. Socioeconomic variables included yearly income (per 100 000 Swedish krona), education (basic to high school vs higher education), and amount of days absent from work in the year preceding the index date (per 10 days). Finally, clinical variables included WHO functional status, tumor size (< 4 cm, 4-6 cm, > 6 cm), history of seizures (no or yes), history of depression (no or yes), comorbidity (using the validated comorbidity scoring system Elixhauser comorbidity index, as defined in Supplementary Table 1), WHO tumor grade, new neurological deficit after surgery, and whether the patient underwent reoperation because of any complication.
Ethics Statement
This study was approved by the regional ethical committee in Västra Götaland region (Dnr: 363-17).
Results
Baseline and treatment characteristics for patients with meningioma are presented in Table 1. In short, mean age was 48 years and 75% of patients were female. The asymptomatic group included 15% of patients, whereas 83% of patients were able to perform at least light work according to WHO functional status. In 87% a complete resection defined as Simpson grades I to III was achieved. New neurologic deficit after surgery was observed in 13% of patients.
Table 1.
Baseline and Treatment Characteristics for Included Patients With Meningioma (n = 956)
| Variable | |
|---|---|
| Age, mean (SD), y | 48.4 (8.5) |
| Female, n (%) | 713 (74.6) |
| Asymptomatic, n (%) | 151 (15.8) |
| WHO functional status, n (%) | |
| 0, fully active | 552 (60.6) |
| 1, light work possible | 206 (22.6) |
| 2, cares for self | 124 (13.6) |
| 3, limited self-care | 21 (2.3) |
| 4, disabled, confined to bed | 8 (0.9) |
| Missing | 45 |
| Tumor laterality, n (%) | |
| Left | 404 (50.6) |
| Right | 335 (42.1) |
| Bilateral | 58 (7.3) |
| Missing | 159 |
| Tumor size, n (%), cm | |
| < 4 | 477 (56.5) |
| 4-6 | 253 (30.0) |
| > 6 | 114 (13.5) |
| Missing | 112 |
| History of seizure, at index date, n (%) | 289 (32.6) |
| Missing | 70 |
| Simpson grade, n (%) | |
| I | 235 (27.8) |
| II | 425(50.3) |
| III | 70 (8.3) |
| IV | 102 (12.1) |
| V | 13 (1.5) |
| Missing | 111 |
| New deficit after surgery, n (%) | 122 (12.9) |
| Missing | 1 |
| Reoperation because of complication, n (%) | 39 (4.0) |
| Missing | 1 |
| WHO grade, n (%) | |
| Grade I | 864 (90.5) |
| Grades II-III | 92 (9.5) |
| Oncological treatment planned, n (%) | 49 (5.2) |
| Missing | 17 |
| Wait time (surgery date, radiological diagnosis), days median (Q1, Q3) | 76 (28, 166) |
| Skull base location | 166 (17.4) |
Abbreviations: Q, quartile; WHO, World Health Organization.
Sick-Leave Compensation
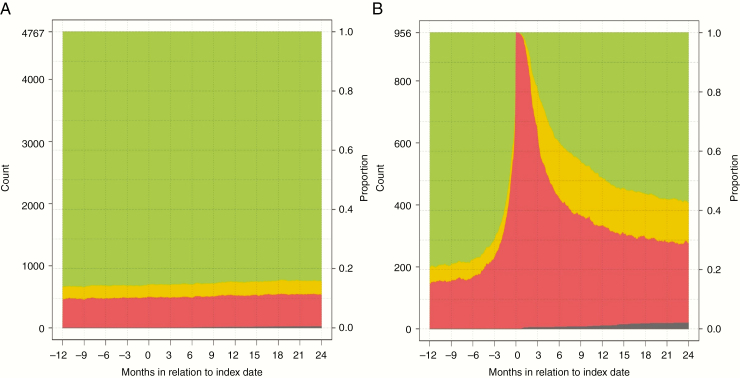
A comparison between patients with meningioma and controls regarding socioeconomic and clinical variables, as well as patterns of sick leave, is summarized in Table 2. Extent of absence from work in the year preceding surgery up to 2 years following surgery is presented in separate graphs for patients (Fig. 2) and controls (Fig. 3). To further study the characteristics of the group of patients on sick leave 1 year before surgery, we have summarized dichotomized data on this group, compared to other patients, in Supplementary Table 3. We also provide separate complementary graphs for subgroups of patients (Supplementary Figures 1 to 10).
Table 2.
Characteristics of Patients and Controls, and Patterns of Compensation
| Meningioma, n = 956 | Controls, n = 4765 | P | |
|---|---|---|---|
| Educational level, n (%) | .93 | ||
| Basic to high school | 545 (64.3) | 2698 (64.1) | |
| Higher education | 302 (35.7) | 1509 (35.9) | |
| Missing | 109 | 558 | |
| Disposable income, n (%) | .65 | ||
| Median (Q1, Q3) | 231k (176 k, 289 k) | 233k (171 k, 298 k) | |
| Missing | 104 | 525 | |
| History of depression, at index date, n (%) | 278 (29.1) | 905 (19.0) | < .001 |
| History of depression (1 y before index date), n (%) | 204 (21.3) | 788 (16.5) | < .001 |
| Elixhauser comorbidities, at index date, n (%) | < .001 | ||
| 0 | 641(67.1) | 3822 (80.2) | |
| 1 | 189 (19.8) | 613 (12.9) | |
| 2 | 66 (6.9) | 180 (3.8) | |
| 3 or more | 60 (6.3) | 150 (3.1) | |
| Number of Elixhauser comorbidities, 1 y prior to index date, n (%) | < .001 | ||
| 0 | 750 (78.5) | 3946 (82.8) | |
| 1 | 117 (12.2) | 549 (11.5) | |
| 2 | 46 (4.8) | 150 (3.1) | |
| 3 or more | 43 (4.5) | 120 (2.5) | |
| Net d absent 365 d prior to index date, median (Q1, Q3) | 35 (5, 131) | 0 (0, 0) | < .001 |
| % on permanent sick leave at index date | 104 (10.9) | 350 (7.3) | < .001 |
| Net d absent 1 y after index date, median (Q1, Q3) | 227 (108, 365) | 0 (0, 0) | < .001 |
| Net d absent 1-2 y after index date, median (Q1, Q3) | 49 (0,343) | 0 (0, 0) | < .001 |
| Without any sick-leave compensation at 1 y before index date, n (%) | 755 (79.0) | 4109 (86.2) | < .001 |
| Without any sick-leave compensation at 1 y after index date, n (%) | 471 (49.3) | 4033 (84.6) | < .001 |
| Without any sick-leave compensation 2 y after index date, n (%) | 548 (57.3) | 4017 (84.3) | < .001 |
| On permanent sick leave 365 d after index date, n (%) | 103 (10.8) | 350 (7.3) | < .001 |
Abbreviation: Q, quartile.
Fig. 2.
Rates Without Sick Leave Compensation (Green), With Partial Compensation (Yellow), and With Full Compensation (Red) From 365 Days Prior to Index Date to 730 Days After Index Date The dark gray stack at the bottom represents deceased patients. A, controls (n = 4767). B, patients (n = 965).
As expected, the educational level and income did not differ between patients and controls. However, 29.1% of patients compared to 19.0% of controls had a history of depression at the index date (P < .001). This difference between patients and controls was present already at 1 year prior to the index date, with 21.3% of patients vs 16.5% of controls (P < .001). In addition, patients showed more preoperative comorbidity than controls according to the Elixhauser comorbidity index (P < .001).
The median number of net days absent from work in the year preceding the index date was zero in the control group and 35 in patients with meningioma (P < .001). In the year following the index date, patients with meningioma had a median 227 net days absent, whereas the control group still had none. The proportion of patients on permanent sick leave at the index date was 10.9% for patients with meningioma and 7.3% for controls (P < .001); this was unchanged 1 year after surgery (10.8% and 7.3%). One year prior to the index date, 79.0% of patients and 86.2% of controls were in full employment (P < .001). One year after surgery the rate of full employment for patients had dropped to 49.3%, whereas 84.6% of the controls retained full work capacity (P < .001). At 2 years after surgery 57.3% of patients vs 84.3% of controls were working full time (P < .001).
Predictors for Returning to Work
A logistic regression model was created to establish predictors for RTW postoperatively (Table 3). For the possible predictors, we also present unadjusted stacked graphs for absence from work in Supplementary Figures 1 to 12. Net days absent in the year preceding surgery (hazard ratio [HR] 0.91, 95% CI 0.89-0.93, P < .001), a history of depression (HR 0.60, 95% CI 0.38-0.94, P = .03), high (vs low) tumor grade (HR 0.46, 95% CI 0.24-0.90, P = .002), and surgically acquired neurological deficits (HR 0.42, 95% CI 0.24-0.76, P = .004) were negatively associated with RTW.
Table 3.
Logistic Regression Model for Return to Work 2 Years After Surgery for Meningioma. Only Baseline and Immediate Tumor and Treatment-Related Covariates Were Allowed in Model
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Covariate | OR (95% CI) | OR | 95% CI | P |
| Index year | 0.96 (0.89-1.03) | 1.02 | 0.89-1.17 | .75 |
| Female (vs male) | 1.09 (0.79-1.49) | 1.37 | 0.83-2.25 | .21 |
| Age (per y) | 0.99 (0.97-1.00) | 1.00 | 0.97-1.02 | .90 |
| Income (per 100 000 SEK) | 2.22 (1.83-2.72) | 1.21 | 0.94-1.56 | .14 |
| Higher education (vs basic to high school) | 2.29 (1.64-3.20) | 1.59 | 1.00-2.54 | .05 |
| Net d absent year before surgery (per 10 d) | 0.90 (0.89-0.91) | 0.91 | 0.89-0.93 | < .01 |
| History of seizure (vs no) | 0.60 (0.44-0.82) | 1.01 | 0.62-1.64 | .97 |
| History of depression (vs no) | 0.30 (0.22-0.40) | 0.59 | 0.38-0.94 | .03 |
| Comorbidity (0, 1, 2, 3, or more) | 0.60 (0.51-0.69) | 0.97 | 0.77-1.24 | .83 |
| Functional level (per WHO category) | 0.54 (0.46-0.64) | 0.87 | 0.68-1.12 | .28 |
| WHO tumor grade II and III (vs grade I tumor) | 0.50 (0.32-0.77) | 0.46 | 0.24-0.90 | .02 |
| Reoperation because of complication (vs no) | 0.40 (0.21-0.76) | 0.57 | 0.19-1.71 | .32 |
| New deficit postoperative (vs no) | 0.45 (0.30-0.66) | 0.42 | 0.24-0.76 | < .01 |
| Tumor size | 0.83 (0.68-1.02) | 0.82 | 0.61-1.09 | .17 |
| Skull base | 0.98 (0.68-1.42) | 1.06 | 0.62-1.81 | .84 |
Abbreviations: OR, odds ratio; SEK, Swedish krona; WHO, World Health Organization.
Discussion
In this nationwide, register-based study spanning from 2009 to 2015, we demonstrate a considerable risk for long-term sick leave up to 2 years after meningioma surgery. The risk was clearly elevated in patients compared to controls. The proportion of meningioma patients working full time 1 year prior to surgery was 79.0%, declining to 49.3% at 1 year after and 57.3% 2 years after surgery, whereas controls remained constant at approximately 84% to 86%. Statistically significant predictors for still receiving sick-leave compensation 2 years postoperatively were a history of depression, higher-grade tumors, more sick-leave days in the year prior to surgery, and surgically acquired neurological deficits. Our results further demonstrate that the extent of sick leave after surgery is strongly time dependent, but that the curve flattens around 2 years postoperatively.
Comparisons across studies are hampered by variations in patient selection and differences in social security systems. Schepers et al reported that 32.9% of patients with meningioma were not able to return to their previous job after intracranial meningioma surgery.11 This percentage is higher than in our cohort, but a direct comparison is difficult to make because their study population was older (mean age, 59 years) and included only 70 patients working prior to surgery. Also, a cross-sectional approach was used by which time from surgery to follow-up, ranging between 2 and 5 years, was not defined for individual patients. The time point of 2 years postoperatively corresponds to the flattening part of our curve (Fig. 2), suggesting that the time-dependent dynamics of RTW will be difficult to judge from that study. Another cross-sectional study of a cohort of 269 patients with skull base meningioma and a median postoperative follow-up of 49 months found that 43% were employed but reported that up to 83% were potentially employable according to follow-up visits, questionnaires, or phone interviews.12 In a study with structured interviews of 91 patients and a mean follow-up of 15 months, 19% were unable to return to previous profession or daily activities at premorbid level after meningioma surgery.13 This magnitude of increase in absence from work is more in line with our findings, even though the population was older (mean age, 56 years). On the other hand, outcome was defined differently (RTW or premorbid level of daily activity). Although age was not a predictor for RTW in our cohort (defined as age 18-60 years), higher age is a well-known risk factor for dropping out of work following stroke.20–22
Prognostic Factors for Return to Work Postoperatively
Patients with meningioma showed a higher absence from work at the year prior to index date compared to controls. Furthermore, patients with a higher level of absence from work during the preoperative year were less likely to RTW within 2 years postoperatively, as can be clearly seen also in Supplementary Figures 1 to 4. This corroborates the association between sick leave prior to and following diagnosis observed in other conditions with brain damage or impairment, such as stroke22 and traumatic brain injury.23 There may be various reasons for absence from work preceding surgery, directly or indirectly related to meningioma. It can be speculated that failure to RTW is due to a heavier symptom burden, more severe comorbidity, professional detachment increasing the threshold for RTW, or a combination of these.2 The failure to RTW after diagnosis could also be influenced by selection bias. For instance, increased absence from work may be related to symptoms of headache or fatigue that increase the likelihood of undergoing neuroimaging, which in turn increases the probability of discovering an asymptomatic meningioma. Such symptoms could further increase the probability that a patient with an otherwise asymptomatic meningioma is deemed eligible for surgery. However, our data are not suitable for drawing causal inference concerning these intriguing questions.
Patients with high-grade meningiomas were less likely to RTW 2 years after meningioma surgery compared to patients with WHO grade I meningiomas. This finding is probably related to increased treatment intensity, including an increased likelihood of undergoing postoperative radiotherapy.3 Also, a more aggressive tumor is more likely to recur and thus affect neurological function and the need for reintervention.24 Psychological aspects related to having a malignant rather than benign tumor diagnosis may also be relevant, as has been shown for breast cancer.25
The presence of a new focal neurological deficit postoperatively was another strong predictor of inability to RTW 2 year after surgery. In fact, this was the only readily modifiable predictor we identified, underpinning the importance of safe surgery and good neurosurgical technique. Whether techniques such as near total safe resection and postoperative stereotactic radiosurgery, directly or at first signs of growth, would improve RTW with acceptable tumor control remains an open question, and this study is not equipped to answer this question.3,26
Patients with meningioma had statistically significant more depression compared to controls. This finding is of particular interest given that a previous history of depression was negatively associated with RTW. It is also noteworthy that the level of sick leave in the year prior to surgery in patients without history of depression was comparable to that of controls, whereas the group with a history of depression was clearly elevated. One explanation for the increased levels of sick leave among patients with a history of depression may be that receiving a diagnosis of meningioma causes secondary depressive symptoms in predisposed individuals. In support of this, we found that patients with depression had smaller tumors and, probably related to the smaller tumor size, longer waiting times from imaging to surgery (data not shown).
Alternatively—and in parallel with prior sick leave as a predictor for RTW—the association could represent detection bias of meningiomas in patients with depression. Patients presenting with depressive symptoms, tiredness, insomnia, anxiety, or chronic pain could have an increased probability of undergoing diagnostic imaging. This interpretation is in line with previous findings that an increased availability of MRI causes an increase in benign brain tumors.27 Further, if a meningioma is diagnosed in such a setting, depressive symptoms may be erroneously attributed to the tumor, or the patient may interpret the finding in this way—and consequently not accept a wait-and-scan strategy. Another explanation is that larger meningiomas, perhaps more so if in frontal locations, could cause psychiatric symptoms, as pointed out by a recent literature review.28 We did not have sufficiently detailed preoperative data to test this further, but our clinical experience tells us that all of the mentioned factors may contribute to the association between depression and meningioma. Importantly, the predictive potential of depressive symptoms suggests that the physician should not only address the meningioma, but also actively treat depression when present. Depression may indeed be an additional modifiable risk factor, if more attention would be given to treatment for depression.
Implications
Our data on the extent of sick leave after meningioma surgery provide valuable information for caregivers making risk-benefit considerations (eg, leaving behind a high-risk remnant). The data may also be used to inform patients more carefully and create realistic expectations, especially if faced with relative indications for surgery, such as small but slowly growing asymptomatic meningiomas, or smaller tumors presenting with seizures but otherwise good seizure control on antiepileptic drugs. For these patients, our results indicate that it is reasonable to consider other alternatives than upfront surgery.
Finally, since preoperative depression is associated with meningioma, an increased awareness of these symptoms may result in more holistic follow-up regimens and treatment. The removal of a small meningioma in a patient with depression and increased symptoms following detection is presumably not optimal medical care, and focus should be on the underlying condition. Further studies are required to elucidate the above-mentioned association and the effect of specific interventions.
Limitations of This Study
Our study has several limitations. It is an observational study, with many possible sources of bias from not being a randomized, comparative study. For instance, our control group was matched for age, sex, educational level, and municipality of residence, but there may be other confounding factors of relevance not captured by this procedure. Concerning the search for relevant predictors in our regression model, we were limited in the number of variables we had access to through this approach.
However, our results represent real-world data. Because we aimed to evaluate the impact of meningioma surgery, it would have been more appropriate to have a control group of matched meningioma patients not undergoing surgery. Instead, our control group consisted of well-matched individuals without meningiomas. We reasoned that our controls represented the “background” population and that our comparison established the excess risk that the meningioma causes with respect to absence from work. Our study used exact first-hand data on sick-leave compensation from the Swedish social insurance agency, allowing us to answer our main objective. However, it should be noted that our results may not be directly translated into ability to work, or quality of life.
A strength of the study is the national coverage, including close to all patients in the target population who underwent meningioma surgery in Sweden during the studied time period.
Conclusion
One year prior to index date there was a small difference in work status between cases and controls. However, at 2 years after surgery considerably more patients than controls had sick leave–related absence from work. This risk increased in case of higher-grade tumor, history of depression, or longer sick leave during the year preceding surgery. The risk for absence from work also increased if surgery resulted in a new neurological deficit.
Funding
This work was supported by a research grant from the Swedish Research Council [2017-00944].
Supplementary Material
Acknowledgments
This project was made possible by the continuous work of the SBTR: Roger Henriksson (chair), Thomas Asklund, Annika Malmström, Lena Damer, Lena Rosenlund, Rickard Sjöberg, Sofia Hylin, Peter Milos, Thomas Blystad, Sara Kinhult, Göran Hesselager, Petra Witt Nyström, Katja Werlenius, Asgeir S. Jakola, Gregor Tomasevic, Magnus Olivecrona, Margret Jensdottir, Michael Bergqvist, Marie Sjögren, Eskil Degsell, Linnea Nilsson, Kerstin Rehn, Kristina Lundqvist, and Lisa Tykosson.
Conflict of interest statement.
None declared.
References
- 1. Kruchko C, Ostrom QT, Boscia A, Truitt G, Gittleman H, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartek J Jr, Sjåvik K, Förander P, et al. Predictors of severe complications in intracranial meningioma surgery: a population-based multicenter study. World Neurosurg. 2015;83(5):673–678. [DOI] [PubMed] [Google Scholar]
- 3. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 4. Cahill KS, Claus EB. Treatment and survival of patients with nonmalignant intracranial meningioma: results from the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Clinical article. J Neurosurg. 2011;115(2):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jakola AS, Gulati M, Gulati S, Solheim O.. The influence of surgery on quality of life in patients with intracranial meningiomas: a prospective study. J Neurooncol. 2012;110(1):137–144. [DOI] [PubMed] [Google Scholar]
- 6. Nanda A, Bir SC, Maiti TK, Konar SK, Missios S, Guthikonda B.. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J Neurosurg. 2017;126(1):201–211. [DOI] [PubMed] [Google Scholar]
- 7. van Alkemade H, de Leau M, Dieleman EM, et al. Impaired survival and long-term neurological problems in benign meningioma. Neuro Oncol. 2012;14(5):658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corell A, Thurin E, Skoglund T, et al. Neurosurgical treatment and outcome patterns of meningioma in Sweden: a nationwide registry-based study. Acta Neurochir (Wien). 2019;161(2):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ra YA, Kim WH. Impact of employment and age on quality of life of individuals with disabilities: a multilevel analysis. Rehabil Couns Bull. 2015;59(2):112–120. [Google Scholar]
- 10. Matérne M, Strandberg T, Lundqvist LO. Change in quality of life in relation to returning to work after acquired brain injury: a population-based register study. Brain Injury. 2018;32(13–14):1731–1739. [DOI] [PubMed] [Google Scholar]
- 11. Schepers VPM, van der Vossen S, Berkelbach van der Sprenkel JW, Visser-Meily JMA, Post MWM. Participation restrictions in patients after surgery for cerebral meningioma. J Rehabil Med. 2018;50(10):879–885. [DOI] [PubMed] [Google Scholar]
- 12. Akagami R, Napolitano M, Sekhar LN. Patient-evaluated outcome after surgery for basal meningiomas. Neurosurgery. 2002;50(5):941–948; discussion 948–949. [DOI] [PubMed] [Google Scholar]
- 13. Krupp W, Klein C, Koschny R, Holland H, Seifert V,Meixensberger J.. Assessment of neuropsychological parameters and quality of life to evaluate outcome in patients with surgically treated supratentorial meningiomas. Neurosurgery. 2009;64(1):40–47; discussion 47. [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. [DOI] [PubMed] [Google Scholar]
- 15. Asklund T, Malmström A, Bergqvist M, Björ O, Henriksson R. Brain tumors in Sweden: data from a population-based registry 1999-2012. Acta Oncol. 2015;54(3):377–384. [DOI] [PubMed] [Google Scholar]
- 16. Elixhauser A, Steiner C, Harris DR, Coffey RM.. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 17. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 18. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rørth R, Wong C, Kragholm K, et al. Return to the workforce after first hospitalization for heart failure: a Danish Nationwide Cohort Study. Circulation. 2016;134(14):999–1009. [DOI] [PubMed] [Google Scholar]
- 20. Nakayama H, Jørgensen HS, Raaschou HO, et al. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 1994;25(4):808–813. [DOI] [PubMed] [Google Scholar]
- 21. Bagg S, Pombo AP, Hopman W. Effect of age on functional outcomes after stroke rehabilitation. Stroke. 2002;33(1):179–185. [DOI] [PubMed] [Google Scholar]
- 22. Westerlind E, Persson HC, Sunnerhagen KS. Return to work after a stroke in working age persons; a six-year follow up. PLoS One. 2017;12(1):e0169759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsson J, Esbjörnsson E, Björkdahl A, Morberg I, Nilsson M, Sunnerhagen KS.. Sick leave after traumatic brain injury. The person or the diagnosis—which has greater impact? Scand J Public Health. 2010;38(5):541–547. [DOI] [PubMed] [Google Scholar]
- 24. Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bai LJ, Liu Q, Wang M, et al. Evaluation of the psychological and biological changes of patients diagnosed with benign and malignant breast tumors. Int J Biol Markers. 2012;27(4):e322–e330. [DOI] [PubMed] [Google Scholar]
- 26. Sun QS, Hawasli AH, Huang J, Chicoine MR, Kim AH. An evidence-based treatment algorithm for the management of WHO Grade II and III meningiomas. Neurosurg Focus. 2015;38(3):E3. [DOI] [PubMed] [Google Scholar]
- 27. Solheim O, Torsteinsen M, Johannesen TB, Jakola AS.. Effects of cerebral magnetic resonance imaging in outpatients on observed incidence of intracranial tumors and patient survival: a national observational study. J Neurosurg. 2014;120(4):827–832. [DOI] [PubMed] [Google Scholar]
- 28. Kessler RA, Loewenstern J, Kohli K, Shrivastava RK.. Is psychiatric depression a presenting neurologic sign of meningioma? A critical review of the literature with causative etiology. World Neurosurg. 2018;112:64–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.