Abstract
The type of Ophiocordyceps unilateralis (Ophiocordycipitaceae, Hypocreales, Ascomycota) is based on an immature specimen collected on an ant in Brazil. The host was identified initially as a leaf-cutting ant (Atta cephalotes, Attini, Myrmicinae). However, a critical examination of the original illustration reveals that the host is the golden carpenter ant, Camponotus sericeiventris (Camponotini, Formicinae). Because the holotype is no longer extant and the original diagnosis lacks critical taxonomic information – specifically, on ascus and ascospore morphology – a new type from Minas Gerais State of south-east Brazil is designated herein. A re-description of the fungus is provided and a new phylogenetic tree of the O. unilateralis clade is presented. It is predicted that many more species of zombie-ant fungi remain to be delimited within the O. unilateralis complex worldwide, on ants of the tribe Camponotini.
Keywords: Atlantic rainforest, Camponotus sericeiventris, carpenter ants, epitype, Ophiocordyceps, phylogeny
INTRODUCTION
Ophiocordyceps unilateralis (Ophiocordycipitaceae: Hypocrea-les) is a fungal pathogen of ants belonging to the tribe Camponotini (Formicinae: Formicidae) with a pantropical distribution (Evans 2001). The fungus alters the behaviour of the ant host causing it to move and die away from the nest, often in an exposed position and, typically, clinging onto and biting into vegetation in a “death-grip” (Hughes et al. 2011). This host manipulation by O. unilateralis is a particularly spectacular and complex example of the extended-phenotype paradigm (Dawkins 1982, Andersen et al. 2009, Hughes 2013, Hughes et al. 2016), which duly garnered the epithet, the zombie-ant fungus (Evans et al. 2011a), and spawned considerable media coverage by the popular press and scientific magazines alike (Kaplan 2011, Costandi 2012, Boddy 2014, Pennisi 2014). In addition, it stimulated on-going research on the nature of the ant-fungal association, as well as on fungal phylogeny, that has generated a wealth of information (reviewed in Hughes et al. 2016). Significant advancement has been made in understanding the mechanisms involved at the molecular level: thus, manipulation of the ant brain by the fungus has been ascribed to two candidate metabolites – guanobutyric acid and sphingosine – previously implicated in neurological diseases and cancer (de Bekker et al. 2014). Using comparative genomics and a mixed transcriptomics approach, it has also been shown that genes unique to the fungus are up-regulated that encode for proteins known to cause neurological and behavioural changes (de Bekker et al. 2015, de Bekker et al. 2017).
Contemporary studies have tended to use the over-arching term O. unilateralis sensu lato for the zombie-ant fungus since it has long been suspected, but only recently established, that this is a species complex. In fact, morphological variations had been noted in collections from around the world from a very early stage (Petch 1924, 1931, 1933, 1935, 1937, Kobayasi 1941, Mains 1958, Evans 1974, 1982, Evans & Samson 1984), but it was concluded that “whilst it is tempting to separate geographic isolates (ecotypes), there is not enough evidence at the moment to conveniently divide the species into varietal units: more information is needed concerning host specificity and the range of variation in temperate, subtropical and tropical specimens” (Evans & Samson 1984). Some three decades later, Evans et al. (2011a) set out to uncover the taxonomic diversity of the newly-termed zombie or brain-manipulating fungus, based on an examination of fresh material collected on infected carpenter ants within a fragment of Atlantic rainforest in Brazil. Four Camponotus species were identified and, following a critical morphological comparison of the freshly-released (mature) ascospores – as well as of the germination process – and of the associated asexual morphs, four Ophiocordyceps species were delimited; leading to the supposition that “each species of the tribe Camponotini may be attacked by a distinct species of Ophiocordyceps” (Evans et al. 2011a), and “that there may be hundreds of species within the complex parasitising formicine ants worldwide” (Evans et al. 2011b). This hypothesis would appear to be holding true based on subsequent publications involving both morphological and molecular evidence, with six new species being described from Thailand (Luangsaard et al. 2011, Kobmoo et al. 2012, Kobmoo et al. 2015), one from Japan (Kepler et al. 2010), three from the Brazilian Amazon (Araújo et al. 2015) and another 14 in the pipeline (Araújo et al. 2018).
Significantly, however, only Kobayasi (1941) appears to have examined the type specimen – named as Torrubia unilateralis on the Brazilian ant Atta cephalotes (Tulasne & Tulasne 1865) – and he noted that it “is now preserved in [the] Paris Entomological Museum [and] is immature”. Unfortunately, repeated attempts to obtain the type for examination of the fungus and identification of the ant host were unsuccessful and it was concluded that the specimen was lost, leading to speculation that this may have gone missing during the Second World War (Evans et al. 2011a). From the latter study, and the confirmation that O. unilateralis represents a species complex, it became necessary to designate a new type, especially since Ophiocordyceps is the type genus of the recently-recognised family Ophiocordycipitaceae which is based on the placement of O. unilateralis within a Bayesian consensus tree (Sung et al. 2007). Ophiocordyceps is a highly diverse genus, with considerable pharmaceutical potential (Berenbaum & Eisner 2008, Paterson 2008, Molnár et al. 2010, Zhang et al. 2012) – species of which have also been identified recently as primary endosymbionts in certain insect hosts (Nishino et al. 2016, Gomez-Polo et al. 2017) – and thus O. unilateralis is central to our understanding of this medically-important group, as well as being considered as a keystone species for unravelling ecosystem functioning and biodiversity of fungi in tropical forests (Evans et al. 2011b).
In his diagnosis, Louis Tulasne described the unilateral position of the fleshy, hemispherical, fertile stroma on the stipe, but failed to provide details of the asci or ascospores, nor did these structures appear in the accompanying illustration by his brother, Charles (Tulasne & Tulasne 1865). This supports the statement of Kobayasi (1941) that the type was immature. Theoretically, the illustration could still stand as the holotype but, because there is no extant material, this would serve as the lectotype and a suitable epitype should be designated (Ariyawansa et al. 2014), not a neotype as Evans et al. (2011a) had originally and mistakenly proposed. The resultant search for a suitable epitype was based on the evidence from the illustration that the host representing the type is a Camponotus ant (Samson et al. 1982): specifically, the golden carpenter ant Camponotus sericeiventris, with its distinctive pronotal plate, and not the leafcutter Atta cephalotes, which is a myrmicine ant having no historical association with O. unilateralis (Evans & Samson 1984, Evans 2001). Cooke (1892) used the same Tulasne illustration to re-describe the so-called “one-sided ant club”, with additional information that the fungus had been “collected by Trail in Brazil”. This specimen is in the RBG Kew fungarium and was found by the English naturalist J.W.H. Trail in 1874 in the Brazilian Amazon, which was examined by Massee (1895) who reported it to be on the same ant species as the type. However, we consider that the type specimen of O. unilateralis was more likely to have originated in the Atlantic rainforest region of south-east Brazil – where several European naturalists were based in the 1860s – and from where the type of Camponotus sericeiventris was collected (Rio de Janeiro) during a series of French expeditions (Guérin-Menéville 1838); specimens from which were deposited in the Paris Entomological Museum, where the type of O. unilateralis was also deposited (Tulasne & Tulasne 1865).
Epitypification has been delayed until now because all the targeted collections of infected C. sericeiventris ants from Atlantic rainforest in south-east Brazil proved to be immature (Evans et al. 2011a). In fact, some newly-infected specimens were marked in situ – whilst others were harvested and incubated in the laboratory – to monitor progress, but none developed to maturity. The present paper is the result of the discovery of specimens with fertile stromata, from the same region of Brazil (Zona da Mata Mineira), enabling a full description of the species, as well as a phylogenetic analysis.
MATERIALS AND METHODS
Field collection
Collecting was concentrated in a vestige of secondary Atlantic rainforest near Viçosa, Minas Gerais, in the Zona da Mata Mineira of south-east Brazil – belonging to the Universidade Federal de Viçosa (UFV) – where ad hoc surveys for zombie-ant fungi had been carried out previously (Evans et al. 2011a, b). Although Camponotus sericeiventris is relatively common in this habitat, it is confined mainly to open, heavily-disturbed areas and the incidence of infected ants was found to be low. All the initial collections proved to be immature and it was decided to follow progress in situ by flagging specimens and monitoring development of the ascostromata through weekly observation. However, none of the five tagged specimens survived, due to predation and loss through heavy rain. Subsequently, additional specimens were bagged but were spoiled by run-off water following storms. Finally, several more immature specimens were harvested together with the vegetation, transferred to a humid chamber in a greenhouse at UFV – with an 8 h misting/16 h dry regime – and monitored. Asexual morphs developed successfully but, because ascostromatal development was slow, the specimens were overgrown by opportunistic fungi before maturation was complete. The taxonomy of the asexual morphs is based on these paratype specimens. The mature epitype was collected by one of us (VRH) from another forest reserve in the Zona da Mata Mineira, some 150 km from the main study site, in the municipality of Juiz de Fora. These specimens were deposited in the fungarium of the Universidade Federal de Viçosa (VIC).
DNA extraction and PCR
We used a BLAST search in the GenBank nucleotide database to ensure the quality of the sequences generated in this study. Sequences that were identified as species not closely related to the species treated in this study were discarded and interpreted to be from a contaminant. All the sequences included here passed the above quality control checks.
The molecular studies were conducted according to Araújo et al. (2018), described below. The DNA templates were obtained directly from two specimens of O. unilateralis infecting Camponotus sericeiventris from the type locality in Minas Gerais (Brazil) that were collected in the field and dried in silica to avoid overgrowth by opportunistic fungi. For DNA extraction, the ants were dissected and the fungal contents (mummified mycelium and hyphal bodies) within their abdomens were placed in 1.5 mL Eppendorf tubes with 100–200 μL of CTAB (2 % CTAB powder, 100 mM Tris pH8, 20 mM EDTA, 1.4 M NaCl) and ground mechanically; 400 μL of CTAB were then added and the tubes were incubated at 60 °C for 20 min and centrifuged for 10 min at 14 000 rpm. The supernatant (approx. 400 μL) was transferred to a new 1.5 mL Eppendorf tube, mixed with 500 μL of 24:1 chloroform: isoamyl-alcohol (Sigma) and mixed by inverting. The mix was then centrifuged for 20 min at 14 000 rpm and the supernatant transferred to a new 1.5 mL Eppendorf tube and further cleaned using the GeneCleanIII kit (MP Biomedicals), following Araújo et al. (2018) modifications.
Four loci were used in the analyses, i.e. small subunit nuclear ribosomal DNA (SSU), large subunit nuclear ribosomal DNA (LSU), translation elongation factor 1-α (tef1) and the largest subunit of RNA polymerase II (rpb1). The final concatenated dataset consisted of 3 795 bp. The primers used were, SSU: NS1 (GTAGTCATATGCTTGTCTC) and NS4 (CTTCCGTCAATTCCTTTAAG) (White et al. 1990); LSU: LR0R (5’-ACCCGCTGAACTTAAGC-3’) and LR5 (5’-TCCTGAGGGAAACTTCG-3’) (Vilgalys & Hester 1990); tef1: EF1-983F (5’-GCYCCYGGHCAYCGTGAYTTYAT-3’) and EF1-2218R (5’- ATGACACCRACRGCRACRGTYTG-3’) (Rehner & Buckley 2005); CRPB1: (5’-CCWGGYTTYATCAAGAARGT-3’) (Castlebury et al. 2004) and RPB1Cr_oph: (5’-CTGVCCMGCRATGTCGTTGTCCAT-3’) (Araújo et al. 2018).
To amplify the target loci, each 25 μL PCR amplification mix contained 4.5 μL of buffer E (Premix E – Epicentre) 0.5 μL of each forward and reverse primers (10 mM), 1 μL of DNA template, 0.1 Platinum Taq polymerase (Invitrogen) and 18.4 μL of ultra-pure distilled water (Gibco). The amplification reactions were placed in a Biometra T300 thermocycler under the following conditions: for SSU and LSU (1) 2 min at 94 °C, (2) 4 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 2 min, followed by (3) 35 cycles of denaturation at 94 °C for 30 s, annealing at 50.5 °C for 1 min, and extension at 72 °C for 2 min and (4) 3 min at 72 °C. For tef1 and rpb1 (1) 2 min at 94 °C, (2) 10 cycles of denaturation at 94 °C for 30 s, annealing at 64 °C for 1 min, and extension at 72 °C for 1 min, followed by (3) 35 cycles of denaturation at 94 °C for 30 s, annealing at 54 °C for 1 min, and extension at 72 °C for 1 min and (4) 3 min at 72 °C. Each 25 μL amplification reaction was cleaned by adding 3.75 μL of Illustra ExoProStar enzymatic PCR clean up (1:1 mix of Exonuclease I and alkaline phosphatase, GE Healthcare Life Sciences), incubated at 37 °C for 1 h and 80 °C for 15 min in the thermocycler. The purified PCR products were sequenced by Sanger DNA sequencing [Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA)] at the Genomics Core Facility service at Penn State University.
Phylogenetic analyses
The raw sequence reads were edited manually using Geneious v. 8.1.8 (Kearse et al. 2012). Individual gene alignments were generated by MUSCLE (Edgar 2004), implemented in Geneious v. 8.1.8 (Kearse et al. 2012). The alignment of every gene was improved manually, annotated and concatenated into a single combined dataset using Geneious v. 8.1.8 (Kearse et al. 2012). Ambiguously aligned regions were manually excluded from phylogenetic analysis and gaps were treated as missing data. The final alignment length was 3 795 bp – 1 071 for SSU, 961 for LSU, 1 011 for tef1 and 752 for rpb1. A Maximum likelihood (ML) analysis was performed with RAxML v. 8.2.4 (Stamatakis 2006) on a concatenated dataset containing all four genes. The dataset consisted of eight data partitions. These included one each for SSU and LSU, and three for each of the three codon positions of the protein coding genes, tef1 and RPB1. The GTRGAMMA model of nucleotide substitution was employed during the generation of 1 000 bootstrap (bs) replicates. The sequences generated for this study were deposited in GenBank (Table 1).
RESULTS
Taxonomy
Ophiocordyceps unilateralis (Tul.) Petch, Trans. Br. Mycol. Soc. 16: 74.1933. emend. H.C. Evans, D.P. Hughes & Araújo. Figs 1–2.
Fig. 1.
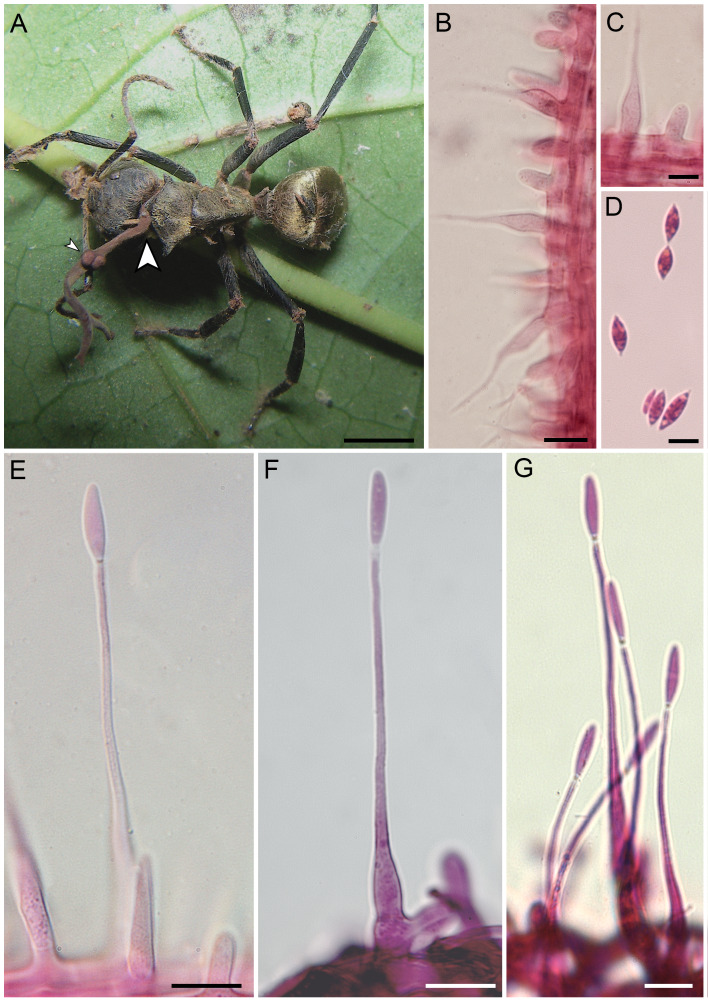
Ophiocordyceps unilateralis, epitype (VIC 44303) on Camponotus sericeiventris. A. Golden carpenter ant biting into a leaf midrib, and the clava arising from the dorsal neck region with the unilateral ascostroma, arrow shows the sporodochium of the asexual morph (Bar = 3 mm); inset, showing details of the ascostroma (Bar = 0.8 mm). B. Section through the ascostroma, showing the crowded, partially erumpent ascomata (Bar = 200 μm). C. Asci en masse (Bar = 40 μm). D–E. Asci with the prominent apical cap and foot region (Bar = 10 μm). F. Ascospore (Bar = 8 μm).
Fig. 2.
Asexual morphs of Ophiocordyceps unilateralis, based on paratype (VIC 44350). A. Camponotus sericeiventris biting into midrib of shrub leaf, showing the clava emerging from the dorsal pronotum (large arrow) and the immature ascostromata forming laterally (small arrow) (Bar = 2.5 mm). B–C. Apical region of clava showing the A-phialides (Bar = 10 μm); D. Limoniform A-conidia (Bar = 7 μm). E. B-phialide from apical region of clava emerging from neck (Bar = 12 μm). F–G. B-phialides from sporodochium emerging from leg joint (Bars = 12 and 20 μm).
Basionym: Torrubia unilateralis Tul., Sel. Fung. Carp. III: 18. 1865.
Synonym: Cordyceps unilateralis (Tul.) Sacc. Syll. Fung. 2: 570. 1883.
Description on host: External mycelium sparse, pale brown; emerging from sutures on body and legs. Clava stromatal, solitary, arising from the dorsal pronotum; cylindrical, brown and hirsute at the base. Ascostroma produced unilaterally, almost encircling the clava; hemisphaerical, 1.5–1.7 × 0.8 μm, dark brown, with roughened surface due to prominent perithecial necks. Ascomata (perithecia) partially erumpent, flask-shaped, 200–250 × 140–160 μm. Asci 8-spored, hyaline, cylindrical, (90–)95–125 × 6–8(–9) μm, swollen centrally tapering to a distinct foot and apical cap region (5–6 × 4–5 μm). Ascospores multiserriate, hyaline, thin-walled, filiform, (70–)75–85 × 2–2.5 μm, 4–5-septate; curved, tapering at both ends.
Lectotype designated here: holotype Brazil, “Atta cephalotes”, Tulasne (1865) Sel. Fung. Carp. III, plate I, fig. 3–4, MBT379723.
Fig. 3.
Phylogeny, host association and geographic distribution of species within the Ophiocordyceps unilateralis complex. Phylogeny of Ophiocordyceps from the ML analysis obtained using RAxML to analyse a concatenated dataset of four loci (SSU, LSU, tef1 and rpb1). The O. unilateralis sensu lato clade is highlighted in orange and the proposed epitype (O. unilateralis sensu stricto) line is bold. The host association and geographical distribution is also presented. Ant images from www.AntWeb.org and the photographers: April Nobile (Camponotus obscuripes, Ca. balzani, Ca. atriceps, Ca. rufipes, Dinomyrmex gigas), Will Ericson (Ca. serivceiventris, Polyrhachis lamellidens), Estella Ortega (Ca. bispinosus), Michael Branstetter (Colobopsis saundersi), Zach Lieberman (Co. leonardi, Po. furcata).
Epitype designated here: Brazil, Minas Gerais, Juiz de Fora, Paraibuna river (700–800 m a.s.l.), on Camponotus sericeiventris (Camponotini: Formicinae: Formicidae), on shrub leaf, 10 Aug. 2014, V.R. Halfeld, I14-1369A (epitype VIC 44303, MBT379722).
Additional materials examined: Brazil, Minas Gerais, Viçosa, Mata do Paraíso (700 m a.s.l.), on Camponotus sericeiventris, on shrub leaf, 26 Apr. 2010, H.C. Evans, MAP-61 (paratype VIC 44354); 12 Aug. 2012, H.C. Evans, MP-426 (paratype VIC 44349); 7 Feb. 2013, H.C. Evans, MP-502 (paratype VIC 44350).
Asexual morph: The asexual morph of the epitype proved to be in poor condition and the diagnosis below is based on the paratype collections.
Apical region of the stromatal clava, smooth, pinkish-brown, tapering to an acute tip; covered by a loose to compact hymenium of scattered to dense phialides. Phialides of two types: with a prominent swollen base (10–12 × 3–3.5 μm), tapering abruptly to a thin neck region (12–15 × 0.5–1 μm), producing hyaline, guttulate, limoniform conidia, 6.5–8 × 2–2.5 μm, apically (= Hirsutella A-type, Evans & Samson 1984); with a cylindrical base (14–16 × 2.5–3 μm), tapering gradually to a long neck (45–50 μm), 1 μm at the tip, producing solitary, hyaline, cylindrical-fusoid conidia, 8–11 × 2.5–3 μm, with a rounded apex and truncate base (= Hirsutella B-type). Hirsutella B-type also produced separately in loose, brown sporodochia arising from the leg joints.
Notes: Other synonyms – Torrubia formicivora, Cordyceps formicivora, C. ridleyi and C. subunilateralis – have been listed by various authors (Petch 1933, Mains 1958, Evans & Samson 1984, Sung et al. 2007): however, because the ant hosts are not identified and the collecting localities of some are outside the geographic range of Camponotus sericeiventris, these can no longer be considered to be synonymous with O. unilateralis s. str. Examination of the types, as well as identification of the Camponotus species involved, will be necessary to clarify their taxonomic status.
The characteristic that distinguishes O. unilateralis from all the other zombie-ant species described, thus far, is the presence of both the A- and B-type phialides within the same hymenium of the stromatal clava (Fig. 2). Cylindrical, pinkish brown synnemata may also arise separately from the body and legs forming both phialide types. In other species, only the A-type phialides are produced in a compact hymenium at the tip of stromatal clava or on separate synnemata. This was named much later as Hirsutella formicarum on specimens from Guyana (Petch 1935): however, the description matches that of the Hirsutella B-type (conidia 9–11 × 2 μm), rather than the significantly smaller, limoniform conidia described by Kobayasi (1941), as well as by Petch (1924) from Asian collections. This led Mains (1951) to question the validity of H. formicarum: “it hardly seems possible that these are all conidial stages of Cordyceps unilateralis”. We can now begin to understand why there was this disparity in the asexual morphs collected on different and geographically-separated ant hosts, as highlighted by subsequent publications (Evans & Samson 1984, Rombach & Roberts 1989). Evans & Samson (1984) also illustrated the asexual morph collected on C. sericeiventris in Honduras and showed that both A- and B-phialides occurred together on the same synnema; although the taxonomic significance of this character was overlooked at the time. The majority of this Honduran collection (~70 specimens) comprised infected ants exhibiting both the A- and B-asexual morphs described herein. These were found around the buttress base of tropical forest trees whilst others were located in the classic death-grip on nearby shrubs. The latter specimens were reported to have only the A-asexual morph, with morphologically distinct phialides and conidia (Evans & Samson 1984). The explanation for this variability of the fungus within a single ant species may lie in the recently-confirmed classification of C. sericeiventris into five subspecies, three of which have a purely Mesoamerican distribution (Bolton et al. 2007). Evidently, therefore, pathogen-host specificity may be even more complex than envisaged previously, but this will only be clarified by more comprehensive collections of infected C. sericeiventris from the Neotropics, specifically from Central America. We are confident, however, that the epitype named here is on the ant, C. sericeiventris sericeiventris (Bolton et al. 2007), whilst it is possible that novel taxa of Ophiocordyceps remain to be discovered on the other five ant subspecies. In addition, fresh material with mature ascostromata is still needed in order to determine the mode of ascospore germination in O. unilateralis s. str., an overlooked but significant taxonomic trait in these fungi (Evans et al. 2011a, b).
Phylogenetic relationships
The topology recovered in this study is in agreement with previous publications (Sung et al. 2007, Quandt et al. 2014, Sanjuan et al. 2015). The Ophiocordyceps unilateralis s. lat. clade was strongly supported (bs = 100 %). The proposed epitype – infecting C. sericeiventris – was strongly resolved, forming a sub-clade (bs = 75 %) with O. camponoti-rufipedis, which is a species native to the same geographic and ecological region as O. unilateralis s. str., the Zona da Mata Mineira in the Atlantic rainforest of south-east Brazil.
DISCUSSION
Our phylogenetic results corroborate previous studies regarding the monophyly of Ophiocordyceps unilateralis core clade (bs = 100 %) (Araújo et al. 2015, 2018, Sanjuán et al. 2015). The clade shares numerous apomorphic traits, including: having ants of the tribe Camponotini as hosts; the ability to manipulate host behaviour resulting in biting into subaxial surfaces of leaves or twigs; producing multiple asexual morphs and; forming capillisporophores and capillispores during ascospore germination (Evans et al. 2011a, b). Besides the morphological evidence that characterises the epitype proposed herein, Ophiocordyceps unilateralis s. str., we also demonstrate that this species is unique at the molecular level. Our analysis shows that O. unilateralis s. str. sits within the New World clade (Fig. 3) sister to another species from the Atlantic rainforest, O. camponoti-rufipedis (bs = 75 %). However, within the New World subclade – composed of species from Atlantic and Amazon rainforests – there is no clustering of species according to the region. Further studies, including more species from different continents, are helping to resolve the relationships within this clade (Araújo et al. 2018).
With the selection and re-description of the epitype of Ophiocordyceps unilateralis, it is now possible to construct a more meaningful phylogenetic tree for the O. unilateralis clade. Previously, trees were constructed using a sequence of the fungus from an unidentified ant in the herbarium of the Oregon State University (OSC 128574) (Sung et al. 2007, Kepler et al. 2010, Araújo et al. 2015, Kobmoo et al. 2015). This will be critical as more new species are identified within the O. unilateralis complex and we begin to understand more about the intricacies of the pathogen-host relationship. None more so than within the type of O. unilateralis on Camponotus sericeiventris, in which the evidence from Honduran collections suggests that different subspecies of the ant occur within the same forest habitat and that this is reflected in different death positions of the infected ants, as well as in morphological variation within the fungal pathogen. In order to coexist, the ant subspecies must occupy different niches within this ecosystem and, therefore, the fungus may also have evolved at the subspecies level with different morphological (spore forms) and physiological (neurotoxins) traits to maximize infection.
Table 1.
Specimen information and GenBank accession numbers for the sequences used in this study.
| Species | Voucher Information1 |
GenBank Accession numbers2 |
|||
|---|---|---|---|---|---|
| SSU | LSU | tef1 | rpb1 | ||
| Ophiocordyceps acicularis | OSC 128580 | DQ522543 | DQ518757 | DQ522326 | DQ522371 |
| Ophiocordyceps agriotidis | ARSEF 5692 | DQ522540 | DQ518754 | DQ522322 | DQ522368 |
| Ophiocordyceps amazonica | HUA 186143 | KJ917562 | KJ917571 | KM411989 | KP212902 |
| Ophiocordyceps annulata | CEM 303 | KJ878915 | KJ878881 | KJ878962 | KJ878995 |
| Ophiocordyceps aphodii | ARSEF 5498 | DQ522541 | DQ518755 | DQ522323 | n/a |
| Ophiocordyceps araracuarensis | HUA 186135 | KC610788 | KC610769 | KC610738 | KF658665 |
| Ophiocordyceps australis | HUA 186147 | KC610784 | KC610764 | KC610734 | KF658678 |
| Ophiocordyceps blattarioides | HUA186093 | KJ917559 | KJ917570 | KM411992 | KP212910 |
| Ophiocordyceps brunneipunctata | OSC 128576 | DQ522542 | DQ518756 | DQ522324 | DQ522369 |
| Ophiocordyceps camponoti-atricipis | ATRI3 | KX713666 | n/a | KX713677 | n/a |
| Ophiocordyceps camponoti-balzani | G104 | KX713660 | KX713593 | KX713689 | KX713703 |
| G143 | KX713658 | KX713595 | KX713690 | KX713705 | |
| Ophiocordyceps camponoti-bispinosi | BISPI2 | KX713665 | KX713588 | n/a | KX713700 |
| OBIS | KX713639 | KX713612 | KX713694 | KX713718 | |
| OBIS3 | KX713638 | KX713614 | KX713695 | n/a | |
| OBIS4 | KX713637 | KX713615 | KX713692 | KX713720 | |
| Ophiocordyceps camponoti-leonardi | TL1 | KJ201515 | n/a | KJ201526 | n/a |
| C36 | KJ201512 | n/a | JN819013 | n/a | |
| Ophiocordyceps camponoti-rufipedis | G108 | KX713659 | KX713594 | KX713679 | KX713704 |
| G177 | KX713657 | KX713596 | KX713680 | n/a | |
| Ophiocordyceps camponoti-saundersi | C19 | n/a | n/a | JN819042 | n/a |
| C40 | n/a | n/a | JN819044 | n/a | |
| Ophiocordyceps communis | NHJ 12582 | EF468975 | EF468830 | EF468771 | n/a |
| NHJ 12581 | EF468973 | EF468831 | EF468775 | n/a | |
| Ophiocordyceps curculionum | OSC 151910 | KJ878918 | KJ878885 | n/a | KJ878999 |
| Ophiocordyceps dipterigena | OSC 151911 | KJ878919 | KJ878886 | KJ878966 | KJ879000 |
| Ophiocordyceps elongata | OSC 110989 | n/a | EF468808 | EF468748 | EF468856 |
| Ophiocordyceps evansii | HUA 186159 | KC610796 | KC610770 | KC610736 | KP212916 |
| Ophiocordyceps formicarum | TNS F18565 | KJ878921 | KJ878888 | KJ878968 | KJ879002 |
| Ophiocordyceps fulgoromorphila | HUA 186139 | KC610794 | KC610760 | KC610729 | KF658676 |
| Ophiocordyceps gracilis | EFCC 3101 | EF468955 | EF468810 | EF468750 | EF468858 |
| Ophiocordyceps halabalaensis | MY1308 | n/a | n/a | GU797109 | n/a |
| MY5151 | n/a | n/a | GU797110 | n/a | |
| Ophiocordyceps heteropoda | EFCC 10125 | EF468957 | EF468812 | EF468752 | EF468860 |
| Ophiocordyceps irangiensis | OSC 128578 | DQ522556 | DQ518770 | DQ522345 | DQ522391 |
| Ophiocordyceps kniphofioides | HUA 186148 | KC610790 | KF658679 | KC610739 | KF658667 |
| Ophiocordyceps longissima | HMAS_199600 | KJ878926 | n/a | KJ878972 | KJ879006 |
| Ophiocordyceps lloydii | OSC 151913 | KJ878924 | KJ878891 | KJ878970 | KJ879004 |
| Ophiocordyceps melolonthae | OSC 110993 | DQ522548 | DQ518762 | DQ522331 | DQ522376 |
| Ophiocordyceps myrmecophila | CEM1710 | KJ878928 | KJ878894 | KJ878974 | KJ879008 |
| Ophiocordyceps neovolkiana | OSC 151903 | KJ878930 | KJ878896 | KJ878976 | KJ879010 |
| Ophiocordyceps nutans | OSC 110994 | DQ522549 | DQ518763 | DQ522333 | DQ522378 |
| Ophiocordyceps polyrhachis-furcata | P39 | KJ201504 | n/a | JN819003 | n/a |
| P51 | KJ201505 | n/a | JN819000 | n/a | |
| Ophiocordyceps ponerinarum | HUA 186140 | KC610789 | KC610767 | KC610740 | KF658668 |
| Ophiocordyceps pruinosa | NHJ 12994 | EU369106 | EU369041 | EU369024 | EU369063 |
| Ophiocordyceps pulvinata | TNS-F 30044 | GU904208 | n/a | GU904209 | GU904210 |
| Ophiocordyceps purpureostromata | TNS F18430 | KJ878931 | KJ878897 | KJ878977 | KJ879011 |
| Ophiocordyceps rami | MY6736 | KM655823 | n/a | KJ201532 | n/a |
| Ophiocordyceps rhizoidea | NHJ 12522 | EF468970 | EF468825 | EF468764 | EF468873 |
| Ophiocordyceps septa | C41 | KJ201525 | n/a | JN819037 | |
| Ophiocordyceps sobolifera | TNS F18521 | KJ878933 | KJ878898 | KJ878979 | KJ879013 |
| Ophiocordyceps sphecocephala | OSC 110998 | DQ522551 | DQ518765 | DQ522336 | DQ522381 |
| Ophiocordyceps stylophora | OSC 111000 | DQ522552 | DQ518766 | DQ522337 | DQ522382 |
| Ophiocordyceps tiputini | QCNE 186287 | KC610792 | KC610773 | KC610745 | KF658671 |
| Ophiocordyceps unilateralis s. str. | VIC 44303 | KX713628 | KX713626 | KX713675 | KX713730 |
| VIC 44354 | KX713627 | n/a | KX713676 | KX713731 | |
| Ophiocordyceps unilateralis var. clavata | INPA 274589 | KX713652 | KX713600 | KX713681 | KX713708 |
| INPA 274590 | KX713651 | n/a | KX713682 | KX713709 | |
| Ophiocordyceps variabilis | OSC 111003 | EF468985 | EF468839 | EF468779 | EF468885 |
| Ophiocordyceps yakusimensis | HMAS_199604 | KJ878938 | KJ878902 | n/a | KJ879018 |
| Stilbella buquetii | HMAS_199617 | KJ878940 | KJ878905 | KJ878985 | KJ879020 |
| Tolypocladium capitatum | OSC 71233 | AY489689 | AY489721 | AY489615 | AY489649 |
| Tolypocladium japonicum | OSC 110991 | DQ522547 | DQ518761 | DQ522330 | DQ522375 |
| Tolypocladium ophioglossoides | OSC 106405 | AY489691 | AY489723 | AY489618 | AY489652 |
1ARSEF, USDA-ARS Collection of Entomopathogenic Fungal Cultures, Ithaca, NY; ATR, BISP, G and OBIS abbreviations from D.P. Hughes personal collection, Penn State University, PA, USA; C, P and TL abbreviations follow those of Kobmoo et al. (2015); CEM from J. W. Spatafora lab collection, Oregon State University, OR, USA; EFCC, Entomopathogenic Fungal Culture Collection, Chuncheon, South Korea; HMAS, Chinese Academy of Sciences, Beijing, China; HUA, Herbarium Antioquia University, Medellin, Colombia; INPA, Herbarium of National Institute of Amazonian Research, Manaus, Brazil; MY, J.J. Luangsa-ard personal collection, BIOTEC, Thailand; NHJ, Nigel Hywel-Jones personal collection; OSC, Oregon State University Herbarium, Corvallis, OR; TNS, National Museum of Science and Nature, Tsukuba, Japan.
2SSU: partial small subunit (18S) nrRNA gene; LSU: partial large subunit (28S) nrRNA gene; tef1: partial translation elongation factor 1-α gene; rpb1: partial fragment of the largest subunit of the RNA polymerase II gene.
Acknowledgments
HCE and JPMA acknowledge financial support from the Brazilian Conselho Nacional de Desenvolvimento Cientifico e Tecnológia (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We would like to thank the anonymous reviewers for their comments and recommendations.
REFERENCES
- Andersen SB, Gerritsma S, Yusah KM, et al. (2009). The life of a dead ant: the expression of an adaptive extended phenotype. American Naturalist 174: 424–433. [DOI] [PubMed] [Google Scholar]
- Araújo JPM, Evans HC, Geiser DM, et al. (2015). Unravelling the diversity behind the Ophiocordyceps unilateralis (Ophiocordycipitaceae) complex: three new species of zombie-ant fungi from the Brazilian Amazon. Phytotaxa 220: 224–238. [Google Scholar]
- Araújo JPM, Kepler R, Evans HC, et al. (2018). Zombie-ant fungus across continents: 14 new species and new combinations within Ophiocordyceps. I. Myrmecophilous hirsutelloid species. Studies in Mycology 90: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyawansa HA, Hawksworth DL, Hyde KD, et al. (2014). Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Diversity 69: 57–91. [Google Scholar]
- Berenbaum MR, Eisner T. (2008). Bugs’ bugs. Science 322: 52–53. [DOI] [PubMed] [Google Scholar]
- Boddy L. (2014). Soils of war. New Scientist 2999: 43–45. [Google Scholar]
- Bolton B, Alpert G, Ward PS, et al. (2007). Bolton’s Catalogue of Ants of the World. Harvard University Press, Cambridge, MA. [Google Scholar]
- Castlebury LA, Rossman AY, Sung G-H, et al. (2004). Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research 108: 864–872. [DOI] [PubMed] [Google Scholar]
- Cooke MC. (1892). Vegetable Wasps and Plant Worms. Society for Promoting Christian Knowledge, London. [Google Scholar]
- Costandi M. (2012). Zombie-ant parasitic fungus kept in check by hyperparasitic fungus. The Guardian (Neurophilosophy), 04/05/2012. [Google Scholar]
- Dawkins R. (1982). The extended phenotype. Oxford University Press, Oxford. [Google Scholar]
- de Bekker C, Quevillon LE, Smith PB, et al. (2014). Species-specific ant brain manipulation by a specialized fungal parasite. BMC Evolutionary Biology 14: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bekker C, Ohm RA, Loreto RG, et al. (2015). Gene expression during zombie ant biting behavior reflects the complexity underlying fungal parasitic behavioral manipulation. BMC Genomics 16: 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bekker C, Ohm RA, Evans HC, et al. (2017). Ant-infecting Ophiocordyceps genomes reveal a high diversity of potential behavioral manipulation genes and a possible major role for enterotoxins. Scientific Reports (Nature) 7: 12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HC. (1974). Natural control of arthropods, with special reference to ants (Formicidae), by fungi in the tropical high forest of Ghana. Journal of Applied Ecology 11: 37–49. [Google Scholar]
- Evans HC. (1982). Entomogenous fungi in tropical forest ecosystems: an appraisal. Ecological Entomology 7: 47–60. [Google Scholar]
- Evans HC. (2001). Entomopathogenic fungi associated with ants (Formicidae): A review. In: Trichomycetes and other fungal groups (Misra JK, Horn BW, eds). Science Publishers, Enfield, USA: 119–144. [Google Scholar]
- Evans HC, Samson RA. (1984). Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems. II. The Camponotus (Formicinae) complex. Transactions of the British Mycological Society 82: 127–150. [Google Scholar]
- Evans HC, Elliot SL, Hughes DP. (2011a). Hidden diversity behind the zombie-ant fungus Ophiocordyceps unilateralis: four new species described from carpenter ants in Minas Gerais, Brazil. PLoS ONE 6: e17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HC, Elliot SL, Hughes DP. (2011b). Ophiocordyceps unilateralis: a keystone species for unraveling ecosystem functioning and biodiversity of fungi? Communicative & Integrative Biology 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Polo P, Ballinger MJ, Lalzar M, et al. (2017). An exceptional family: Ophiocordyceps-allied fungus dominates the microbiome of soft scale insects (Hemiptera: Sternorrhyncha: Coccidae). Molecular Ecology 26: 5855–5868. [DOI] [PubMed] [Google Scholar]
- Guérin-Méneville FE. (1838). Première division. Crustacés, arachnides et insectes. In: Voyage Autour du Monde, executé par ordre du Roi, sur la corvette de sa Majesté, La Coquille, pendant les années (Duperrey LI, ed), Zoologie. Tome Deuxième, Part 2. H. Bertrand, Paris: 1822, 1823, 1824 et 1825: 9–320. [Google Scholar]
- Hughes DP. (2013). Pathways to understanding the extended phenotype of parasites in their hosts. Journal of Experimental Biology 216: 142–147. [DOI] [PubMed] [Google Scholar]
- Hughes DP, Wappler T, Labandeira C. (2011). Ancient death-grip leaf scars reveal ant-fungal parasitism. Biology Letters 7: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DP, Araújo JPM, Loreto RG, et al. (2016). From so simple a beginning: the evolution of behavioral manipulation by fungi. Advances in Genetics 94: 437–469. [DOI] [PubMed] [Google Scholar]
- Kaplan M. (2011). Befriending the body snatchers. New Scientist 2827: 37–41. [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler RM, Kaitsu Y, Tanaka E, et al. (2010). Ophiocordyceps pulvinata sp. nov., a pathogen of ants with a reduced stroma. Mycoscience 52: 39–47. [Google Scholar]
- Kobayasi Y. (1941). The genus Cordyceps and its allies. Science Reports, Tokyo Bunrika Daigaku, Sect. B 5: 53–260. [Google Scholar]
- Kobmoo N, Mongkolsamrit S, Tasanathai K, et al. (2012). Molecular phylogenies reveal host-specific divergence of Ophiocordyceps unilateralis sensu lato following its host ants. Molecular Ecology 21: 3022–3031. [DOI] [PubMed] [Google Scholar]
- Kobmoo N, Mongkolsamrit S, Wutikhun T, et al. (2015). New species of Ophiocordyceps unilateralis, an ubiquitous pathogen of ants from Thailand. Fungal Biology 119: 44–52. [DOI] [PubMed] [Google Scholar]
- Luangsaard JJ, Ridkaew R, Tasanathai K, et al. (2011). Ophiocordyceps halabalaensis: a new species of Ophiocordyceps pathogenic to Camponotus gigas in Hala Bala Wildlife Sanctuary, Southern Thailand. Fungal Biology 115: 608–614. [DOI] [PubMed] [Google Scholar]
- Mains EB. (1951). Entomogenous species of Hirsutella, Tilachlidium and Synnematium. Mycologia 43: 691–718. [Google Scholar]
- Mains EB. (1958). North American entomogenous species of Cordyceps. Mycologia 50: 169–222. [Google Scholar]
- Massee G. (1895). A revision of the genus Cordyceps. Annals of Botany 9: 1–44. [Google Scholar]
- Molnár I, Gibson DM, Krasnoff SB. (2010). Secondary metabolites from entomopathogenic Hypocrealean fungi. Natural Products Reports 27: 1233–1372. [DOI] [PubMed] [Google Scholar]
- Nishino T, Tanahashi M, Lin C-P, et al. (2016). Fungal and bacterial endosymbionts of eared leafhoppers of the subfamily Ledrinae (Hemiptera: Cicadellidae). Applied Entomology & Zoology 51: 465–477. [Google Scholar]
- Paterson RRM. (2008). Cordyceps – a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 69: 1469–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E. (2014). Parasitic puppeteers begin to yield their secrets. Science 343: 239. [DOI] [PubMed] [Google Scholar]
- Petch T. (1924). Studies in entomogenous fungi. IV. Some Ceylon Cordyceps. Transactions of the British Mycological Society 10: 28–45. [Google Scholar]
- Petch T. (1931). Notes on entomogenous fungi. Transactions of the British Mycological Society 16: 55–75. [Google Scholar]
- Petch T. (1933). Notes on entomogenous fungi. Transactions of the British Mycological Society 18: 48–75. [Google Scholar]
- Petch T. (1935). Notes on entomogenous fungi. Transactions of the British Mycological Society 19: 161–194. [Google Scholar]
- Petch T. (1937). Notes on entomogenous fungi. Transactions of the British Mycological Society 21: 34–67. [Google Scholar]
- Quandt CA, Kepler RM, Gams W, et al. (2014). Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus 1: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehner SA, Buckley E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Rombach MC, Roberts DW. (1989). Hirsutella species (Deuteromycotina; Hyphomycetes) on Philippine insects. Philippine Entomologist 7: 491–518. [Google Scholar]
- Samson RA, Evans HC, Hoekstra ES. (1982). Notes on entomogenous fungi from Ghana. VI. The genus Cordyceps. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, Series C 85: 589–605. [Google Scholar]
- Sanjuán TI, Franco-Molano AE, Kepler RM, et al. (2015). Five new species of entomopathogenic fungi from the Amazon and evolution of neotropical Ophiocordyceps. Fungal Biology 119: 901–916. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Sung G-H, Hywel-Jones NL, Sung J-M, et al. (2007). Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology 57: 1–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulasne LR, Tulasne C. (1865). Selecta Fungorum Carpologia III. Imperial Press, Paris. [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA from Several Cryptococcus Species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A Guide to Methods and Applications (Innis MA, Gelfand DH, Sninsky JJ, et al. eds). Academic Press, New York: 315–322. [Google Scholar]
- Zhang Y, Li E, Wang C, et al. (2012). Ophiocordyceps sinensis, the flagship fungus of China: terminology, life strategy and ecology. Mycology 3: 2–10. [Google Scholar]