Abstract
Background
We assessed glioma incidence and disparities in postglioma survival rate in the Olmsted County, Minnesota, population.
Methods
This population-based study assessed the incidence of pathologically confirmed primary gliomas between January 1, 1995, and December 31, 2014. Age- and sex-adjusted incidence rates per 100 000 person-years were calculated and standardized to the US white 2010 population. We compared incidence trends of glioma during our study period with previously published Olmsted County data from 1950 to 1990. We assessed postglioma survival rates among individuals with different socioeconomic status (SES), which was measured by a validated individual HOUsing-based SES index (HOUSES).
Results
We identified 135 incident glioma cases (93% white) with 20 pediatric (50% female) and 115 adult cases (44% female). Overall incidence rate during our study period, 5.51 per 100 000 person-years (95% CI: 4.56-6.46), showed no significant changes and was similar to that seen in 1950 to 1990, 5.5 per 100 000 person-years. The incidence of pediatric (age < 20 years) glioma was 2.49 (95% CI: 1.40-3.58), whereas adult glioma incidence was 6.47 (95% CI: 5.26-7.67). Among those with grade II to IV gliomas, individuals with lower SES (< median HOUSES) had significantly lower 5-year survival rates compared to those with higher SES, adjusted hazard ratio 1.61 (95% CI: 1.01-2.85).
Conclusion
In a well-defined North American population, long-term glioma incidence appears stable since 1950. Significant socioeconomic disparities exist for postglioma survival.
Keywords: astrocytoma, glioblastoma, mortality, pediatric, socioeconomic
Gliomas are the most common primary malignant brain tumor in adults1,2 and the most common primary brain tumor in children.1,2 Gliomas carry significant morbidity and mortality, most noticeable in middle-aged adults with grade IV glioma (which are most commonly glioblastomas).1,2 Understanding glioma epidemiology, including longitudinal incidence, addresses public health concerns, provides insight into the pathogenesis by identifying risk factors, and offers researchers, clinicians, and patients more detailed prognostic information. Several population-based studies have been published assessing the long-term incidence of gliomas in the United States using tumor registry data from Surveillance, Epidemiology, and End Results (SEER), the Central Brain Tumor Registry of the United States (CBTRUS), and the New York State Cancer Registry, with most showing a change in incidence rates of glial tumors3,4 and/or certain glioma subtypes.2–8 However, there is limited research assessing these data in well-defined populations using extensive clinical annotation. To the best of our knowledge only a few previous studies have been published that used extensive clinical annotation in well-defined populations, and all were performed on the same population as in our study setting.9–11 Those studies found the all-ages glioma incidence rate from 1950 to 1990 was 5.5 per 100 000 person-years (calculated from 5.0 for symptomatic gliomas plus 0.7 for asymptomatic gliomas minus 0.2 for ependymomas), with the risk noticeably increasing after age 45 years, particularly for grade IV gliomas.11 Since then, the incidence in this population has not been assessed and updating the incidence of glioma provides a unique opportunity to further understand the epidemiology of glioma over time. It also addresses public health concerns, particularly in the setting of modern environmental changes like widespread cell phone use,12 but also establishes a baseline in a changing health-care environment (eg, the introduction of the Affordable Care Act occurred near the end of our study period).
Along these lines, there has been limited literature addressing disparities in postprimary brain tumor survival by race/ethnicity and socioeconomic status (SES) in populations. Specifically, given the impact of SES on health outcomes at multiple levels,13 assessing socioeconomic disparities in postglioma survival addresses an important unmet need of patients. One US study using SEER data from 2003 to 2012 showed SES disparities in glioma therapies used and survival.14 Another US study showed increased glioma incidence and also increased survival in residents of counties with higher SES.15 Both of these studies used aggregate data (such as census block groups or county data) to determine SES.14,15 Although access to surgery and radiation therapy is likely to be an important outcome determinant,14 SES is a key element of health and a person’s ability to access resources.16 However, in health research it is often difficult to assess a person’s SES accurately because SES is frequently unavailable in commonly used data sources for research. To overcome the unavailability of SES for research, we developed and validated an individual HOUsing-based SES index called HOUSES, which has been extensively used in health disparities and social epidemiology research.17–27 Briefly, unlike aggregate-level SES measures, which are commonly used in research, HOUSES is an individual-level SES measure derived from real property data of an individual housing unit, which are publicly available from an assessor’s office of local government.
In summary, we aim to assess longitudinal glioma incidence rates and disparities in postglioma survival using a population-based study in a well-defined US population using comprehensive medical record reviews including biopsy data. We also compare our longitudinal incidence data to previous glioma incidence data from the same population, that of Olmsted County, Minnesota.11 This offers a unique and comparative assessment of glioma incidence across several decades.
Design and Methods
Study Setting
We performed a population-based study using residents from the mixed urban-rural population of Olmsted County, Minnesota. The unique epidemiological advantages of Olmsted County make it an ideal study setting for population-based studies such as this,28 as elegantly outlined in a paper by Kurland et al.10 Essentially, the population of Olmsted County is relatively isolated from other urban centers, acting as a self-contained health-care environment. Despite its isolation, it has a major medical center with comprehensive capabilities for managing brain tumors. Furthermore, the records system for nearly all Olmsted County medical providers and their individual patients is linked (through the Rochester Epidemiology Project [REP], a National Institutes of Health–funded medical record linkage system) and fully complete, going back decades.29 On registering with any health-care provider in Olmsted County, patients and/or parents or guardians are asked to refuse or grant consent for their medical records to be used in research, and this research authorization is granted for more than 95% of patients. The medical records are easy to access and include essentially all formal medical and surgical encounters through medical index search codes even before the availability of International Classification of Diseases codes. In essence, the entire population uses Mayo Clinic exclusively for all brain tumor management, with all the relevant medical records easily accessible, and with full neuro-oncology capabilities, including neurology, neuroradiology, neuropathology, and neurosurgery. This makes Olmsted County an ideal population among which to perform population-based studies on neurological diseases, including brain tumors, because it captures all incident cases of brain tumors. Previous glioma incidence studies have been performed on this same population, dating back to 1950,11 providing a unique opportunity to compare our recent data with glioma incidence rates over the past several decades.
Study Design
We conducted a population-based, retrospective cohort study that assessed the incidence of all glioma cases both in adults and children and compared postglioma survival rates among individuals with different race and SES as measured by HOUSES index during the study period January 1, 1995, through December 31, 2014. Because previous studies assessed the incidence of glioma between 1950 and 1990, our present study results allow us to naturally assess the long-term incidence of glioma since then, as well as assess stability or trends during our study period.
Study Participants and Case Ascertainment
The Mayo Clinic Tumor Registry and the REP were used to identify Olmsted County, Minnesota, residents with an International Classification of Diseases, Ninth Revision diagnosis code for glioma occurring during the study period (January 1, 1995, through December 31, 2014). Patients were excluded from the study if they did not have a research authorization at each medical office they used, which almost exclusively consisted of Mayo Clinic and the Olmsted County Medical Centers. The medical records of patients with research consent were reviewed to determine whether they had a primary glioma occurring during the study period, if they had a tissue-based pathology report, if they had a glioma-related syndrome (eg, neurofibromatosis, and others as described as follows in exclusion criteria), and (also using the REP data) if they were residents of Olmsted County at the time of the pathology-based diagnosis and 1 year prior (to exclude referral cases). The review occurred in a similar manner as the earlier incidence study described above.11 World Health Organization grading was used based on tissue pathology reports. The eligibility criteria were as follows:
Research authorization on file both at Mayo Clinic and Olmsted Medical Center
Primary glioma, not a recurrence, with the diagnosis occurring during our study period of January 1, 1995, through December 31, 2014
Glioma diagnosis based on tissue pathology, which includes only astrocytomas, oligodendrogliomas, mixed oligoastrocytomas, gliosarcomas, and gliomas not otherwise specified (NOS)
Residency within Olmsted County at the time of diagnosis and 1 year prior to diagnosis date
Patients were excluded if they met any of the following exclusion criteria:
Absence of research authorization at Mayo Clinic or Olmsted Medical Center
Recurrent glioma, or a glioma diagnosis occurring outside the range of our study dates
An imaging-only diagnosis (without a pathology diagnosis)
Nontumors and nongliomas (including a diagnosis of an ependymoma, metastatic tumor, choroid plexus tumor, meningioma, neuronal or mixed glial-neural tumor (ganglioglioma), dysembryoplastic neuroepithelial tumor, germinoma, pineal tumor, or embryonal tumor)
The presence of a glioma-associated genetic syndrome (including neurofibromatosis types 1 and 2, tuberous sclerosis complex, nevoid basal cell carcinoma syndrome, adenomatous polyposis syndrome, linear nevus sebaceus syndrome, and Li-Fraumeni cancer family syndrome or inherited p53 mutations)
Residency outside Olmsted County at the time of the diagnosis or 1 year prior to diagnosis (this criterion excludes referral cases)
An insufficient medical record (ie, lacking any data required to meet the inclusion criteria as described above)
Study Outcomes
We assessed 2 main study outcomes: the incidence of glioma and postglioma 5-year survival rate. We assessed whether postglioma survival rates differ by race and SES as measured by HOUSES index, limiting our analysis to grades II to IV, because grade I glioma has distinctive pathophysiological characteristics and associated better survival rates.1,2 We categorized race into binary variable (white vs nonwhite) because the study participants were predominantly white and detailed ethnic information was not uniformly available during the study period.
Predictor Variables
The main predictor variables for postglioma 5-year survival rate were race and SES. We obtained race data from the REP that were reported by patients at the time of registration. SES was measured by HOUSES index, and the development, initial testing, and validation of this index have been previously reported.17 Briefly, the street addresses of eligible study participants were geocoded and matched to real property data of individual housing units from the county assessor’s office. Principal components factor analysis identified 4 real property variables (housing value, square footage of housing unit, number of bedrooms, and number of bathrooms) that were then formulated into a standardized HOUSES index score through summation of the z score for each variable. A higher HOUSES (z score) means a higher SES. A broad range of health outcomes both in adults and children have been previously reported to be associated with HOUSES, which include risk of low birth weight, obesity, smoking exposure at home, asthma control status, vaccination, pneumococcal disease, postmyocardial infarction mortality, falls, rheumatoid arthritis, and multiple chronic conditions.17–27 Also, we included other demographic variables, such as age and sex, in the analysis.
Data Analysis
Overall age- and sex-adjusted incidence rates of glioma during the study period were calculated based on the Olmsted County population and standardized to the 2010 US white population. Ninety-five percent CIs for the incidence rates were calculated under the Poisson distribution. Incidence rates of the most common subtypes (grade IV astrocytomas for adult analysis, and grade I astrocytomas for pediatric analysis) were also calculated. Poisson regression of the age- and sex-adjusted incidence rates per calendar year, for adults (age 20 years and older) and children (age < 20 years), was used to investigate temporal, age, and sex trends.
The Kaplan-Meier method was used to estimate 5-year survival rates among all ages. Individuals diagnosed at autopsy were excluded from the survival analysis. Among all-ages grade II to IV glioma cases, we assessed the impact of SES (as measured by HOUSES index and categorized into 2 groups: above and below median) on postglioma survival rates adjusting for age, sex, and race (white vs nonwhite) using a Cox proportional hazard model.
Ethics Statement
The study was approved by our institutional review board.
Results
Characteristics of Study Participants
A total of 567 patients were found in the Mayo Clinic Tumor Registry or the REP with a diagnosis code for glioma. Of these, 432 were excluded based on the inclusion and exclusion criteria described in the Methods section. Specifically, the reasons for exclusion were metastatic tumor (106), nonglioma tumor (102), not a resident of Olmsted County at the time of diagnosis or 1 year prior (77), imaging-only diagnosis (43), recurrent glioma or index date outside our study period (38), nontumor lesions (28), lack of research consent (21), presence of glioma-associated genetic syndrome (13), and insufficient medical record (4). A total of 135 Olmsted County residents (93% white), composed of 115 adults (51 female) and 20 children (10 female), were identified as having been diagnosed by tissue pathology with a primary glioma during the study period. Unless specified as “all ages,” the summarized data are given as 2 separate groups: children or pediatric (ages 0-19 years) and adults (ages 20 years and older), as shown in Table 1. The overall age- and sex-adjusted incidence rate for all ages was 5.5 per 100 000 person-years (95% CI: 4.56-6.46).
Table 1.
Demographics and Glioma Characteristics
| Adults, Ages 20 y and Older | Children, Ages 0 to 19 y | |
|---|---|---|
| Glioma cases | 115 | 20 |
| Female | 51 (44%) | 10 (50%) |
| Age, y | Median age (interquartile range) | Mean age, age range |
| 58 (41.9 to 69.5) | 10.8, 2.4 to 18.6 | |
| Subtypes | 98 (85%) astrocytoma | 19 (95%) astrocytomas |
| 6 (6%) grade I | 12 (63%) grade I pilocytic | |
| 20 (20%) grade II/III | 5 (26%) grade II/III | |
| 72 (73%) grade IV | 2 (11%) grade IV | |
| 8 (7%) oligodendroglioma (grade II/III) | 1 (5%) glioma NOS | |
| 7 (6%) mixed oligoastrocytoma (grade II/III) | 1 (100%) grade I | |
| 2 (2%) glioma NOS (grade IV) | ||
| Grade | 6 (5%) grade I | 13 (65%) grade I |
| 35 (30%) grade II/III | 5 (25%) grade II/III | |
| 74 (64%) grade IV | 2 (10%) grade IV |
Abbreviation: NOS, not otherwise specified.
Glioma in Adults
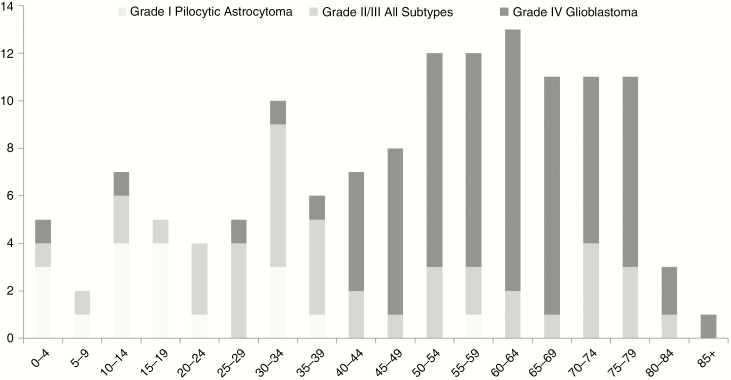
Males comprised 56% of adult (ages 20 years and older) glioma patients. The median (interquartile range) age for adult patients was 58 years (range, 41.9-69.5 years). For subtypes of adult glioma, astrocytoma accounted for the majority of adult cases (98 of 115) at 85%. Of the 98 astrocytomas, 72 (73%) were grade IV, 20 (20%) were grades II/III, and 6 (6%) were grade I. The remainder of the adult gliomas included 8 oligodendrogliomas (all grade II/III), 7 mixed oligoastrocytomas (all grades II/III), and 2 grade IV gliomas NOS. In terms of tumor grade, grade IV accounted for the majority of adult cases at 64%. Grade IV astrocytoma (glioblastoma) became the predominant tumor type after age 40 years (77%), though occurring in only 12% of adults younger than 40 years, as seen in Fig. 1. Also seen in Fig. 1 is that the majority (68%) of tumors in patients ages 20 to 39 years were grades II/III (which included astrocytomas, oligodendrogliomas, or mixed oligoastrocytomas).
Fig. 1.
Number of Glioma Patients (With Glioma Grade and Subtype) per Age Group, y The majority (77%) of gliomas after age 40 years were grade IV glioblastoma, which occurred in only 12% of adults younger than 40 years. The majority (68%) of gliomas in patients ages 20 to 39 years were grades II/III. The majority (60%) of gliomas in patients ages 0 to 19 years were grade I pilocytic astrocytomas. Unspecified gliomas are excluded.
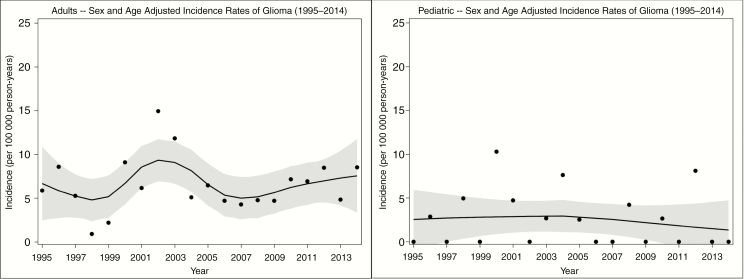
The overall age- and sex-adjusted incidence rate for gliomas in adults was 6.47 per 100 000 person-years (95% CI: 5.26-7.67). There was not a significant trend over the 20-year time period, as seen in Fig. 2. Adult males, 7.87 (95% CI 5.90-9.84), had a higher rate than adult females, 5.23 (95% CI 3.78-6.69). The incidence rate for grade IV astrocytoma in adults was 4.31 (95% CI 3.31-5.32), as seen in Table 2.
Fig. 2.
Adult (Left) and Childhood (Right) Glioma Incidence by Year Sex- and age-adjusted glioma incidence rate of adults (left) and children (right) per year from 1995 to 2014 with Loess smoother (black line) with 95% confidence intervals for the Loess smooths (dotted lines). All incidence rates are per 100 000 person-years.
Table 2.
Glioma Incidence in Adults (Ages ≥ 20 y) per Age Group
| Age Groups, y | |||||||
|---|---|---|---|---|---|---|---|
| 20 to 29 | 30 to 39 | 40 to 49 | 50 to 59 | 60+ | Total | ||
| Totala | (9) | (16) | (15) | (25) | (50) | (115) | |
| 2.11 (0.96-4.00) | 3.83 (2.19-6.22) | 3.76 (2.10-6.20) | 7.61 (4.93-11.24) | 11.51 (8.54-15.18) | 6.47 (5.26-7.67) | ||
| Sex | Maleb | (5) | (9) | (7) | (15) | (28) | (64) |
| 2.56 (0.83-5.96) | 4.44 (2.03-8.43) | 3.64 (1.46-7.49) | 9.59 (5.37-15.82) | 14.72 (9.78-21.27) | 7.87 (5.90-9.84) | ||
| Femaleb | (4) | (7) | (8) | (10) | (22) | (51) | |
| 1.73 (0.47-4.42) | 1.73 (0.47-4.42) | 3.87 (1.67-7.62) | 5.81 (2.79-10.69) | 9.01 (5.65-13.65) | 5.23 (3.78-6.69) | ||
| Tumor type | WHO IV astrocytomaa | (1) | (2) | (12) | (19) | (38) | (72) |
| 0.23 (0.01-1.30) | 0.48 (0.06-1.73) | 3.01 (1.55-5.25) | 5.79 (3.48-9.04) | 8.75 (6.19-12.01) | 4.31 (3.31-5.32) | ||
| All other typesa | (8] | (14) | (3) | (6) | (12) | (43) | |
| 1.87 (0.81-3.69) | 3.35 (1.83-5.63) | 0.75 (0.15-2.20) | 1.83 (0.67-3.98) | 2.76 (1.43-4.83) | 2.15 (1.49-2.81) | ||
Abbreviation: WHO, World Health Organization.
Data listed as (count) incidence rate (95% CI). All incidence rates are per 100 000 person-years.
aAge-specific, sex-adjusted incidence rates (except for final Total column, which is age- and sex-adjusted).
bAge-specific incidence rates (except for final Total column, which is age-adjusted).
Comparing adult age groups, as seen in Table 2, the incidence rates increased from 2.11 per 100 000 person-years for the 20- to 29-year age group to its peak of 11.51 for the 60 years and older age group, most noticeably increasing after age 50 years.
Glioma in Children
Twenty gliomas were diagnosed in children (age < 20 years), age range 2.4 to 18.6 years (mean, 10.8 years), with sex equally affected (10 boys, 10 girls). Nineteen gliomas were identified as astrocytomas (12 were grade I and pilocytic, 5 were grades II/III, and 2 were grade IV) and 1 was a grade I glioma NOS. There were no oligodendrogliomas or oligoastrocytomas.
The age- and sex-adjusted incidence rate of childhood glioma was 2.49 per 100 000 person-years (95% CI: 1.40-3.58). There was not a significant trend over the 20-year time period, as seen in Fig. 2. Incidence rate of boys was 2.43 (95% CI: 0.93-3.94) and for girls it was 2.55 (95% CI: 0.97-4.12), as seen in Table 3. The incidence of grade I pilocytic astrocytoma in children was 1.49 (95% CI: 0.65-2.34), which occurred in 12 (60%) of the 20 childhood cases. The remaining 8 cases had an incidence rate of 0.99 (95% CI: 0.31-1.68).
Table 3.
Glioma Incidence in Children (Ages < 20 y)
| Totala | (20) | 2.49 | (1.40 | 3.58) | |
|---|---|---|---|---|---|
| Sex | Maleb | (10) | 2.43 | (0.93 | 3.94) |
| Femaleb | (10) | 2.55 | (0.97 | 4.12) | |
| Tumor type | WHO I astrocytomaa | (12) | 1.49 | (0.65 | 2.34) |
| All othersa | (8) | 0.99 | (0.31 | 1.68) |
Abbreviation: WHO, World Health Organization.
Data listed as (count) incidence rate (95% CI). All incidence rates are per 100 000 person-years.
aAge- and sex-adjusted incidence rates.
bAge-adjusted incidence rates.
Postglioma Survival Rates and Disparities for All Ages
Survival estimates for all ages can be seen in Table 4, which excludes 2 patients who were diagnosed at autopsy (ages 13 and 78 years, both grade IV, one above the median SES and one below the median SES). Only 6.2% of grade IV astrocytoma patients had survived at 5 years. Patients with grade I gliomas, which had 100% survival at 5 years, were excluded from further survival analysis.
Table 4.
Kaplan-Meier 5-Year Survival Estimates for All Ages by Glioma Grade and Subtype
| Grade | No. | 5-y Estimated Survival (95% CI) | Subtype | No. | 5-y Estimated Survival (95% CI) |
|---|---|---|---|---|---|
| I | 19 | 100.0% (100.0%-100.0%) | Astrocytoma | 18 | 100.0% (100.0%-100.0%) |
| Not otherwise specified | 1 | 100.0% (100.0%-100.0%) | |||
| II | 20 | 84.2% (69.3%-100%) | Astrocytoma | 9 | 77.8% (54.9%-100.0%) |
| Oligodendroglioma and mixed oligoastrocytoma | 11 | 90.1 (73.2%-100.0%) | |||
| III | 20 | 40.6% (23.2%-71.1%) | Astrocytoma | 16 | 27.8% (12.2-63.3) |
| Oligodendroglioma and mixed oligoastrocytoma | 4 | 100% (100%-100%) | |||
| IV | 74 | 6.0% (2.1%-16.9%) | Astrocytoma | 72 | 6.2% (2.2%-17.4%) |
| Not otherwise specified | 2 | 0.0% (0.0%-0.0%) |
Data exclude 2 patients who were diagnosed at autopsy (ages 13 and 78 years, both grade IV).
Survival varied based on tumor type for grade II/III gliomas, with oligodendroglioma and mixed oligoastrocytoma having better survival compared to astrocytoma; hazard ratio is 0.16 (95% CI: 0.04-0.72).
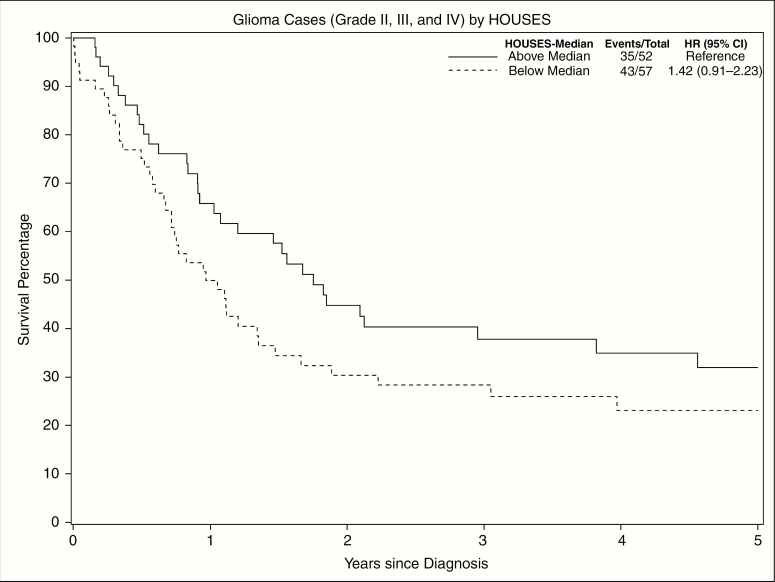
Among grade II to IV glioma patients, lower SES had higher mortality, although not statistically significant, as seen in Fig. 3 (unadjusted hazard ratio 1.42, 95% CI: 0.91-2.23). When controlling for age, sex, grade, and race, lower SES had statistically significantly higher mortality compared to higher SES, adjusted hazard ratio 1.61 (95% CI: 1.01-2.85).
Fig. 3.
Disparities in Postglioma Survival Rates (Grades II, III, and IV) Among Patients With Different SES as Measured by HOUSES (Above vs Below Median HOUSES Index) The Kaplan-Meier estimator for grade II to IV glioma patients shows increased survival times for those with higher SES. HOUSES indicates HOUsing-based SES index; HR, hazard ratio; SES, socioeconomic status.
Discussion
The age- and sex-adjusted incidence rate of primary gliomas in Olmsted County, Minnesota, during January 1, 1995, through December 31, 2014, for all ages was 5.51 per 100 000 person-years. This rate is stable from an earlier study on the same population of Olmsted County from 1950 to 1990,11 which reported an all-ages incidence rate of 5.5 per 100 000 person-years. Despite many changes in environment during the study period, the incidence rate for each calendar year over the 20-year period of 1995 to 2014 did not show a significant change in either adults or children, as seen in Fig. 2. Though the incidence rate of gliomas has been stable over the past 65 years, there were significant disparities in the postglioma survival rate among patients with different SES for those with grades II to IV glioma (patients with lower SES had a significantly shorter survival rate than those with higher SES).
Comparison to Other Population-Based Glioma Incidence Studies
The glioma incidence study in Olmsted County from 1950 to 1990, as described in Background, estimated an age- and sex-adjusted incidence rate of 5.5 per 100 000 person-years for all ages. This is essentially the same incidence rate in our study, which included symptomatic and asymptomatic gliomas, and excluded ependymomas.
Comparing the all-ages incidence rate to other population-based studies gives varying, but relatively similar, results. The Lothian region in Scotland showed an all-ages rate of 7.7 during 1989 to 1990.30 The rate was only 4.2 and 4.1 for the Māori and non-Māori populations, respectively, in New Zealand during 1993 to 2003.31 CBTRUS data from 2008 to 2012 showed a glioma incidence rate (when using the same gliomas as in this study, ie, excluding ependymomas and neuroepithelial tumors, and using the white population data to match our study population, which was mostly white) of 6.01,1 and in 2011 to 2015 it was 5.96.2
When we focused on adult gliomas (ages 20 years and older), the age- and sex-adjusted incidence rate was 6.5 per 100 000 person-years, which is relatively similar to other glioma incidence studies. For example, the rate found in the regions of Lancashire and South Cumbria, Britain, during 2006 to 2010 for patients age 15 years and older was 7.1.32
The pediatric incidence rate was 2.49 for all gliomas, and 1.49 for grade I astrocytoma, which accounted for 60% of pediatric gliomas. Similar to adult gliomas, the pediatric gliomas did not show a significant trend when viewed over the 20-year period, as seen in Fig. 2. But unlike with adult gliomas, young male and female patients were equally affected. The incidence rate that we found is relatively similar to other reported studies. The study conducted in the Lothian region in Scotland showed a rate of 3.5 for those younger than 15 years.30 The Netherlands study showed a rate of 2 for children.33 For US studies, CBTRUS data from 2008 to 2012 (when using the same glioma types as in our study, ie, excluding ependymomas and neuroepithelial tumors, and using the white population data to match our study population, which was mostly white) showed a glioma incidence rate of 2.35 for children ages 0 to 19 years,1 and CBTRUS data from 2011 to 2015 showed a rate of 2.45.2
Effects of Age and Sex
Although gliomas can occur at any age, there is an increased risk for developing a glioma as age increases, as well as a risk for developing a higher grade of glioma. The median age of adult glioma diagnosis in our study was approximately 58 years. The highest incidence rate was seen in the oldest age group, that of 60 years and older. And the incidence rate escalated, essentially doubling itself, between the age group of 40 to 49 to the age group of 50 to 59, as seen in Table 2.
Comparing age groups is noteworthy because different age groups are affected by different tumors, as seen in Fig. 1. Grade IV glioblastoma became the increasingly predominant tumor type after age 40 years, accounting for 77% of gliomas. The incidence of glioma in adults noticeably increased after age 50, which parallels the increase in grade IV gliomas. This finding is consistent with data from an incidence study on glioblastoma multiforme in Los Angeles County from 1974 to 1999, where the rate rose substantially after age 40, peaking at the age group of 70 to 79 years.34 The relationship with age seen in our data is also consistent with the data from Olmsted County from 1950 to 1990,11 which showed an increasing risk of glioma as age increases in adults, most noticeably after age 45 years in that study. With that comparison we can conclude that grade IV glioblastomas are not occurring at a younger age.
Younger ages also have predilections for certain tumor types. Although seen at all ages, grade II/III gliomas (of any subtype) were most commonly seen in young adults (age groups of 20-39 years), for whom they comprised the majority (68%) of gliomas. For children (younger than 20 years) the most common tumor was a grade I pilocytic astrocytoma, which accounted for 60% of pediatric gliomas in our study. In summary, as illustrated in Fig. 1, our data reliably show older adults are primarily affected by grade IV astrocytomas, younger adults are primarily affected by grade II/III gliomas, and children are predominantly affected by grade I astrocytomas.
Astrocytomas were the most common subtype seen in all age groups, except for the 20- to 29-year age group, in whom mixed oligoastrocytoma was the most common subtype. Overall, astrocytomas accounted for 85% of adult gliomas and 95% of pediatric gliomas.
Sex is also a risk factor for glioma in adults. Men had a higher incidence, 7.87 vs 5.23, compared to women. Sex difference has been previously shown in a population-based study from the Netherlands during 1989 to 2003, which showed an increased rate in men compared to women, 6 and 4, respectively.33 Other studies have shown a sex difference as well.1,2,34
Factors Associated With Postglioma Survival Rate
Our study showed tumor type and tumor grade affected survival. Higher tumor grades led to shortened survival, which has been shown before.1,2 Similarly, grade II/III oligodendroglioma and mixed oligoastrocytoma had better survival times than grade II/III astrocytoma. It should be noted that glioma nomenclature has changed in recent years, and the term oligoastrocytoma is no longer used. Molecular genetics is being incorporated into glioma nomenclature, particularly isocitrate dehydrogenase (IDH) status (ie, IDH mutant or IDH wild-type) and the presence or absence of 1p/19q codeletion. IDH and 1p/19q status were not uniformly available during the time period of our study.
In addition to tumor characteristics having an effect on mortality, our study showed significant disparities in postglioma survival based on patients’ SES as measured by HOUSES. For example, patients with a lower SES had a significantly lower 5-year survival rate, compared to those with higher SES for grades II, III, and IV tumors. The effect of SES on survival rate was comparable to effects of using radiotherapy or chemotherapy.35–38 SES’s effect on glioma survival has been previously seen in the United States, where aggregate data were used to determine SES.14,15 For example, when analyzing SEER data, Deb et al showed that higher SES had more radiation and surgery compared to lower SES, as well as slightly improved survival times.14 One hypothesis is that higher SES allows better access to care and thus more and earlier radiation and surgical treatments, leading to longer survival.14 Outside the United States, studies on glioblastoma multiforme in Australia showed SES did not affect survival outcome, but rather participation in a clinical trial did.39–41 Alternatively, different health literacy among patients with different SES might affect the timing of glioma detection (eg, among patients with lower SES, delayed identification of glioma and its ensuing advanced stage may lead to poor survival). In this respect, HOUSES index as an individual-level SES measure can be a useful nonclinical prognostic marker for postglioma survival because it represents an important social determinant of a patient’s health that affects multiple factors and mechanisms underlying postglioma survival.13 To reduce and ultimately eliminate disparities in postglioma survival rates among individuals with different SES, it is important for a health-care system and for clinicians to recognize that patients with lower SES might have limited health literacy and access to survival-determining interventions. Thus, implementing support systems for this underserved population from the beginning of clinical care (right after the diagnosis of glioma) needs to be considered, and in this context the HOUSES index will be an important tool to identify such an underserved population in oncology practice.
Strengths and Limitations
The main strength of this study is the population-based method of determining glioma incidence rate based on pathology report. The incidence rates determined in our study can then be compared to other population-based studies as well as tumor registry data. Importantly, our data can be compared to previous data from Olmsted County, giving a unique look at glioma trends over several decades. Also, our study setting had unique epidemiological advantages such as the REP and a self-contained health-care environment, both of which allowed us to conduct this population-based study capturing all eligible incident cases of glioma. Our study used an innovative, individual-level SES measure (HOUSES index) to assess socioeconomic disparities in postglioma survival rates. Our study has inherent limitations as a retrospective study. However, given the low incidence of glioma, retrospective studies are often the only way to assess the incidence estimate. Because of the limited number of incident glioma cases during the study period, we were not able to fully address the incidence of glioma among people with different race/ethnic backgrounds. Because our study setting has a predominantly white population, our study findings might have limitations in being generalizable to other study settings with differential racial/ethnic compositions. We did not include a specific genotype of glioma or medications or interventions in the assessment of disparities of postglioma survival, and these elements need to be studied in the future. Another limitation of our study is that it includes a relatively small amount of pediatric cases.
Conclusion
Despite many changes in environment, demographics, and health-care delivery in recent years, the overall all-ages incidence of glioma over the past several decades (since 1950) in a North American population has been stable. Though our present study corroborates previously reported risk factors for glioma and its outcome (eg, age, sex, grade, and subtype), we found significant socioeconomic disparities in the postglioma survival rate. The HOUSES index can be a useful tool for identifying high-risk underserved populations at the time of glioma diagnosis. Further studies are necessary to understand the nature of socioeconomic disparities observed in our study and to develop interventions eliminating such disparities.
Funding
This work was supported by the National Institutes of Health [R01 HL126667] and the Mayo Foundation.
Conflict of interest statement. None declared.
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17(suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977-2000. Cancer. 2004;101(10):2293–2299. [DOI] [PubMed] [Google Scholar]
- 4. Li K, Lu D, Guo Y, et al. Trends and patterns of incidence of diffuse glioma in adults in the United States, 1973-2014. Cancer Med. 2018;7(10):5281–5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKinley BP, Michalek AM, Fenstermaker RA, Plunkett RJ. The impact of age and sex on the incidence of glial tumors in New York State from 1976 to 1995. J Neurosurg. 2000;93(6):932–939. [DOI] [PubMed] [Google Scholar]
- 6. McCarthy BJ, Propp JM, Davis FG, Burger PC.. Time trends in oligodendroglial and astrocytic tumor incidence. Neuroepidemiology. 2008;30(1):34–44. [DOI] [PubMed] [Google Scholar]
- 7. Gittleman HR, Ostrom QT, Rouse CD, et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. 2015;121(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Achey RL, Khanna V, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Incidence and survival trends in oligodendrogliomas and anaplastic oligodendrogliomas in the United States from 2000 to 2013: a CBTRUS report. J Neurooncol. 2017;133(1):17–25. [DOI] [PubMed] [Google Scholar]
- 9. Kurland LT. The frequency of intracranial and intraspinal neoplasms in the resident population of Rochester, Minnesota. J Neurosurg. 1958;15(6):627–641. [DOI] [PubMed] [Google Scholar]
- 10. Kurland LT, Schoenberg BS, Annegers JF, Okazaki H, Molgaard CA. The incidence of primary intracranial neoplasms in Rochester, Minnesota, 1935-1977. Ann N Y Acad Sci. 1982;381:6–16. [DOI] [PubMed] [Google Scholar]
- 11. Radhakrishnan K, Mokri B, Parisi JE, O’Fallon WM, Sunku J, Kurland LT.. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37(1):67–73. [DOI] [PubMed] [Google Scholar]
- 12. Yang M, Guo W, Yang C, et al. Mobile phone use and glioma risk: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0175136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deb S, Pendharkar AV, Schoen MK, Altekruse S, Ratliff J, Desai A.. The effect of socioeconomic status on gross total resection, radiation therapy and overall survival in patients with gliomas. J Neurooncol. 2017;132(3):447–453. [DOI] [PubMed] [Google Scholar]
- 15. Cote DJ, Ostrom QT, Gittleman H, et al. Glioma incidence and survival variations by county-level socioeconomic measures. Cancer. 2019;125(19):3390–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56(4):769–784. [DOI] [PubMed] [Google Scholar]
- 17. Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health. 2011;88(5):933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris MN, Lundien MC, Finnie DM, et al. Application of a novel socioeconomic measure using individual housing data in asthma research: an exploratory study. NPJ Prim Care Respir Med. 2014;24:14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson MD, Urm SH, Jung JA, et al. Housing data-based socioeconomic index and risk of invasive pneumococcal disease: an exploratory study. Epidemiol Infect. 2013;141(4):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bang DW, Manemann SM, Gerber Y, et al. A novel socioeconomic measure using individual housing data in cardiovascular outcome research. Int J Environ Res Public Health. 2014;11(11):11597–11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghawi H, Crowson CS, Rand-Weaver J, Krusemark E, Gabriel SE, Juhn YJ.. A novel measure of socioeconomic status using individual housing data to assess the association of SES with rheumatoid arthritis and its mortality: a population-based case-control study. BMJ Open. 2015;5(4):e006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wi CI, St Sauver JL, Jacobson DJ, et al. Ethnicity, socioeconomic status, and health disparities in a mixed rural-urban US community—Olmsted County, Minnesota. Mayo Clin Proc. 2016;91(5):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahashi PY, Ryu E, Hathcock MA, et al. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health. 2016;70(3):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryu E, Juhn YJ, Wheeler PH, et al. Individual housing-based socioeconomic status predicts risk of accidental falls among adults. Ann Epidemiol. 2017;27(7):415–420.e2. [DOI] [PubMed] [Google Scholar]
- 25. Ryu E, Olson JE, Juhn YJ, et al. Association between an individual housing-based socioeconomic index and inconsistent self-reporting of health conditions: a prospective cohort study in the Mayo Clinic Biobank. BMJ Open. 2018;8(5):e020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wi CI, Gauger J, Bachman M, et al. Role of individual-housing-based socioeconomic status measure in relation to smoking status among late adolescents with asthma. Ann Epidemiol. 2016;26(7):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hammer R, Capili C, Wi CI, Ryu E, Rand-Weaver J, Juhn YJ.. A new socioeconomic status measure for vaccine research in children using individual housing data: a population-based case-control study. BMC Public Health. 2016;16(1):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwon HJ, Bang DW, Kim EN, et al. Asthma as a risk factor for zoster in adults: a population-based case-control study. J Allergy Clin Immunol. 2016;137(5):1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ III.. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Counsell CE, Collie DA, Grant R. Incidence of intracranial tumours in the Lothian region of Scotland, 1989-90. J Neurol Neurosurg Psychiatry. 1996;61(2):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexander H, Irwin C, Purdie G, Hunn M.. Incidence and management of high grade glioma in Māori and non-Māori patients. J Clin Neurosci. 2010;17(9):1144–1147. [DOI] [PubMed] [Google Scholar]
- 32. Sehmer EA, Hall GJ, Greenberg DC, et al. Incidence of glioma in a northwestern region of England, 2006-2010. Neuro Oncol. 2014;16(7):971–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Houben MP, Aben KK, Teepen JL, et al. Stable incidence of childhood and adult glioma in the Netherlands, 1989–2003. Acta Oncol. 2006;45(3):272–279. [DOI] [PubMed] [Google Scholar]
- 34. Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104(12):2798–2806. [DOI] [PubMed] [Google Scholar]
- 35. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 36. Keime-Guibert F, Chinot O, Taillandier L, et al. ; Association of French-Speaking Neuro-Oncologists Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. [DOI] [PubMed] [Google Scholar]
- 37. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 38. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 39. Field KM, Drummond KJ, Yilmaz M, et al. Clinical trial participation and outcome for patients with glioblastoma: multivariate analysis from a comprehensive dataset. J Clin Neurosci. 2013;20(6):783–789. [DOI] [PubMed] [Google Scholar]
- 40. Sia Y, Field K, Rosenthal M, Drummond K.. Socio-demographic factors and their impact on the number of resections for patients with recurrent glioblastoma. J Clin Neurosci. 2013;20(10):1362–1365. [DOI] [PubMed] [Google Scholar]
- 41. Field KM, Rosenthal MA, Yilmaz M, Tacey M, Drummond K.. Comparison between poor and long-term survivors with glioblastoma: review of an Australian dataset. Asia Pac J Clin Oncol. 2014;10(2):153–161. [DOI] [PubMed] [Google Scholar]