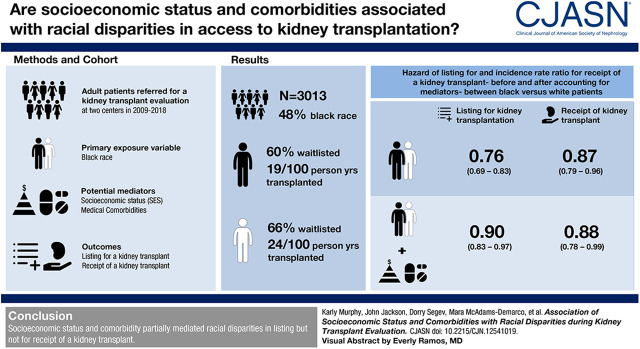
Visual Abstract
Keywords: transplantation, end stage kidney disease, kidney transplantation, risk factors, Incidence, Prospective Studies, African Americans, Social Class, Continental Population Groups, Renal Insufficiency, Employment, Comorbidity, Cohort Studies
Abstract
Background and objectives
Black patients referred for kidney transplantation have surpassed many obstacles but likely face continued racial disparities before transplant. The mechanisms that underlie these disparities are unclear. We determined the contributions of socioeconomic status (SES) and comorbidities as mediators to disparities in listing and transplant.
Design, setting, participants, & measurements
We studied a cohort (n=1452 black; n=1561 white) of patients with kidney failure who were referred for and started the transplant process (2009–2018). We estimated the direct and indirect effects of SES (self-reported income, education, and employment) and medical comorbidities (self-reported and chart-abstracted) as mediators of racial disparities in listing using Cox proportional hazards analysis with inverse odds ratio weighting. Among the 983 black and 1085 white candidates actively listed, we estimated the direct and indirect effects of SES and comorbidities as mediators of racial disparities on receipt of transplant using Poisson regression with inverse odds ratio weighting.
Results
Within the first year, 876 (60%) black and 1028 (66%) white patients were waitlisted. The relative risk of listing for black compared with white patients was 0.76 (95% confidence interval [95% CI], 0.69 to 0.83); after adjustment for SES and comorbidity, the relative risk was 0.90 (95% CI, 0.83 to 0.97). The proportion of the racial disparity in listing was explained by SES by 36% (95% CI, 26% to 57%), comorbidity by 44% (95% CI, 35% to 61%), and SES with comorbidity by 58% (95% CI, 44% to 85%). There were 409 (42%) black and 496 (45%) white listed candidates transplanted, with a median duration of follow-up of 3.9 (interquartile range, 1.2–7.1) and 2.8 (interquartile range, 0.8–6.3) years, respectively. The incidence rate ratio for black versus white candidates was 0.87 (95% CI, 0.79 to 0.96); SES and comorbidity did not explain the racial disparity.
Conclusions
SES and comorbidity partially mediated racial disparities in listing but not for transplant.
Introduction
Black adults are 3.7-fold more likely to develop kidney failure than white adults (1). Racial disparities persist throughout the kidney transplantation process (2−4), even as post-transplant outcome disparities are decreasing (5). Black adults who overcome disparities in referral for transplant (4,6–9) likely reflect a select population. Yet, they face continued disparities in listing for transplant (4,6,7,10–13) and receipt of transplant (3,4,6,7,13–16). This underscores the importance of identifying potential causal mechanisms underlying racial disparities at distinct stages of the kidney transplant process.
Both socioeconomic status (SES) and comorbidity likely influence access to kidney transplantation (17). National studies of patients who initiated dialysis suggest that medical factors do not fully explain racial disparities (14,15). Among adults referred for kidney transplantation, worse neighborhood-level poverty is associated with lower listing rates (4,12,13). Yet among a Veterans Affairs (VA) population, where patients receive universal health care coverage and other support (e.g., travel and lodging), there are no racial disparities for listing (18). This suggests that differences in SES may contribute to disparities in listing observed in the non-VA population (18). Because low SES is associated with greater comorbidity (19,20), it is important to identify the contributions of SES in the context of comorbidity and access to transplantation.
Among patients referred for transplant, we used causal mediation analysis, an approach to identify mechanisms and meaningful intervention targets (17,21,22), to test whether SES and comorbidity act as mediators to explain racial disparities in access to listing and transplant.
Materials and Methods
Study Design
We studied a cohort of patients with kidney failure (n=3985) recruited for a prospective cohort on aging and kidney failure at Johns Hopkins Hospital, Baltimore, Maryland, and University of Michigan, Ann Arbor, Michigan (November 2009 to June 2018). Eligible participants were English speaking and aged ≥18 years at the first visit to transplant clinic (23). We excluded participants missing all SES (n=136) or comorbidity (n=508) data. Excluded (versus included) participants were more likely to be of black race (56% versus 48%; P=0.001) but did not differ by age, sex, or waitlist mortality. We collected demographic, SES, and comorbidity data at first visit to transplant clinic.
We linked the cohort to the Scientific Registry of Transplant Recipients (SRTR). The SRTR includes data on all donors, waitlist candidates, and transplant recipients in the United States, submitted by members of the Organ Procurement and Transplantation Network (OPTN) (24). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. We had no participants lost to follow-up, and data were linked to SRTR. The Johns Hopkins University and University of Michigan Institutional Review Boards approved the study, and participants providing written informed consent. The research conduct was consistent with the Declaration of Helsinki and Declaration of Istanbul.
Ascertainment of Race
Race was identified by self-report (white, black, Hispanic, Asian/Asian-American, Native Hawaiian/Pacific Islander, other) by questionnaire. We only included participants who self-reported as white or black race.
SES Mediators
Participants were asked, “What is the highest level of education you attained?” (grade school, high school, 2-year technical degree, college, graduate school); we dichotomized education at the high school level. Participants were also asked, “What is your combined household annual income” (<$50,000, $50,000–$100,000, >$100,000); we dichotomized income at the >$50,000 level. Finally, participants reported whether they were employed or unemployed. If a participant declined to answer a question, this was coded as a separate category (25).
Comorbidity Mediators
We recorded body mass index and tobacco use (current, former, never smokers). On the basis of the Charlson Comorbidity Index for kidney failure (26), we ascertained comorbidities from self-report and electronic medical chart abstraction by trained research assistants overseen by a physician (23). Missing data were coded as “unavailable” as a separate category (25).
Outcomes
For the outcome of listing, we followed participants from the time of initial visit to transplant clinic until first active listing (status 7 or status 1 listing) at either study site, administrative censoring on June 1, 2018, or 1 year to meet the proportional hazards assumption. For the outcome of transplant, we limited the analysis to participants listed at either study site. We followed participants from date of first active listing until transplant or censoring for waitlist mortality, deactivation, or administrative censoring on June 1, 2018. Death was ascertained from SRTR.
Statistical Analyses
We estimated the observed racial disparity for time to listing using Cox proportional hazards models, adjusted for age, sex, and transplant center. We checked proportional hazards assumptions with complementary log-log plots and Schoenfeld residuals. Time to listing was limited to 1 year to meet proportional hazard assumptions. Among waitlisted candidates, we estimated the observed racial disparity for transplant using Poisson regression models, adjusted for age, sex, transplant center, blood type, and cause of kidney failure. We used only active waitlist time for the calculation of person-time and censored candidates for waitlist mortality.
Mediation of Racial Disparities in Listing for Transplant and Receipt of Transplant.
For mediation analyses, we used the causal inference method of inverse odds ratio weighting (IORW) (27,28) to estimate the direct and indirect effects of SES and comorbidities on racial disparities in listing or receipt of transplant (Figure 1). This method accommodates multiple mediators without prespecification of the order of mediators (27). It is valid in the presence or absence of exposure-mediator interactions (27). Our primary interest was the composite mediation provided by SES and comorbidity (19,20).
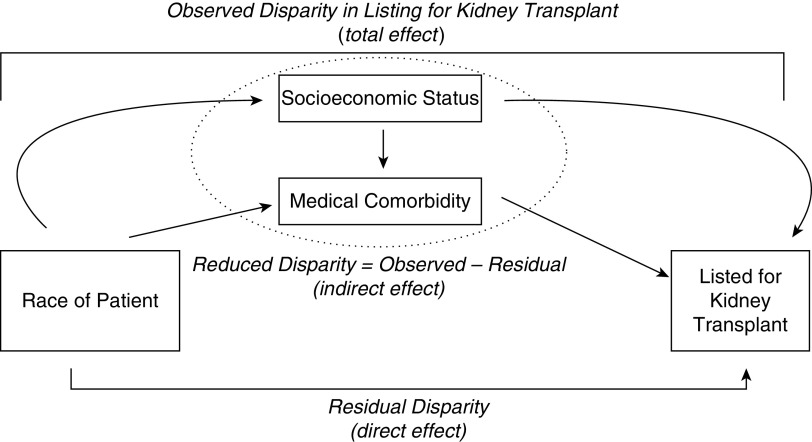
Figure 1.
A conceptual mediation model of the observed disparity in listing for kidney transplant as decomposed into the reduced disparity and residual disparity. The reduced disparity (indirect effect) represents the racial disparity in listing for transplant when socioeconomic status (SES) and comorbidity are accounted for as mediating variables. The residual disparity (direct effect) represents the remaining racial disparities in after accounting for SES and/or comorbidities.
IORW uses logistic regression to model the predicted probability of race membership adjusted for age, sex, and transplant center, and mediator factors of interest (i.e., SES, comorbidities). We used the predicted values to estimate IORW weights. Then, we fitted a Cox proportional hazards regression model given age, sex, and transplant center, with and without IORW weights (27,28). The unweighted regression model estimates the total effect, which is the observed disparity of race in listing (Figure 1). The weighted regression analysis estimates the direct effect, which is the residual disparity that would remain if the distribution of mediating factors (SES and comorbidity) were equal between white and black patients. We determined if SES and comorbidity were mediators, if the indirect effect was statistically significant with P<0.05. We calculated this indirect effect, which is conceptually the reduced disparity (amount by which the disparity would change), as the difference between the observed and residual disparity. We calculated the percent attenuated of the effect estimate on the additive hazards scale by ([HR−1]−[HR*−1])/(HR−1) when HR represents the observed disparity and HR* represents the residual disparity. We derived SEMs with bootstrap analysis. We used a Poisson regression model for receipt of transplant, with and without IORW weights, with analyses adjusted for age, sex, transplant center, cause of kidney failure, and blood type.
Sensitivity Analyses.
Sensitivity analyses were conducted by excluding patients with a history of cancer and/or dementia, as transplantation may be contraindicated and we were unable to determine timing of cancer; excluding patients at University of Michigan; adjusting for time on dialysis and dialysis modality; receipt of deceased donor transplant; using total time on waitlist (including active and inactive time) with Cox proportional hazards models, adjusting for year in study; waitlisting after new kidney allocation system on December 14, 2014; and donor status (living versus deceased). Dialysis time was only available for candidates who were listed. Among waitlisted candidates, we used Fine and Gray competing risk models (29) to estimate the risk (subdistribution hazard ratio) of waitlist mortality and accounting for transplant as a competing risk. We conducted a stratified analysis by income because prior work suggests an interaction between race and income (12). Nonetheless, our weighting approach remains valid in the presence of any exposure-mediator interactions, even if not specified in the models (27). To further address missing data (“declined to answer” or “unavailable”), we assumed missingness at random and used multiple chained imputation equations (27). All analyses were performed using Stata, version 15 (College Station, TX).
Results
Participant Characteristics
Among 3013 patients with kidney failure, 1452 (48%) patients reported black race (Table 1). Among the black participants, the mean age was 53 years (SD 13) and 43% were women. Among the white participants, the mean age was 57 years (SD 13) and 39% were women. Black patients were more likely to report lower SES and have greater comorbidity (Table 1). In our cohort, 24% of white patients and 35% of black patients declined to answer annual income; 31% of white patients and 28% of black patients had indeterminate heart failure status (Supplemental Table 1, Table 1). All other mediators had <1% missing data.
Table 1.
Characteristics of adult patients with kidney failure, stratified by race, who have started the transplant evaluation process and at time of initial transplant clinic visit to Johns Hopkins Hospital and University of Michigan (n=3013)
| Characteristics | White (n=1561) | Black (n=1452) |
|---|---|---|
| Age, mean yr (SD) | 57 (13) | 53 (13) |
| Women, n (%) | 611 (39) | 628 (43) |
| Johns Hopkins Hospital Center, n (%) | 1352 (87) | 1385 (95) |
| Dialysis modality, n (%) | ||
| Hemodialysis | 654 (42) | 920 (64) |
| Peritoneal dialysis | 175 (11) | 162 (11) |
| None (preemptive transplant) | 717 (46) | 357 (25) |
| Socioeconomic status | ||
| Highest level education attained, n (%) | ||
| Grade school | 44 (3) | 129 (9) |
| High school | 529 (34) | 605 (42) |
| Two-year technical degree | 140 (9) | 115 (8) |
| College | 530 (34) | 394 (27) |
| Graduate school | 312 (20) | 201 (14) |
| Declined to answer | 2 (0.1) | 6 (0.4) |
| Annual income, n (%) | ||
| <$50,000 | 405 (26) | 502 (35) |
| $50,000–100,000 | 379 (24) | 268 (19) |
| ≥$100,000 | 401 (26) | 202 (13) |
| Declined to answer | 376 (24) | 480 (33) |
| Employment status, n (%) | ||
| Not employed | 926 (59) | 974 (67) |
| Employed | 629 (40) | 473 (33) |
| Declined to answer | 6 (0.4) | 5 (0.3) |
| Comorbidities | ||
| Body mass index in kg/m2, mean (SD) | 29 (6) | 30 (6) |
| Obese (body mass index ≥30 kg/m2), n (%) | 602 (39) | 630 (43) |
| Tobacco use, n (%) | ||
| Never | 995 (65) | 977 (67) |
| Former | 458 (29) | 330 (23) |
| Current | 100 (6) | 140 (10) |
| History of myocardial infarction, n (%) | 180 (12) | 113 (8) |
| History of congestive heart failure, n (%) | 122 (8) | 187 (13) |
| Diabetes, n (%) | 627 (40) | 659 (45) |
| Diabetes with complications, n (%) | 400 (26) | 413 (28) |
| Peripheral vascular disease, n (%) | 114 (7) | 86 (6) |
| Cerebral vascular disease, n (%) | 96 (6) | 91 (6) |
| Chronic obstructive pulmonary disease, n (%) | 105 (7) | 79 (5) |
| Rheumatologic disease, n (%) | 89 (6) | 111 (8) |
| Peptic ulcer disease, n (%) | 62 (4) | 44 (3) |
| Liver disease (moderate-severe), n (%) | 44 (3) | 61 (4) |
| HIV, n (%) | 7 (0.5) | 91 (6) |
| Dementia, n (%) | 7 (0.4) | 5 (0.3) |
| History of leukemia, n (%) | 7 (0.5) | 3 (0.2) |
| History of lymphoma, n (%) | 22 (1) | 7 (0.5) |
| History of metastatic cancer, n (%) | 19 (1) | 15 (1) |
Listing for Transplant by Race
In the first year after evaluation, 66% (n=1028) of white patients and 60% (n=876) of black patients were listed. Median time to listing was 3.7 months (interquartile range [IQR], 1.6–12.0 months) for white patients and 5.2 months (IQR, 2.0–12.0 months) for black patients (P<0.001). After adjustment, black patients were 0.76 times (95% confidence interval [95% CI], 0.69 to 0.83; P<0.001) as likely to be listed as white patients (Table 2). On the relative scale, black patients were 24% less likely to be listed.
Table 2.
Testing of mediation of individual SES or comorbidity measures on listing for transplant within 1 year between black versus white patients with kidney failure (n=3013) or receipt of transplant between black versus white kidney transplant candidates on the waitlist (n=2068), using inverse odds ratio weighting and among a population who have started the transplant evaluation process
| Potential Mediator | Listing for Kidney Transplant | Receipt of Kidney Transplant | ||
|---|---|---|---|---|
| Adjusted HR (95% CI) Associated with Black Race | Proportion of Racial Disparity Attributed to Mediator (95% CI)a | Adjusted IRR (95% CI) Associated with Black Race | Proportion of Racial Disparity Attributed to Mediator (95% CI)a | |
| Base model (no potential mediator)b | 0.76 (0.69 to 0.83) | — | 0.87 (0.79 to 0.96) | — |
| SES | ||||
| Education | 0.80 (0.72 to 0.87) | 16 (11 to 26)* | 0.88 (0.78 to 0.98) | 5 (−2 to 47) |
| Income | 0.82 (0.75 to 0.90) | 28 (20 to 44)* | 0.87 (0.78 to 0.97) | 0.7 (−4 to 36) |
| Employment | 0.81 (0.74 to 0.89) | 23 (17 to 35)* | 0.87 (0.78 to 0.97) | 3 (−2 to 35) |
| Comorbidity | ||||
| Obesity | 0.78 (0.71 to 0.86) | 10 (8 to 16) | 0.87 (0.78 to 0.97) | 3 (−2 to 34) |
| Tobacco use | 0.78 (0.71 to 0.86) | 10 (7 to 14) | 0.86 (0.76 to 0.96) | −7 (−8 to 3) |
| Myocardial infarction | 0.77 (0.71 to 0.85) | 7 (5 to 11) | 0.86 (0.78 to 0.96) | −5 (−6 to 6) |
| Congestive heart failure | 0.86 (0.80 to 0.92) | 41 (35 to 51)* | 0.89 (0.81 to 1.00) | 22 (9 to 100) |
| Diabetes | 0.79 (0.72 to 0.87) | 14 (10 to 22)* | 0.88 (0.79 to 0.98) | 11 (3 to 59) |
| Diabetes with complications | 0.78 (0.71 to 0.86) | 10 (7 to 15) | 0.87 (0.78 to 0.96) | −3 (−5 to 10) |
| Peripheral vascular disease | 0.78 (0.72 to 0.86) | 10 (7 to 16) | 0.87 (0.78 to 0.97) | 2 (−2 to 28) |
| Cerebrovascular disease | 0.78 (0.72 to 0.86) | 11 (8 to 16) | 0.87 (0.78 to 0.97) | 3 (−2 to 34) |
| Chronic obstructive pulmonary disease | 0.78 (0.71 to 0.85) | 9 (7 to 13) | 0.87 (0.78 to 0.97) | 2 (−3 to 29) |
| Rheumatologic disease | 0.78 (0.72 to 0.86) | 11 (8 to 17) | 0.87 (0.78 to 0.97) | 2 (−2 to 29) |
| Peptic ulcer disease | 0.78 (0.72 to 0.86) | 11 (8 to 17) | 0.87 (0.78 to 0.97) | 4 (−1 to 35) |
| Liver disease, moderate-severe | 0.78 (0.71 to 0.86) | 10 (8 to 16) | 0.87 (0.79 to 0.97) | 4 (−1 to 37) |
| HIV | 0.80 (0.73 to 0.88) | 18 (13 to 30)* | 0.86 (0.77 to 0.96) | −6 (−8 to 8) |
| Dementia | 0.78 (0.71 to 0.85) | 9 (7 to 14) | 0.87 (0.78 to 0.97) | 3 (−2 to 33) |
| History of leukemia | 0.78 (0.72 to 0.86) | 11 (8 to 18) | 0.87 (0.79 to 0.97) | 4 (−1 to 37) |
| History of lymphoma | 0.79 (0.72 to 0.86) | 13 (9 to 20)* | 0.87 (0.78 to 0.97) | 0.1 (−3 to 23) |
| Metastatic cancer | 0.78 (0.71 to 0.86) | 10 (7 to 15) | 0.87 (0.79 to 0.96) | 2 (−2 to 30) |
The observed disparity (total effect) model is the association of race on outcome without accounting for individual mediator variables. The subsequent models show the residual disparity (direct effect) on the outcome after accounting for mediator(s) listed in the first column. SES, socioeconomic status; HR, hazard ratio; 95% CI, 95% confidence interval; IRR, incidence rate ratio; —, not applicable.
The percent (%) attributed to the mediator estimates how much of the observed disparity acts through the mediator and was calculated by ([HR−1] −[HR*−1])/(HR−1), where HR indicates observed disparity and HR* indicates residual disparity. Bootstrap analysis was used to derive SEMs. Mediators of statistical significance (P<0.05) are denoted with an asterisk (*). Interpretation of individual mediator variables limited by having potential unmeasured confounding.
Base model adjusted for age, sex, and transplant center. Receipt of transplant is also adjusted for cause of kidney failure and ABO status.
SES as Mediator of Racial Disparities in Listing.
Education, income, and employment partially attenuated the observed racial disparity in listing, such that they could account for some but not all of the racial disparities in listing (Table 2). Accounting for differences in SES attenuated the observed racial disparity by 36% (Table 3). When all measured SES factors were accounted for, black patients were 0.84 times (95% CI, 0.77 to 0.93; P<0.001) as likely to be listed as white patients. After accounting for differences in SES, black patients were 16% less likely to be listed.
Table 3.
Testing of mediation of SES, comorbidity, or SES and comorbidity on listing for kidney transplant within 1 year between black versus white patients with kidney failure (n=3013) or receipt of transplant between black versus white kidney transplant candidates on the waitlist (n=2068), using inverse odds ratio weighting and among a population who have started the transplant evaluation process
| Potential Mediator | Listing for Kidney Transplant | Receipt of Kidney Transplant | ||
|---|---|---|---|---|
| Adjusted HR (95% CI) Associated with Black Race | Proportion of Racial Disparity Attributed to Mediator (95% CI)a | Adjusted IRR (95% CI) Associated with Black Race | Proportion of Racial Disparity Attributed to Mediator (95% CI)a | |
| Base model (no potential mediator)b | 0.76 (0.69 to 0.83) | — | 0.87 (0.79 to 0.96) | — |
| SES | 0.84 (0.77 to 0.93) | 36 (26 to 57)* | 0.87 (0.78 to 0.98) | 0.7 (−5 to 42) |
| Comorbidity | 0.86 (0.80 to 0.93) | 44 (35 to 61)* | 0.87 (0.77 to 0.97) | −2 (−6 to 25) |
| SES and comorbidity | 0.90 (0.83 to 0.97) | 58 (44 to 85)* | 0.88 (0.78 to 0.99) | 4 (−6 to 68) |
The observed disparity (total effect) model is the association of race on outcome without accounting for individual mediator variables. The subsequent models show the residual disparity (direct effect) on the outcome after accounting for mediator(s) listed in the first column. SES, socioeconomic status; HR, hazard ratio; 95% CI, 95% confidence interval; IRR, incidence rate ratio; —, not applicable.
The percent (%) attributed to the mediator estimates how much of the observed disparity acts through the mediator and was calculated by ([HR−1] −[HR*−1])/(HR−1), where HR indicates observed disparity and HR* indicates residual disparity. Bootstrap analysis was used to derive SEMs. Mediators of statistical significance (P<0.05) are denoted with an asterisk (*).
Base model adjusted for age, sex, and transplant center. Receipt of kidney transplant is also adjusted for cause of kidney failure and ABO status.
Comorbidity as Mediator of Racial Disparities in Listing.
Comorbidity partially attenuated the observed racial disparity in listing. Heart failure, diabetes, HIV, or lymphoma history partially attenuated the observed disparity; other comorbidities alone did not (Table 2). Accounting for differences in comorbidity attenuated the observed racial disparity by 44% before accounting for SES (Table 3). After accounting for all comorbid conditions, black patients were 0.86 times (95% CI, 0.80 to 0.93; P<0.001) as likely to be listed as white patients. After accounting for differences in comorbidity, black patients were 14% less likely to be listed.
Combined Role of SES and Comorbidity as Mediator of Racial Disparities in Listing.
Accounting for differences in SES and comorbidity attenuated the observed racial disparity by 58%. After accounting for partial mediation by SES and comorbidity, black patients were 0.90 times (95% CI, 0.83 to 0.97; P=0.01) as likely to be listed as white patients (Table 3). After accounting for differences in SES and comorbidity, black patients were 10% less likely to be listed compared with white patients.
Transplant Rates by Race
Of the 1085 white candidates and 983 black candidates who were listed, 496 (45%) white candidates and 409 (42%) black candidates received a transplant; furthermore, 214 (20%) white candidates and 48 (5%) black candidates received a living donor transplant. The incidence rate of transplant was 24 per 100 person-years for white candidates and 19 per 100 person-years for black candidates. The median follow-up time was 2.8 years (IQR, 0.8–6.3 years) for white candidates and 3.9 years (IQR, 1.2–7.1 years) for black candidates. Of those who received a transplant, the median time to transplant was 0.8 years (IQR, 0.4–1.7 years) for white candidates and 1.0 year (IQR, 0.5–2.1 years) for black candidates (P=0.001). The median time to living donor transplant was 0.7 years (IQR, 0.3–1.6 years) for white candidates and 0.6 years (IQR, 0.4–1.6 years) for black candidates (P=0.6). The mean inactive waiting time was 1.6 (SD 1.4) years for black candidates and 1.3 (SD 1.3) years for white candidates (P<0.001). We observed differences in inactive waiting time for education, income, employment, diabetes, peripheral vascular disease, and history of lymphoma (Supplemental Table 2). All listed candidates were transplanted, died, or were administratively censored. Mortality rates were 7.7 deaths per 100 person-years for white candidates and 6.0 deaths per 100 person-years for black candidates. Waitlist mortality did not differ between white and black candidates (adjusted subdistribution hazard ratio, 0.98; 95% CI, 0.78 to 1.24).
After adjustment, black candidates were 0.87 times (95% CI, 0.79 to 0.96; P=0.005) as likely to receive a transplant as white candidates. That is, black candidates were 13% less likely to receive a transplant compared with white candidates (Table 2).
SES as Mediator of Racial Disparities in Transplant Rates.
Accounting for differences in education, income, or employment status did not attenuate the observed racial disparity in receipt of transplant (Tables 2 and 3). After accounting for all measured SES factors, black candidates were 0.87 times (95% CI, 0.78 to 0.98; P=0.02) as likely to receive a transplant as white candidates. The residual racial disparity for receipt of transplant was 13% after accounting for differences in SES.
Comorbidity as Mediator of Racial Disparities in Transplantation Rates.
Accounting for differences in comorbidity did not attenuate the observed racial disparity in receipt of transplant before accounting for SES (Tables 2 and 3). After accounting for all comorbidities, black candidates were 0.87 times (95% CI, 0.77 to 0.97; P=0.01) as likely to receive a transplant as white candidates (Table 3), suggesting that candidate’s race appeared to have a 13% residual disparity on receipt of transplant after accounting for differences in comorbidity.
Combined SES and Comorbidity as Mediator of Racial Disparities in Transplantation Rates.
Accounting for differences in SES and comorbidity did not attenuate racial disparities for receipt of transplant (Table 3). After accounting for potential mediation by SES and comorbidity, black candidates were 0.88 times (95% CI, 0.78 to 0.99; P=0.03) as likely to receive a transplant as white candidates. The residual disparity suggests that black candidates were 12% less likely to have access to transplant after accounting for differences in SES and comorbidity.
Sensitivity Analyses
Findings for receipt of transplant did not differ between those listed during entire study period compared with those listed in the first year (Supplemental Table 3). We observed no disparities in receipt of transplant when we adjusted for donor status (aIRR, 0.94; 95% CI, 0.86 to 1.02), and SES and comorbidity were not significant mediators (Supplemental Table 3). For all other sensitivity analyses, including total time on the waitlist, our inferences remained consistent with the primary results. When we analyzed missing data with the original cohort using multiple chained imputation equations, findings were similar (Supplemental Table 3). On stratified analysis by income, measured SES and comorbidities attenuated 51% of the observed listing disparities for those earning ≤$50,000 per year and 40% for those earning >$50,000 per year (Supplemental Table 4).
Discussion
In this cohort of 3013 patients with kidney failure presenting for transplant evaluation, black candidates were 24% less likely to be listed for transplant compared with white candidates. SES attenuated 36%, comorbidity attenuated 44%, and the combination of SES and comorbidity attenuated 58% of the observed disparity. Among the 2068 candidates listed for transplant, black candidates were 13% less likely to receive a transplant. Accounting for SES and comorbidity did not attenuate the observed racial disparity in receipt of transplant.
To our knowledge, our study is the first to use causal inference strategies (27,28) to identify the individual and overlapping contributions of measured SES and comorbidity on racial disparities within distinct stages of the transplantation process. Previously, Hall et al. (14) and Purnell et al. (15) attributed 13%–18% of racial disparities to contextual poverty; however, these retrospective studies looked at patients across multiple stages, from onset of kidney failure to transplantation. We extended this work to two distinct stages of the kidney transplant process and among a population who have overcome referral-related disparities (4,6–9). It is at this point that transplant centers engage with candidates and have opportunities to influence the coordination of the transplant process (18,30). Our observed disparity in listing of 0.76 is consistent with studies among referred patients, where observed racial disparities ranged from 0.52 to 0.88 (10,11,13,16).
By accounting for differences in SES and comorbidity, we observed a reduction in disparities in listing between white and black patients. Our study may help explain why income was not associated with likelihood of listing in VA settings (18). The VA, by offering medical care with financial support in the form of medical tests, travel, and lodging during evaluation, may be mitigating differences in SES (18), and thereby reducing racial disparities in kidney transplantation (31). Candidates with greater comorbidity, who are often of low SES, may require extensive evaluation. Additional testing, although appropriate, is time-consuming and often requires financial resources (11,17,18,30). Furthermore, prior studies of listing (4,12,13) or receipt of transplant (14,15) measured neighborhood-level poverty indices. Our study complements this work by using individual-level SES measures, including self-reported income, which national databases do not capture. Our stratified analysis by income was consistent with work that suggests poverty level may act as an effect modifier of the association between race and transplant access (12).
Our study also suggests that among those who are listed, SES and comorbidity may have less influence on receipt of transplant. This is consistent with work that suggests immunologic concerns and availability of donor organs are important predictors for transplant (2,17). Prior national policies have focused on allocation stage of organ transplantation (32,33), yet disparities in transplantation may be widening between 1995 and 2014 (3) and within donor evaluation process (34).
Even after accounting for differences in SES and comorbidity, racial disparities in listing persisted. We based our conceptual framework on the causal inference literature, which places race upstream of adult SES and comorbidity (35). This interpretation, although potentially simplified, uses race as a social construct and includes perceived skin color, genetics, and sociocultural context (35). The residual disparity may represent racial bias from implicit and explicit biases from providers and transplant committee (36). Perceived discrimination and implicit bias have been associated with reduced treatment adherence, engagement in care, and clinical decision-making (37−39). Implicit bias may appear as a differential weight that a transplant committee may consider for a given factor (e.g., severity of heart failure) for a black candidate versus a white candidate. Similarly, structural racism, in which institutions and health care systems reinforce inequity through beliefs, values, and distribution of resources (36,40), may contribute to the residual disparity through neighborhood- and system-level factors (12,17,41).
Major study strengths include its longitudinal design, person-level data for SES, a kidney failure population that has been less well studied, and use of a causal inference strategy that accommodates multiple mediators simultaneously (27).
These results should be considered in light of important limitations. Our cohort is a select group of candidates who have overcome significant barriers to be referred to and seen at transplant clinic (8,9). A majority of black candidates were recruited at one site and our sample size is modest (n=3013). We had >20% of missing data for income and heart failure status and used categorical variables to indicate “unavailable,” an approach that is advocated in population-based studies using socioeconomic data but is subject to residual confounding (25,42). However, results were similar on sensitivity analysis using a missing at random approach. We did not account for dialysis time, which differed by race, in our main analysis as this information was unavailable for the 37% (716 out of 1911) of participants on dialysis. However, inclusion of this covariate on sensitivity analyses did not change primary findings. Given the relatively low follow-up time for white candidates and differences in live donor transplantation rates, our work may not represent the wider transplant population or may have residual confounding. Although identification of a living donor may have influenced the likelihood of listing, we observed no difference in the median time to living donor transplant. Most likely, observed disparities with donor status reflect wider disparities in access to transplantation (43). We were unable to determine reason for inactivation and observed differences in inactive time between black and white candidates, SES and comorbidity; yet our findings were unchanged when we included total time on the waitlist.
We also note our results are observational in nature and only infer correlation. Although mediation analysis assumes no unmeasured confounding (44), our study did not measure social support, substance use history, depression, household size, and insurance, all of which has been associated with lower listing rates (45,46). Bias analyses can estimate the magnitude of bias from unmeasured confounding; (47,48) however, these approaches only have been developed for standard regression or single mediators (47−51). Similarly, our measured SES mediators likely do not fully capture all facets of SES and are subject to misclassification. However, inverse odds weighting is less sensitive is unmeasured confounding along the causal pathway (50). Our estimated contributions of comorbidity to racial disparities may suffer from confounding by SES, nonetheless, we feel our step-wise analyses highlight the complex connections between SES and comorbidity.
In a cohort of patients with kidney failure evaluated for transplant, accounting for SES and comorbidity significantly attenuated observed racial disparities in listing but not for receipt of transplant. The patients represent a population who have overcome multiple barriers (10) yet face disparities in the transplant process. Transplant centers and health systems have influence over how the evaluation process unfolds and what support is available to assist patients with this process. Efforts to eliminate racial disparities in listing for transplant may wish to focus on barriers that candidates with low SES and high comorbidity will face.
Disclosures
Dr. Segev reports other funding from CSL Behring, Novartis, and Sanofi, outside the submitted work. Dr. Chu, Dr. Crews, Dr. Haugen, Dr. Jackson, Dr. McAdams-DeMarco, Dr. Murphy, Dr. Norman, Dr. Purnell, and Dr. Shaffer have nothing to disclose.
Funding
This study was funded by National Institutes of Health grants R01-AG042504 (principal investigator [PI]: Dr. Segev), K24-DK101828 (PI: Dr. Segev), and R01-AG055781 (PI: Dr. McAdams-DeMarco), and by Agency for Healthcare Research and Quality grant K01HS024600 (PI: Dr. Purnell). Dr. Murphy was supported by National Heart, Lung, and Blood Institute grant 2T32HL007180-41A. Dr. Jackson was supported by National Heart, Lung, and Blood Institute grant K01HL145320. Dr. Shaffer was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant F30DK116658. Dr. Haugen was supported by National Institute of Aging grant F32AG05302.
Supplementary Material
Acknowledgments
Dr. Murphy, Dr. Jackson, and Dr. McAdams-DeMarco designed the study. Dr. Murphy carried out the analysis and drafted the manuscript. Dr. Murphy, Dr. Jackson, Dr. Purnell, Purnell, Dr. Haugen, Dr. Chu, Dr. Crews, and Dr. McAdams-DeMarco contributed to the interpretation of data. All authors critically revised and approved the final version of the manuscript.
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. The data reported here have been supplied by the Hennnepin Healthcare Research Institute as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
SRTR Registry staff performed all Social Security number linkages to Social Security Death Master File and Centers for Medicare & Medicaid Services data to ensure confidentiality of Social Security number data provided to the Organ Procurement and Transplantation Network.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12541019/-/DCSupplemental.
Supplemental Table 1. Characteristics of adult patients with kidney failure, stratified by race, at time of initial transplant clinic visit.
Supplemental Table 2. Difference for inactive time on waitlist with SD.
Supplemental Table 3. Sensitivity analyses testing of mediation of socioeconomic status and comorbidity on listing for kidney transplant within 1 year between black versus white patients with kidney failure or receipt of kidney transplant between black versus white kidney transplant candidates on the waitlist using inverse odds ratio weighting.
Supplemental Table 4. Stratified analysis testing mediation of socioeconomic status and comorbidity on listing for kidney transplant within 1 year between black and white patients with kidney failure using inverse odds ratio weighting.
References
- 1.US Renal Data System: 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 2.Harding K, Mersha TB, Pham PT, Waterman AD, Webb FA, Vassalotti JA, Nicholas SB: Health disparities in kidney transplantation for African Americans. Am J Nephrol 46: 165–175, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purnell TS, Luo X, Cooper LA, Massie AB, Kucirka LM, Henderson ML, Gordon EJ, Crews DC, Boulware LE, Segev DL: Association of race and ethnicity with live donor kidney transplantation in the United States from 1995 to 2014. JAMA 319: 49–61, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Purnell TS, Luo X, Kucirka LM, Cooper LA, Crews DC, Massie AB, Boulware LE, Segev DL: Reduced racial disparity in kidney transplant outcomes in the United States from 1990 to 2012. J Am Soc Nephrol 27: 2511–2518, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM: The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med 341: 1661–1669, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weissman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM: Racial disparities in access to renal transplantation—Clinically appropriate or due to underuse or overuse? N Engl J Med 343: 1537–1544, 2 p preceding 1537, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patzer RE, Plantinga LC, Paul S, Gander J, Krisher J, Sauls L, Gibney EM, Mulloy L, Pastan SO: Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA 314: 582–594, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gander JC, Zhang X, Plantinga L, Paul S, Basu M, Pastan SO, Gibney E, Hartmann E, Mulloy L, Zayas C, Patzer RE: Racial disparities in preemptive referral for kidney transplantation in Georgia. Clin Transplant 32: e13380, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng FL, Joffe MM, Feldman HI, Mange KC: Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis 46: 734–745, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Monson RS, Kemerley P, Walczak D, Benedetti E, Oberholzer J, Danielson KK: Disparities in completion rates of the medical prerenal transplant evaluation by race or ethnicity and gender. Transplantation 99: 236–242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM: Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol 20: 1333–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU: Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol 6: 1760–1767, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Hall YN, Choi AI, Xu P, O’Hare AM, Chertow GM: Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol 22: 743–751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purnell TS, Xu P, Leca N, Hall YN: Racial differences in determinants of live donor kidney transplantation in the United States. Am J Transplant 13: 1557–1565, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe RA, Ashby VB, Milford EL, Bloembergen WE, Agodoa LY, Held PJ, Port FK: Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis 36: 1025–1033, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Purnell TS, Hall YN, Boulware LE: Understanding and overcoming barriers to living kidney donation among racial and ethnic minorities in the United States. Adv Chronic Kidney Dis 19: 244–251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman MA, Pleis JR, Bornemann KR, Croswell E, Dew MA, Chang CH, Switzer GE, Langone A, Mittal-Henkle A, Saha S, Ramkumar M, Adams Flohr J, Thomas CP, Myaskovsky L: Has the department of veterans affairs found a way to avoid racial disparities in the evaluation process for kidney transplantation? Transplantation 101: 1191–1199, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB: State of disparities in cardiovascular health in the United States. 111: 1233–1241, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Diez Roux AV, Mujahid MS, Hirsch JA, Moore K, Moore LV: The impact of neighborhoods on CV risk. Glob Heart 11: 353–363, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson JW, VanderWeele TJ: Decomposition analysis to identify intervention targets for reducing disparities. Epidemiology 29: 825–835, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson JW: On the interpretation of path-specific effects in health disparities research. Epidemiology 29: 517–520, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Warsame F, Haugen CE, Ying H, Garonzik-Wang JM, Desai NM, Hall RK, Kambhampati R, Crews DC, Purnell TS, Segev DL, McAdams-DeMarco MA: Limited health literacy and adverse outcomes among kidney transplant candidates. Am J Transplant 19: 457–465 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massie AB, Kucirka LM, Segev DL: Big data in organ transplantation: Registries and administrative claims. Am J Transplant 14: 1723–1730, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Egerter S, Cubbin C, Takahashi ER, Braveman P: Potential implications of missing income data in population-based surveys: An example from a postpartum survey in California. Public Health Rep 122: 753–763, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen Tchetgen EJ: Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am J Epidemiol 181: 349–356, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchetgen Tchetgen EJ: Inverse odds ratio-weighted estimation for causal mediation analysis. Stat Med 32: 4567–4580, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 30.Basu M, Petgrave-Nelson L, Smith KD, Perryman JP, Clark K, Pastan SO, Pearson TC, Larsen CP, Paul S, Patzer RE: Transplant center patient navigator and access to transplantation among high-risk population: A randomized, controlled trial. Clin J Am Soc Nephrol 13: 620–627, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myaskovsky L, Kendall K, Li X, Chang CH, Pleis JR, Croswell E, Ford CG, Switzer GE, Langone A, Mittal-Henkle A, Saha S, Thomas CP, Adams Flohr J, Ramkumar M, Dew MA: Unexpected race and ethnicity differences in the US national Veterans Affairs kidney transplant program. Transplantation 103: 2701–2714, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashby VB, Port FK, Wolfe RA, Wynn JJ, Williams WW, Roberts JP, Leichtman AB: Transplanting kidneys without points for HLA-B matching: Consequences of the policy change. Am J Transplant 11: 1712–1718, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Hall EC, Massie AB, James NT, Garonzik Wang JM, Montgomery RA, Berger JC, Segev DL: Effect of eliminating priority points for HLA-B matching on racial disparities in kidney transplant rates. Am J Kidney Dis 58: 813–816, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Kumar K, Tonascia JM, Muzaale AD, Purnell TS, Ottmann SE, Al Ammary F, Bowring MG, Poon A, King EA, Massie AB, Chow EKH, Thomas AG, Ying H, Borja M, Konel JM, Henderson M, Cameron AM, Garonzik-Wang JM, Segev DL: Racial differences in completion of the living kidney donor evaluation process. Clin Transplant 32: e13291, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanderWeele TJ, Robinson WR: On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology 25: 473–484, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arriola KJ: Race, racism, and access to renal transplantation among African Americans. J Health Care Poor Underserved 28: 30–45, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Chapman EN, Kaatz A, Carnes M: Physicians and implicit bias: How doctors may unwittingly perpetuate health care disparities. J Gen Intern Med 28: 1504–1510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehon E, Weiss N, Jones J, Faulconer W, Hinton E, Sterling S: A systematic review of the impact of physician implicit racial bias on clinical decision making. Acad Emerg Med 24: 895–904, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Myaskovsky L, Almario Doebler D, Posluszny DM, Dew MA, Unruh M, Fried LF, Switzer GE, Kim S, Chang CC, Ramkumar M, Shapiro R: Perceived discrimination predicts longer time to be accepted for kidney transplant. Transplantation 93: 423–429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT: Structural racism and health inequities in the USA: Evidence and interventions. Lancet 389: 1453–1463, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Crews DC, Pfaff T, Powe NR: Socioeconomic factors and racial disparities in kidney disease outcomes. Semin Nephrol 33: 468–475, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP; CRIC and H-CRIC Study Groups: CKD in hispanics: Baseline characteristics from the CRIC (chronic renal insufficiency cohort) and hispanic-CRIC studies. Am J Kidney Dis 58: 214–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigue JR, Kazley AS, Mandelbrot DA, Hays R, LaPointe Rudow D, Baliga P; American Society of Transplantation: Living donor kidney transplantation: overcoming disparities in live kidney donation in the US--recommendations from a consensus conference. Clin J Am Soc Nephrol 10: 1687–1695, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keyes K, Galea S: What matters most: Quantifying an epidemiology of consequence. Ann Epidemiol 25: 305–311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudley CR, Johnson RJ, Thomas HL, Ravanan R, Ansell D: Factors that influence access to the national renal transplant waiting list. Transplantation 88: 96–102, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Mucsi I, Bansal A, Jeannette M, Famure O, Li Y, Novak M, Kim SJ: Mental health and behavioral barriers in access to kidney transplantation: A Canadian cohort study. Transplantation 101: 1182–1190, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Vanderweele TJ, Arah OA: Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology 22: 42–52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arah OA, Chiba Y, Greenland S: Bias formulas for external adjustment and sensitivity analysis of unmeasured confounders. Ann Epidemiol 18: 637–646, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Nelson S, Albert JM: Estimation of causal mediation effects for a dichotomous outcome in multiple-mediator models using the mediation formula. Stat Med 32: 4211–4228, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VanderWeele TJ, Vansteelandt S: Mediation analysis with multiple mediators. Epidemiol Methods 2: 95–115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith LH, VanderWeele TJ: Mediational E-values: Approximate sensitivity analysis for unmeasured mediator-outcome confounding. Epidemiology 30: 835–837, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.