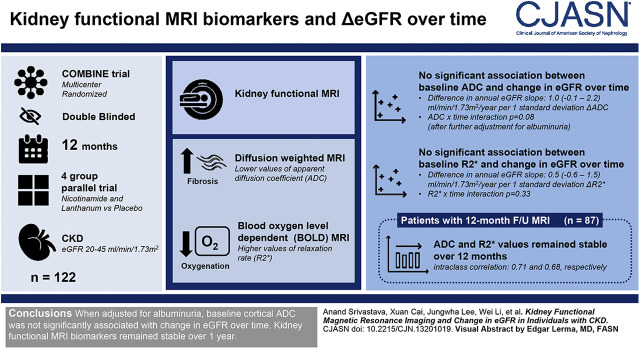
Visual Abstract
Keywords: MRI, hypoxia, fibrosis, chronic kidney disease, ESRD, glomerular filtration rate, lanthanum carbonate, albuminuria, Niacinamide, Follow-Up Studies, Diffusion Magnetic Resonance Imaging, Renal Insufficiency, Chronic, Magnetic Resonance Imaging, Lanthanum, Biomarkers, Oxygen
Abstract
Background and objectives
Kidney functional magnetic resonance imaging (MRI) requires further investigation to enhance the noninvasive identification of patients at high risk of CKD progression.
Design, setting, participants, & measurements
In this exploratory study, we obtained baseline diffusion-weighted and blood oxygen level–dependent MRI in 122 participants of the CKD Optimal Management with Binders and Nicotinamide trial, which was a multicenter, randomized, double-blinded, 12-month, four-group parallel trial of nicotinamide and lanthanum carbonate versus placebo conducted in individuals with eGFR 20–45 ml/min per 1.73 m2. Lower values of apparent diffusion coefficient (ADC) on diffusion-weighted MRI may indicate increased fibrosis, and higher values of relaxation rate (R2*) on blood oxygen level–dependent MRI may represent decreased oxygenation. Because there was no effect of active treatment on eGFR over 12 months, we tested whether baseline kidney functional MRI biomarkers were associated with eGFR decline in all 122 participants. In a subset of 87 participants with 12-month follow-up MRI data, we evaluated whether kidney functional MRI biomarkers change over time.
Results
Mean baseline eGFR was 32±9 ml/min per 1.73 m2, and mean annual eGFR slope was −2.3 (95% confidence interval [95% CI], −3.4 to −1.1) ml/min per 1.73 m2 per year. After adjustment for baseline covariates, baseline ADC was associated with change in eGFR over time (difference in annual eGFR slope per 1 SD increase in ADC: 1.3 [95% CI, 0.1 to 2.5] ml/min per 1.73 m2 per year, ADC×time interaction P=0.04). This association was no longer significant after further adjustment for albuminuria (difference in annual eGFR slope per 1 SD increase in ADC: 1.0 (95% CI, −0.1 to 2.2) ml/min per 1.73 m2 per year, ADC×time interaction P=0.08). There was no significant association between baseline R2* and change in eGFR over time. In 87 participants with follow-up functional MRI, ADC and R2* values remained stable over 12 months (intraclass correlation: 0.71 and 0.68, respectively).
Conclusions
Baseline cortical ADC was associated with change in eGFR over time, but this association was not independent of albuminuria. Kidney functional MRI biomarkers remained stable over 1 year.
Clinical Trial registry name and registration number
CKD Optimal Management with Binders and Nicotinamide (COMBINE), NCT02258074.
Introduction
Kidney functional magnetic resonance imaging (MRI) offers a noninvasive imaging modality to quantify fibrosis and tissue hypoxia without the need for potentially nephrotoxic contrast media (1). By assessing the displacement of water molecules in tissue, diffusion-weighted MRI may detect kidney fibrosis (2−5). Lower levels of cortical apparent diffusion coefficient (ADC) on diffusion-weighted MRI may indicate greater fibrosis (3). Because of the varying magnetic properties of the oxygenated and deoxygenated forms of hemoglobin, blood oxygen level–dependent (BOLD) MRI evaluates the relative tissue oxygenation status of the kidney (6,7). Higher cortical relaxation rate (R2*) on BOLD MRI may represent reduced oxygenation (7). In patients with CKD stages 2–4 undergoing diffusion-weighted MRI before a native kidney biopsy, lower cortical ADC values were associated with higher percentages of fibrosis quantified on biopsy specimens (8). A prior cross-sectional study demonstrated lower kidney cortex oxygenation, as assessed by BOLD MRI, in patients with CKD stages 2–4 compared with healthy volunteers, and found a negative correlation between cortical R2* measurements and eGFR (9). However, longitudinal studies evaluating whether kidney functional MRI biomarkers may inform risk of kidney function decline, beyond baseline eGFR, are limited and come from single-center studies (10,11). Further evidence is required to support the incorporation of kidney functional MRI biomarkers into longitudinal, multicenter clinical trials to enhance selection of high-risk patients with CKD for testing of novel therapies.
The CKD Optimal Management with Binders and Nicotinamide (COMBINE) trial (12,13) was a multicenter, randomized clinical trial that tested the effects of nicotinamide and lanthanum carbonate on serum fibroblast growth factor 23 and phosphate levels in 205 individuals with CKD stages 3b and 4. In an exploratory analysis, we performed baseline kidney functional MRI in 122 participants to test the hypothesis that higher levels of fibrosis, as assessed by lower ADC on diffusion-weighted MRI, and cortical tissue hypoxia, as quantified by higher R2* on BOLD MRI, were associated with change in eGFR over 12 months in patients with CKD stages 3b and 4. In a subset of 87 participants who returned for a follow-up functional MRI at month 12, we further hypothesized that levels of cortical fibrosis and tissue hypoxia would increase over 12 months in the setting of eGFR decline.
Materials and Methods
Source Population
The rationale, design, and primary results of the COMBINE trial (Clinicaltrials.gov identifier NCT02258074) have been published previously (12,13). Briefly, the COMBINE trial was a randomized, double-blinded, 12-month, four-group parallel trial of nicotinamide and lanthanum carbonate versus placebo in 205 participants with CKD stages 3b and 4. Inclusion criteria required eGFR 20–45 ml/min per 1.73 m2, serum phosphate concentration ≥2.8 mg/dl, platelet count ≥125,000/mm3, and ability to provide informed consent. Key exclusion criteria included known allergy to nicotinamide or lanthanum carbonate, secondary hyperparathyroidism, liver disease, recent blood or platelet transfusion, severe anemia, and hypoalbuminemia. A complete list of inclusion and exclusion criteria has been previously described (13). Participants were recruited from seven clinical centers in the United States (Denver Nephrology Research, Denver, CO; George Washington University, Washington, DC; NorthShore University HealthSystem, Evanston, IL; Northwestern University, Chicago, IL; Salt Lake City Veterans Affairs Hospital, Salt Lake City, UT; University of California San Diego, San Diego, CA; University of Utah, Salt Lake City, UT). The COMBINE trial was conducted with approvals from the respective institutional review boards, and all participants provided written informed consent. Primary results of the COMBINE trial showed no significant effects of treatment on the primary outcomes of changes in serum phosphate or fibroblast growth factor 23 and no significant effects of treatment on eGFR over 1 year (13).
Kidney Functional MRI Substudy
Of the 205 participants, 165 consented to participate in the kidney functional MRI ancillary study. Twenty-six participants were excluded owing to polycystic kidney disease (n=3), large body habitus (n=3), metal implants (n=6), logistical issues (n=11), claustrophobia (n=2), and patient refusal (n=1). Kidney functional MRI studies from 14 additional participants were of unsatisfactory quality because of breath-holding or magnetic susceptibility differences. Of the 125 participants with baseline kidney functional MRI measurements, individuals who were lost to follow-up (n=3) were excluded, which resulted in the 122 participants included in this study (Supplemental Figure 1). Of these, 87 participants returned for the month 12 kidney functional MRI. The mean time between kidney functional MRI scans was 11.9±0.9 months.
MRI Acquisition and Analysis
All participants were instructed to avoid nonsteroidal anti-inflammatory drugs for 3 days prior, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers for 1 day prior, and loop diuretics on the day of the functional MRI. Participants were instructed to fast for at least 4 hours before the MRI scans at baseline and follow-up. All scans were performed on 3T whole-body scanners (Trio, Verio/SkyraFit, Skyra; Siemens Healthcare, Erlangen, Germany). A multiple-echo gradient-recalled-echo sequence was used to acquire BOLD images in the coronal plane during breath-holding at end expiration (9). Diffusion-weighted imaging was performed using spin-echo planar imaging. Diffusion-sensitizing gradients were applied along three different directions for calculating diffusion trace, which is a direction-independent measure. Images using five different diffusion-sensitizing factors were acquired during free-breathing, and the acquisitions were averaged to improve signal to noise ratio and to minimize motion artifacts. Diffusion maps were generated in-line using a single exponential fit on the scanner. Kidney functional MRI acquisition parameters are included in Supplemental Table 1.
The NorthShore University HealthSystem served as the core image laboratory to analyze kidney functional MRI measurements from all clinical sites. R2* maps were generated using a custom image-processing toolbox using Python (Python Software Foundation) (14). The toolbox also facilitated region of interest analysis of ADC maps. Regions of interest were manually drawn free-hand in the cortex (14,15). A single reader (W.L.), with over 25 years of experience in MRI analysis, performed all kidney functional MRI analyses unaware of any participants’ clinical information.
Exposures and Outcomes
The primary exposures were baseline cortical ADC measurements on diffusion-weighted MRI and cortical R2* measurements on BOLD MRI. Regions of interest in the kidney cortex were averaged between left and right kidneys and over all of the image slices, resulting in a single representative value for cortical ADC and cortical R2*, respectively (14,15).
The primary outcome was the rate of change per 12 months in eGFR. We used the creatinine-based CKD Epidemiology Collaboration equation to calculate eGFR (16). Serum creatinine was measured at baseline and follow-up visits every month for the first 3 months and every 3 months until the end of the study. For individuals that reached kidney failure (need for dialysis or kidney transplantation) or died during follow-up, we used the last eGFR available from the electronic medical record. If no eGFR value was available, we imputed a value of 10 ml/min per 1.73 m2. Including the baseline value, participants had 6.3±1.0 eGFR values during the course of the study. All participants had at least three eGFR values.
Measurements and Assessment of Covariates
At baseline, we collected participant information, including demographics, diabetes mellitus status, urine albumin-to-creatinine ratio (UACR), and eGFR. Serum creatinine, urine albumin, and urine creatinine values were measured in a central laboratory with isotope dilution mass spectrometry standardization (Spectra Clinical Research, Rockleigh, NJ). Serum and urine creatinine were measured using a Jaffe-based method, and urine albumin was measured by an immunoturbidometric method.
Statistical Analyses
Descriptive statistics were summarized as mean±SD or median with interquartile range for continuous variables, and frequency distribution was presented with percentages for categorical variables. We used t tests to evaluate differences in kidney functional MRI measurements between active treatment and placebo groups at baseline. We evaluated the association of baseline kidney functional MRI measurements with annual patient-specific eGFR slope using Pearson correlation coefficient. To calculate annual patient-specific eGFR slope, we used mixed-effects models with eGFR treated as the dependent variable and time of eGFR measurement (years since baseline) as the independent variable. The model included random intercepts and slopes for participants. To evaluate the association between baseline kidney functional MRI biomarkers and mean annual eGFR slope, we used mixed-effects models that included a random intercept for each participant and a random slope for time as a continuous variable. We evaluated unadjusted models and models adjusted for age, sex, race, diabetes status, treatment group (active treatment versus placebo), study center, baseline natural log-transformed UACR, baseline eGFR, and the natural log-transformed UACR×time interaction term. The functional MRI biomarker×time interaction term tested whether the functional MRI biomarker was associated with annual eGFR slope.
In additional analyses, we repeated the primary analysis with baseline measurements of whole-kidney ADC, whole-kidney R2*, and medullary R2*. We dichotomized participants according to whether they experienced kidney failure or death or whether they experienced rapid progression. We labeled participants as rapid progressors if they experienced an annual eGFR decline >3 ml/min per 1.73 m2 per year. This threshold was chosen to indicate a clinically important decline in kidney function, as described in prior studies (10,17,18). We fit multivariable logistic regression models to examine associations of functional MRI biomarkers with each dichotomous outcome. Unadjusted models were further adjusted for baseline eGFR and natural log-transformed UACR. At baseline, we evaluated the association between cortical R2* and ADC measurements with Pearson correlation coefficient. In the subset of 87 participants with month 12 functional MRI data, we performed intraclass correlation to determine the stability of cortical R2* and ADC measurements within participants from baseline to month 12. Statistical tests were two-sided, and P<0.05 was considered significant. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Study Population Characteristics
Baseline characteristics of the 122 participants with baseline kidney functional MRI data are presented in Table 1. A total of 89 participants (73%) were randomized to active treatment (lanthanum carbonate and nicotinamide, lanthanum carbonate and nicotinamide placebo, or lanthanum carbonate placebo and nicotinamide) and 33 (27%) were randomized to double placebo treatment. The mean age was 65±12 years, 63 participants (52%) had diabetes mellitus, mean eGFR was 32±9 ml/min per 1.73 m2, and median UACR was 171 (interquartile range, 23–601) mg/g creatinine.
Table 1.
Baseline characteristics of participants with baseline kidney functional MRI
| Characteristic | All Patients, n=122 | Active Treatment, n=89 | Placebo, n=33 |
|---|---|---|---|
| Women, % | 43 (35) | 32 (36) | 11 (33) |
| Age, yr | 65±12 | 65±13 | 66±10 |
| Race, % | |||
| White | 71 (58) | 55 (62) | 16 (49) |
| Black | 38 (31) | 24 (27) | 14 (42) |
| Other | 13 (11) | 10 (11) | 3 (9) |
| Diabetes mellitus, % | 63 (52) | 50 (56) | 13 (39) |
| Cause of CKD, % | |||
| Diabetic kidney disease | 50 (41) | 37 (42) | 13 (40) |
| Hypertensive nephrosclerosis | 26 (21) | 19 (21) | 7 (21) |
| Other | 14 (11) | 10 (11) | 4 (12) |
| Unknown | 10 (8) | 7 (8) | 3 (9) |
| GN | 9 (7) | 4 (4) | 5 (15) |
| Obstructive uropathy | 5 (4) | 5 (6) | 0 (0) |
| Other interstitial nephritis | 4 (3) | 4 (4) | 0 (0) |
| Renal artery stenosis | 2 (2) | 2 (2) | 0 (0) |
| Hereditary nephritis | 2 (2) | 1 (1) | 1 (3) |
| Serum creatinine, mg/dl | 2.1±0.5 | 2.1±0.5 | 2.2±0.5 |
| eGFR, ml/min per 1.73 m2 | 32±9 | 32±9 | 32±9 |
| UACR, mg/g | 171 [23–601] | 158 [21–543] | 187 [24–647] |
| Hemoglobin, g/dl | 12.9±1.7 | 12.8±1.7 | 13.0±1.7 |
| Cortical ADC, ×10−3 mm2/s | 1.45±0.17 | 1.44±0.16 | 1.49±0.17 |
| Cortical R2*, s−1 | 20.3±3.17 | 20.3±3.35 | 20.2±2.67 |
Participants in the active treatment group received lanthanum carbonate and nicotinamide, lanthanum carbonate and nicotinamide placebo, or lanthanum carbonate placebo and nicotinamide. Participants in the placebo group received double placebo treatment. Values for categorical variables presented as count (percentage); values for continuous variables presented as mean±SD or median [interquartile range]. MRI, magnetic resonance imaging; UACR, urine albumin-to-creatinine ratio; ADC, apparent diffusion coefficient; R2*, relaxation rate.
Effect of Intervention on eGFR
The mean annual eGFR slope was −2.3 (95% confidence interval [95% CI], −3.4 to −1.1) ml/min per 1.73 m2 per year. There was no significant difference in mean annual eGFR slope by treatment group (active treatment: −2.3 [95% CI, −7.0 to 2.4] versus placebo: −2.2 [95% CI, −4.4 to −0.1] ml/min per 1.73 m2 per year; P=0.94), and active treatment had no effect on change in eGFR over time (treatment×time interaction P=0.94). Because there was no treatment effect on change in eGFR over time, we analyzed the data in all participants as a single group.
Associations of Diffusion-Weighted and BOLD MRI Biomarkers with Annual eGFR Slope
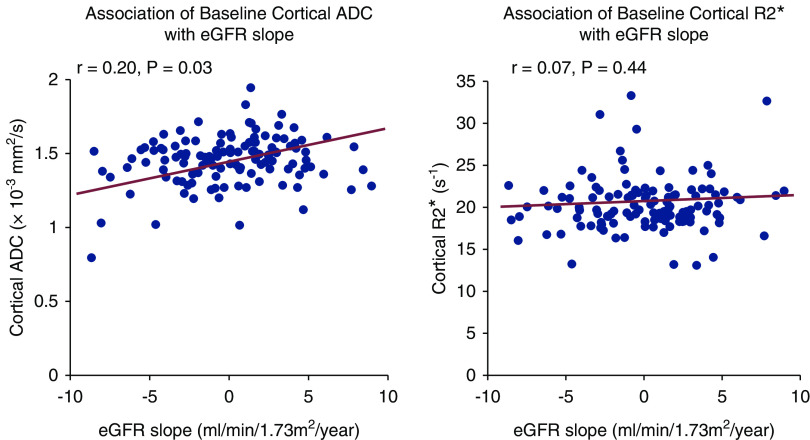
Baseline cortical ADC values directly correlated with annual patient-specific eGFR slope (r=0.20; P=0.03; Figure 1). After adjustment for baseline covariates, baseline ADC was independently associated with change in eGFR over time (ADC×time interaction P=0.04) (Table 2). Further adjustment for the interaction of natural log-transformed UACR×time attenuated the association between cortical ADC and change in eGFR over time (P=0.08). Baseline cortical R2* measurements did not correlate with annual patient-specific eGFR slope (r=0.07; P=0.44; Figure 1). Cortical R2* did not have a statistically significant relationship with change in eGFR over time in multivariable adjusted models (R2*×time interaction P=0.33) (Table 2). There were no differences in mean annual eGFR slope by tertiles of kidney functional MRI biomarkers (Supplemental Table 2). The associations between baseline kidney functional MRI biomarkers and change in eGFR over time were not appreciably different when both MRI biomarkers were included in the same model (Supplemental Table 3).
Figure 1.
Cortical ADC, as assessed by diffusion-weighted MRI, correlates with annual patient-specific eGFR slope. Scatterplots demonstrate a positive correlation between cortical apparent diffusion coefficient (ADC) measurements and annual patient-specific eGFR (r=0.20; P=0.03) and no association between cortical relaxation rate (R2*) measurements and annual patient-specific eGFR slope (r=0.07; P=0.44).
Table 2.
Association of baseline kidney functional MRI measurements with change in eGFR over time
| Kidney Functional MRI Biomarker | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI)a | P Value | β (95% CI)a | P Value | β (95% CI)a | P Value | |
| Cortical ADC | 1.2 (−0.002 to 2.4) | 0.05 | 1.3 (0.1 to 2.5) | 0.04 | 1.0 (−0.1 to 2.2) | 0.08 |
| Cortical R2* | 0.6 (−0.6 to 1.7) | 0.32 | 0.6 (−0.6 to 1.7) | 0.33 | 0.5 (−0.6 to 1.5) | 0.40 |
Model 1 is unadjusted. Model 2 is adjusted for age, sex, race, diabetes status, treatment group (active treatment versus placebo), study center, baseline hemoglobin, baseline log-transformed UACR, and baseline eGFR. Model 3 is model 2 and further adjusts for the interaction of natural log-transformed UACR×time. MRI, magnetic resonance imaging; 95% CI, 95% confidence interval; ADC, apparent diffusion coefficient; R2*, relaxation rate; UACR, urine albumin-to-creatinine ratio.
The β estimate is the difference in annual eGFR slope (ml/min per 1.73 m2 per year) per 1 SD increase in the MRI biomarker of interest from the respective MRI biomarker×time interaction term. P values are from the interaction term of each respective MRI biomarker×time. Cortical ADC and R2* are reported in ×10−3 mm2/s and s−1, respectively. Time is measured in years.
Additional Analyses
We obtained similar results when using baseline whole-kidney ADC, whole-kidney R2*, or medullary R2* (Supplemental Table 4). Six participants (5%) experienced kidney failure or death, and 25 (21%) participants experienced rapid progression (annual eGFR loss >3 ml/min per 1.73 m2 per year) during the 12-month study. Supplemental Table 5 demonstrates the associations of kidney functional MRI biomarkers with kidney failure or death and with rapid progression. Lower levels of ADC were associated with kidney failure or death in unadjusted models (odds ratio [OR], 2.41; 95% CI, 1.19 to 4.87), which was similar after adjustment for baseline eGFR and UACR (adjusted OR, 2.37; 95% CI, 1.03 to 5.48). Lower levels of ADC were not associated with rapid progression in unadjusted (OR, 1.43; 95% CI, 0.93 to 2.21) or adjusted models (adjusted OR, 1.29; 95% CI, 0.82 to 2.04). There was no significant association of cortical R2* with kidney failure, death, or rapid progression. There was no association between cortical R2* and ADC at baseline (r=0.10; P=0.28).
Change in MRI Biomarkers
In the subset of 87 participants with kidney functional MRI measurements at month 12, cortical ADC levels increased minimally in the active treatment group and decreased in the placebo group (0.05±0.11×10−3 mm2/s versus −0.02±0.15×10−3 mm2/s; P=0.02), with the magnitude of the change not considered to be meaningful. From baseline to month 12, cortical R2* values increased slightly in both the active treatment and placebo groups, but the changes in cortical R2* values did not differ significantly between groups (0.1±2.7 s−1 versus 0.4±2.4 s−1; P=0.64) (Supplemental Table 6). The intraclass correlation was 0.71 and 0.68 for cortical ADC and R2* measurements, respectively.
Discussion
In this exploratory analysis of data from a prospective multicenter trial of participants with eGFR 20–45 ml/min per 1.73 m2, lower baseline cortical ADC was associated with more rapid loss of eGFR over 12 months. However, the association between cortical ADC and change in eGFR over time was attenuated after adjustment for albuminuria. Although kidney cortical hypoxia may contribute to CKD progression (10,11), we did not observe statistically significant associations between baseline cortical R2* and change in eGFR over time. In the subset of participants who returned for a follow-up functional MRI, we found that cortical ADC and R2* values remained stable over 1 year, which may be because of relatively small changes in eGFR over time in the study population. The results from our hypothesis-generating analyses suggest that the associations of kidney functional MRI biomarkers with change in kidney function over time require additional investigation in larger prospective studies with longer follow-up.
Multiple cross-sectional studies demonstrated that patients with lower cortical ADC values correlated with lower eGFR and higher albuminuria (1,11,19). Lower ADC values also strongly correlate with increased semiquantitative histopathologic assessments of fibrosis (1,4,5,8,19–21). In contrast, a recent study did not find a significant association between lower baseline cortical ADC values and eGFR decline over 5 years among patients with mild-to-moderate CKD (eGFR 49±29 ml/min per 1.73 m2) (11). Our findings largely support a possible relationship between lower cortical ADC and annual eGFR decline, but it is important to note the inconsistencies in some of our results. The magnitude of association between lower cortical ADC and annual eGFR decline was modest and no longer significant after further adjustment for the effect of albuminuria on change in eGFR over time. Although albuminuria confounded the association between cortical ADC and annual eGFR decline, another explanation is that noninvasive imaging assessments of cortical fibrosis have overlapping information with traditional risk factors for CKD progression, such as eGFR and albuminuria. In addition, it is not known whether albuminuria is on the causal pathway to CKD progression as a consequence of cortical fibrosis (22). If albuminuria is collinear with cortical fibrosis or a causal mediator along the pathway to eGFR decline, then its inclusion in models with cortical fibrosis could be an overadjustment, which requires further exploration in future larger studies of diffusion-weighted MRI. Our findings suggest that additional studies are required to understand the association of cortical ADC with eGFR decline and support the efforts of ongoing multicenter initiatives to optimize and standardize protocols for kidney functional MRI measurements, as being undertaken by the Biomarker Enterprise to Attack Diabetic Kidney Disease (BEAt-DKD) trial (NCT03716401) and PARENCHIMA (23,24).
The chronic hypoxia hypothesis suggests that early impaired tissue oxygen delivery has a pro-fibrogenic effect, which leads to extracellular matrix deposition and fibrosis that further obliterates the microvasculature and propagates scarring and progressive loss of kidney function (25,26). Prior studies report that there is reduced kidney cortical oxygenation, as evidenced by higher cortical R2*, in patients with CKD stages 2–4 compared with healthy volunteers (8,9,15). Recent single-center studies (10,11) found that reduced cortical oxygenation was associated with annual eGFR decline in patients with CKD stages 2–4, which differs from our current findings in patients with CKD stages 3b and 4. One potential explanation for our finding is that the chronic hypoxia hypothesis is more relevant in patients with mild-to-moderate CKD, which is consistent with the patients included in the prior studies (eGFR 55±29 and 49±29 ml/min per 1.73 m2) (10,11) compared with our study participants who had more advanced CKD (eGFR 32±9 ml/min per 1.73 m2). Our participants might have reached the point where reduced oxygen consumption from decreased glomerular filtration and tubular sodium transport led to our inability to detect an association between cortical hypoxia and annual eGFR slope (27).
In a subset of participants, we performed kidney functional MRI at two time points across seven clinical sites, and we found high intraclass correlations in cortical ADC and R2*. We interpret the results to indicate stability in repeated kidney functional MRI measurements over the course of 1 year, which likely reflects the relatively small changes in eGFR over time. This finding suggests that kidney functional MRI measurements may not change substantially in the presence of small changes in eGFR, and larger changes in eGFR are required to evoke significant changes in kidney functional MRI measurements.
Our study has multiple strengths. All of our participants had at least three eGFR measurements to estimate eGFR slope. We were able to perform kidney functional MRI in a multicenter clinical trial and obtain follow-up MRI in a subset of study participants. This study also has several limitations. We successfully recruited participants with CKD stage 3b and 4, but this limits the generalizability of the results to other patients with less severe CKD. We were unable to determine the underlying cause of CKD or determine associations of functional MRI findings with underlying histopathologic lesions as patients did not undergo kidney biopsies. The exploratory nature of our study with a small sample size and short duration of follow-up limited our ability to define the association between kidney functional MRI and eGFR decline. The number of participants who received a furosemide challenge was low (15), which limited our ability to test whether tubular sodium reabsorption activity, as assessed by BOLD MRI, is associated with eGFR decline. We did not control for dietary sodium intake, glycemia, or the use of intravenous iron, which could influence BOLD MRI results (28–30). We used hand-drawn ROIs, which may not be objective, and future studies should incorporate automated segmentation on high-contrast images that co-localize BOLD and diffusion-weighted MRI data. We did not assess kidney perfusion by functional MRI techniques, such as arterial spin labeling, which could provide further insight into the importance of microvascular dysfunction in CKD progression as suggested by a recent study (31).
In conclusion, our study demonstrates that kidney functional MRI can be incorporated into multicenter clinical trials. Our findings suggest the need for larger multicenter prospective studies with longer follow-up to test kidney functional MRI as a noninvasive method to identify patients at high risk of CKD progression.
Disclosures
Dr. Block reports grants from National Institutes of Health (NIH) during conduct of the study, other funding from Reata Pharmaceuticals, grants, personal fees, and nonfinancial support from Akebia, Inc., and Ardelyx, Inc., personal fees and nonfiancial support from Keryx, Inc., Kyowa Hakko Kirin, and OPKO, outside the submitted work. Dr. Carr reports other financial support from Circle and GE Healthcare, and grants from Bayer, Guerbet, and Siemens, outside the submitted work. Dr. Cheung reports grants from NIH, during the conduct of the study. Dr. Chonchol reports grants from Otsuka, Sanofi, and Kadmon, outside the submitted work, and grants from NIH/NIDDK during conduct of the study. Dr. Isakova reports nonfinancial support from Shire during the conduct of the study, and personal fees from Kirin and Guidepoint Global, outside the submitted work. Dr. Ix reports grants from NIDDK during the conduct of the study and grants from Baxter International, outside the submitted work. Dr. Middleton reports grants and personal fees from Relypsa, personal fees from AstraZeneca, Tricida, and NIDDK, outside the submitted work. Dr. Raphael reports grants from NIH and U.S. Department of Veterans Affairs, during the conduct of the study. Dr. Sprague reports grants from NIH, during the conduct of the study. Dr. Srivastava reports personal fees from Horizon Pharma, PLC, and CVS Caremark, outside the submitted work. Dr. Wolf reports personal fees from Akebia, Amag, Amgen, Ardelyx, Diasorin, Keryx, Lutipold, and Sanofi, and grants from Shire, outside the submitted work. All remaining authors have nothing to disclose.
Funding
The COMBINE trial is supported by NIH grants U01DK099877, U01DK097093, U01DK099930, U01DK099933, U01DK099924, and R01DK102438 (to Dr. Isakova). Work also supported in part by NIH grant R01DK093793 (to Dr. Prasad). Dr. Isakova is also supported by NIH grants R01DK110087, U01DK099930, and K24HL150235. Dr. Srivastava is supported by NIH grant K23DK120811, the Dixon Translational Research Grants Initiative at Northwestern Medicine and the Northwestern University Clinical and Translational Sciences Institute (UL1TR001422), and a Pilot & Feasibility Grant from the Center for Kidney Research and Therapeutics at Northwestern University Feinberg School of Medicine. Dr. Isakovaand Dr. Srivastava are supported by core resources from the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY; NIH grant P30DK114857).
Supplementary Material
Data Sharing Statement
The COMBINE Study Protocol, Manual of Operations, Statistical Analysis Plan, Case Report Forms, data dictionary, and study database data elements for all participants who consented to the COMBINE study will be available at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Repository by 2021 and will be available indefinitely. Detailed instructions for requesting data from the NIDDK Repository are available at https://repository.niddk.nih.org/.
Acknowledgments
Dr. Srivastava, Dr. Prasad, and Dr. Isakova designed the study. Dr. Srivastava, Ms. Cai, Dr. Lee, and Dr. Isakova were responsible for statistical analysis. Dr. Prasad and Dr. Isakova were responsible for supervision or mentorship. All authors were responsible for data acquisitions, and data analysis and interpretation. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Because Dr. Chonchol is a Deputy Editor of CJASN, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Part of this work was presented as a poster at the American Society of Nephrology Scientific Session on October 25, 2018 in San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Can COMBINED Magnetic Resonance Imaging Measure the Progression of Kidney Disease?,” on pages 747–749.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13201019/-/DCSupplemental.
Supplemental Table 1. Kidney functional MRI acquisition parameters.
Supplemental Table 2. Mean annual eGFR slope by tertiles of baseline kidney functional MRI biomarkers.
Supplemental Table 3. Association of baseline cortical ADC and cortical R2* with change in eGFR over time.
Supplemental Table 4. Associations of whole-kidney ADC, whole-kidney R2*, and medullary R2* with change in eGFR over time.
Supplemental Table 5. Associations of kidney functional MRI measurements with rapid CKD progression.
Supplemental Table 6. Kidney functional MRI measurements by treatment group among participants with baseline and follow-up functional MRI.
Supplemental Figure 1. Flow diagram for inclusion/exclusion of participants in kidney functional MRI substudy of the COMBINE trial.
References
- 1.Caroli A, Schneider M, Friedli I, Ljimani A, De Seigneux S, Boor P, Gullapudi L, Kazmi I, Mendichovszky IA, Notohamiprodjo M, Selby NM, Thoeny HC, Grenier N, Vallée JP: Diffusion-weighted magnetic resonance imaging to assess diffuse renal pathology: A systematic review and statement paper. Nephrol Dial Transplant 33[Suppl 2]: ii29–ii40, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M: Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168: 497–505, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Togao O, Doi S, Kuro-o M, Masaki T, Yorioka N, Takahashi M: Assessment of renal fibrosis with diffusion-weighted MR imaging: Study with murine model of unilateral ureteral obstruction. Radiology 255: 772–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao W, Zhou J, Zeng M, Ding Y, Qu L, Chen C, Ding X, Wang Y, Fu C, Gu F: Intravoxel incoherent motion diffusion-weighted imaging for the assessment of renal fibrosis of chronic kidney disease: A preliminary study. Magn Reson Imaging 47: 118–124, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Yu Y, Wen J, Zhang M, Chen J, Cheng D, Zhang L, Liu Z: Combination of functional magnetic resonance imaging and histopathologic analysis to evaluate interstitial fibrosis in kidney allografts. Clin J Am Soc Nephrol 14: 1372–1380, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad PV: Evaluation of intra-renal oxygenation by BOLD MRI. Nephron Clin Pract 103: c58–c65, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Prasad PV, Edelman RR, Epstein FH: Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation 94: 3271–3275, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, Watanabe Y, Takenaka T, Katayama S, Tanaka J, Suzuki H: Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol 22: 1429–1434, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad PV, Thacker J, Li LP, Haque M, Li W, Koenigs H, Zhou Y, Sprague SM: Multi-Parametric evaluation of chronic kidney disease by MRI: A preliminary cross-sectional study. PLoS One 10: e0139661, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruijm M, Milani B, Pivin E, Podhajska A, Vogt B, Stuber M, Burnier M: Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int 93: 932–940, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama K, Inoue T, Kozawa E, Ishikawa M, Shimada A, Kobayashi N, Tanaka J, Okada H: Reduced oxygenation but not fibrosis defined by functional magnetic resonance imaging predicts the long-term progression of chronic kidney disease [published online ahead of print Nov 12, 2018]. Nephrol Dial Transplant doi: 10.1093/ndt/gfy324 [DOI] [PubMed] [Google Scholar]
- 12.Isakova T, Ix JH, Sprague SM, Raphael KL, Fried L, Gassman JJ, Raj D, Cheung AK, Kusek JW, Flessner MF, Wolf M, Block GA: Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 26: 2328–2339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ix JH, Isakova T, Larive B, Raphael KL, Raj DS, Cheung AK, Sprague SM, Fried LF, Gassman JJ, Middleton JP, Flessner MF, Block GA, Wolf M: Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: The COMBINE trial. J Am Soc Nephrol 30: 1096–1108, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thacker JM, Li LP, Li W, Zhou Y, Sprague SM, Prasad PV: Renal blood oxygenation level-dependent magnetic resonance imaging: A sensitive and objective analysis. Invest Radiol 50: 821–827, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad PV, Li W, Raj DS, Carr J, Carr M, Thacker J, Li LP, Wang C, Sprague SM, Ix JH, Chonchol M, Block G, Cheung AK, Raphael K, Gassman J, Wolf M, Fried LF, Isakova T; CKD Optimal Management with BInders and NicotinamidE (COMBINE) study group: Multicenter study evaluating intrarenal oxygenation and fibrosis using magnetic resonance imaging in individuals with advanced CKD. Kidney Int Rep 3: 1467–1472, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL 3rd, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidney Disease Improving Global Outcomes: Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl (2011) 3: 63–72, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, Rifkin D, Sarnak MJ: Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 20: 2625–2630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchanan CE, Mahmoud H, Cox EF, McCulloch T, Prestwich BL, Taal MW, Selby NM, Francis ST: Quantitative assessment of renal structural and functional changes in chronic kidney disease using multi-parametric magnetic resonance imaging [published online ahead of print Jun 29, 2019]. Nephrol Dial Transplant doi: 10.1093/ndt/gfz129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Li J, Zhang L, Chen Y, Zhang M, Yan F: Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: A preliminary clinical study. Eur J Radiol 83: 756–762, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Wang ZJ, Liu M, Zhu J, Zhang X, Zhang T, Li S, Li Y: Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol 69: 1117–1122, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Jefferson JA, Shankland SJ, Pichler RH: Proteinuria in diabetic kidney disease: A mechanistic viewpoint. Kidney Int 74: 22–36, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Selby NM, Blankestijn PJ, Boor P, Combe C, Eckardt KU, Eikefjord E, Garcia-Fernandez N, Golay X, Gordon I, Grenier N, Hockings PD, Jensen JD, Joles JA, Kalra PA, Krämer BK, Mark PB, Mendichovszky IA, Nikolic O, Odudu A, Ong ACM, Ortiz A, Pruijm M, Remuzzi G, Rørvik J, de Seigneux S, Simms RJ, Slatinska J, Summers P, Taal MW, Thoeny HC, Vallée JP, Wolf M, Caroli A, Sourbron S: Magnetic resonance imaging biomarkers for chronic kidney disease: A position paper from the European Cooperation in Science and Technology Action PARENCHIMA. Nephrol Dial Transplant 33[Suppl 2]: ii4–ii14, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caroli A, Pruijm M, Burnier M, Selby NM: Functional magnetic resonance imaging of the kidneys: Where do we stand? The perspective of the European COST Action PARENCHIMA. Nephrol Dial Transplant 33[Suppl 2]: ii1–ii3, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fine LG, Orphanides C, Norman JT: Progressive renal disease: The chronic hypoxia hypothesis. Kidney Int Suppl 65: S74–S78, 1998 [PubMed] [Google Scholar]
- 26.Fine LG, Norman JT: Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Neugarten J: Renal BOLD-MRI and assessment for renal hypoxia. Kidney Int 81: 613–614, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, Burnier M, Vogt B: Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension 55: 1116–1122, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Vakilzadeh N, Zanchi A, Milani B, Ledoux JB, Braconnier P, Burnier M, Pruijm M: Acute hyperglycemia increases renal tissue oxygenation as measured by BOLD-MRI in healthy overweight volunteers. Diabetes Res Clin Pract 150: 138–143, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Pruijm M, Mendichovszky IA, Liss P, Van der Niepen P, Textor SC, Lerman LO, Krediet CTP, Caroli A, Burnier M, Prasad PV: Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: A statement paper and systematic review. Nephrol Dial Transplant 33[Suppl 2]: ii22–ii28, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad PV, Li LP, Thacker JM, Li W, Hack B, Kohn O, Sprague SM: Cortical perfusion and tubular function as evaluated by magnetic resonance imaging correlates with annual loss in renal function in moderate chronic kidney disease. Am J Nephrol 49: 114–124, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.