Visual Abstract
Keywords: hemodialysis; cardiovascular disease; Bradycardia; Incidence; Arrhythmias, ; Cardiac Conduction System Disease; hypotension; Tachycardia, Ventricular
Abstract
Background and objectives
Patients receiving maintenance hemodialysis (HD) have a high incidence of cardiac events, including arrhythmia and sudden death. Intradialytic hypotension (IDH) is a common complication of HD and is associated with development of reduced myocardial perfusion, a potential risk factor for arrhythmia.
Design, setting, participants, & measurements
We analyzed data from the Monitoring in Dialysis study, which used implantable loop recorders to detect and continuously monitor electrocardiographic data from patients on maintenance HD (n=66 from the United States and India) over a 6-month period (n=4720 sessions). Negative binomial mixed effects regression was used to test the association of IDH20 (decline in systolic BP >20 mm Hg from predialysis systolic BP) and IDH0–20 (decline in systolic BP 0–20 mm Hg from predialysis systolic BP) with clinically significant arrhythmia (bradycardia≤40 bpm for ≥6 seconds, asystole≥3 seconds, ventricular tachycardia ≥130 bpm for ≥30 seconds, or patient-marked events) during HD.
Results
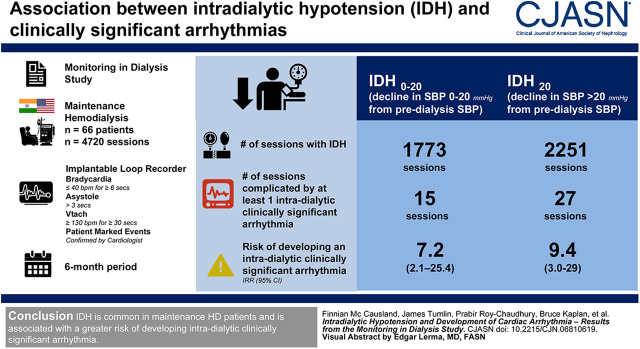
The median age of participants was 58 (25th–75th percentile, 49–66) years; 70% were male; and 65% were from the United States. IDH occurred in 2251 (48%) of the 4720 HD sessions analyzed, whereas IDH0–20 occurred during 1773 sessions (38%). The number of sessions complicated by least one intradialytic clinically significant arrhythmia was 27 (1.2%) where IDH20 occurred and 15 (0.8%) where IDH0–20 occurred. Participants who experienced IDH20 (versus not) had a nine-fold greater rate of developing an intradialytic clinically significant arrhythmia (incidence rate ratio, 9.4; 95% confidence interval, 3.0 to 29.4), whereas IDH0–20 was associated with a seven-fold higher rate (incidence rate ratio, 7.2; 95% confidence interval, 2.1 to 25.4).
Conclusions
IDH is common in patients on maintenance HD and is associated with a greater risk of developing intradialytic clinically significant arrhythmia.
Introduction
The annual mortality rate for patients on hemodialysis (HD) in the United States approaches 20%, with a median life expectancy of approximately 36 months (1). Cardiovascular disease remains the leading cause of mortality in patients on maintenance HD, accounting for up to 41% of deaths, of which half are ascribed to sudden cardiac death (1). Within this complex disease category, it is likely that a large proportion of these events are related to development of fatal cardiac arrhythmia (2,3).
Mortality rates are clearly higher during the days that HD is performed, compared with non-HD days, (4) which is suggestive of an adverse effect associated with the HD procedure itself. In this respect it is notable that up to one-third of outpatient HD treatments are complicated by episodes of intradialytic hypotension (IDH). Prior reports have noted that some adverse associations of IDH include myocardial hypo-perfusion and stunning (5,6), which in turn may predispose to the development of cardiac arrhythmia. It is important to determine if such an association exists, as IDH represents a potentially modifiable risk factor that could be targeted in dedicated prospective studies.
The Monitoring in Dialysis (MiD; NCT01779856) (7) study was a prospective, cohort study that used implantable loop recorders to characterize arrhythmia in patients undergoing thrice-weekly HD over a 6-month period. Detailed recording of intradialytic treatment parameters enabled us to test the hypothesis that IDH is associated with a greater risk of the subsequent development of a clinically significant arrhythmia during the remainder of the HD session.
Materials and Methods
Study Design and Population
The MiD study was a prospective, multicenter study of implantable loop recorders (Reveal XT or Reveal LINQ; Medtronic, Minneapolis, MN), in participants aged 21 years or more, designed to determine the frequency and composition of arrhythmia in patients on maintenance HD from the United States (n=43) and India (n=23). The design and primary outcome have been reported (7,8). Notable exclusion criteria included use of pacemakers or implantable defibrillators; expected survival or duration of maintenance HD <6 months; and recent infections, thoracic surgery, or catheters that could interfere with device placement. Informed consent was obtained from all subjects, and the study was approved by local institutional review boards.
Exposures and Outcomes
Per protocol, each site was instructed to measure the pre-HD systolic BP in a seated position after 5 minutes of rest, and to measure seated intradialytic BP every 30 minutes (the protocol did not mandate use of any particular type of BP measurement device). The primary exposure of interest was the development of IDH, defined as any occurrence of an intradialytic systolic BP decline of >20 mm Hg from the predialysis measurement (IDH20). Effect estimates were also ascertained for systolic BP decline between 0 and 20 mm Hg (IDH0–20), with no change or an increase in intradialytic systolic BP serving as the reference group. On the basis of the data distribution, a secondary exposure (IDH25%) was considered for sessions where the minimum intradialytic systolic BP was less than the 25th percentile of all recorded measurements (≤104 mm Hg). Finally, a third definition was considered (IDHnadir) where IDH was defined as any nadir<100 mm Hg if the pre-HD systolic BP was ≥160 mm Hg, or any nadir<90 mm Hg if the pre-HD systolic BP was <160 mm Hg (9). BP, dialysis prescription, and treatment parameters were measured per standard unit protocols and collected prospectively at every HD session during the first 6 months of the MiD study. Of note, as this was primarily an observational study, the protocol did not mandate specific dialysate temperature or other dialysate prescriptions. Of the 4761 total HD sessions there was insufficient data to calculate IDH20 or IDHnadir in 41 sessions and in 26 sessions for IDH25%. The median number of HD sessions per participant was 75 (25th–75th percentile, 71–77).
The primary outcome of interest was the development of a clinically significant arrhythmia during the same HD session. Clinically significant arrhythmia was the prespecified primary outcome of the MiD study and is defined as any occurrence of bradycardia (≤40 bpm for ≥6 seconds), asystole (≥3 seconds), ventricular tachycardia (≥130 bpm for ≥30 seconds), or patient-marked events (patients were free to record a “mark” for any symptoms, but only those coinciding with a reviewer-confirmed arrhythmia met the definition of clinically significant arrhythmia). For the purposes of these analyses, in sessions with an arrhythmic event, the presence of IDH was determined according to the pre-HD and nadir systolic BP before the event. Sessions with an arrhythmia before the first intradialytic BP measurement were excluded from the analyses.
Study Data
Study data were obtained from participants by means of interview, chart review, and questionnaires. Participants transmitted loop recorder data immediately before all HD sessions and after each session associated with a study blood draw during the 6-month period of the primary study. Blood was drawn both before and after HD: twice weekly for 4 weeks and once weekly thereafter through 6 months. For comparative purposes in the present analyses, baseline laboratory and HD session characteristics of participants were calculated from HD sessions during the first 2 weeks of the study.
Statistical Analyses
Continuous variables were examined graphically and recorded as mean (±SD) for normally distributed data, or median (with 25th and 75th percentile) for non–normally distributed data. Categoric variables were examined by frequency distribution and recorded as proportions.
The association of various definitions of IDH with the frequency of subsequent clinically significant arrhythmia at each HD session was examined by mixed effects negative binomial regression. Initially, unadjusted models were fit. Subsequently, manual backward selection models were employed using predefined variables according to the original analytic approach used to report the primary outcome (5,6). These included age, sex, race, dialysis vintage, and vascular access, with P<0.05 required for retention in the model. A random intercept was included to account for repeated measures within subjects, imposing a compound symmetric covariance structure, with an offset to indicate the time within each period (at-risk time for a clinically significant arrhythmia was considered to include the whole dialysis session). In exploratory analyses, models with adjustment for baseline systolic BP and models adjusted for the pre-HD systolic BP were fit.
Two sensitivity analyses were performed: (1) repeated measures logistic regression models were fit to determine the association of various definitions of IDH with any subsequent development of clinically significant arrhythmia during the corresponding HD sessions; and (2) in the negative binomial models, the at-risk time was restricted to the period after the occurrence of an IDH event (n=4665 sessions available for analysis). Nominal two-sided P values of <0.05 were considered statistically significant. All analyses were performed using SAS v9.3 (Cary, NC).
Results
Baseline Characteristics
The median age of all participants was 58 (25th–75th percentile, 49–66) years, 70% were male, 53% were black, and 64% had diabetes. Those with IDH20 in the baseline period were more likely to be black, heavier, and have a higher body mass index than those without IDH20 (Table 1), although they were more likely to be dialyzed against a dialysate calcium of 2.5 mEq/L and high-flux membrane (Table 2), and to have higher pre-HD serum sodium, creatinine, phosphate, and albumin, but have lower B-type natriuretic peptide (Table 3).
Table 1.
Baseline characteristics according to the presence or absence of any intradialytic hypotensive event (average systolic BP decline >20 mm Hg)
| Characteristica | All Subjects (n=66) | IDH (n=35) | No IDH (n=31) |
|---|---|---|---|
| Age, yr | 58 (49–66) | 54 (43–65) | 61 (53–67) |
| Female, % | 30 | 31 | 29 |
| Race, % | |||
| Asian | 35 | 14 | 58 |
| Black | 53 | 69 | 36 |
| Other | 1 | 3 | 0 |
| White | 11 | 14 | 6 |
| Vintage, yr | 2.4 (1.2–5.3) | 2.4 (1.1–5.7) | 2.4 (1.3–5.2) |
| Access, % | |||
| AV fistula | 69 | 65 | 74 |
| AV graft | 26 | 32 | 19 |
| Catheter | 5 | 3 | 7 |
| Diabetes, % | 64 | 69 | 58 |
| Hyperlipidemia, % | 61 | 71 | 48 |
| Hypertension, % | 85 | 91 | 77 |
| Ischemic heart disease, % | 48 | 40 | 58 |
| Heart failure, % | 26 | 34 | 16 |
| Coronary artery bypass graft, % | 14 | 9 | 19 |
| Arrhythmia, % | 32 | 43 | 19 |
| Smoking, % | |||
| Current | 8 | 11 | 3 |
| Never | 70 | 60 | 81 |
| Past | 23 | 29 | 16 |
| Weight, kg | 82 (68–95) | 89 (75–120) | 74 (65–90) |
| BMI, kg/m2 | 27 (24, 32) | 29 (26, 36) | 26 (22, 28) |
| Systolic BP, mm Hg | 141±23 | 143±26 | 138±21 |
| LVEF (%) | 55 (55–60) | 57 (55–60) | 55 (50–63) |
| Prior myocardial infarction, % | 9 | 9 | 10 |
| Beta blocker use, % | 64 | 62 | 67 |
| Calcium channel blocker use, % | 60 | 53 | 70 |
Values for continuous variables are given as mean±SD or median (25th–75th percentile). Percentages may not add up to 100 due to rounding. IDH, intradialytic hypotension; AV, arteriovenous; BMI, body mass index; LVEF, left ventricular ejection fraction.
Baseline refers to the average values over the first 2 weeks of the study.
Table 2.
Baseline hemodialysis session characteristics according to presence or absence of any intradialytic hypotensive event (average systolic BP decline >20 mm Hg)
| Characteristica | All Subjects | IDH | No IDH |
|---|---|---|---|
| Session length, h | 4.0 (3.5–4.0) | 4.0 (3.5–4.0) | 4.0 (3.5–4.0) |
| Dry weight target, kg | 81 (65–94) | 85 (73–120) | 71 (64–89) |
| Over dry weight target, kg | 4.2 (2.7–5.2) | 4.5 (2.7–5.9) | 4.1 (2.4–5.0) |
| UF rate (ml/kg per h) | 10.9 (7.4–15.9) | 10.5 (7.4–15.9) | 12.5 (7.1–15.9) |
| Dialysate temperature, % | |||
| 35.5 | 2 | 3 | 0 |
| 36.0 | 4 | 9 | 0 |
| 36.5 | 8 | 6 | 10 |
| 37.0 | 86 | 83 | 90 |
| Dialysate potassium, % | |||
| 1.0 | 2 | 0 | 3 |
| 2.0 | 80 | 80 | 81 |
| 3.0 | 17 | 17 | 16 |
| 4.0 | 2 | 3 | 0 |
| Dialysate calcium, % | |||
| 1.5 | 6 | 6 | 6 |
| 1.6 | 14 | 6 | 23 |
| 2.0 | 2 | 3 | 0.0 |
| 2.5 | 58 | 74 | 39 |
| 3.0 | 12 | 9 | 16 |
| 3.5 | 4 | 3 | 6 |
| 6.0 | 4 | 0.0 | 10 |
| Dialysate sodium, % | |||
| 135 | 10 | 6 | 15 |
| 138 | 10 | 9 | 11 |
| 140 | 80 | 85 | 74 |
| Dialysate bicarbonate, % | |||
| ≤30 | 11 | 9 | 13 |
| 31–34 | 18 | 14 | 23 |
| 35–37 | 51 | 49 | 55 |
| ≥38 | 20 | 29 | 10 |
| Sodium modeling, % | 14 | 11 | 16 |
| High flux, % | 64 | 83 | 42 |
| Membrane reuse, % | 27 | 31 | 23 |
| Cellulose membrane, % | 8 | 6 | 10 |
Percentages may not add up to 100 due to rounding. Values for continuous variables are given as median (25th–75th percentile). IDH, intradialytic hypotension; UF, ultrafiltration.
Baseline refers to the average values over the first 2 weeks of the study.
Table 3.
Baseline hemodialysis session characteristics according to presence or absence of any intradialytic hypotensive event (average systolic BP decline >20 mm Hg)
| Characteristica | All | IDH | No IDH |
|---|---|---|---|
| Sodium, mEq/L | 138 (135–140) n=59 | 139 (136–141) n=31 | 137 (131–139) n=28 |
| Potassium, mEq/L | 4.7 (4.2–5.4) n=58 | 4.5 (4.2–5.5) n=31 | 4.8 (4.4–5.4) n=27 |
| Bicarbonate, mEq/L | 22±4 n=59 | 23±4 n=31 | 21±4 n=28 |
| BUN, mg/dl | 55 (47–73) n=59 | 62 (50–73) n=31 | 55 (44–74) n=28 |
| Creatinine, mg/dl | 9.6 (7.2–12.4) n=59 | 11.1 (7.5–13.5) n=31 | 8.4 (6.6–11.1) n=28 |
| Calcium, mg/dl | 8.8 (8.2–9.3) n=59 | 9.0 (8.4–9.3) n=31 | 8.5 (8.1–9.3) n=28 |
| Phosphate, mg/dl | 5.1 (4.3–6.3) n=59 | 5.6 (5.0–7.3) n=31 | 4.8 (4.0–5.4) n=28 |
| Magnesium, mg/dl | 2.3 (2.0–2.7) n=59 | 2.2 (2.0–2.5) n=31 | 2.3 (2.1–2.8) n=28 |
| Albumin, g/dl | 3.9±0.3 n=59 | 4.0±0.2 n=31 | 3.8±0.4 n=28 |
| PTH, pg/ml | 379 (227–607) n=41 | 310 (214–601) n=28 | 481 (233–671) n=13 |
| Hb, g/dl | 10.6±1.2 n=56 | 10.8±1.0 n=28 | 10.4±1.4 n=28 |
| Ferritin, ng/ml | 1093±348 n=27 | 1031±364 n=18 | 1217±293 n=9 |
| HbA1c (%) | 5.9 (5.4–6.9) n=41 | 6.2 (5.6–7.1) n=28 | 5.6 (4.8–6.1) n=13 |
| hsCRP, mg/L | 5.9 (3.0–14.4) n=40 | 6.9 (3.0–14.1) n=28 | 5.9 (2.8–24.6) n=12 |
| spKt/V (Daugirdas) | 1.5 (1.2–1.7) n=59 | 1.5 (1.2–1.7) n=31 | 1.5 (1.2–1.7) n=28 |
| Troponin T, ng/ml | 0.1 (0.0–0.1) n=29 | 0.1 (0.0–0.1) n=22 | 0.1 (0.1–0.1) n=7 |
| BNP, pg/ml | 178 (98–423) n=42 | 136 (77–280) n=29 | 423 (177–567) n=13 |
Values for continuous variables are given as median (25th–75th percentile) or mean±SD. IDH, intradialytic hypotension; PTH, parathyroid hormone; Hb, hemoglobin; hsCRP, high-sensitivity C-reactive protein; spKt/V, single pool Kt/V; BNP, B-type natriuretic peptide.
Baseline refers to the average values over the first 2 weeks of the study.
Associations of IDH20 with Clinically Significant Arrhythmia
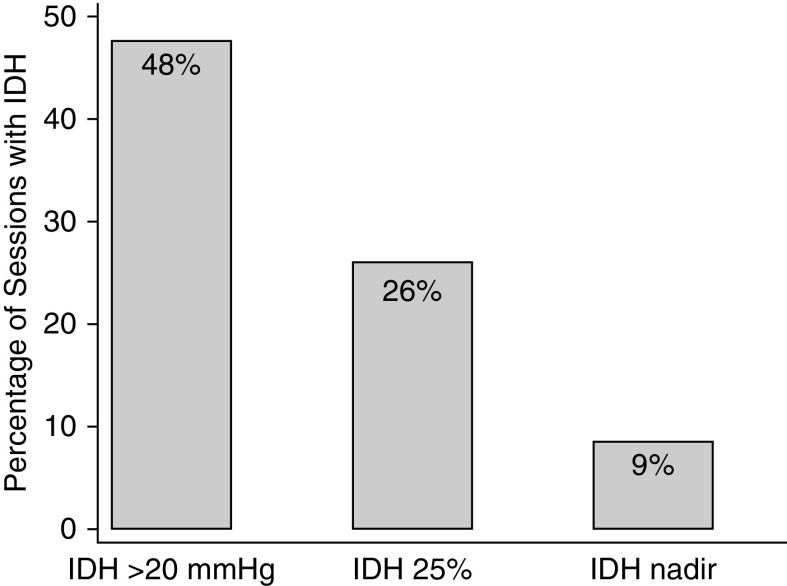
IDH20 occurred during 2251 sessions (48%); IDH0–20 occurred during 1773 sessions (38%); whereas systolic BP was unchanged or increased during the remaining 696 (15%) sessions (Figure 1). The number of sessions complicated by least one intradialytic clinically significant arrhythmia was 27 (1.2%) where IDH20 occurred, 15 (0.8%) where IDH0–20 occurred, and one (0.1%) where intradialytic systolic BP was unchanged or increased. The median time from IDH20 to onset of the clinically significant arrhythmia was 1.2 (25th–75th percentile, 0.9–1.6) minutes.
Figure 1.
Frequency of intradialytic hypotension according to different definitions.
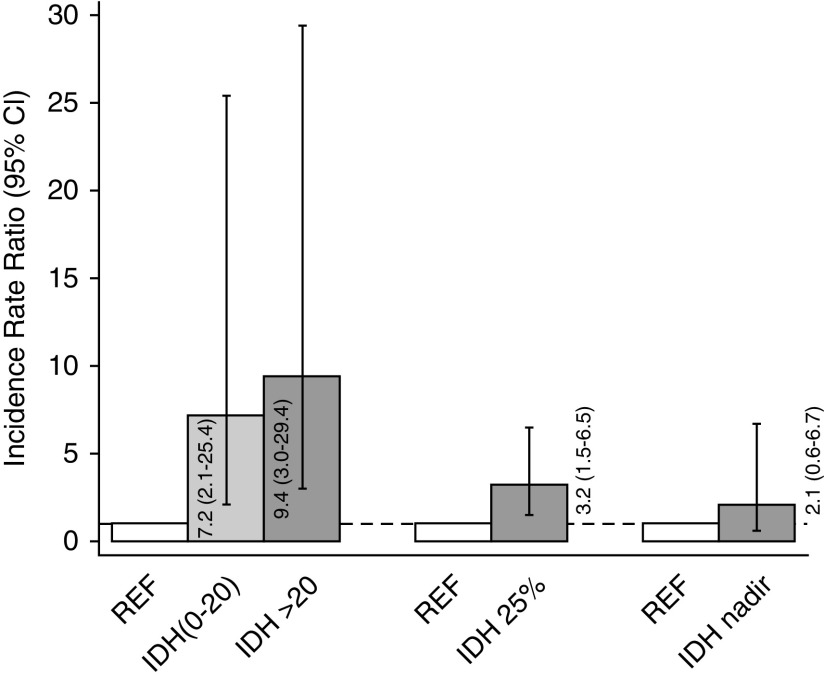
In unadjusted analyses, IDH20 was associated with a 9.4-fold higher rate of subsequent clinically significant arrhythmia (incidence rate ratio [IRR], 9.4; 95% confidence interval [95% CI], 3.0 to 29.4; P<0.001), whereas IDH0–20 was associated with a 7.2-fold higher rate of clinically significant arrhythmia (IRR, 7.2; 95% CI, 2.1 to 25.4; P=0.001), compared with sessions without IDH (Figure 2). In the backward selection model, the effect estimates were marginally attenuated (IRR, 7.6; 95% CI, 3.9 to 14.9; P<0.001 for IDH20; and IRR, 6.2; 95% CI, 2.8 to 13.8; P<0.001 for IDH0–20). In exploratory analyses, upon adjustment for baseline systolic BP, IDH20 was associated with a 9.4-fold higher rate of subsequent clinically significant arrhythmia (IRR, 9.4; 95% CI, 3.0 to 28.9; P<0.001), whereas IDH0–20 was associated with a 7.4-fold higher rate of clinically significant arrhythmia (IRR, 7.4; 95% CI, 2.2 to 25.2; P=0.001), compared with sessions without IDH. Upon adjustment for the pre-HD systolic BP at each session, IDH20 was associated with an 11.0-fold higher rate of subsequent clinically significant arrhythmia (IRR, 11.0; 95% CI, 4.0 to 30.0; P<0.001), whereas IDH0–20 was associated with a 7.3-fold higher rate of clinically significant arrhythmia (IRR, 7.3; 95% CI, 2.3 to 23.7; P=0.004), compared with sessions without IDH.
Figure 2.
Rates of clinically significant arrhythmia according to different definitions of intradialytic hypotension.
In sensitivity analyses, when unadjusted models were fit using logistic regression, similar patterns of association were noted (odds ratio [OR], 4.4; 95% CI, 0.8 to 24.5; P=0.09 for IDH20; and 4.1; 95% CI, 0.9 to 19.3; P=0.08 for IDH0–20). In sensitivity analyses with negative binomial models, when the at-risk time was limited to the period after an IDH event, IDH20 was associated with a 12.5-fold higher rate of subsequent clinically significant arrhythmia (IRR, 12.5; 95% CI, 3.6 to 43.9; P<0.001), whereas IDH0–20 was associated with an 8.5-fold higher rate of clinically significant arrhythmia (IRR, 8.5; 95% CI, 2.3 to 32.3; P<0.001), compared with sessions without IDH.
When analyzed as a continuous variable, there was no significant association of systolic BP decline with clinically significant arrhythmia (data not shown).
Associations of IDH25% with Clinically Significant Arrhythmia
IDH25% occurred in 1238 of 4735 sessions (26%), of which 24 (2%) were complicated by at least one intradialytic clinically significant arrhythmia (Figure 1). Of the remaining 3497 sessions where IDH25% did not occur, 19 (0.5%) were complicated by at least one clinically significant arrhythmia.
In unadjusted analyses there was a 3.2-fold greater rate of subsequent clinically significant arrhythmia (IRR, 3.2; 95% CI, 1.5 to 6.5; P=0.002) in sessions with IDH25% versus those without (Figure 2). In the backward selection model, the effect estimate was accentuated (IRR, 6.3; 95% CI, 1.8 to 21.4; P=0.004). When analogous unadjusted models were fit using logistic regression, similar patterns of association were noted (OR, 3.3; 95% CI, 1.6 to 6.6; P=0.001).
In exploratory analyses, upon adjustment for baseline systolic BP, there was a 3.4-fold greater rate of subsequent clinically significant arrhythmia (IRR, 3.4; 95% CI, 1.7 to 6.8; P<0.001) in sessions with IDH25% versus those without. In analyses adjusted for pre-HD systolic BP at each session there was a 3.4-fold greater rate of subsequent clinically significant arrhythmia (IRR, 3.4; 95% CI, 1.9 to 6.1; P<0.001) in sessions with IDH25% versus those without.
Associations of IDHnadir with Clinically Significant Arrhythmia
IDHnadir occurred in 404 of 4720 sessions (9%; Figure 1). The number of sessions complicated by least one intradialytic clinically significant arrhythmia was 11 (3%) where IDHnadir occurred and 32 (0.7%) where IDHnadir did not occur.
Although effect estimates were qualitatively similar to IDH20 and IDH25%, the unadjusted and adjusted associations of IDHnadir with clinically significant arrhythmia did not meet statistical significance. In unadjusted analyses there was a 2.1-fold higher rate of subsequent clinically significant arrhythmia (IRR, 2.1; 95% CI, 0.6 to 6.7; P=0.22) in sessions with IDHnadir versus those without (Figure 2). In the backward selection model, the effect estimate was accentuated (IRR, 2.6; 95% CI, 0.9 to 7.5; P=0.08). There was no association of IDHnadir with clinically significant arrhythmia in unadjusted logistic regression models (OR, 1.4; 95% CI, 0.3 to 6.0; P=0.62).
In exploratory models, upon adjustment for baseline systolic BP, there was a 2.1-fold higher rate of subsequent clinically significant arrhythmia (IRR, 2.1; 95% CI, 0.5 to 9.0; P=0.73) in sessions with IDHnadir versus those without. In analyses adjusted for pre-HD systolic BP at each session there was a 2.8-fold higher rate of subsequent clinically significant arrhythmia (IRR, 2.8; 95% CI, 0.8 to 10.0; P=0.10) in sessions with IDHnadir versus those without.
Discussion
In this post hoc analysis of the MiD study, which used implantable loop recorders to detect cardiac arrhythmia from individuals on maintenance HD, we report that development of IDH is common and is associated with a higher risk of developing clinically significant arrhythmic events during the remainder of the affected HD session.
Despite major advances in diagnosis and treatment of heart and vascular disease in the non-HD population, cardiovascular diseases remain the leading cause of death for individuals dependent on maintenance HD therapy (7). Interestingly, the rates of cardiovascular death and hospitalization are higher on days during which HD therapy occurs, compared with non-HD days, and appear to peak after the longer 2-day interval (4). This pattern was previously noted to exist for sudden deaths (2,3), which are estimated to account for up to half of all cardiovascular deaths, highlighting a temporal association with HD therapy. The classification of sudden death encompasses a spectrum of potential diagnoses, ranging from malignant arrhythmia, to massive pulmonary emboli, stroke, and others. In a prior autopsy study of sudden deaths in patients on HD, only half of the 35 cases had an identifiable cause, e.g., aortic dissection, cerebral hemorrhage (10). Despite performance of an autopsy, the true contribution from arrhythmia is difficult to determine accurately. It is therefore possible that many of the remainder could have been related to malignant arrhythmic events.
Prior studies have reported the occurrence of several electrocardiographic abnormalities during the HD procedure. These include a higher frequency of QT prolongation, QT dispersion, premature ventricular complexes, and atrial fibrillation. Consistent with prior results, the MiD study found that two-thirds of enrolled patients experienced at least one arrhythmia during follow-up. Interestingly, although atrial fibrillation was the most common overall arrhythmia detected, bradyarrhythmia and asystole were relatively more common than ventricular arrhythmias (7), an observation that has been confirmed in other studies using implantable loop recorders (11,12). Of note, the definition of clinically significant arrhythmia was a specific prespecified end point for the MiD study, designed to capture events that are associated with syncope or cardiac arrest, or to result in symptoms of hypoperfusion (7,8).
In light of the observed temporal association of adverse cardiovascular outcomes with HD sessions, many authorities have focused on identifying pathophysiologic pathways by which HD may predispose to such events. Although prior reports have suggested a role for excessive calcium (13), potassium (14,15), and magnesium (15) flux, the potential for ischemia-driven causes of arrhythmia should not be forgotten, as they appear to be a major etiologic factor for out-of-hospital cardiac arrest in the general population (16). Repeated episodes of IDH are known to be associated with development of myocardial hypo-perfusion and stunning, and with the longer-term risk of mortality (6,9,17); therefore, it is plausible that such IDH events could predispose to ischemia-driven arrhythmias. Depending on the definition, IDH is estimated to affect approximately one-third of outpatient HD sessions (9). In our current analyses of the MiD study, we report that the frequency of IDH ranges from as high as 48% to as low as 9% (using IDH20 and IDHnadir, respectively). We purposefully restricted our analyses to the development of clinically significant arrhythmia after the IDH event occurred, but still within the same HD session, in order to more confidently determine temporal associations. Despite the modest number of clinically significant arrhythmia events (n=43), we found that IDH20 and IDH25% were associated with a higher risk of subsequent clinically significant arrhythmia in both unadjusted and adjusted models. The effect estimates for IDHnadir were qualitatively similar, although they failed to achieve statistical significance. Overall, these results suggest that IDH is temporally associated with development of clinically significant arrhythmia, and may be an important intermediate on the causal pathway linking intermittent HD with arrhythmic death. Although the window we examined most directly reflects short-term temporal associations between acute hypotension and near-term events likely to be directly caused by hypoperfusion-related myocardial ischemia or changes in autonomic tone, longer-term adverse consequences with microinfarction leading to scar and long-term disruption of conduction pathways and increase in the chronic risk of arrhythmia are also plausible. Indeed, a prior study reported an association of development of regional wall motion abnormalities with a higher frequency of premature ventricular complexes during HD (18). Additional investigations examining the burden of IDH events during a baseline period with the long-term burden of arrhythmia are warranted. Alternatively, changes in sympathetic and parasympathetic tone or other neurocardiogenic reflexes in response to IDH may play a role. Although the overall absolute risk of IDH-associated clinically significant arrhythmia was modest in this study, further investigation in larger groups of patients (especially IDH-prone) with a higher burden of cardiovascular risk factors is warranted. If confirmed, then IDH may be a potentially modifiable risk factor to reduce future adverse arrhythmic events.
The major strengths of our study include the utilization of detailed loop recorder data from the MiD study to continuously detect arrhythmia over a 6-month monitoring period, the prospective collection of demographic and dialysis-related data, the use of a prespecified definition of clinically significant arrhythmia, and detailed data on the timing of arrhythmia events within each dialysis session. This uniquely allowed our analyses to focus on the specific intradialytic period, ensuring that the IDH exposure came before the clinically significant arrhythmia event. However, several limitations should be noted. Despite the large number of HD sessions analyzed, the population was relatively young, all patients were on thrice-weekly HD, granular recording of the frequency and timing of patient symptoms was not available, and there were a limited number of clinically significant arrhythmia events that occurred after an IDH episode. In addition, a common BP device was not mandated for all sites, and neither was protocol-based lowering of the dialysate temperature, which is known to reduce the frequency of IDH (19). These issues limited our power and ability to perform multivariable adjusted analyses, as well as the generalizability of our findings. As already discussed, this trade-off was necessary to ascertain temporal associations, and certainly obligates further larger studies with a more diverse sample to confirm our results. In addition, the median time on dialysis before study entry exceeded 1 year. Risk of sudden death is highest during the first few months of HD, and it is possible that associations between IDH and clinically significant arrhythmia could be even stronger during the first few months of dialysis when hemodynamic instability and risk of sudden death are highest. Unfortunately, electrocardiographic evidence of ischemia was not available for these analyses. Finally, there is no clear consensus on the definition of IDH. We attempted to address this by examining several definitions, including IDH25%, which reflects the BP data contributed by MiD study participants. It is reassuring that the associations were consistent across three distinct definitions utilized, although we accept that some misclassification of physiologically important episodes of hypo-perfusion remains possible. Nevertheless, we were unable to adequately discriminate between symptomatic versus asymptomatic episodes of hypotension, and are cognizant that the frequency and strength of associations with IDH events may be different with different IDH definitions or study populations, and that they may vary by geographic location and practice patterns.
In summary, using detailed data from the MiD study, we report an association of IDH with development of clinically significant arrhythmia during the remainder of the HD session. Confirmation of these findings and exploration of electrolyte shifts and the role of myocardial ischemia and autonomic function in larger studies are necessary. However, there is cause for optimism, because IDH represents a potentially modifiable risk factor which, if minimized, could reduce the risk of malignant arrhythmia in patients on HD.
Disclosures
All of the authors, with the exception of Dr. Mc Causland, received significant research support and/or consulting fees from Medtronic in relationship to the design and conduct of the study. Dr. Charytan noted the following disclosures: expert witness fees related to dialysate composition, Fresenius (significant); research support, Medtronic; research support and consulting fees related to services as national investigator, trial steering committee, or data monitoring committee, Allena Pharmaceuticals, Astra Zeneca (modest), Gilead Pharmaceuticals, Janssen Pharmaceuticals, Novo Nordisk, and Zoll Medical; consulting fees and travel support, Amgen, Daichi Sankyo, Fresenius (modest), Medtronic/Covidien, and Merck. Dr. Costea is on the speaker’s bureau for Biotronik and Biosense Webster. Dr. Kher reported the following disclosures: research funding, Astellas India, Novartis India, and Sanofi Aventis India; honoraria, Astellas India, Novartis India, Reddy’s India, Roche India, and Torrent India; scientific advisor, Biocon India, Medtronis, Novartis India, Reddy’s India, Roche India, Sanofi Aventis, and Torrent; speaker’s bureau, Biocon India, Intas India, Medtronic, Novartis India, Panacea India, Pfizer, Roche India, and Sanofi Aventis India. Dr. Roy-Chaudhury disclosed the following: consultant or advisory board for, Akebia, Bayer, BD-Bard, Cormedix, Humacyte, Medtronic, Vifor-Relypsa, and WL Gore. Dr. Williamson is the Chief Operating Officer for American Renal Associates. Dr. Koplan, Dr. Mc Causland, Dr. Pokhariyal, and Dr. Tumlin have nothing additional to disclose.
Funding
The Monitoring in Dialysis study was funded by Medtronic and designed by Medtronic in collaboration with an advisory committee that included the authors. Medtronic was responsible for design of the study database, the data collection instruments, and funding the statistical analysis. The authors were responsible for data interpretation and writing the manuscript. Dr. Mc Causland was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK102511.
Supplementary Material
Acknowledgments
The authors would like to thank Ven Manda, John Burnes, and Amy Roettger from Medtronic for support and collaboration on Monitoring in Dialysis and Candace McClure from NAMSA for statistical support.
Footnotes
*MiD Investigators and Committees: Nephrology Investigators—Don Williamson, MD (Southeastern Clinical Research Institute, Augusta, GA), Prabir Roy-Chaudhury, MD, (University of Cincinnati Medical Center Cincinnati, OH; University of Arizona Tuscon, AZ), James Tumlin, MD (NephroNet Clinical Research Institute, Atlanta, GA), Vijay Kher, MD (Medanta - The Medicity- Kidney & Urology Institute, Gurgaon, India), Vikranth Reddy, MD (CARE Hospital Hyderabad, India), Kowdle Chandrasekhar Prakash, MD, (Apollo Hospitals–Chennai, India), David Charytan, MD MSc (Brigham & Women’s Hospital, Boston, MA), Suresh Chandra Tiwari, MD (Fortis Vasant Kunj Hospital Delhi, India), Saurabh Pokhariyal, MD (Fortis Memorial Research Institute Gurgaon, India), Amber Podoll, MD (University of Texas-Houston, Houston, TX), Sanjeev Jasuja, MD (Apollo Hospitals–Delhi, Delhi, India). Cardiology Investigators—G. Leslie Walters, MD (Augusta Cardiology Clinic, Augusta, GA), Kraig Wangsnes, MD (Cardiovascular Associates, Augusta, GA), Alexandru Costea, MD (University of Cincinnati Medical Center, Cincinnati, OH), Selcuk Tombul, MD (Diagnostic Cardiology Group, Chattanooga, TN), Balbir Singh, MD (Medanta - The Medicity-Heart Institute, Gurgaon, India), Brajesh Mishra, MD (Medanta-The Medicity-Heart Institute, Gurgaon, India), Sachin Yalagudri, MD (CARE Hospital, Hyderabad, India), Abhijeet Shelke, MD (CARE Hospital Hyderabad, India), Calambur Narasimhan, MD (CARE Hospital, Hyderabad, India), A.M. Karthigesan, MD (Apollo Hospitals–Chennai, Chennai, India), Abraham Oomman, MD (Apollo Hospitals–Chennai, Chennai, India), K P Pramod Kumar, MD (Apollo Hospitals–Chennai, Chennai, India), Bruce Koplan, MD (Brigham & Women’s Hospital, Boston, MA), Upendra Kaul, MD (Fortis Vasant Kunj Hospital, Delhi, India), Tapan Ghose, MD (Fortis Vasant Kunj Hospital, Delhi, India), Ripen Gupta, MD (Fortis Vasant Kunj Hospital, Delhi, India), Arvind Sethi, MD (Fortis Escorts Hospital, Delhi, India), Nikhil Kumar, MD (Fortis Memorial Research Institute, Gurgaon, India), Ramesh Hariharan, MD, (University of Texas-Houston, Houston, TX), Rajnish Sardana, MD (Apollo Hospitals–Delhi, Delhi, India), Arif Wahab, MD (Apollo Hospitals–Delhi, Delhi, India) N.N Khanna, MD (Apollo Hospitals–Delhi, Delhi, India). Nephrology Co-Investigators—Mark Smith, MD (Southeastern Clinical Research Institute, Augusta, GA), Suresh Kamath, MD (University of Cincinnati Medical Center, Cincinnati, OH), Claude Galphin, MD (South East Renal Research Institution (SERRI), Chattanooga, TN), Puneet Sodhi, MD (Medanta-The Medicity-Heart Institute, Gurgaon, India), Rajsekara Chakravarthy, MD (CARE Hospital, Hyderabad, India), Subba Rao Budithi, MD (Apollo Hospitals–Chennai, Chennai, India), Finnian McCausland, MB, MMSc (Brigham & Women’s Hospital, Boston, MA), Sanjeev Gulati, MD (Fortis Vasant Kunj Hospital, Delhi, India), Munawer Dijoo, MD (Fortis Vasant Kunj Hospital, Delhi, India), Upendra Singh, MD (Fortis Escorts Hospital, Delhi, India), Salil Jain, MD (Fortis Memorial Research Institute, Gurgaon, India), Vishal Saxena, MD (Fortis Memorial Research Institute, Gurgaon, India), Gaurav Sagar, MD (Apollo Hospitals – Delhi, Delhi, India). Advisory Committee—David Charytan, MD, MSc, (Brigham & Women’s Hospital, Boston, MA), Rachel Fissell, MD (Vanderbilt University, Nashville, TN), Robert Foley, MD (Hennepin County Medical Center, Minneapolis, MN), Charles A. Herzog, MD (Hennepin County Medical Center, University of Minnesota, Minneapolis, MN), Peter McCullough, MD (Baylor University Medical Center, Baylor Heart and Vascular Institute, Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas), John D. Rogers, MD (Scripps Clinic-Torrey Pines, La Jolla, CA), James A. Tumlin, MD (South East Renal Research Institution (SERRI), Chattanooga, TN), Peter Zimetbaum, MD (Beth Israel Deaconess Medical Center, Boston, MA). Adverse Events Committee—Manish Assar, MD (Baylor University Medical Center, Dallas, TX), Mark Kremers, MD (Mid Carolina Cardiology Charlotte, North Carolina), Wolfgang C. Winkelmayer, MD ScD (Baylor College of Medicine, Houston, TX).
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06810619/-/DCSupplemental.
Supplemental Appendix. MiD Investigators and Committees.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Albertus P, Ayanian J, Balkrishnan R, Bragg-Gresham J, Cao J, Chen JLT, Cope E, Dharmarajan S, Dietrich X, Eckard A, Eggers PW, Gaber C, Gillen D, Gipson D, Gu H, Hailpern SM, Hall YN, Han Y, He K, Hebert P, Helmuth M, Herman W, Heung M, Hutton D, Jacobsen SJ, Ji N, Jin Y, Kalantar-Zadeh K, Kapke A, Katz R, Kovesdy CP, Kurtz V, Lavalee D, Li Y, Lu Y, McCullough K, Molnar MZ, Montez-Rath M, Morgenstern H, Mu Q, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Pearson J, Pisoni R, Plattner B, Port FK, Potukuchi P, Rao P, Ratkowiak K, Ravel V, Ray D, Rhee CM, Schaubel DE, Selewski DT, Shaw S, Shi J, Shieu M, Sim JJ, Song P, Soohoo M, Steffick D, Streja E, Tamura MK, Tentori F, Tilea A, Tong L, Turf M, Wang D, Wang M, Woodside K, Wyncott A, Xin X, Zang W, Zepel L, Zhang S, Zho H, Hirth RA, Shahinian V: US Renal Data System 2016 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 69: A7–A8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G: Characteristics of sudden death in hemodialysis patients. Kidney Int 69: 2268–2273, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bleyer AJ, Russell GB, Satko SG: Sudden and cardiac death rates in hemodialysis patients. Kidney Int 55: 1553–1559, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Gilbertson DT, Murray T, Collins AJ: Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 365: 1099–1107, 2011 [DOI] [PubMed] [Google Scholar]
- 5.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CSR, Camici PG: Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 4: 1925–1931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy-Chaudhury P, Tumlin JA, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM; MiD investigators and committees: Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int 93: 941–951, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Charytan DM, Foley R, McCullough PA, Rogers JD, Zimetbaum P, Herzog CA, Tumlin JA; MiD Investigators and Committees: Arrhythmia and sudden death in hemodialysis patients: Protocol and baseline characteristics of the monitoring in dialysis study. Clin J Am Soc Nephrol 11: 721–734, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM: Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 26: 724–734, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeda K, Harada A, Okuda S, Fujimi S, Oh Y, Hattori F, Motomura K, Hirakata H, Fujishima M: Sudden death in chronic dialysis patients. Nephrol Dial Transplant 12: 952–955, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Roberts PR, Zachariah D, Morgan JM, Yue AM, Greenwood EF, Phillips PC, Kalra PA, Green D, Lewis RJ, Kalra PR: Monitoring of arrhythmia and sudden death in a hemodialysis population: The CRASH-ILR Study. PLoS One 12: e0188713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacher F, Jesel L, Borni-Duval C, De Precigout V, Lavainne F, Bourdenx J-P, Haddj-Elmrabet A, Seigneuric B, Keller A, Ott J, Savel H, Delmas Y, Bazin-Kara D, Klotz N, Ploux S, Buffler S, Ritter P, Rondeau V, Bordachar P, Martin C, Deplagne A, Reuter S, Haissaguerre M, Gourraud J-B, Vigneau C, Mabo P, Maury P, Hannedouche T, Benard A, Combe C: Cardiac rhythm disturbances in hemodialysis patients: Early detection using an implantable loop recorder and correlation with biological and dialysis parameters. JACC Clin Electrophysiol 4: 397–408, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Pun PH, Horton JR, Middleton JP: Dialysate calcium concentration and the risk of sudden cardiac arrest in hemodialysis patients. Clin J Am Soc Nephrol 8: 797–803, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redaelli B, Locatelli F, Limido D, Andrulli S, Signorini MG, Sforzini S, Bonoldi L, Vincenti A, Cerutti S, Orlandini G: Effect of a new model of hemodialysis potassium removal on the control of ventricular arrhythmias. Kidney Int 50: 609–617, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Pun PH, Middleton JP: Dialysate potassium, dialysate magnesium, and hemodialysis risk. J Am Soc Nephrol 28:3441–3451, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spaulding CM, Joly LM, Rosenberg A, Monchi M, Weber SN, Dhainaut JF, Carli P: Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 336: 1629–1633, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Dasselaar JJ, Slart RHJA, Knip M, Pruim J, Tio RA, McIntyre CW, de Jong PE, Franssen CFM: Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant 24: 604–610, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Burton JO, Korsheed S, Grundy BJ, McIntyre CW: Hemodialysis-induced left ventricular dysfunction is associated with an increase in ventricular arrhythmias. Ren Fail 30: 701–709, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Selby NM, McIntyre CW: A systematic review of the clinical effects of reducing dialysate fluid temperature. Nephrol Dial Transplant 21: 1883–1898, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.