Visual Abstract
Keywords: chronic kidney disease, chronic metabolic acidosis, pediatrics, renal progression, bicarbonates, phosphates, alkalis, renal insufficiency, chronic, longitudinal studies, proteinuria, glomerular filtration rate, renal replacement therapy, anemia, acidosis, hypertension, demography
Abstract
Background and objectives
Studies of adults have demonstrated an association between metabolic acidosis, as measured by low serum bicarbonate levels, and CKD progression. We evaluated this relationship in children using data from the Chronic Kidney Disease in Children study.
Design, setting, participants, & measurements
The relationship between serum bicarbonate and a composite end point, defined as 50% decline in eGFR or KRT, was described using parametric and semiparametric survival methods. Analyses were stratified by underlying nonglomerular and glomerular diagnoses, and adjusted for demographic characteristics, eGFR, proteinuria, anemia, phosphate, hypertension, and alkali therapy.
Results
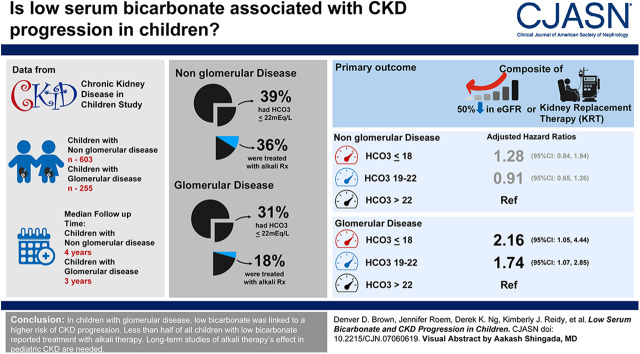
Six hundred and three participants with nonglomerular disease contributed 2673 person-years of follow-up, and 255 with a glomerular diagnosis contributed 808 person-years of follow-up. At baseline, 39% (237 of 603) of participants with nonglomerular disease had a bicarbonate level of ≤22 meq/L and 36% (85 of 237) of those participants reported alkali therapy treatment. In participants with glomerular disease, 31% (79 of 255) had a bicarbonate of ≤22 meq/L, 18% (14 of 79) of those participants reported alkali therapy treatment. In adjusted longitudinal analyses, compared with participants with a bicarbonate level >22 meq/L, hazard ratios associated with a bicarbonate level of <18 meq/L and 19–22 meq/L were 1.28 [95% confidence interval (95% CI), 0.84 to 1.94] and 0.91 (95% CI, 0.65 to 1.26), respectively, in children with nonglomerular disease. In children with glomerular disease, adjusted hazard ratios associated with bicarbonate level ≤18 meq/L and bicarbonate 19–22 meq/L were 2.16 (95% CI, 1.05 to 4.44) and 1.74 (95% CI, 1.07 to 2.85), respectively. Resolution of low bicarbonate was associated with a lower risk of CKD progression compared with persistently low bicarbonate (≤22 meq/L).
Conclusions
In children with glomerular disease, low bicarbonate was linked to a higher risk of CKD progression. Resolution of low bicarbonate was associated with a lower risk of CKD progression. Fewer than one half of all children with low bicarbonate reported treatment with alkali therapy. Long-term studies of alkali therapy’s effect in patients with pediatric CKD are needed.
Introduction
Approximately 15% of the United States population is affected by CKD (1). Although the prevalence in children is lower, pediatric CKD exacts a large clinical and economic toll (1). Children with CKD have higher risk for hospitalizations, metabolic abnormalities, cardiovascular disease, growth restriction, and cognitive impairment (1–5). Morbidity and mortality further increases when there is progression to ESKD (6–8). Thus, identifying modifiable risk factors for disease progression is important.
Metabolic acidosis is an early, and frequent, complication of pediatric CKD, likely due to the higher prevalence of congenital abnormalities of the kidney and urinary tract, and associated tubular dysfunction (2). In the Chronic Kidney Disease in Children (CKiD) study, Furth et al. (2) demonstrated that the overall mean serum bicarbonate level in children with CKD was 22 meq/L, compared with 26.7 meq/L in healthy children in the National Health and Nutrition Examination Survey III. Rodig et al. (4) found that as little as one third of children with low bicarbonate in CKiD reported treatment with alkali therapy.
Suboptimal treatment is important because observational studies in animals and adults have documented the potential role of metabolic acidosis in CKD progression, and the fact that treatment may slow disease decline (9–16). The proposed pathogenesis is complement-mediated tubulointerstitial injury caused by increased ammonia production per nephron, because of reduced kidney mass (11,17,18). Other mechanisms include increased levels of endothelin and aldosterone (19–21). There is a paucity of studies examining low bicarbonate and kidney disease progression in children. The few investigations have been of limited duration, focused on multiple risk factors (22), or did not encompass a heterogeneous population (23). We sought to describe and characterize the longitudinal relationship between low serum bicarbonate, a surrogate for metabolic acidosis, and CKD progression in children enrolled in the CKiD study. We hypothesized that low serum bicarbonate would be associated with faster kidney disease progression and that resolution would be associated with slower disease progression.
Materials and Methods
Study Population
The CKiD study is a prospective cohort of children aged 6 months to 16 years old with mild to moderate CKD (stages 2–3 by eGFR) from 54 tertiary care pediatric nephrology programs across North America. Briefly, data collected include blood and urine markers of CKD progression, general health, and prescribed therapies. The CKiD study design and methods have been described previously (24). All participating centers had approval from their respective institutional review boards, and all participants and families provided informed consent. Inclusion criteria for our study were age ≥1 year old, and at least one serum bicarbonate and eGFR measurement. Infants were excluded because acceptable values for bicarbonate differ from older children (25). Of the 955 CKiD participants, 97 (10%) were excluded for missing either bicarbonate measurements or complete baseline data (Supplemental Figure 1).
Measurements and Definitions of Covariates
Covariates were selected on the basis of known risk factors or markers of CKD progression. Additionally, we selected covariates used in a previous study of baseline bicarbonate and CKD progression (22) so that comparisons could be drawn. Covariates included age, sex, race, eGFR (26), proteinuria (26), anemia (defined as hemoglobin <5th percentile for age, sex, and race) (27), phosphate (centered at 4.5 mg/dl) (28,29), hypertension (defined as systolic or diastolic BP ≥95th percentile for age, sex, height, or prior medical diagnosis) (30), and alkali therapy (sodium bicarbonate and citrate/citric acid agents). We also included clinically relevant data such as alkali therapy adherence (defined by a “yes” or “no” response to missing at least one dose of prescribed alkalinizing agent in the past week) and reported growth hormone use. For 8% of the data, measurements from the previous visit were carried forward for missing covariates.
Primary Outcome
The primary outcome was CKD progression defined as the earliest of either a 50% decline in baseline eGFR or initiation of KRT (dialysis or transplant). The time of 50% eGFR decline was interpolated between two visits in which a 50% decline was known to occur, assuming a linear decline in eGFR during that time. Dates of dialysis or transplant were obtained by participant interview and/or clinical chart review. Participants were censored at 6 months after their last study visit if they did not reach the composite end point.
Exposure
The primary exposure was serum bicarbonate, measured at the local clinical sites. On average, participants had four (interquartile range [IQR], 2 to 7) serum bicarbonate measurements (five in children with nonglomerular disease [IQR, 3 to 7] and four in children with glomerular disease [IQR, 2 to 5]). Bicarbonate values were both dichotomized as ≤22 meq/L and >22 meq/L, and categorized as ≤18 meq/L (very low), 19–22 meq/L (low), and >22 meq/L (normal). Bicarbonate was treated as both time-fixed (i.e., baseline) and time-varying. In the first time-to-event analyses, serum bicarbonate was dichotomized “acidosis-free” if measured bicarbonate was >22 meq/L and “ever acidosis” if at least one measured bicarbonate was ≤22 meq/L. Participants who entered the study as acidosis-free contributed to that group until the first visit where serum bicarbonate was ≤22 meq/L. After this point, the participant contributed to the “ever acidosis” group throughout the remainder of the analyses (Supplemental Figure 2).
In the second time-to-event analyses, using only participants from the ever acidosis group, we characterized time-varying bicarbonate to describe the association of acidosis resolution and the composite outcome. After the visit where a participant first became acidotic (bicarbonate ≤22 meq/L), the participant was considered “resolved” if at follow-up visits serum bicarbonate measured >22 meq/L or “unresolved” if bicarbonate was ≤22 meq/L (Supplemental Figure 3). If bicarbonate fluctuated during follow-up, participants contributed data to the appropriate bicarbonate group at each follow-up.
Statistical Analyses
Because of evidence suggesting differences in progression trajectories, we primarily stratified analyses by primary CKD diagnosis: nonglomerular or glomerular (22,31). We present results from analyses of the entire cohort in the Supplemental Materials (Supplemental Tables 4–5). Demographic and clinical baseline characteristics of the study population overall, and by serum bicarbonate categories (very low, low, and normal), are described.
When bicarbonate was dichotomized (ever acidosis versus acidosis-free, and unresolved versus resolved), Kaplan–Meier survival methods were used to estimate time to the composite event. Parametric survival models were fit to estimate relative time differences as the metric of risk related to bicarbonate groups for specific percentiles. There were variable numbers of children in each group who reached the composite event so percentiles were selected on the basis of the nonparametric survival in order to avoid extrapolation beyond observed data. The relative percentile measure of association summarizes the difference in time (in this application, in years) for the Pth percentile of the exposed group (e.g., those with acidosis) compared with the unexposed group (e.g., those without acidosis) (32). Parametric models were chosen using likelihood ratio tests to determine the models of best fit (see Supplemental Material for full model selection description). When bicarbonate was treated as ever acidosis versus acidosis-free, the time origin was the baseline visit. In analyses using ever acidosis participants only, the time origin was the subsequent visit after the serum bicarbonate was ≤22 meq/L. In these analyses, the acidosis persistency exposure (unresolved versus resolved) was allowed to be time-varying.
Finally, we analyzed time to the composite end point by serum bicarbonate categories using semiparametric Cox proportional hazards models. In these analyses, the time origin was the baseline visit. Serum bicarbonate levels were considered as both time-fixed and time-varying independent variables in separate models. We report unadjusted and adjusted hazard ratios (HRs), and 95% confidence intervals (95% CIs). In the adjusted models using baseline serum bicarbonate, all covariates were also measured at baseline. For the adjusted models with time-varying serum bicarbonate, proteinuria, anemia, phosphate, hypertension, and alkali therapy were treated as time-varying. For adjusted models, the total missing data were <10% for both diagnosis groups, thus we employed a complete case analysis that assumed data were missing at random. Longitudinal results were censored for death and participants lost to follow-up.
As a sensitivity analysis, in the first time-to-event analyses where serum bicarbonate was dichotomized as ever acidosis versus acidosis-free, we added a third classification of “confirmed acidosis,” which required at least two low serum bicarbonate measurements. The time origin was the second visit where bicarbonate was measured. Among this same population, in separate models, we analyzed time to the composite end point by time-varying serum bicarbonate categories. We utilized semiparametric Cox proportional hazards models with the same covariate adjustments as presented above with the addition of the previous (lagged) bicarbonate level. In these models, the time origin was the second visit where bicarbonate was measured. HRs for the composite outcome were interpreted as the risk associated with low bicarbonate after adjusting for lagged serum bicarbonate.
Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc, Cary, North Carolina) and Stata 15 (StataCorp, 2017, College Station, TX). Statistical significance was evaluated at the 5% level.
Results
Baseline Demographics and Bicarbonate Treatment
The study population comprised 858 participants: 603 children with nonglomerular disease and 255 children with glomerular disease. Tables 1 and 2 describe baseline demographic and clinical characteristics. Overall, there was a predominance of boys and white participants, and low bicarbonate was associated with lower eGFR and more significant proteinuria. There were a total of five deaths (Supplemental Table 1). For participants with nonglomerular disease, 39% had a baseline bicarbonate of ≤22 meq/L and 36% of those participants were treated with alkali therapy. During the median follow-up time of 4 years, alkali treatment rates increased to 46% (Supplemental Table 2). For participants with glomerular disease, 31% had a baseline bicarbonate of ≤22 meq/L and 18% were treated with alkali therapy. During the median follow-up time of 3 years, reported treatment rates increased to 23% (Supplemental Table 3).
Table 1.
Baseline demographic and clinical characteristics for 603 children with nonglomerular disease in the CKiD study
| Characteristicsa | Overall (n=603) | Normal (>22 meq/L) (n=366) | Low (19–22 meq/L) (n=190) | Very Low (≤18 meq/L) (n=47) |
|---|---|---|---|---|
| Demographics | ||||
| Median age (IQR), yr | 10 (6, 14) | 11 (7, 14) | 9 (5, 13) | 11 (8, 14) |
| Median CKD duration at study entry (IQR), yr | 9 (5, 13) | 10 (6, 13) | 8 (4, 11) | 10 (6, 14) |
| Male sex, n (%) | 404 (67) | 243 (66) | 133 (70) | 28 (60) |
| Race, n (%) | ||||
| White | 430 (71) | 258 (70) | 140 (74) | 32 (68) |
| Black | 111 (18) | 70 (19) | 33 (17) | 8 (17) |
| Other | 62 (10) | 38 (10) | 17 (9) | 7 (15) |
| Hispanic ethnicity, n (%) | 80 (13) | 39 (11) | 32 (17) | 9 (20) |
| Clinical characteristics, n (%) | ||||
| Median height (IQR), z-score | −0.6 (−1.4, 0.2) | −0.4 (−1.2, 0.4) | −0.8 (−1.5, −0.1) | −1.4 (−2.1, −0.6) |
| Hypertensionb | 306 (51) | 166 (45) | 115 (61) | 25 (53) |
| Median eGFR (IQR), ml/min per 1.73 m2c | 50 (36, 62) | 54 (41, 66) | 44 (35, 56) | 34 (28, 44) |
| eGFR, ml/min per 1.73 m2 | ||||
| ≥60 | 171 (28) | 132 (36) | 36 (19) | 3 (6) |
| ≥45–60 | 183 (30) | 119 (33) | 57 (30) | 7 (15) |
| ≥30–45 | 178 (30) | 79 (22) | 78 (41) | 21 (45) |
| <30 | 71 (12) | 36 (10) | 19 (10) | 16 (34) |
| Protein/creatinine ratio, mg/mg | ||||
| <0.5 | 375 (62) | 252 (69) | 107 (56) | 16 (34) |
| 0.5–2.0 | 178 (30) | 97 (27) | 58 (31) | 21 (45) |
| ≥2.0 | 50 (8) | 17 (5) | 25 (13) | 10 (21) |
| Median serum albumin (IQR), g/dl | 4.4 (4.2, 4.6) | 4.4 (4.2, 4.6) | 4.4 (4.2, 4.6) | 4.4 (4.0, 4.5) |
| Hypoalbuminemiad | 12 (2) | 5 (1) | 4 (2) | 3 (6) |
| Anemiae | 135 (22) | 66 (18) | 49 (26) | 20 (43) |
| Hyperphosphatemiaf | 75 (12) | 36 (10) | 28 (15) | 11 (23) |
| Hyperkalemiag | 45 (7) | 16 (4) | 21 (11) | 8 (17) |
| Hypocalcemiah | 8 (1) | 6 (2) | 0 (0) | 2 (4) |
| Medications, n (%) | ||||
| Alkali therapy usei | 183 (30) | 98 (27) | 65 (34) | 20 (43) |
| Adherencej | 148/174 (85) | 80/92 (87) | 53/63 (84) | 15/19 (79) |
| Growth hormone use | 68 (11) | 30 (8) | 28 (15) | 10 (21) |
Missing data include CKD onset date: n=4, Hispanic: n=8, height z-score: n=18, and potassium: n=1.
Hypertension was defined as either systolic or diastolic BP ≥95th percentile for age, sex, height, or prior medical diagnosis.
eGFR on the basis of the 2012 CKD in Children study equation.
Hypoalbuminemia was defined as albumin <3.8 g/dl.
Anemia was defined as hemoglobin <5th percentile for age, sex, and race.
Phosphate was centered around 4.5 mg/dl.
Hyperkalemia was defined as potassium >5.2 mmol/L.
Hypocalcemia was defined as calcium <8.5 mg/dl.
Alkali therapy includes sodium bicarbonate and citrate/citric acid alkalizing agents.
Adherence defined as missing more than one dose of prescribed medication in the past 7 days.
Table 2.
Baseline demographic and clinical characteristics for 255 children with glomerular disease in the CKiD study
| Characteristicsa | Overall (n=255) | Normal (>22 meq/L) (n=176) | Low (19–22 meq/L) (n=61) | Very Low (≤18 meq/L) (n=18) |
|---|---|---|---|---|
| Demographics | ||||
| Median age (IQR), yr | 14 (11, 16) | 14 (12, 16) | 13 (8, 16) | 15 (14, 17) |
| Median CKD duration at study entry (IQR), yr | 4 (1, 8) | 4 (1, 9) | 4 (2, 6) | 4 (2, 6) |
| Male sex, n (%) | 138 (54) | 93 (53) | 36 (59) | 9 (50) |
| Race, n (%) | ||||
| White | 135 (53) | 99 (56) | 32 (52) | 4 (22) |
| Black | 77 (30) | 57 (32) | 14 (23) | 6 (33) |
| Other | 43 (17) | 20 (11) | 15 (25) | 8 (44) |
| Hispanic ethnicity | 40 (16) | 19 (11) | 17 (29) | 4 (22) |
| Clinical characteristics, n (%) | ||||
| Median height (IQR), z-score | −0.2 (−1.0, 0.6) | 0.0 (−0.8, 0.7) | −0.5 (−1.6, 0.2) | −1.0 (−1.6, −0.2) |
| Hypertensionb | 154 (60) | 98 (56) | 43 (70) | 13 (72) |
| Median eGFR (IQR), ml/min per 1.73 m2c | 61 (45, 77) | 65 (51, 82) | 56 (35, 64) | 38 (32, 49) |
| eGFR, ml/min per 1.73 m2 | ||||
| ≥60 | 135 (53) | 108 (61) | 25 (41) | 2 (11) |
| ≥45–60 | 56 (22) | 38 (22) | 13 (21) | 5 (28) |
| ≥30–45 | 45 (18) | 23 (13) | 15 (25) | 7 (39) |
| <30 | 19 (7) | 7 (4) | 8 (13) | 4 (22) |
| Protein/creatinine ratio, mg/mg | ||||
| <0.5 | 112 (44) | 81 (46) | 25 (41) | 6 (33) |
| 0.5–2.0 | 82 (32) | 61 (35) | 19 (31) | 2 (11) |
| ≥2.0 | 61 (24) | 34 (19) | 17 (28) | 10 (56) |
| Median serum albumin (IQR), g/dl | 4.2 (3.7, 4.4) | 4.2 (3.8, 4.5) | 4.1 (3.6, 4.4) | 3.6 (2.9, 4.3) |
| Hypoalbuminemiad | 69 (27) | 43 (24) | 17 (28) | 9 (50) |
| Anemiae | 93 (36) | 57 (32) | 27 (44) | 9 (50) |
| Hyperphosphatemiaf | 62 (24) | 35 (20) | 16 (26) | 11 (61) |
| Hyperkalemiag | 20 (8) | 5 (3) | 10 (16) | 5 (28) |
| Hypocalcemiah | 31 (12) | 12 (7) | 10 (16) | 9 (50) |
| Medications, n (%) | ||||
| Alkali therapyi use | 29 (11) | 15 (9) | 10 (16) | 4 (22) |
| Adherencej | 24/27 (89) | 13/14 (93) | 9/10 (90) | 2/3 (67) |
| Growth hormone use | 7 (3) | 3 (2) | 4 (7) | 0 (0) |
Missing data include CKD onset date: n=7, Hispanic: n=4, height z-score: n=3, and potassium: n=2.
Hypertension was defined as either systolic or diastolic BP ≥95th percentile for age, sex, height, or prior medical diagnosis.
eGFR on the basis of the 2012 CKD in Childhood study equation.
Hypoalbuminemia was defined as albumin <3.8 g/dl.
Anemia was defined as hemoglobin <5th percentile for age, sex, and race.
Phosphate was centered around 4.5 mg/dl.
Hyperkalemia was defined as potassium >5.2 mmol/L.
Hypocalcemia was defined as calcium <8.5 mg/dl.
Alkali therapy includes sodium bicarbonate and citrate/citric acid alkalizing agents.
Adherence defined as missing more than one dose of prescribed medication in the past 7 days.
Low Serum Bicarbonate and Association with CKD Progression: Unadjusted
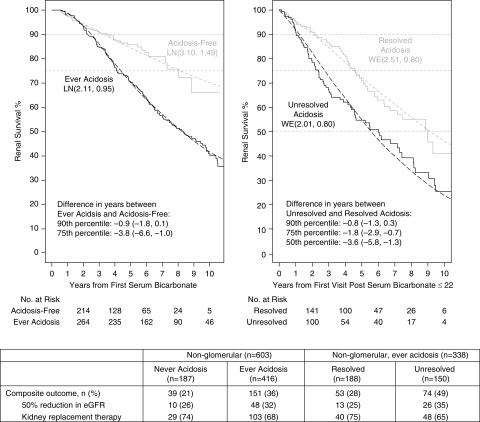
Figure 1 displays the time to the composite end point for the ever acidosis and acidosis-free groups among children with nonglomerular disease. Participants classified as ever acidosis had a shorter time to KRT or a 50% eGFR decline compared with acidosis-free participants. Specifically, 25% of the ever acidosis participants experienced the event 3.8 years earlier than 25% of the acidosis-free group. In the children with nonglomerular disease who were classified as ever acidosis, those whose low bicarbonate (≤22 meq/L) persisted throughout follow-up had shorter times to disease progression (Figure 1): 25% had the event 1.8 years earlier than 25% of the resolved children.
Figure 1.
Survival time to 50% decline in eGFR or KRT (transplant or dialysis) by “ever acidosis” following the first bicarbonate measurement among 603 participants with non-glomerular disease (left). Event-free time by acidosis persistence following the first visit after participants become acidotic among 338 participants with non-glomerular disease who were ever acidotic (right). Parametric Weibull or Lognormal distributions for each group with location (β) and scale (σ) and denoted as WE(β, σ) or LN(β, σ), respectively, are shown by dashed lines. Percentiles provided due to differences in the proportion of participants who reached the composite event. The relative percentile measure of association summarizes the difference in time (years) for the pth percentile of the exposed group (e.g., those with acidosis) compared to the unexposed group (e.g., those without acidosis).
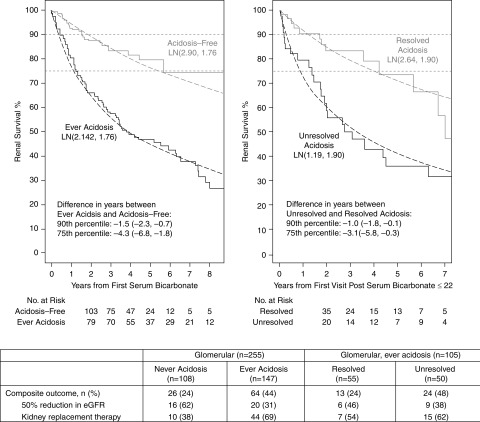
Similarly, among children with glomerular disease, ever acidosis participants experienced progression 4.3 years earlier than those who were acidosis-free (Figure 2). During follow-up, the children with persisting unresolved low bicarbonate reached the composite end point 3.1 years earlier than those who had resolution of their low bicarbonate (Figure 2).
Figure 2.
Survival time to 50% decline in eGFR or KRT (transplant or dialysis) by “ever acidosis” following the first bicarbonate measurement among 255 participants with glomerular disease (left). Event-free time by acidosis persistence following the first visit after participants become acidotic among 105 participants with glomerular disease who were ever acidotic (right). Parametric Lognormal distributions for each group with location (β) and scale (σ) and denoted as LN(β, σ) are represented by dashed lines. Percentiles provided due to differences in the proportion of participants who reached the composite event. The relative percentile measure of association summarizes the difference in time (years) for the pth percentile of the exposed group (e.g., those with acidosis) compared to the unexposed group (e.g., those without acidosis).
Sensitivity Analysis
When we included a confirmed acidosis category requiring two low serum bicarbonate levels (≤22 meq/L), the Kaplan–Meier survival curves of the 511 children with nonglomerular disease and the 213 with glomerular disease were similar to analyses requiring at least 1 bicarbonate measurement (Supplemental Figure 4). Regardless of CKD diagnosis, lower bicarbonate was associated with faster CKD progression.
Multivariable Associations between Low Serum Bicarbonate and CKD Progression in Nonglomerular and Glomerular CKD
Extending the survival analysis, semiparametric Cox proportional hazards models were used, unadjusted and adjusted, for covariates of interest. In these analyses, serum bicarbonate was categorized as very low (≤18 meq/L), low (19–22 meq/L), and normal (>22 meq/L), and treated as both time-fixed and time-varying. In participants with nonglomerular disease (Table 3), unadjusted baseline and time-varying bicarbonate levels ≤18 meq/L and 19–22 meq/L were associated with a significantly higher risk for the composite end point, compared with bicarbonate >22 meq/L. With adjustments for all other covariates of interest (model 4), bicarbonate ≤18 meq/L was associated with a greater hazard for CKD progression at baseline (adjusted HR 1.95; 95% CI, 1.23 to 3.09) and longitudinally (adjusted HR 1.28; 95% CI, 0.84 to 1.94), although statistical significance was lost with the latter.
Table 3.
Hazard ratios (95% confidence intervals) for development of 50% decline in eGFR or KRT (transplant or dialysis) among 603 children with nonglomerular disease contributing 2673 person-years of follow-up and 190 composite events
| Serum Bicarbonate | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Baseline bicarbonate | ||||
| >22 meq/L | 1 | 1 | 1 | 1 |
| 19–22 meq/L, HR (95% CI) | 1.85 (1.35 to 2.52)a | 1.17 (0.84 to 1.62) | 1.14 (0.82 to 1.58) | 1.13 (0.80 to 1.59) |
| ≤18 meq/L, HR (95% CI) | 5.60 (3.65 to 8.58)a | 2.20 (1.40 to 3.43)a | 1.91 (1.21 to 3.02)a | 1.95 (1.23 to 3.09)a |
| Time-varying bicarbonate | ||||
| >22 meq/L | 1 | 1 | 1 | 1 |
| 19–22 meq/L, HR (95% CI) | 1.71 (1.24 to 2.35)a | 1.13 (0.81 to 1.57) | 0.89 (0.64 to 1.25) | 0.91 (0.65 to 1.26) |
| ≤18 meq/L, HR (95% CI) | 4.01 (2.70 to 5.95)a | 1.87 (1.45 to 2.82)a | 1.29 (0.85 to 1.96) | 1.28 (0.84 to 1.94) |
Model 1: unadjusted model. Model 2: model 1 plus adjustment for baseline age (centered at 10), male sex, white race, baseline eGFR (<30, 30–45, 45–60, or ≥60 ml/min per 1.73 m2), and proteinuria (< 0.5, 0.5–2.0, or ≥2.0 mg/mg). Model 3: model 2 plus adjustment for anemia and phosphate (centered at 4.5 mg/dl). Model 4: model 3 plus adjustment for hypertension and alkali therapy use. HR, hazard ratio; 95% CI, 95% confidence interval.
aIndicates significance.
For participants with glomerular disease (Table 4), baseline bicarbonate 19–22 meq/L was associated with a higher hazard for CKD progression in unadjusted analyses (HR 1.79; 95% CI, 1.14 to 2.81). When using time-varying serum bicarbonate, bicarbonate ≤18 meq/L and 19–22 meq/L were associated with a higher unadjusted risk of the composite end point (HR 8.92; 95% CI, 4.84 to 16.45 and HR 2.83; 95% CI, 1.80 to 4.47, respectively). In all adjusted models (models 2–4), both very low and low time-varying serum bicarbonate were associated with CKD progression. In analyses of the entire cohort, we observed a higher risk of CKD progression with time-varying bicarbonate ≤18 meq/L (Supplemental Table 4) . We also found a higher risk for the composite event with alkali therapy use (Supplemental Table 5). In sensitivity analyses adjusting for previous (lagged) serum bicarbonate level (Supplemental Table 6), children with nonglomerular disease had similar HRs (to nonlagged analyses) for very low and low time-varying serum bicarbonate. In children with glomerular disease, the association was slightly stronger in the fully adjusted model (sensitivity model 4) for bicarbonate ≤18 meq/L (HR 2.68; 95% CI, 1.07 to 6.71) than in models that did not account for previous bicarbonates (HR 2.16; 95% CI, 1.05 to 4.44).
Table 4.
Hazard ratios (95% confidence intervals) for development of 50% decline in eGFR or KRT (transplant or dialysis) among 255 children with glomerular disease contributing 808 person-years of follow-up and 90 composite events
| Serum Bicarbonate | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Baseline bicarbonate | ||||
| >22 meq/L | 1 | 1 | 1 | 1 |
| 19–22 meq/L, HR (95% CI) | 1.79 (1.14 to 2.81)a | 1.28 (0.81 to 2.04) | 1.28 (0.80 to 2.05) | 1.28 (0.80 to 2.05) |
| ≤18 meq/L, HR (95% CI) | 1.94 (0.92 to 4.09) | NE | NE | NE |
| Time-varying bicarbonate | ||||
| >22 meq/L | 1 | 1 | 1 | 1 |
| 19–22 meq/L, HR (95% CI) | 2.83 (1.80 to 4.47)a | 2.17 (1.35 to 3.48)a | 1.86 (1.15 to 3.02)a | 1.74 (1.07 to 2.85)a |
| ≤18 meq/L, HR (95% CI) | 8.92 (4.84 to 16.45)a | 3.27 (1.65 to 6.49)a | 2.21 (1.08 to 4.56)a | 2.16 (1.05 to 4.44)a |
Model 1: unadjusted model. Model 2: model 1 plus adjustment for baseline age (centered at 10), male sex, white race, baseline eGFR (<30, 30–45, 45–60, or ≥60 ml/min per 1.73 m2), and proteinuria (<0.5, 0.5–2.0, or ≥2.0 mg/mg). Model 3: model 2 plus adjustment for anemia and phosphate (centered at 4.5 mg/dl). Model 4: model 3 plus adjustment for hypertension and alkali therapy use. Baseline models 2, 3, and 4 included 237 children with 82 composite events. HR, hazard ratio; 95% CI, 95% confidence interval; NE: not estimated in adjusted models due to small sample size (n=18, 8 events).
Indicates significance.
Discussion
Using annual assessments of serum bicarbonate over a long duration of follow-up, our data suggest an association between low serum bicarbonate and CKD progression. More specifically, after adjusting for multiple covariates, bicarbonate ≤22 meq/L was associated with earlier CKD progression in children with glomerular disease. Bicarbonate ≤18 meq/L was linked to a greater risk for CKD progression in participants with nonglomerular disease. Outcome differences between disease groups and with use of baseline versus time-varying bicarbonate values may be explained by differences in sample size between the primary disease groups, and the fact that the two groups may not be similarly affected by CKD comorbidities (22). We also found that many participants with low baseline serum bicarbonate did not report treatment with alkali therapies. Reported treatment increased during follow-up but remained <50%. This is important because in our investigation, normalization of low serum bicarbonate (to >22 meq/L), was linked to slower CKD progression.
Our findings are consistent with the longitudinal results from the Comorbidity in Children with CKD (4C) study (23). Similar to our study, there was a preponderance of boys enrolled, the majority had congenital abnormalities of the kidney and urinary tract, and disease progression was defined as a 50% decline in eGFR or development of ESKD (23). Different from our study, this cohort was entirely European, included children with severe CKD, and participants were not analyzed by disease category (23). In the 4C cohort, after adjusting for potential clinical confounders, serum bicarbonate <18 meq/L was associated with a twofold higher risk of CKD progression, compared with a bicarbonate of >22 meq/L (23). Lastly, there was a small but significant effect of alkali therapy on disease progression in the 4C study (adjusted HR 0.995 per mg/kg per day bicarbonate equivalent; 95% CI, 0.99 to 1) (23), which was not observed in our study.
Whereas our study represents one of the first to examine the association between acidosis and CKD progression in children, this relationship has been documented in observational studies of adults (12–14,33). In a study of >5000 participants, after adjusting for risk factors of disease progression, serum bicarbonate ≤22 meq/L was associated with a 54% higher risk of CKD progression (9). In the Chronic Renal Insufficiency Cohort study, there was a significant decrease in the risk of kidney disease progression per 1 meq/L higher serum bicarbonate (10). In a study of transplant recipients, participants with time-varying serum bicarbonate <22 meq/L had a greater risk of graft loss, even after adjusting for confounders such as eGFR and acute rejection (34).
In our study, slower kidney function decline in participants with resolved acidosis suggests that treatment could attenuate this decline. However, in our data, alkali therapy use was associated with the composite event. Because alkali therapy is usually prescribed when bicarbonate is low, the association could have been subject to confounding by indication or other unmeasured confounders. In animal studies, alkali therapy was associated with preservation of kidney function (11,35–37). Investigations describing the benefits of an alkaline diet on kidney function further contribute to the idea that CKD progression may be slowed with the correction of acidosis (33,38–42). The promising effects of acidosis treatment on CKD progression have been shown in small, randomized controlled clinical trials of adults (15,16,43,44). Currently, there is an ongoing multicenter, randomized trial of alkali therapy use to preserve allograft function in adult transplant participants (45). There are no trials of alkali therapy exclusively in children. Larger clinical investigations are needed to better delineate the effect of acidosis correction on pediatric kidney function.
This study also highlights the continued potential under treatment of metabolic acidosis (4) and disparities in treatment between the primary diagnosis groups. We believe this is noteworthy because, in addition to the potential link to CKD progression, metabolic acidosis has been negatively associated with other pediatric outcomes such as growth (2,4,23,46–48). Rodig et al. found that children with serum bicarbonates of <18 meq/L (compared with ≥22 meq/L) had lower height SD scores (0.67; 95% CI, 0.31 to 1.03) (4). Similarly our baseline data show lower height z-scores with low bicarbonate (Tables 1 and 2). Alkali treatment improved growth in children with renal tubular acidosis (RTA) and normal kidney function (46,48,49), but this is understudied in CKD. Longitudinal investigations of the contribution of metabolic acidosis to poor growth in participants with impaired kidney function may better inform treatment of the growth failure commonly seen in pediatric CKD.
A limitation of our study is the use of annual bicarbonate values versus more frequent clinical measurements. Additionally, there is potential variability of bicarbonate measurement using local laboratories but that is balanced by the risk of erroneous results, which may occur with delays that can happen when measuring bicarbonate at one centralized site. We do not have data on calcium carbonate use. We believe in adjusting for hyperphosphatemia and we accounted for some of its effect, although we recognize this as a limitation. We used low serum bicarbonate as a proxy for acidosis, but bicarbonate levels do not always correlate with serum pH. Lastly, we were unable to make causal inferences about the effect of acidosis on progression to ESKD. Our analyses strongly suggest that bicarbonate control should be an important clinical consideration, especially in the presence of effective and available treatment options (i.e., alkali therapy). However, even with adjusting for the previous year’s bicarbonate value when assessing risk of CKD progression, we acknowledge our analyses cannot rule out reverse causality or residual confounding. Future efforts should include blood gases and consideration of other markers of acid/base status (e.g., urine ammonium) (50). Despite these limitations, our study had significant strengths that included a large sample size and prospective, systematic, and longitudinally collected data.
In conclusion, we found that low serum bicarbonate was associated with more rapid progression of CKD in children, even after adjustment for clinical confounders. This is important because we also demonstrated that alkali therapy use in pediatric CKD may be underutilized. Future studies should evaluate determinants of acidosis and prospectively evaluate, in a randomized fashion, whether treatment of metabolic acidosis improves outcomes, including kidney function and growth, in pediatric CKD.
Disclosures
Dr. Abramowitz reports personal fees from Tricida, Inc., outside the submitted work. Dr. Melamed is on the Honorarium Nephrology Exam Committee of the American Board of Internal Medicine, receives nonfinancial support from the New York Society of Nephrology, and personal fees from Icon Medical Imaging. Dr. Reidy reports receiving other support from Advicienne and Complexa, outside the submitted work. Dr. Schwartz reports personal fees from AstraZeneca and Tricida, Inc., outside the submitted work. Dr. Brown, Dr. Furth, Dr. Kaskel, Dr. Kumar, Dr. Mak, and Dr. Ng have nothing to disclose.
Funding
This work was supported by the Chronic Kidney Disease in Children (CKiD) study with clinical coordinating centers at Children’s Mercy Hospital (Dr. Warady) and Children’s Hospital of Philadelphia (Dr. Furth), data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Dr. Roem and Dr. Ng), and the Central Biochemistry Laboratory at the University of Rochester Medical Center (Dr. Schwartz). The CKiD study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Heart, Lung, and Blood Institute (grants U01-DK-66143, U01-DK-66174, U24-DK-082194, and U24-DK-66116). Dr. Brown was previously supported by Developmental and Translational Nephrology Training grant T32 DK007110. Dr. Kumar is supported by NICHD grant R01 HD091185. Dr. Reidy reports NIDDK grants R03 DK105242 and R01 DK118015, during the conduct of the study.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07060619/-/DCSupplemental.
Supplemental Table 1. Causes of death by primary CKD diagnosis.
Supplemental Table 2. Alkali therapy treatment throughout the follow-up period for participants with nonglomerular disease.
Supplemental Table 3. Alkali therapy treatment throughout the follow-up period for participants with glomerular disease.
Supplemental Table 4. Hazard ratios (95% confidence intervals) for development of 50% decline in eGFR or KRT among all participants (not separated by underlying CKD cause).
Supplemental Table 5. Hazard ratios (95% confidence intervals) for development of a 50% decline in eGFR or KRT using baseline and time-varying values.
Supplemental Table 6. Time-varying sensitivity analyses including adjustment for lagged bicarbonate (i.e., controlling for bicarbonate level from the previous year) among all participants contributing at least two serum bicarbonate measurements.
Supplemental Figure 1. Case selection from the CKD in Childhood cohort.
Supplemental Figure 2. Descriptions of “acidosis-free” and “ever acidosis” categorizations.
Supplemental Figure 3. Descriptions of “resolved” and “unresoved” categorizations.
Supplemental Figure 4. Survival time to 50% decline in eGFR or KRT between acidosis categories: acidosis free, ever acidosis, and confirmed acidosis (i.e. two consecutive serum bicarbonate measurements ≤22 meq/L).
Parametric Model Selection Description. Description of the way in which parametric models were selected for use.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung JT, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine CE, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Kurella Tamura M, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, Shahinian V: US Renal Data System 2018 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 73: A7–A8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA: Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease [published correction appears in Clin J Am Soc Nephrol 9: 997–998, 2014]. Clin J Am Soc Nephrol 6: 2132–2140, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethna CB, Ng DK, Jiang S, Saland J, Warady BA, Furth S, Meyers KE: Cardiovascular disease risk among children with focal segmental glomerulosclerosis: A report from the chronic kidney disease in children study. Pediatr Nephrol 34: 1403–1412, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodig NM, McDermott KC, Schneider MF, Hotchkiss HM, Yadin O, Seikaly MG, Furth SL, Warady BA: Growth in children with chronic kidney disease: A report from the Chronic Kidney Disease in Children Study. Pediatr Nephrol 29: 1987–1995, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper SR, Gerson AC, Butler RW, Gipson DS, Mendley SR, Lande MB, Shinnar S, Wentz A, Matheson M, Cox C, Furth SL, Warady BA: Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol 6: 1824–1830, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Renal Data System (USRDS) : 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 7.McDonald SP, Craig JC; Australian and New Zealand Paediatric Nephrology Association : Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Parekh RS, Carroll CE, Wolfe RA, Port FK: Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr 141: 191–197, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Shah SN, Abramowitz M, Hostetter TH, Melamed ML: Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am J Kidney Dis 54: 270–277, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, Hostetter T, Jaar B, Lora CM, Nessel L, Ojo A, Scialla J, Steigerwalt S, Teal V, Wolf M, Rahman M; CRIC Investigators : Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 62: 670–678, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nath KA, Hostetter MK, Hostetter TH: Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon V, Tighiouart H, Vaughn NS, Beck GJ, Kusek JW, Collins AJ, Greene T, Sarnak MJ: Serum bicarbonate and long-term outcomes in CKD. Am J Kidney Dis 56: 907–914, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S: Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda E, Ai M, Yoshida M, Kuriyama R, Shiigai T: High serum bicarbonate level within the normal range prevents the progression of chronic kidney disease in elderly chronic kidney disease patients. BMC Nephrol 14: 4, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Iorio BR, Bellasi A, Raphael KL, Santoro D, Aucella F, Garofano L, Ceccarelli M, Di Lullo L, Capolongo G, Di Iorio M, Guastaferro P, Capasso G; UBI Study Group : Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: The UBI Study. J Nephrol 32: 989–1001, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson DP: Control of hydrogen ion homeostasis and renal acidosis. Medicine (Baltimore) 50: 503–541, 1971. [DOI] [PubMed] [Google Scholar]
- 18.Torres VE, Cowley BD Jr., Branden MG, Yoshida I, Gattone VH: Long-term ammonium chloride or sodium bicarbonate treatment in two models of polycystic kidney disease. Exp Nephrol 9: 171–180, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Wesson DE, Simoni J: Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int 78: 1128–1135, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Goraya N, Simoni J, Jo CH, Wesson DE: Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH, Flynn J, Moxey-Mims MM, Furth S: Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: The Chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis 65: 878–888, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harambat J, Kunzmann K, Azukaitis K, Bayazit AK, Canpolat N, Doyon A, Duzova A, Niemirska A, Sözeri B, Thurn-Valsassina D, Anarat A, Bessenay L, Candan C, Peco-Antic A, Yilmaz A, Tschumi S, Testa S, Jankauskiene A, Erdogan H, Rosales A, Alpay H, Lugani F, Arbeiter K, Mencarelli F, Kiyak A, Dönmez O, Drozdz D, Melk A, Querfeld U, Schaefer F; 4C Study Consortium : Metabolic acidosis is common and associates with disease progression in children with chronic kidney disease. Kidney Int 92: 1507–1514, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tietz N: Textbook of Clinical Chemistry, Philadelphia, PA, WB Saunders Company, 1994 [Google Scholar]
- 26.Furth SL, Pierce C, Hui WF, White CA, Wong CS, Schaefer F, Wühl E, Abraham AG, Warady BA; Chronic Kidney Disease in Children (CKiD); Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) Study Investigators : Estimating time to ESRD in children with CKD [published correction appears in Am J Kidney Dis 74: 144, 2019]. Am J Kidney Dis 71: 783–792, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C; Centers for Disease Control and Prevention, National Center for Health Statistics: Hematological and iron-related analytes--reference data for persons aged 1 year and over: United States, 1988-94. Vital Health Stat 11 247: 1–156, 2005 [PubMed] [Google Scholar]
- 28.National Kidney Foundation : K/DOQI Clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis 46[Suppl 1]: S1–S122, 2005 [PubMed] [Google Scholar]
- 29.KDOQI Work Group : 2008 update. Executive summary. Am J Kidney Dis 53[Suppl 2]: S11–S104, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM; Subcommittee on Screening and Management of High Blood Pressure in Children : Clinical practice guideline for screening and management of high blood pressure in children and adolescents [published correction appears in Pediatrics 142: e20181739, 2018]. Pediatrics 140: e20171904, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Pierce CB, Cox C, Saland JM, Furth SL, Muñoz A: Methods for characterizing differences in longitudinal glomerular filtration rate changes between children with glomerular chronic kidney disease and those with nonglomerular chronic kidney disease. Am J Epidemiol 174: 604–612, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng DK, Moxey-Mims M, Warady BA, Furth SL, Muñoz A: Racial differences in renal replacement therapy initiation among children with a nonglomerular cause of chronic kidney disease. Ann Epidemiol 26: 780–787.e1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goraya N, Wesson DE: Clinical evidence that treatment of metabolic acidosis slows the progression of chronic kidney disease. Curr Opin Nephrol Hypertens 28: 267–277, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Kang E, Park S, Kim YC, Han SS, Ha J, Kim DK, Kim S, Park SK, Han DJ, Lim CS, Kim YS, Lee JP, Kim YH: Metabolic acidosis and long-term clinical outcomes in kidney transplant recipients. J Am Soc Nephrol 28: 1886–1897, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gadola L, Noboa O, Márquez MN, Rodriguez MJ, Nin N, Boggia J, Ferreiro A, García S, Ortega V, Musto ML, Ponte P, Sesser P, Pizarrosa C, Ravaglio S, Vallega A: Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int 65: 1224–1230, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Tanner GA: Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J Am Soc Nephrol 9: 1242–1248, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Toblli JE, DeRosa G, Lago N, Angerosa M, Nyberg C, Pagano P: Potassium citrate administration ameliorates tubulointerstitial lesions in rats with uric acid nephropathy. Clin Nephrol 55: 59–68, 2001. [PubMed] [Google Scholar]
- 38.Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE: Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int 73: 192–199, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Wesson DE, Simoni J: Increased tissue acid mediates a progressive decline in the glomerular filtration rate of animals with reduced nephron mass. Kidney Int 75: 929–935, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Wesson DE, Nathan T, Rose T, Simoni J, Tran RM: Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int 71: 210–217, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Goraya N, Wesson DE: Dietary management of chronic kidney disease: Protein restriction and beyond. Curr Opin Nephrol Hypertens 21: 635–640, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Banerjee T, Crews DC, Wesson DE, Tilea AM, Saran R, Ríos-Burrows N, Williams DE, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : High dietary acid load predicts ESRD among adults with CKD. J Am Soc Nephrol 26: 1693–1700, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE: Short- and long-term effects of alkali therapy in chronic kidney disease: A systematic review. Am J Nephrol 35: 540–547, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiegand A, Ritter A, Graf N, Arampatzis S, Sidler D, Hadaya K, Müller TF, Wagner CA, Wüthrich RP, Mohebbi N: Preservation of kidney function in kidney transplant recipients by alkali therapy (Preserve-Transplant study): Rationale and study protocol. BMC Nephrol 19: 177, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McSherry E, Morris RC Jr.: Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J Clin Invest 61: 509–527, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caldas A, Broyer M, Dechaux M, Kleinknecht C: Primary distal tubular acidosis in childhood: Clinical study and long-term follow-up of 28 patients. J Pediatr 121: 233–241, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Sharma AP, Singh RN, Yang C, Sharma RK, Kapoor R, Filler G: Bicarbonate therapy improves growth in children with incomplete distal renal tubular acidosis. Pediatr Nephrol 24: 1509–1516, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F; ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Raphael KL, Carroll DJ, Murray J, Greene T, Beddhu S: Urine ammonium predicts clinical outcomes in hypertensive kidney disease. J Am Soc Nephrol 28: 2483–2490, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.