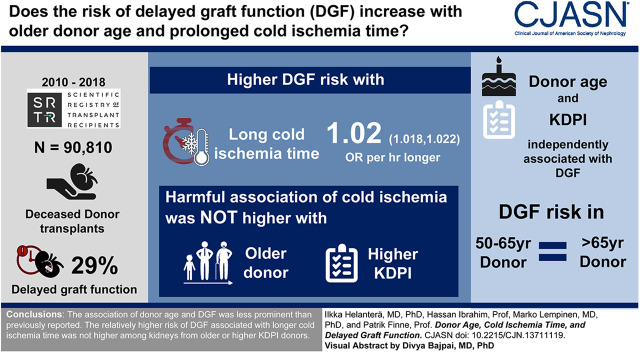
Visual Abstract
Keywords: Epidemiology and outcomes, transplant outcomes, cadaver organ transplantation, delayed graft function, Cold Ischemia, kidney transplantation, creatinine, Retrospective Studies, Transplant Recipients, risk factors, Cause of Death, Body Mass Index, renal dialysis, Tissue Donors, Kidney Diseases, Registries, diabetes mellitus, hypertension, Cohort Studies
Abstract
Background and objectives
Increased donor age is one of the most important risk factors for delayed graft function (DGF), and previous studies suggest that the harmful effect of cold ischemia time is increased in kidneys from older donors. Our aim was to study the association of increased donor age and cold ischemia time with the risk of delayed graft function in a large cohort kidney transplants from the current era.
Design, setting, participants, & measurements
The Scientific Registry of Transplant Recipients was used for this observational, retrospective registry analysis to identify all deceased donor kidney transplantations in the United States between 2010 and September 2018, who were on dialysis pretransplantation (n=90,810). The association of donor age and cold ischemia time with the risk of DGF was analyzed in multivariable models adjusted for recipient characteristics (age, race, sex, diabetes, calculated panel-reactive antibodies, pretransplant dialysis duration) and donor characteristics (cause of death, sex, race, body mass index, creatinine, donation after circulatory death status, history of hypertension, and HLA mismatch).
Results
Cold ischemia time and donor age were independently associated with the risk of DGF, but the risk of DGF was not statistically significantly lower in donor age categories between 50 and 64 years, compared with donors ≥65 years. The harmful association of cold ischemia time was not higher in kidneys from older donors in any age category, not even among donation after circulatory death donors. When donor risk was assessed with kidney donor profile index, although a statistically significant interaction with cold ischemia time was found, no practically meaningful increase in cold-ischemia susceptibility of kidneys with a high kidney donor profile index was found.
Conclusions
We were unable to demonstrate an association between donor age and DGF. The association of longer cold ischemia time with the risk of DGF was not magnified in older or more marginal donors.
Introduction
Delayed graft function (DGF) is estimated to occur in approximately 20%–40% of patients after deceased donor kidney transplantation (1–3), and is associated with increased length of hospital stay and costs after transplantation (4,5). DGF is also associated with an increased risk of acute rejection (6) and impaired long-term outcome after kidney transplantation (6,7), although some studies have failed to show harmful long-term effects of DGF (8,9).
DGF is thought to originate from ischemia-reperfusion injury to the graft, and the most important risk factors for DGF are described to be increased donor age and increased cold ischemia time (1,10,11). Similarly, other factors that compromise the functional or reparative capacity of the graft have been associated with the risk of DGF, such as donation after circulatory death (12), especially uncontrolled donation after circulatory death (13), presence of donor-specific antibodies (14), highly sensitized patients (10), or kidney dysfunction in the donor (10,11). The use of machine perfusion, on the other hand, has been associated with a lower incidence of DGF (15). In the recent years, the quality of deceased donors in the United States has been assessed with the kidney donor profile index (KDPI) (16), and similar to high donor age, high KDPI has been associated with increased risk of DGF (17).
Several studies have shown that older kidneys may be more susceptible to the damage caused by cold storage (18–20), whereas no data exist about the possible more harmful effect of cold ischemia time on marginal kidneys when assessed with the KDPI scale.
Our aim was to study the association between donor age and the risk of DGF in the current era in a large cohort of deceased donor kidney transplant recipients, and to study whether the harmful association of longer cold ischemia with the risk of DGF is magnified in kidneys from older donors or kidneys from high-KDPI donors.
Materials and Methods
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. Standard Analysis Files (Q3 2018 release) were used. This study is a retrospective observational registry analysis. This study had the approval of the institutional review board of Helsinki University Hospital (HUS/333/2019). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Patients
From the SRTR data, we identified all deceased donor kidney transplantations between 2010 and September 2018. The primary outcome was DGF, defined by the need of dialysis during the first post-transplant week, and therefore only patients with pretransplant dialysis treatment were included (n=90,810) in the primary analyses. Altogether, 93 patients had the data about DGF missing and were excluded from the analyses, resulting in a cohort of 90,717 patients for the primary analyses.
Statistical Analyses
Multivariable, binary logistic regression was used to examine risk factors for DGF, with post-transplant dialysis during the first post-transplant week as the primary outcome. As our primary focus was to study the association of donor age and cold ischemia time with the risk of DGF, these variables were the primary effect variables in the models. Adjustment was made with covariates, which were significant in univariable logistic regression (P<0.05), or which were identified as risk factors for DGF in the previous literature. For the logistic regression models, the last available calculated panel-reactive antibody (cPRA) level was categorized to <80% (reference group), from 80% to <95%, from 95% to <99%, and ≥99%. The association of donor age with the risk of DGF was compared with age ≥65 years (reference group), and categorized between 5-year increments until the age categories 12–19, 5–11, and 0–4 years. Cold ischemia time was categorized to 0–11 hours (reference group), 12–15, 16–19, 20–23, and ≥24 hours. KDPI values were calculated as described for year 2017 (16). For the logistic regression models, KDPI was categorized to quartiles, with the lowest quartile as the reference value. Variables were categorized because the association with DGF was not linear throughout the distribution in the variables. In addition, donor age was modeled nonlinearly as a continuous variable, using fourth-degree polynomial functions. When KDPI was included in the models, all of the other donor factors used to calculate KDPI were left out of the model because of possible multicollinearity (age, race, body mass index [BMI], history of hypertension, history of diabetes, and cause of death, donation after circulatory death status). The interaction terms cold ischemia time×donor age and cold ischemia×KDPI were used to analyze whether the risk associated with cold ischemia differed according to age category or category of KDPI. In addition, the odds ratios (ORs) associated with different cold ischemia time categories were analyzed separately in different age categories, and in different KDPI categories. As the use of machine perfusion has been associated with lower incidence of DGF and significant interaction was detected between donor age and the use of machine perfusion, analyses were performed also separately among kidneys transplanted after machine perfusion. The United Network for Organ Sharing and OPTN implemented a new kidney allocation system (KAS) on December 4, 2014, which may affect our findings. Therefore, sensitivity analyses were performed also separately in kidneys transplanted before or after implementation of the KAS.
Complete data were available for all other variables used for analysis, except for donor history of hypertension (missing from 587 patients), recipient diabetes history (missing from five), number of HLA mismatches (missing from 357), cold ischemia time (missing from 776), and cPRA (missing from 20 patients). Patients with missing data were left out of the respective analyses. The calculations were performed with IBM SPSS statistical software (version 24.0; IBM Corporation, Somers, NY). Two-sided P values <0.05 were considered statistically significant.
Results
A total of 90,717 recipients of deceased donor kidney transplantation were included in the analyses. The frequency of DGF was 29% in the whole cohort, 45% in kidneys from donors after circulatory death, and 25% in kidneys from donors after brain death (P<0.001). Patients with and without DGF are compared in Table 1.
Table 1.
Characterization of patients included in the study, transplanted between 2010 and September 2018 in the United States
| Variable | Patients with DGF (n=26,066) | Patients without DGF (n=64,651) |
|---|---|---|
| Recipient age, yr | 54±13 | 50±16 |
| Recipient male | 17,545 (67%) | 37,743 (58%) |
| Recipient diabetes | 11,019 (42%) | 21,072 (33%) |
| Black recipients | 10,122 (39%) | 21,037 (33%) |
| cPRA, % | ||
| 0–79 | 21,928 (84%) | 53,358 (83%) |
| 80–94 | 1791 (7%) | 5384 (8%) |
| 95–98 | 924 (4%) | 2607 (4%) |
| ≥99 | 1416 (5%) | 3288 (5%) |
| Donor age categories, yr | ||
| 0–29 | 6531 (25%) | 24,067 (37%) |
| 30–44 | 7366 (28%) | 17,546 (27%) |
| 45–64 | 9971 (38%) | 18,574 (28%) |
| ≥65 | 2198 (8%) | 4464 (7%) |
| Donor male | 16,362 (63%) | 38,849 (60%) |
| Donor body mass index | 29±7 | 27±7 |
| Donation after circulatory death donors | 7216 (28%) | 8980 (14%) |
| Machine perfusion | 9584 (37%) | 24,034 (37%) |
| Black donor | 3728 (15%) | 9734 (15%) |
| Expanded criteria donor | 4496 (17%) | 8335 (13%) |
| Donor serum creatinine >1.5 mg/dl | 7037 (27%) | 9328 (14%) |
| Donor history of hypertension | 8894 (34%) | 15,822 (25%) |
| KDPI>80% | 4738 (18%) | 8179 (13%) |
| Cold ischemia time, h | ||
| <12 | 5748 (22%) | 20,934 (33%) |
| 12–15 | 4726 (18%) | 12,642 (20%) |
| 16–19 | 4882 (19%) | 11,334 (18%) |
| 20–23 | 4087 (16%) | 8084 (13%) |
| ≥24 | 6444 (25%) | 11,089 (18%) |
| Number of HLA mismatches | 4±2 | 4±2 |
Mean±1 SD, unless otherwise indicated. DGF, delayed graft function; cPRA, calculated panel-reactive antibodies; KDPI, kidney donor profile index.
Association of Donor Age with the Risk of DGF
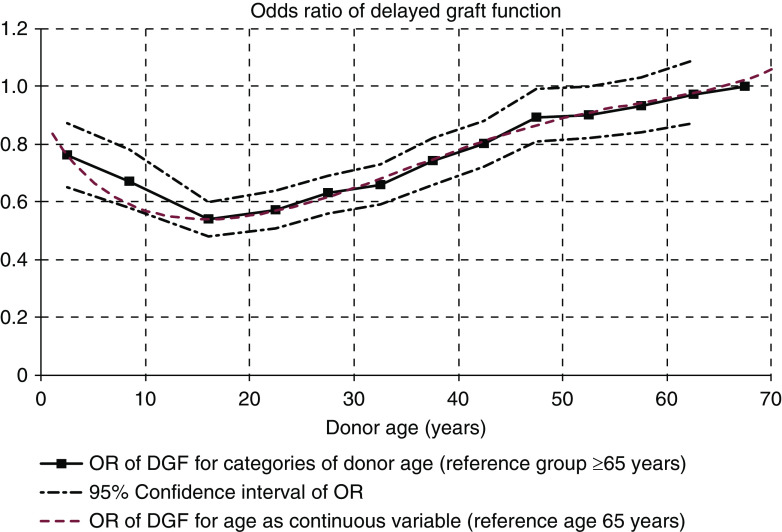
Table 2 shows the results of the multivariable model analyzing the risk factors for DGF with regard to cold ischemia time and donor age. The final model was adjusted for recipient factors (age, race, sex, diabetes, last available cPRA, duration of pretransplant dialysis) and donor factors (cause of death, sex, race, BMI, creatinine, donation after circulatory death status, history of hypertension, and HLA mismatch). Detailed results of the multivariable model are shown in Supplemental Table 1. In the multivariable model, cold ischemia time (OR, 1.02 per 1-hour increase; 95% confidence interval [95% CI], 1.01 to 1.03) was an independent predictor of DGF. For the final model, cold ischemia time was categorized to 0–11 hours (reference group), 12–15, 16–19, 20–23, and ≥24 hours. To assess the statistical significance of the association of increased donor age with the risk of DGF, the oldest age group (donor age ≥65 years) was used as the reference group and the risk was analyzed with 5-year increments until age categories 12–19, 5–11, and 0–4 years. Although a clear association was seen between donor age and the risk of DGF, statistically significantly lower risk associated with younger donor age was not seen until with donor age <50 years, and the risk continued to be lower within each age category until donor age <12 years (Table 2). The nonlinear association of donor age with the risk of DGF is graphically characterized in Figure 1.
Table 2.
The risk of delayed graft function associated with donor age and cold ischemia time, among the 90,717 patients transplanted between 2010 and September 2018
| Variable | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Donor age, yr (reference ≥65 yr) | ||
| 60–64 | 0.97 | 0.87 to 1.09 |
| 55–59 | 0.93 | 0.84 to 1.03 |
| 50–54 | 0.90 | 0.82 to 1.00 |
| 45–49 | 0.89 | 0.81 to 0.99 |
| 40–44 | 0.80 | 0.72 to 0.88 |
| 35–39 | 0.74 | 0.66 to 0.82 |
| 30–34 | 0.66 | 0.59 to 0.73 |
| 25–29 | 0.63 | 0.56 to 0.69 |
| 20–24 | 0.57 | 0.51 to 0.64 |
| 12–19 | 0.54 | 0.48 to 0.60 |
| 5–11 | 0.67 | 0.58 to 0.78 |
| 0–4 | 0.76 | 0.65 to 0.87 |
| Cold ischemia time, h (reference <12 h) | ||
| 12–15 | 1.20 | 1.15 to 1.26 |
| 16–19 | 1.27 | 1.21 to 1.33 |
| 20–23 | 1.47 | 1.40 to 1.55 |
| ≥24 | 1.70 | 1.63 to 1.78 |
Model adjusted for donation after circulatory death status, recipient age and sex, recipient diabetes, recipient race, calculated panel-reactive antibodies, pretransplant dialysis duration, donor cause of death, donor race, donor history of hypertension, donor creatinine, donor body mass index, and number of HLA mismatches.
Figure 1.
The risk of delayed graft function associated with donor age does not increase linearly. Risk of delayed graft function associated with donor age is expressed as odds ratios for categories of donor age (black line, 95% confidence intervals expressed as dashed lines; reference group age >65 years).In addition, odds ratios for donor age as a continuous variable using fourth-degree polynomial logistic regression equations are used to describe the nonlinear association between donor age and the risk of delayed graft function (red line; reference age >65 years). Associations were analyzed in a multivariable logistic regression model adjusted for cold ischemia time, calculated panel-reactive antibodies, donor after circulatory death status, recipient age and sex, recipient diabetes, recipient race, pretransplant dialysis duration, donor race, donor sex, donor history of hypertension, donor creatinine, donor body mass index, donor cause of death, and number of HLA mismatches. DGF, delayed graft function; OR, odds ratio.
Association of Machine Perfusion with Donor Age
Machine perfusion is potentially a confounding factor when the association of donor age is analyzed, as the frequency of machine perfusion utilization increased with donor age and machine perfusion may be used more frequently in marginal donors with the highest risk of DGF. Among the whole study cohort, machine perfusion was used in 33,656 kidney transplantations (37%). The frequency was 26% in donors aged <20 years, 43% among donors aged between 45 and 64 years, and 49% among kidneys from donors aged ≥65 years. When the use of machine perfusion was included in the multivariable model, the use of machine perfusion was associated with a lower risk of DGF (OR, 0.58; 95% CI, 0.56 to 0.60; P<0.001). All other independent risk factors remained significant and the magnitude of effect remained within similar range. A statistically significant interaction was detected between donor age and the use of machine perfusion (P=0.001). Therefore, the association of donor age was analyzed separately among kidney transplants with or without machine perfusion in a similar multivariable model, shown in Supplemental Table 2. Among kidneys transplanted without machine perfusion, the association of donor age remained similar as in the whole cohort (Supplemental Table 2). Among kidneys transplanted with machine perfusion, statistically significantly lower risk of DGF was detected already in donors between age 50 and 54 years, whereas no statistically significant difference in the risk of DGF was detected between kidneys from donors aged between 55 and 64 years compared with donors aged ≥65 years.
Association of Cold Ischemia with Kidneys from Older Donors
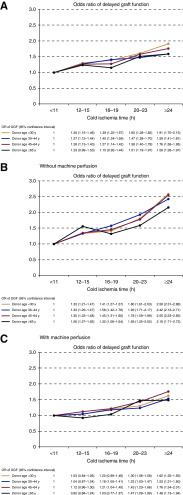
The association of increased cold ischemia time with the risk of DGF was not higher in kidneys from older donors in any donor age category. No significant interaction was detected in the multivariable logistic regression between donor age and cold ischemia time (P=0.27). Frequencies of DGF in different donor age and cold ischemia categories are shown in Table 3. Although both higher donor age and longer cold ischemia times were associated with higher risk of DGF, the risk associated with longer cold ischemia times was not higher among the kidneys from older donors compared with younger donors. This was confirmed in a multivariable model (including the same variables as in Table 2), where the ORs within the various cold ischemia categories remained virtually similar in all donor age categories, as well as in kidneys transplanted with or without machine perfusion (Figure 2).
Table 3.
The frequency of delayed graft function in categories of cold ischemia time and donor age
| Cold Ischemia Time, h | Donor Age 0–29 yr | Donor Age 30–44 yr | Donor Age 45–64 yr | Donor Age ≥65 yr |
|---|---|---|---|---|
| <12 | 15% | 23% | 27% | 27% |
| 12–15 | 20% | 28% | 33% | 33% |
| 16–19 | 23% | 32% | 36% | 32% |
| 20–23 | 26% | 35% | 39% | 37% |
| ≥24 | 30% | 37% | 43% | 38% |
Figure 2.
The harmful association between cold ischemia time and the risk of delayed graft function is similar in various donor age categories. The risk of delayed graft function in categories of donor age and cold ischemia time is expressed as odds ratios (colored lines for different donor age categories; odds ratios and 95% confidence intervals in the table), analyzed in a multivariable logistic regression model adjusted for calculated panel-reactive antibodies, donor after circulatory death status, recipient age and sex, recipient diabetes, recipient race, pretransplant dialysis duration, donor race, donor sex, donor history of hypertension, donor creatinine, donor body mass index, donor cause of death, and number of HLA mismatches. (A) All patients, (B) kidneys transplanted without machine perfusion, and (C) kidneys transplanted with machine perfusion.
Association of KDPI and Cold Ischemia Time with DGF
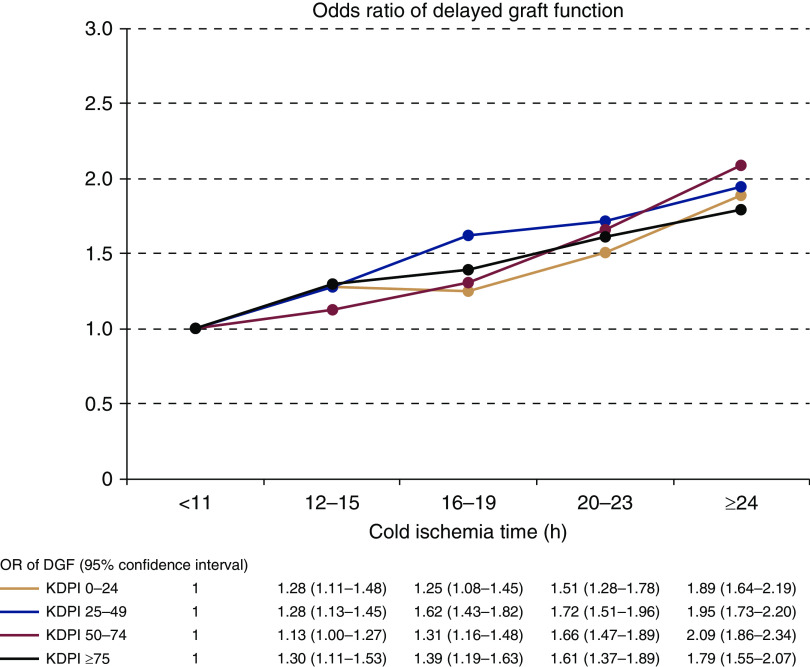
When KDPIs were included in the logistic regression model (categorized to 0%–24% as the reference, compared with 25%–49%, 50%–74%, and ≥75%) to replace variables included in the KDPI, KDPI and cold ischemia time were both independently associated with higher risk of DGF (Table 4). Other risk factors for DGF (high cPRA, recipient age, gender, race, and diabetes, donor gender, and duration of pretransplant dialysis) remained significant (Supplemental Table 3). There was a statistically significant interaction between KDPI and cold ischemia time (P=0.008). The frequencies of DGF in different cold ischemia time and KDPI categories are shown in Table 5. Although the frequency of DGF was higher with both KDPI and with increasing cold ischemia times, no clinically relevant increase in the association between cold ischemia time and DGF risk could be observed among the kidneys from high-KDPI donors. This was confirmed in a multivariable model (including the same variables as in Table 4), where the ORs of the various cold ischemia categories remained in the same range in all KDPI categories (Figure 3).
Table 4.
The association of kidney donor profile index (KDPI) and cold ischemia time with the risk of delayed graft function
| Variable | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| KDPI, % (reference <25%) | ||
| 25–49 | 1.58 | 1.51 to 1.65 |
| 50–74 | 2.03 | 1.94 to 2.12 |
| ≥75 | 2.15 | 2.05 to 2.26 |
| Cold ischemia time, h (reference <12 h) | ||
| 12–15 | 1.29 | 1.24 to 1.36 |
| 16–19 | 1.45 | 1.38 to 1.52 |
| 20–23 | 1.71 | 1.62 to 1.79 |
| ≥24 | 1.95 | 1.87 to 2.04 |
Model adjusted for calculated panel-reactive antibodies, cold ischemia time, recipient age and sex, recipient diabetes, recipient race, pretransplant dialysis duration, donor sex, number of HLA mismatches, and KDPI categories.
Table 5.
The frequency of delayed graft function in categories of cold ischemia time and kidney donor profile index (KDPI)
| Cold ischemia time, h | KDPI 0%–24% | KDPI 25%–49% | KDPI 50%–74% | KDPI 75%–100% |
|---|---|---|---|---|
| <12 | 14% | 22% | 27% | 29% |
| 12–15 | 18% | 28% | 31% | 35% |
| 16–19 | 20% | 31% | 34% | 36% |
| 20–23 | 22% | 33% | 39% | 40% |
| ≥24 | 25% | 35% | 43% | 42% |
Figure 3.
The harmful association between cold ischemia time and the risk of delayed graft function is similar in various kidney donor profile index (KDPI) categories. The risk of delayed graft function in categories of KDPI and cold ischemia time is expressed as odds ratios (colored lines for different KDPI categories; odds ratios and 95% confidence intervals in the table), analyzed in a multivariable logistic regression model adjusted for calculated panel-reactive antibodies, cold ischemia time, recipient age and sex, recipient diabetes, recipient race, pretransplant dialysis duration, donor sex, and number of HLA mismatches.
Sensitivity Analyses
To investigate the most important findings of this study in more detail, the same analyses were repeated in different subgroups as sensitivity analyses. As the effect of donor age may be different among donors after circulatory death, we studied the association of donor age with DGF separately among donation after circulatory death and donation after brain death donor kidneys. Among kidneys from donors after brain death, the results of the multivariable, logistic regression model (including the same variables as in Table 2) were similar enough to draw the same conclusions as from the whole cohort (Supplemental Table 4). Among the 16,213 kidneys from donors after circulatory death, the association of increased donor age was comparable with the whole cohort, as the risk of DGF was not statistically significantly lower until donors aged <35 years compared with donors aged ≥65 years (OR, 0.54; 95% CI, 0.30 to 0.96; P=0.04 for donors aged 30–34 years; Supplemental Table 4). When kidneys transplanted before or after the KAS were analyzed separately, the association of donor age with the risk of DGF supported our findings from the whole cohort. Among kidneys transplanted before KAS, lower risk of DGF associated with younger donor age reached statistical significance only among donors aged <45 years (OR, 0.82; 95% CI, 0.72 to 0.94; P=0.005 for donors aged 40–44 years) compared with donors aged ≥65 years, whereas among kidneys transplanted after KAS, a statistically significantly lower risk of DGF associated with donor age was seen among kidneys transplanted from donors aged <50 years (OR, 0.80; 95% CI, 0.69 to 0.93; P=0.005). The other risk factors associated with the risk of DGF remained significant in the models, and the association between cold ischemia time and DGF remained similar in all age and KDPI categories (Supplemental Table 5).
During the study period, a total of 12,383 patients received a deceased donor kidney transplantation preemptively without preceding dialysis treatment. As the definition of DGF in this study was the need for dialysis during the first post-transplant week, patients with preemptive transplantation were excluded from the primary analyses. Among the 12,383 patients with preemptive transplantation, the frequency of DGF with the current definition was 7% (1027 patients). When these patients with preemptive transplantation were included in the cohort, the results of the multivariable model remained unchanged with regard to the association of donor age and cold ischemia time with risk of DGF (Supplemental Table 6).
Discussion
In this study analyzing a large number of kidney transplants from the current era in the United States, our main finding was that although higher donor age is independently associated with higher risk of DGF, the effect of age becomes less pronounced when donor age exceeds 50 years. In fact, the risk of DGF for donor age groups between 50 and 64 years were not statistically different compared with the reference group of donor age of >65 years. In addition, no increased susceptibility of older kidneys to harmful effect of cold storage was found in terms of DGF risk. Similarly, when low organ quality was assessed by means of KDPI value instead of donor age, although a statistically significant interaction with cold ischemia time was found, we did not observe a practically meaningful increase in the susceptibility of lower-quality kidneys to cold ischemia.
In all studies addressing the risk of DGF after deceased donor kidney transplantation, increased cold ischemia time and high donor age have been major risk factors (7), and prolonged cold storage has been considered especially harmful to kidneys from older donors (18–20). Within transplant organizations such as Eurotransplant Senior Program, organ allocation policies have been designed to minimize cold ischemia time in older donors on the basis of this assumption (21). The aim of our study was to examine the association of cold ischemia and DGF in detail, and in contrast to previous literature, our findings do not support higher risk of DGF for older donor or marginal kidneys with prolonged cold ischemia time. Although donor age is an important risk factor for DGF, the differences were not statistically significant beyond donor age of 50 years, compared with the oldest age group. The reasons for the difference in the association of donor age with the risk of DGF in our study and previous literature can only be speculated. Average life expectancy increases in all developed countries, allowing individuals to live healthier for longer. Therefore, other factors than biologic age, most importantly comorbidities, may play a more important role in the risk of DGF. Indeed, donor history of hypertension, donor kidney function, and donor BMI were all associated with increased risk of DGF in our study, possibly diluting the individual effect of donor age. However, although the association was not amplified in old or marginal donors, longer cold ischemia time was still associated with higher risk of DGF, and this may have potential effect on long-term survival of the grafts or graft function. Therefore, organ allocation policies may still need to be directed to prevent this cumulative damage to more compromised kidneys from older or marginal donors.
Average donor age has not increased in the United States in the recent years, and <25% of deceased donors have been aged >50 years (22). This is in sharp contrast with many European countries: within the Eurotransplant in 2017, 47% of deceased kidney donors were aged >55 years and 23% of donors were aged ≥65 years (23). Only 7% of the deceased donors in our study were aged ≥65 years, and the lower relative utilization of older donors in the United States possibly leads to selection of healthier old donors with less comorbidities. Difference in age distribution between various kidney transplant cohorts may be one factor explaining the varying effect of donor age on the risk of DGF in earlier studies. Therefore, the results may not be directly applicable to other transplant cohorts.
Although DGF commonly complicates deceased donor kidney transplantation, relatively little research focus has been directed to risk factors of DGF in recent years, not to mention the optimal treatment strategies after occurrence of DGF. Although the effect of DGF on long-term graft prognosis has been questioned (8,9), DGF is associated with higher risk of acute rejection, morbidity, length of hospital stay, and higher costs after transplantation (4–6). The frequency or duration of DGF can be addressed from several directions, including optimal treatment of the deceased donor (24,25), strategies to minimize cold ischemia times (26), the optimal utilization of machine perfusion (27), or optimal dialysis strategy after occurrence of DGF (28).
The use of induction therapy, especially lymphocyte-depleting induction, has been associated with lower incidence of DGF in several studies (29–31), although a recent Cochrane systematic review failed to show any benefit of induction therapy in terms of DGF risk (32). Our analyses were not adjusted for data regarding induction therapy, which may limit our conclusions. On the other hand, according to the OPTN/SRTR 2016 Annual Data Report, induction therapy was used on approximately 90% of kidney transplantations in the United States, and T cell–depleting agents in >70% of patients (22). Similarly, some studies have failed to show any difference in the risk of DGF between IL-2R antagonists and lymphocyte-depleting agents (33). Therefore, the possible confounding effect of induction therapy in our analyses is likely small. However, the increasing use of induction therapy compared with earlier studies may be one factor contributing to the lower importance of donor age on the risk of DGF.
This study has some limitations of note. Several studies have shown that longer cold ischemia time is associated with higher risk of graft failure (34,35), independent of the occurrence of DGF. We did not, however, analyze the effect of donor age on graft survival, as our aim was to analyze risk factors associated only with DGF, not long-term prognosis, and to focus specifically on the association of donor age and cold ischemia time. KDPI was used to characterize the marginality of donors in this study, although KDPI and KAS were only introduced in 2014. For a significant proportion of patients in this study, KDPI is only calculated retrospectively, and was not used in organ allocation at the time of transplantation, which may limit the generalizability of the results. Lastly, causality between explanatory variables and DGF cannot be concluded from this observational registry analysis, as there may be known or unknown confounding factors that were not adjusted for.
In conclusion, the association of old donor age with the risk of DGF was less prominent than previously reported, and the relative increase in the risk of DGF associated with longer cold ischemia time was not higher among kidneys from older or higher KDPI donors. Our data suggest that other factors than just old donor age play a role in the development of DGF after kidney transplantation in the current era.
Disclosures
Dr. Finne reports personal fees from Baxter, outside the submitted work. Dr. Helanterä reports grants from Finska Läkaresällskapet, during the conduct of the study; personal fees from Novartis, outside the submitted work. Dr. Ibrahim and Dr. Lempinen have nothing to disclose.
Funding
This study was supported by Finska Läkaresällskapet and Finnish Government Research Funds for Health Sciences (Valtion rahoitus yliopistotasoiseen terveyden tutkimukseen) grant TYH2019214 (to Dr. Helanterä).
Supplementary Material
Acknowledgments
An abstract including part of the results of this study were presented as a poster presentation in American Transplant Congress in Boston, Masschusetts, June 1–5, 2019.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as a contractor for the Scientific Registry of Transplant Recipients (SRTR). The data that support the findings of this study are available from SRTR. Restrictions apply to the availability of these data, which were used under license for this study. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Donor Characteristics and Short-Term Kidney Allograft Outcomes: Opportunities to Expand Utilization,” on pages 750–751.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13711119/-/DCSupplemental.
Supplemental Table 1. Results of the multivariable model of risk factors associated with delayed graft function after deceased donor kidney transplantation.
Supplemental Table 2. The risk of delayed graft function associated with donor age in kidneys transplanted with or without machine perfusion.
Supplemental Table 3. Results of the multivariable model of risk factors associated with delayed graft function after deceased donor kidney transplantation, including kidney donor profile index in the model.
Supplemental Table 4. The risk of delayed graft function associated with donor age in kidneys transplanted from (A) donors after brain death, or (B) donors after circulatory death (B).
Supplemental Table 5. The risk of delayed graft function in categories of cold ischemia time and donor age, or categories of cold ischemia time and kidney donor profile index (KDPI), analyzed in a multivariable model, expressed as odds ratios and 95% confidence intervals (adjusted for calculated panel-reactive antibodies, donation after circulatory death status, recipient age and sex, recipient diabetes, recipient race, donor race, donor history of hypertension, donor creatinine, donor body mass index, donor cause of death, and number of HLA mismatches).
Supplemental Table 6. The risk of delayed graft function associated with donor age and cold ischemia time, among the 103,100 patients transplanted between 2010 and September 2018, including 12,383 patients transplanted preemptively without preceding dialysis treatment.
References
- 1.Siedlecki A, Irish W, Brennan DC: Delayed graft function in the kidney transplant. Am J Transplant 11: 2279–2296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollmén ME, Kyllönen LE, Inkinen KA, Lalla ML, Salmela KT: Urine neutrophil gelatinase-associated lipocalin is a marker of graft recovery after kidney transplantation. Kidney Int 79: 89–98, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Mannon RB: Delayed graft function: The AKI of kidney transplantation. Nephron 140: 94–98, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagenmeyer EG, Häussler B, Hempel E, Grannas G, Kaló Z, Kilburg A, Nashan B: Resource use and treatment costs after kidney transplantation: Impact of demographic factors, comorbidities, and complications. Transplantation 77: 1545–1550, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Serrano OK, Vock DM, Chinnakotla S, Dunn TB, Kandaswamy R, Pruett TL, Feldman R, Matas AJ, Finger EB: The relationships between cold ischemia time, kidney transplant length of stay, and transplant-related costs. Transplantation 103: 401–411, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Yarlagadda SG, Coca SG, Formica RN Jr., Poggio ED, Parikh CR: Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant 24: 1039–1047, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC: A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant 10: 2279–2286, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Boom H, Mallat MJ, de Fijter JW, Zwinderman AH, Paul LC: Delayed graft function influences renal function, but not survival. Kidney Int 58: 859–866, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Kayler LK, Srinivas TR, Schold JD: Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant 11: 2657–2664, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Irish WD, McCollum DA, Tesi RJ, Owen AB, Brennan DC, Bailly JE, Schnitzler MA: Nomogram for predicting the likelihood of delayed graft function in adult cadaveric renal transplant recipients. J Am Soc Nephrol 14: 2967–2974, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Chapal M, Le Borgne F, Legendre C, Kreis H, Mourad G, Garrigue V, Morelon E, Buron F, Rostaing L, Kamar N, Kessler M, Ladrière M, Soulillou JP, Launay K, Daguin P, Offredo L, Giral M, Foucher Y: A useful scoring system for the prediction and management of delayed graft function following kidney transplantation from cadaveric donors. Kidney Int 86: 1130–1139, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Snoeijs MG, Winkens B, Heemskerk MB, Hoitsma AJ, Christiaans MH, Buurman WA, van Heurn LW: Kidney transplantation from donors after cardiac death: A 25-year experience. Transplantation 90: 1106–1112, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Del Río F, Andrés A, Padilla M, Sánchez-Fructuoso AI, Molina M, Ruiz Á, Pérez-Villares JM, Peiró LZ, Aldabó T, Sebastián R, Miñambres E, Pita L, Casares M, Galán J, Vidal C, Terrón C, Castro P, Sanroma M, Coll E, Domínguez-Gil B; Spanish Group for the Study of Donation after Circulatory Death: Kidney transplantation from donors after uncontrolled circulatory death: The Spanish experience. Kidney Int 95: 420–428, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Peräsaari JP, Kyllönen LE, Salmela KT, Merenmies JM: Pre-transplant donor-specific anti-human leukocyte antigen antibodies are associated with high risk of delayed graft function after renal transplantation. Nephrol Dial Transplant 31: 672–678, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, van Kasterop-Kutz M, van der Heide JJ, Squifflet JP, van Heurn E, Kirste GR, Rahmel A, Leuvenink HG, Paul A, Pirenne J, Ploeg RJ: Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 360: 7–19, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Organ Procurement and Transplantation Network : A guide to calculating and interpreting the kidney donor profile index, 2018. Available at: https://optn.transplant.hrsa.gov/resources/allocation-calculators/kdpi-calculator/. Accessed March 22, 2019
- 17.Zens TJ, Danobeitia JS, Leverson G, Chlebeck PJ, Zitur LJ, Redfield RR, D’Alessandro AM, Odorico S, Kaufman DB, Fernandez LA: The impact of kidney donor profile index on delayed graft function and transplant outcomes: A single-center analysis. Clin Transplant 32: e13190, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CM, Carter JT, Randall HB, Hiose R, Stock PG, Melzer JS, Dafoe DC, Freise CE, Alfrey EJ: The effect of age and prolonged cold ischemia times on the national allocation of cadaveric renal allografts. J Surg Res 91: 83–88, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Krüger B, Zülke C, Fischereder M, Leingärtner T, Kammerl M, Fürst A, Graeb C, Anthuber M, Jauch KW, Krämer BK: Early experience with the ET Senior Program “Old For Old”; better to be number one? Transpl Int 15: 541–545, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Denecke C, Biebl M, Fritz J, Brandl A, Weiss S, Dziodzio T, Aigner F, Sucher R, Bösmüller C, Pratschke J, Öllinger R: Reduction of cold ischemia time and anastomosis time correlates with lower delayed graft function rates following transplantation of marginal kidneys. Ann Transplant 21: 246–255, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Frei U, Noeldeke J, Machold-Fabrizii V, Arbogast H, Margreiter R, Fricke L, Voiculescu A, Kliem V, Ebel H, Albert U, Lopau K, Schnuelle P, Nonnast-Daniel B, Pietruck F, Offermann R, Persijn G, Bernasconi C: Prospective age-matching in elderly kidney transplant recipients--a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant 8: 50–57, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, Wainright JL, Haynes CR, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2016 annual data report: Kidney. Am J Transplant 18[Suppl 1]: 18–113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eurotransplant: Statistical report, 2017. Available at: https://www.eurotransplant.org/wp-content/uploads/2019/12/Annual-Report-2017-HR.pdf. Accessed January 15, 2019
- 24.Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, Broglio K, Hirose R, Roberts JP, Malinoski D: Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med 373: 405–414, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Schnuelle P, Gottmann U, Hoeger S, Boesebeck D, Lauchart W, Weiss C, Fischereder M, Jauch KW, Heemann U, Zeier M, Hugo C, Pisarski P, Krämer BK, Lopau K, Rahmel A, Benck U, Birck R, Yard BA: Effects of donor pretreatment with dopamine on graft function after kidney transplantation: A randomized controlled trial. JAMA 302: 1067–1075, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Shrestha S, Bradbury L, Boal M, Blackmur JP, Watson CJ, Taylor CJ, Forsythe JL, Johnson R, Marson LP: Logistical factors influencing cold ischemia times in deceased donor kidney transplants. Transplantation 100: 422–428, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Gill J, Dong J, Eng M, Landsberg D, Gill JS: Pulsatile perfusion reduces the risk of delayed graft function in deceased donor kidney transplants, irrespective of donor type and cold ischemic time. Transplantation 97: 668–674, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Yan T, Peng W, Lv J, Wu J, Chen J: Hemodialysis or peritoneal dialysis, which is better for patients with delayed graft function? Kidney Blood Press Res 43: 1813–1821, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Sandrini S: Use of IL-2 receptor antagonists to reduce delayed graft function following renal transplantation: A review. Clin Transplant 19: 705–710, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Kyllönen LE, Eklund BH, Pesonen EJ, Salmela KT: Single bolus antithymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: Efficacy and safety. Transplantation 84: 75–82, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Guirado L: Does rabbit antithymocyte globulin (Thymoglobuline®) have a role in avoiding delayed graft function in the modern era of kidney transplantation? J Transplant 2018: 4524837, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill P, Cross NB, Barnett AN, Palmer SC, Webster AC: Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev 1: CD004759, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D; Thymoglobulin Induction Study Group: Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 355: 1967–1977, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Debout A, Foucher Y, Trébern-Launay K, Legendre C, Kreis H, Mourad G, Garrigue V, Morelon E, Buron F, Rostaing L, Kamar N, Kessler M, Ladrière M, Poignas A, Blidi A, Soulillou JP, Giral M, Dantan E: Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int 87: 343–349, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Roodnat JI, Mulder PG, Van Riemsdijk IC, IJzermans JN, van Gelder T, Weimar W: Ischemia times and donor serum creatinine in relation to renal graft failure. Transplantation 75: 799–804, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.