Abstract
Rationale:
The availability and abuse of synthetic analogues of cathinone have increased dramatically around the world. Synthetic cathinones, such as 3,4-methylenedioxypyrovalerone [MDPV] and α-pyrrolidinopentiophenone [α-PVP], are cocaine-like inhibitors of monoamine transporters and common constituents of “bath salts” or “flakka” preparations. Studies in rats suggest that MDPV and α-PVP are 3 to 4-fold more effective reinforcers than cocaine; however, comparisons of the relative reinforcing effectiveness of MDPV and α-PVP have not been reported in other species.
Objectives:
Accordingly, in the present study, 4 adult male rhesus monkeys responding under a progressive ratio schedule of reinforcement were used to characterize the reinforcing effects of MDPV and α-PVP and to compare directly these effects to those of cocaine and methamphetamine.
Results:
MDPV was the most potent reinforcer, followed by α-PVP, methamphetamine, and cocaine. α-PVP was the most effective reinforcer, followed by MDPV, cocaine, and methamphetamine. In addition to making more responses to obtain MDPV and α-PVP, monkeys also responded for longer periods of time when MDPV or α-PVP were available compared to when either cocaine or methamphetamine were available for infusion.
Conclusions:
These studies confirm recent reports from rodents, and provide strong evidence that the synthetic cathinones MDPV and α-PVP are capable of maintaining high levels of responding for prolonged periods of time, and that they function as more effective reinforcers than either cocaine or methamphetamine. The relative strength of these reinforcing effects may account for the high rates of “bath salts” use reported in humans.
Keywords: synthetic cathinones, MDPV, α-PVP, cocaine, methamphetamine, rhesus monkey, self-administration
INTRODUCTION
Worldwide estimates suggest that the abuse of stimulant drugs (e.g., cocaine, amphetamine-type stimulants, “ecstasy”) is second only to cannabinoids (UNODC, 2018). Although cocaine and methamphetamine remain the most widely used stimulant drugs, synthetic cathinones, often referred to as “bath salts” or “flakka”, represent a growing, and ever evolving threat to public health. Indeed, recent estimates suggest that high school age students in the United States are more likely to use synthetic cathinones than either heroin or methamphetamine (Johnston et al. 2019; Palamar et al. 2019). The abuse of synthetic cathinones is associated with a variety of adverse effects, including abuse, excited delirium, acute psychosis, aggressive/violent behavior, cardiovascular complications, and death (Benzie et al. 2011; Spiller et al. 2011; Prosser et al. 2012; Miotto et al. 2013; Johnson and Johnson, 2014). Since their emergence in 2009, the number of synthetic cathinones identified on the world illicit drug market has increased steadily, with a total of 148 unique cathinone derivatives identified in 2017 (UNODC, 2018), 14 of which have been placed under Schedule I regulations by the United States Drug Enforcement Administration.
Similar to other stimulant drugs, synthetic cathinones exert their abuse-related and toxic effects through interactions with monoaminergic (e.g., dopamine [DAT], norepinephrine [NET], and serotonin [SERT]) transporters, where they can function as cocaine-like uptake inhibitors (e.g., 3,4-methylenedioxypyrovalerone [MDPV], or α-pyrrolidinopentiophenone [α-PVP]), or amphetamine-like substrates (e.g., 3,4-methylenedioxy-N-methylcathinone [methylone], or 4-methylmethcathinone [mephedrone]) to increase extracellular levels of dopamine, norepinephrine, and serotonin (Baumann et al. 2013; Eshleman et al. 2013, 2017; Simmler et al. 2013; Gannon et al. 2018a). Despite similarities in their mechanism of action, human users report that synthetic cathinones are powerful stimulants, with subjective effects that are similar to or greater than those produced by drugs such as cocaine and methamphetamine (Winstock et al. 2011; Carhart-Harris et al. 2011; Johnson & Johnson, 2014). It is important to note, however, that the chemical constituents of “bath salts” and related preparations varies across “brands”, and within “brand” across time (e.g., Brandt et al. 2010; Spiller et al. 2011; Schneir et al. 2014), making it difficult for users to accurately predict which synthetic cathinone(s) they are using at any given time. Although this shifting composition likely contributes to variability in the euphoric and subjective effects reported in humans (e.g., Winstock et al. 2011; Johnson & Johnson, 2014), mounting evidence from preclinical studies suggests that the abuse-related effects (e.g., locomotor stimulatory, discriminative stimulus, and reinforcing effects) of synthetic cathinones exist on a continuum.
For instance, early studies in rodents indicated that MDPV was more effective than methamphetamine (0.05 or 0.25 mg/kg/inf) at maintaining responding under a progressive ratio (PR) schedule of reinforcement in rats (Aarde et al. 2013; Watterson et al. 2014), whereas behavioral economic studies suggest that cathinones such as methylone and 4-methyl-N-ethylcathinone (4-MEC) are less effective than cocaine or methamphetamine (Huskinson et al. 2017; Gannon et al. 2019). These findings have been confirmed and extended to clearly show that MDPV and α-PVP maintain final ratios approximately 3- to 5-fold larger, whereas methylone maintains final ratios approximately 4-fold smaller than those maintained by either cocaine or methamphetamine (Gannon et al. 2017a; 2018a; 2018b). Consistent with previous reports linking the reinforcing effects of cocaine to its potency to inhibit uptake at DAT (Ritz et al. 1987; Bergman et al. 1989), the potency of a series of structurally-related, pyrrolidine-containing cathinones (e.g., MDPV, MDPBP, α-PVP, α-PPP) to function as reinforcers is similarly correlated with their potency to inhibit uptake at DAT (Gannon et al. 2018a; 2018c). Although these findings suggest that the reinforcing effects of MDPV and related cathinones are primarily mediated by their capacity to increase dopamine signaling, their reinforcing effects appear to be negatively modulated by actions at SERT (Aarde et al. 2013; 2015; Dolan et al. 2018; Gannon et al. 2017a; 2017b; 2018a; 2018c; 2018d; Huskinson et al. 2017; Motbey et al. 2013; Watterson et al. 2014). Indeed, a recent study in rats found that the relative reinforcing effectiveness of a series of synthetic cathinones was positively correlated with their selectivity to inhibit uptake at DAT relative to SERT (Gannon et al. 2018a), strongly suggesting that the abuse potential of stimulant drugs (e.g., cocaine, methamphetamine, and synthetic cathinones) is determined by their capacity to stimulate dopamine systems, with increases in serotonergic activity serving to negatively modulate, or dampen their reinforcing effectiveness. Although a handful of studies have demonstrated that cathinone functions as a reinforcer in rhesus monkey (Schuster & Johanson, 1979; Yanagita, 1979; Johanson & Schuster, 1981; Woolverton & Johansen, 1984; Yanagita, 1986), relatively little is known about the abuse-related effects of synthetic cathinones. For instance, although a pair of studies (Smith et al. 2017a; Smith et al. 2017b) recently demonstrated that 5 common synthetic cathinones (MDPV, α-PVP, methcathinone, methylone, and mephedrone) have cocaine-like discriminative stimulus effects, as has been reported in rats (Gatch et al. 2013; 2015; Collins et al. 2016; Gannon and Fantegrossi, 2016), it is currently unclear if the differences in reinforcing effectiveness observed in rats (e.g., α-PVP > MDPV > cocaine = methamphetamine), also translate to non-human primates. Accordingly, the present studies used a PR schedule of reinforcement to compare directly the reinforcing effects (potency and effectiveness) of two common synthetic cathinones (MDPV and α-PVP) to two widely abused stimulant drugs (cocaine and methamphetamine) in four adult male rhesus monkeys.
METHODS
Subjects.
Four adult male rhesus monkeys (7.8-11.7 kg) participated in these studies. All monkeys were individually housed in an environmentally controlled vivarium under a 14 h/10 h light/dark cycle with continuous access to water. Monkeys were fed primate chow (Harlan Teklad, High Protein Monkey Diet, Madison, WI), fresh fruit, and peanuts daily in the morning, approximately 4 h before the start of their daily experimental session. Although experimental histories differed among these monkeys, all four had a history of cocaine self-administration prior to initiating these studies. All monkeys were maintained, and all experiments were performed, in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the Guide for the Care and Use of Laboratory Animals (National Research Council 2011).
Surgical Preparation & Apparatus
Monkeys were initially anesthetized with 10 mg/kg of ketamine (SC; Henry Schein, Dublin, OH) prior to being intubated, and maintained on 2 l/min oxygen and 2% isoflurane anesthesia (Butler Animal Health Supply, Grand Prairie, TX, USA) to allow for an indwelling venous catheter to be placed. Catheters exited the monkeys in the mid-scapular region, and monkeys were fit with a mesh primate jacket (Lomir Biomedical Inc., Malone, NY, USA) connected to a stainless steel tether through which the catheter was passed and connected to an 18-ga fluid swivel (Lomir Biomedical Inc., Malone, NY, USA). The swivel was secured to the back wall of the cage to allow free movement within the home cage. An instrument panel was located on one side of the cage which contained two or three depressible levers (Model 121-07, BRS-LVE, Laurel, Maryland, USA), separated by stainless steel dividers to reduce the likelihood of pressing multiple levers with the same hand. A stimulus light that could be illuminated red or green was located above each lever. Silicone tubing connected the swivel to an infusion pump (PHM-100; Med-Associates, Georgia, Vermont, USA) that was located behind the cage. A computer running Med-PC IV software (Med-Associates, Georgia, Vermont, USA) that was located behind the cage controlled experimental events.
Self-Administration.
Similar to previous studies performed in chaired monkeys (Gerak et al. 2016; Collins & France, 2018), the current study allowed monkeys to self-administer drug under a PR schedule of reinforcement in which the initial ratio was set to 32, and the response requirement incremented with each infusion according to the equation: ratio=[5e^(infusion number+9)*0.2)]-5). This resulted in the following series of ratio values: 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, 737, 901, etc. A catheter loading infusion was delivered approximately 1 h before the start of the session in order to fill the catheter with the appropriate concentration of the drug that would be available for responding. The start of the session was signaled by the illumination of the green light above the active lever (counterbalanced across monkeys) which also served as the discriminative stimulus that drug infusions were available for responding on that lever. Drug infusions were delivered in conjunction with a 5-sec presentation of the red light above the active lever (i.e., infusion-associated stimuli), and followed by a 30-sec timeout during which the discriminative and infusion-associated stimuli were extinguished and responses were recorded but had no scheduled consequence. Illumination of the green light above the active lever signaled the end of the timeout, and indicated that drug was available for responding. Responses on the inactive lever were recorded but had no scheduled consequence. The maximum session duration was 20 h; however, sessions were terminated if a ratio was not completed within 2 h (i.e., 2 h limited hold). The order of testing was cocaine (0.0032-0.32 mg/kg/inf), methamphetamine (0.0032-0.1 mg/kg/inf), MDPV (0.001-0.01 mg/kg/inf), and α-PVP (0.001-0.01 mg/kg/inf). For each drug, the doses were evaluated in random order, with each dose available until the stability criterion was met (number of infusions differed by no more than 2 from day to day). Full dose-response curves for each drug were determined in duplicate before moving on the next drug. Saline served as a negative control, and was occasionally substituted for drug on at least 4 occasions to ensure that responding was being maintained by drug infusions. In order to determine if sensitivity to the reinforcing effects of cocaine was affected by the sequence of testing, the cocaine dose-response curve was determined a third time after all other dosing conditions had been evaluated.
Drugs.
(−)-Cocaine HCl was provided by the National Institute of Drug Abuse Drug Supply Program, and d-methamphetamine HCl was purchased from Sigma-Aldrich (St. Louis, MO). (+/−)-MDPV HCl, and (+/−)-α-PVP HCl were synthesized by Agnieszka Sulima and Kenner Rice at the Drug Design and Synthesis Section of the Molecular Targets and Medications Branch on the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism (Bethesda, MD). All drugs were dissolved in physiologic saline, with doses expressed as the salt in mg per kg body weight.
Statistical Analyses.
At the group level, the mean number of infusions (± 1 SEM), mean final ratio, and mean session duration in minutes (± 1 SEM) are shown for each drug; variance around the mean final ratio is not reported. At the individual subject level, the mean number of infusions and final ratio completed are shown for the two determinations for each drug. Normalized PR dose-response curves were used to obtain estimates of reinforcing potency [dose estimated to produce a 50% (ED50) of maximal responding for a given drug] and effectiveness [maximal number of infusions (Emax), regardless of dose] for individual subjects. Briefly, dose-response curves for each drug were normalized to the dose of that drug that maintained the greatest number of infusions (Emax), and saline (i.e., Emax = 100%, and infusions of saline = 0%), with ED50 values obtained by linear regression of the portion of the dose-response curve spanning the 20%–80% effective levels (i.e., inclusive of no more than one dose above 80% and no more than one dose below 20%). Mean ED50 (95% confidence intervals) provide an estimate of the reinforcing potency for a given drug, whereas mean Emax values (± 1 SEM) provide a dose-independent estimate of the reinforcing effectiveness for a given drug. One-way, repeated measures ANOVA followed by post-hoc Tukey’s test for multiple comparisons to determine if Emax values or maximum session durations varied significantly among the drugs, whereas differences in ED50 values were considered statistically significant if the confidence intervals did not overlap. Prism 7.04 software (GraphPad Software, Inc., La Jolla, CA) was used to generate figures and conduct statistical analyses.
RESULTS
Depicted in figure 1 are dose-response curves for the mean (± 1 SEM) data for the total number of infusions earned (figure 1; top panel) and corresponding session duration (figure 1; bottom panel) for 4 monkeys responding under a PR schedule of reinforcement for cocaine, methamphetamine, MDPV, or α-PVP. Estimates for relative reinforcing potency (ED50), and dose-independent measures of relative reinforcing effectiveness (Emax infusions, and Emax final ratio), as well as the maximum session duration for each of the drugs are reported in Table 1. The rank order for potency was MDPV = α-PVP > methamphetamine > cocaine, with MDPV and α-PVP being ~4- to 5-fold more potent than cocaine, and methamphetamine being ~2.5-fold more potent than cocaine. A repeated measures, one-way ANOVA of the maximal number of infusions earned revealed a significant main effect of drug (F[3,9] = 20.4; p<0.001), with post-hoc Tukey’s tests indicating that cocaine, MDPV, and α-PVP each maintained more infusions than methamphetamine, whereas α-PVP, but not MDPV, maintained significantly more infusions than cocaine at the group level. A similar relationship was observed with respect to the maximal final ratio completed, with a significant main effect of drug (F[3,9] = 10.2; p<0.01), and post-hoc tests indicating that the final ratios completed were each larger for MDPV, and α-PVP compared with methamphetamine, with the final ratio completed for α-PVP, but not MDPV, being significantly greater than for cocaine. Maximal session duration also varied as a function of the drug that was available for infusion (F[3,9] = 6.1; p<0.05), with MDPV maintaining responding for significantly longer periods of time than either cocaine or methamphetamine.
Figure 1.
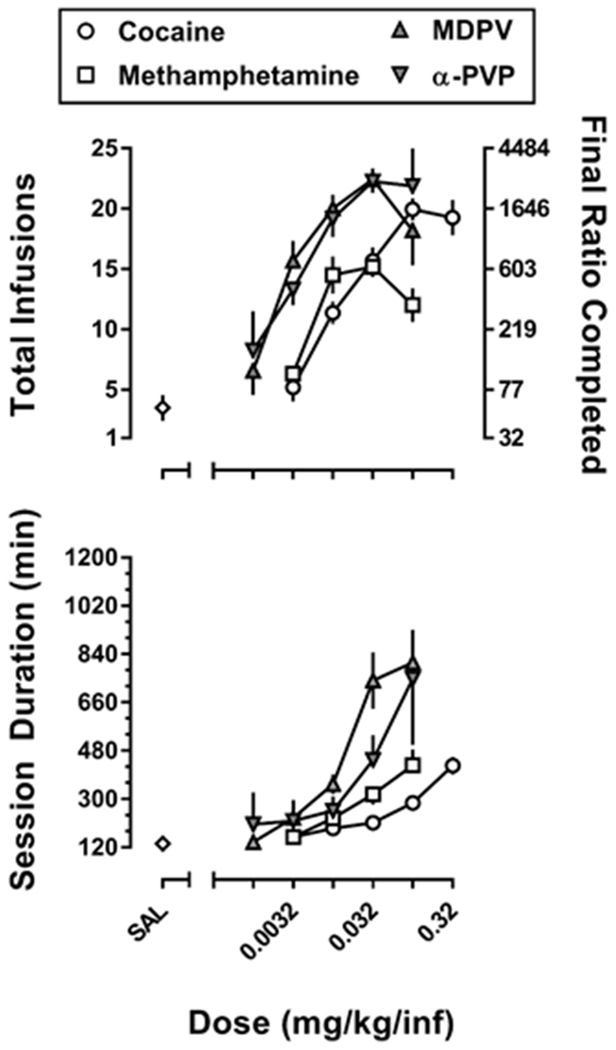
Dose-response curves for the self-administration of cocaine, methamphetamine, MDPV, and α-PVP in rhesus monkeys (n=4) responding under a progressive ratio (PR) schedule of reinforcement. Top Panel: Data represent the mean (± 1 SEM) for the total number of infusions earned for cocaine (0.0032-0.32 mg/kg/inf), methamphetamine (0.0032-0.1 mg/kg/inf), MDPV (0.001-0.1 mg/kg/inf) and α-PVP (0.001-0.1 mg/kg/inf; left ordinate), as well as the corresponding final ratio completed (right ordinate). Bottom Panel: Data represent the mean (± 1 SEM) for the session duration in minutes for each dose of each drug.
Table 1.
Relative reinforcing effects of cocaine, methamphetamine, MDPV, and α-PVP in rhesus monkeys.
| ED50 mg/kg/inf (95% CI) | Emax | Max Duration min (SEM) | ||
|---|---|---|---|---|
| Infusions (SEM) | Final Ratio (SEM) | |||
| cocainea | 0.012 (0.01-0.014) | 20.1 (1.0)# | 1936 (270) | 436 (28) |
| cocaineb | 0.011 (0.007-0.017) | 20.7 (0.6)# | 2133 (229) | 483 (40) |
| methamphetamine | 0.0045 (0.0033-0.0062) | 15.9 (1.1) | 913 (168) | 447 (39) |
| MDPV | 0.0025 (0.0016-0.0040) | 22.8 (1.0)# | 3133 (668)# | 841 (131)*,# |
| α-PVP | 0.0029 (0.0016-0.0052) | 24.3 (1.1)*,# | 4260 (917)*,# | 755# (121) |
initial evaluation of cocaine;
terminal evaluation of cocaine
significant difference from cocaine as determined by one-way ANOVA with post-hoc Tukey’s tests;
significant difference from methamphetamine as determined by one-way ANOVA with post-hoc Tukey’s tests
Dose-response curves for individual subject data are shown in Figure 2. Consistent with the group level data, MDPV and α-PVP tended to maintain more responding than cocaine; however, for one subject (M4), cocaine, MDPV, and α-PVP each maintained comparable levels of responding, suggesting that for this monkey they were equally effective reinforcers. It is also worth noting that even though dose-response curves for most drugs either reached asymptotes (difference in total number of infusions earned differed by less than 2 from one dose to the next), or began to display an inverted U shape (i.e., decreased responding with increasing dose), for two subjects (M2 and M3), it was not possible to accurately estimate the maximal effect level for α-PVP. Thus, rather than risk the safety of the monkeys by evaluating larger doses, infusion data from the largest dose were used to estimate the Emax for α-PVP. Maximal effect levels for methamphetamine, MDPV, and α-PVP were also normalized to the maximal effect of cocaine for individual subjects (1-4), and expressed change scores (Figure 3).
Figure 2.
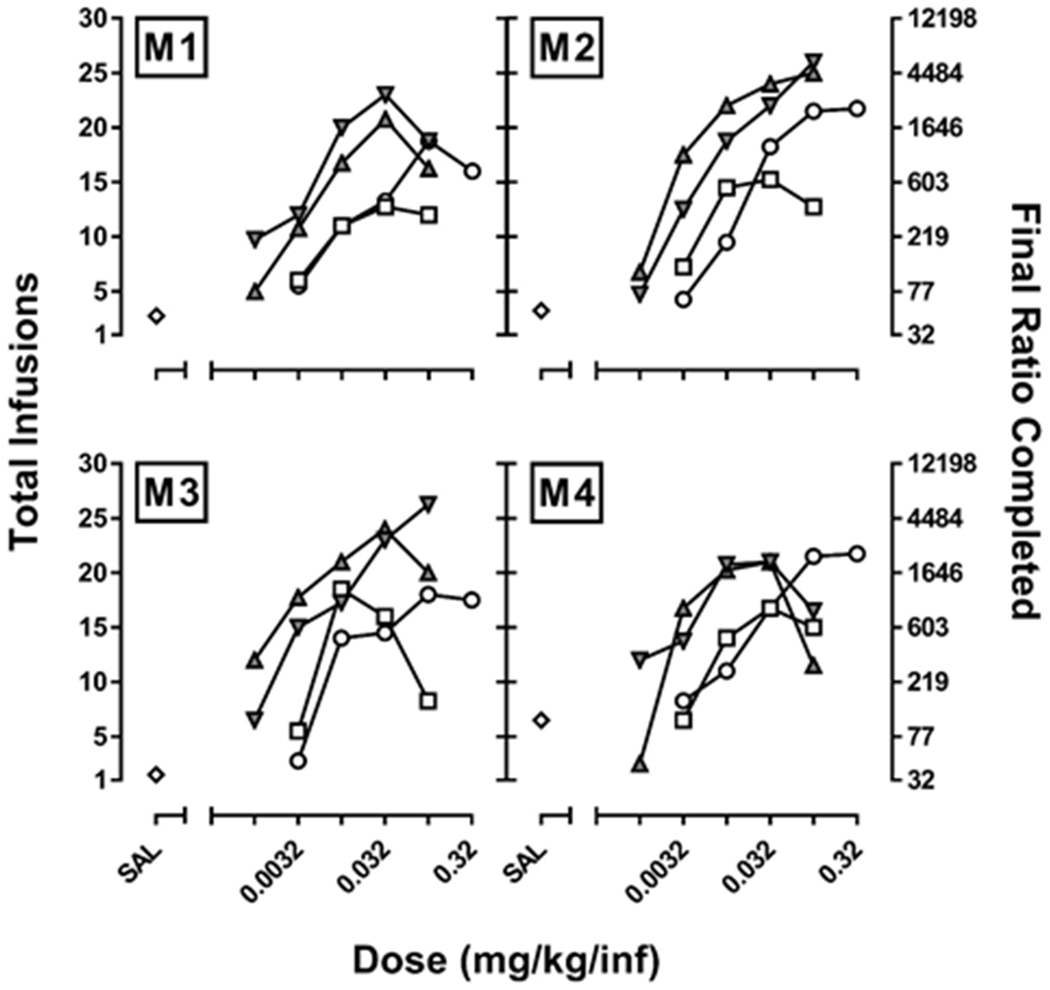
Dose-response curves for the self-administration of cocaine, methamphetamine, MDPV, and α-PVP in each of 4 rhesus monkeys (M1-M4) responding under a progressive ratio (PR) schedule of reinforcement. Data represent the mean (± 1 SEM) for the total number of infusions earned for cocaine (0.0032-0.32 mg/kg/inf), methamphetamine (0.0032-0.1 mg/kg/inf), MDPV (0.001-0.1 mg/kg/inf) and α-PVP (0.001-0.1 mg/kg/inf; left ordinate), as well as the corresponding final ratio completed (right ordinate).
Figure 3.
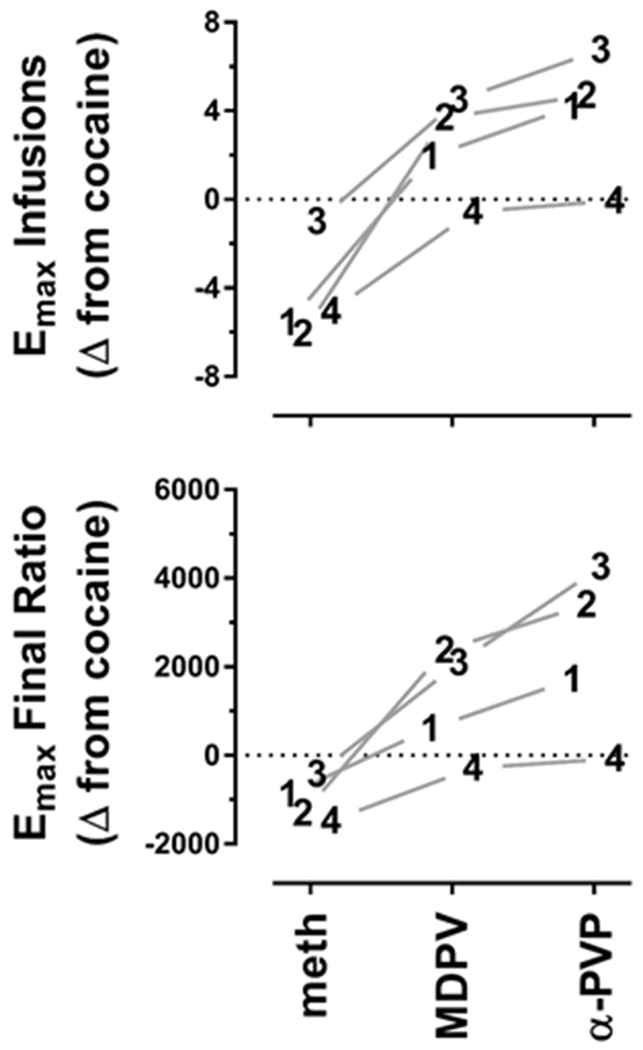
Estimates of reinforcing effectiveness obtained for methamphetamine, MDPV, and α-PVP, normalized to cocaine for each of 4 rhesus monkeys (identified as 1, 2, 3, or 4). Top Panel: Data represent the maximum number of infusions of methamphetamine, MDPV, and α-PVP earned, normalized to the maximum number of infusions earned for cocaine for each monkey. Bottom Panel: Data represent the final ratio completed for the dose that maintained the maximum number of infusions of methamphetamine, MDPV, and α-PVP earned, normalized to the final ratio completed for cocaine for each monkey.
As shown in Table 1, the reinforcing potency and effectiveness of cocaine did not change over the course of the study with initial estimates not different from those obtained after evaluating methamphetamine, MDPV, and α-PVP in duplicate.
DISCUSSION
Studies in rodents suggest that the locomotor and discriminative stimulus effects of synthetic MDPV and α-PVP are similar to those of cocaine and methamphetamine (Marusich et al. 2012; 2014; Baumann et al. 2013; Fantegrossi et al. 2013; Aarde et al. 2013; 2015; Gatch et al. 2013; Berquist et al. 2016; 2017; Collins et al. 2016; Gannon et al. 2016); however, mounting evidence suggests that both MDPV and α-PVP function as more effective reinforcers than either cocaine or methamphetamine (Aarde et al. 2013; 2015; Watterson et al. 2014; Gannon et al. 2017a; 2018a). Although less is known about the abuse-related effect of cathinone and synthetic analogues of cathinone in rhesus monkeys, evidence suggests that the reinforcing effectiveness of cathinone is comparable to cocaine (Woolverton & Johanson, 1984), and that the discriminative stimulus effects of MDPV, α-PVP, and related synthetic cathinones overlap with those of cocaine (Smith et al. 2017a; 2017b). The current studies confirm and extend these findings by demonstrating that not only do MDPV and α-PVP function as reinforcers in rhesus monkeys, but that they are more effective than either cocaine (in 3 of 4 monkeys) or methamphetamine (in all monkeys) at maintaining responding under a PR schedule. These findings are consistent with the results of rodent studies, and anecdotal reports from humans suggesting that abuse-related effects of synthetic cathinones, such as MDPV and α-PVP, are greater than the prototypical stimulant drugs of abuse (e.g., cocaine, methamphetamine), and provide further, strong evidence that MDPV and α-PVP have particularly high abuse potential.
The current study used a PR schedule of reinforcement to quantify and compare the reinforcing effects of two common synthetic cathinones, MDPV and α-PVP, to the reinforcing effects of cocaine and methamphetamine. This schedule was chosen because it results in monotonically increasing dose-response curves for most drugs of abuse, which make it ideal for making quantitative comparisons of reinforcing potency (i.e., ED50s) and reinforcing effectiveness (i.e., Emax) among drugs. Importantly, the relative measures of potency and effectiveness for cocaine and methamphetamine obtained in the current study align with those reported in a previous study that used this PR schedule to compare the reinforcing effects of cocaine and methamphetamine in rhesus monkeys (Lile et al. 2013). In addition, the rank order for reinforcing potency in rhesus monkeys (MDPV = α-PVP > methamphetamine > cocaine) is consistent with recent comparisons of these drugs in rats (Gannon et al. 2017a; 2018a). Although this relationship is in general agreement with their relative potency to inhibit uptake at human DAT (Eshleman et al. 2013; 2017), studies in rats have reported a larger difference in potency between MDPV and cocaine (~10-fold; Gannon et al. 2017a; 2018a) than was observed in rhesus monkeys (~5-fold). Likewise, although the rank order for reinforcing effectiveness (α-PVP >= MDPV > cocaine >= methamphetamine) is largely consistent across species, the magnitude of these differences (final ratio completed) was ~2-fold larger in rats (α-PVP ~4-fold > cocaine, MDPV ~2-fold > cocaine; Gannon et al. 2017a; 2018a) than was observed in rhesus monkeys (α-PVP ~2-fold > cocaine, MDPV ~1.5-fold > cocaine). Importantly, there are several possible explanations for differences between the reinforcing effects of MDPV and α-PVP between rats and rhesus monkeys, including schedule constraints in the current study, differences in the functional selectivities of these drugs at DAT, NET, and SERT, and/or pharmacokinetic profiles between rats and rhesus monkeys.
First, given that the dose-response curves for MDPV and α-PVP did not reach an asymptote in 2 monkeys (M2 and M3), it is possible that the current studies underestimated the Emax values for MDPV and α-PVP, and that evaluation of a larger dose would have resulted in further increases in the maximum number of infusions earned (or reductions in the number of infusions as was observed at the 0.1 mg/kg/inf dose in the other monkeys). Importantly that M2 and M3 both exhibited extremely high levels of responding for the largest dose of α-PVP (28 infusions; final ratio completed = 8175) on several occasions, suggests that both monkeys could obtain more infusions than the 26 that constituted their stable effect level for 0.1 mg/kg/inf α-PVP. Although speculative, given that sessions in which large doses of MDPV and α-PVP (0.1 mg/kg/inf) were available lasted on average 18 h for monkeys M2 and M3 (compared to <7 h for Emax doses of cocaine or methamphetamine), it is possible that changes in sleep and/or activity interfered with our ability to capture a “true” maximal effect for MDPV and α-PVP in all monkeys. In addition, it should be pointed out that unlike monkeys M1-M3 who all responded for MDPV and α-PVP at levels significantly greater than cocaine or methamphetamine, the reinforcing effectiveness of MDPV and α-PVP appeared to be comparable to cocaine (but greater than methamphetamine) in monkey M4. Although the factors that contributed to this differential response are unknown, this finding highlights the importance of considering individual subject data when interpreting the behavioral effects of drugs. Moreover, while the schedule parameters used in the current studies appear to be well suited for evaluating drugs such as cocaine and methamphetamine, refining these procedures (e.g., larger starting ratio, more rapid increases in ratio size, shorter limited hold, etc.) may be necessary when evaluating drugs, such as MDPV and α-PVP, which appear to function as significantly more effective reinforcers. In addition, it is also possible that the use of alternative approaches for scaling reinforcing effectiveness (e.g., behavioral economic demand curve analyses) would have resulted in a more accurate assessment of the relative reinforcing effects of cocaine, methamphetamine, MDPV, and α-PVP. However, it should be pointed out that a recent study in rats used these two methods (PR and demand curve analyses) to characterize the reinforcing effects of a series of structurally related, pyrrolidine-containing synthetic cathinones (e.g., MDPV, MDPPP, α-PVP, α-PPP), and found a high degree of correlation between the estimates of reinforcing effectiveness obtained from PR schedules and demand curve analyses (Gannon et al. 2019). When taken together with the results of previous studies in rats (Aarde et al. 2013; 2015; Watterson et al. 2014; Gannon et al. 2017a; 2018a), this study provides strong evidence to suggest that α-PVP and MDPV function as exceptionally powerful reinforcers capable of maintaining significantly greater levels of responding than either cocaine or methamphetamine.
Second, although the functional profiles of MDPV and α-PVP have not been evaluated in assays expressing DAT, NET, and SERT from the rhesus monkey, differences in the relative potency and effectiveness for MDPV, α-PVP and cocaine between rats and rhesus monkeys are consistent with differences in the functional profiles obtained for rat versus human DAT, NET, and SERT (Eshleman et al. 2013; 2017; Gannon et al. 2018a). For example, the potency difference between MDPV and cocaine to inhibit uptake at DAT is greater for rats (~65-fold; Gannon et al. 2018a) than human (~30-fold; Eshleman et al. 2013). While these difference could account for the differences in relative reinforcing potency observed between MDPV and cocaine in rats (e.g., Gannon et al. 2017a, 2018a) and rhesus monkeys, differences in the functional selectivity of MDPV to inhibit uptake at DAT relative to SERT in rats (~750-fold; Gannon et al. 2018x) and humans (~110-fold; Eshleman et al. 2013) could also account for differences in relative reinforcing effectiveness observed between rats and rhesus monkeys. Interestingly, unlike MDPV, the functional selectivity of α-PVP to inhibit uptake at DAT relative to SERT appears to be much more similar between rats (~3800-fold; Gannon et al. 2018a) and humans (~2900-fold; Eshleman et al. 2013). Thus, despite slight differences in the relative reinforcing potency and effectiveness of cocaine, methamphetamine, MDPV, and α-PVP between rats and rhesus monkeys, the current findings provide additional evidence linking reinforcing potency of stimulant drugs to their potency to inhibit uptake at DAT, and reinforcing effectiveness to their selectivity to inhibit DAT relative to SERT, as has recently been established for these drugs in rats (Gannon et al. 2018a, 2018c). Importantly, although this relationship between DAT/SERT selectivity and reinforcing effectiveness appears adequate to describe the reinforcing effects of monoamine uptake inhibitors, such as cocaine, MDPV, and α-PVP, the fact that methamphetamine was the least reinforcing drug in all four monkeys suggests that the reinforcing effectiveness of monoamine releasing drugs is influenced by other pharmacological properties (e.g., release of 5-HT).
Third, in addition to these apparent species differences in the functional profiles of MDPV and α-PVP, there is also evidence to suggest that the pharmacokinetic profiles of MDPV and α-PVP differ between rats and rhesus monkeys. For instance, when administered to rats at functionally equivalent doses, the locomotor effects of MDPV and α-PVP are similar in terms of their duration of action (Gatch et al. 2013; 2015); however, in rhesus monkeys, the discriminative stimulus effects of MDPV appear to be significantly longer lived than an equivalent dose of α-PVP (~300 versus ~60 min; Smith et al. 2017a). Although such large differences in duration of action could also account for differences in the relative reinforcing effectiveness of observed for MDPV and α-PVP (e.g., due to drug accumulation), a more rigorous evaluation of the pharmacokinetic profiles of MDPV and α-PVP in rhesus monkeys is necessary to support such claims.
In summary, synthetic cathinones represent a serious and growing threat to public health. Despite their use being linked to high levels of toxicity, and recent evidence suggesting that high school students in the United States are more likely to use synthetic cathinones, and α-PVP in particular, than either heroin or methamphetamine (Johnston et al. 2019; Palamar et al. 2019), little is known about the abuse-related effects of these drugs in non-human primates. The present study is the first to describe the reinforcing effects of MDPV and α-PVP in non-human primates, and provides a direct comparison of the relative reinforcing potency and effectiveness of these synthetic cathinones to those of the most widely used stimulant drugs of abuse, cocaine and methamphetamine. Consistent with mounting evidence from studies in rats, MDPV and α-PVP maintained significantly greater levels of responding than either methamphetamine or cocaine, suggesting that they function as exceptionally highly effective reinforcers. Moreover, when taken together with the finding that MDPV and α-PVP were capable of maintaining these high rates of responding under the PR schedule for exceedingly long periods of time (e.g., 18-20 h), these studies provide strong evidence in support of the notion that MDPV and α-PVP have particularly high potential for abuse.
Acknowledgements:
The Authors would like to thank Jade Juarez, Krissian Martinez, Emily Spoliarch, and Samuel Womak for their excellent technical assistance in completing these studies. Research was supported by a National Institutes of Health research grant from the National Institute on Drug Abuse (R01DA039146 [GTC]), the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism provided support for the work conducted by the Molecular Targets and Medications Discovery Branch (KCR, AS), and by the Welch Foundation (Grant AQ-0039 [CPF]). Funding sources had no involvement beyond financial support of this study.
Nonstandard abbreviations
- MDPV
3,4-methylenedioxypyrovalerone
- α-PVP
α-pyrrolidinopentiophenone
- DAT
dopamine transporter
- NET
norepinephrine transporter
- SERT
serotonin transporter
- PR
progressive ratio
- TO
timeout
- ANOVA
analysis of variance
Footnotes
CONFLICT OF INTEREST STATEMENT
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Gregory T. Collins, Department of Pharmacology, University of Texas Health Science Center at San Antonio, San Antonio, Texas, 78229, USA; Addiction Research, Treatment & Training Center of Excellence, University of Texas Health Science Center at San Antonio, San Antonio, Texas, 78229, USA; South Texas Veterans Health Care System, San Antonio, Texas, 78229, USA
Agnieszka Sulima, Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, Bethesda, Maryland, 20850, USA.
Kenner C. Rice, Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, Bethesda, Maryland, 20850, USA
Charles P. France, Department of Pharmacology, University of Texas Health Science Center at San Antonio, San Antonio, Texas, 78229, USA; Addiction Research, Treatment & Training Center of Excellence, University of Texas Health Science Center at San Antonio, San Antonio, Texas, 78229, USA; Department of Psychiatry, University of Texas Health Science Center at San Antonio, San Antonio, Texas, 78229, USA
REFERENCES
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, and Taffe MA (2013) The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, and Taffe MA (2015) Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 232: 1867–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, and Schindler CW (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38: 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie F, Hekman K, Cameron L, Wade DR, Miller C, Smolinske S, and Warrick B (2011) Emergency department visits after use of a drug sold as “ bath salts ” — Michigan, November 13, 2010-March 31, 2011. Morbidity and Mortality Weekly Report 60: 624–627. [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, and Spealman RD (1989) Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther 251: 150–155. [PubMed] [Google Scholar]
- Brandt SD, Sumnall HR, Measham F, and Cole J. Analyses of second-generation “legal highs” in the UK: initial findings (2010) Drug Testing and Analysis 2: 377–382. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, and Nutt DJ (2011) A web-based survey on mephedrone. Drug and Alcohol Dependence 118: 19–22. [DOI] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, and France CP (2016) Discriminative stimulus effects of binary drug mixtures: studies with cocaine, MDPV, and caffeine. J Pharmacol Exp Ther 359: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT and France CP (2018) Effects of lorcaserin and buspirone, administered alone and as a mixture, on cocaine self-administration in male and female rhesus monkeys. Exp Clin Psychopharmacol 26: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SB, Chen Z, Huang R, and Gatch MB (2018) “Ecstasy” to addiction: Mechanisms and reinforcing effects of three synthetic cathinone analogs of MDMA. Neuropharmacology 133: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. (2013) Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 85: 1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, and Janowsky A (2017) Structure-activity relationships of substituted cathinones, with transporter binding, uptake, and release. J Pharmacol Exp Ther 360: 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM and Fantegrossi WE (2016) Cocaine-Like Discriminative stimulus effects of mephedrone and naphyrone in mice. J Drug Alcohol Res 5: pii: 236009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, and Collins GT (2017a) Individual differences in the relative reinforcing effects of 3,4-Methylenedioxypyrovalerone under fixed and progressive ratio schedules of reinforcement in rats. J Pharmacol Exp Ther. 361: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Rice KC, and Collins GT (2017b) Reinforcing effects of abused ‘bath salts’ constituents 3,4-methylenedioxypyrovalerone and α-pyrrolidinopentiophenone and their enantiomers. Behav Pharmacol 28: 578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Baumann MH, Walther D, Jimenez-Morigosa C, Sulima A, Rice KC, and Collins GT (2018a) The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 43: 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Rice KC, Collins GT (2018b) Reinforcing effects of binary mixtures of common bath salt constituents: studies with 3,4-Methylenedioxypyrovalerone (MDPV), 3,4-methylenedioxymethcathinone (methylone), and caffeine in rats. Neuropsychopharmacology. 43: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, and Collins GT (2018c) Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 134: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Sulima A, Rice KC, and Collins GT (2018d) Inhibition of cocaine and 3,4-methylenedioxypyrovalerone (MDPV) self-administration by lorcaserin is mediated by 5-HT2C receptors in rats. J Pharmacol Exp Ther 364: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Mesmin MP, Sulima A, Rice KC, and Collins GT (2019) Behavioral economic analysis of the reinforcing effects of “bath salts” mixtures: studies with MDPV, methylone, and caffeine in male Sprague-Dawley rats. Psychopharmacology (Berl) E-pub ahead of print. doi: 10.1007/s00213-018-5046-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, and Forster MJ (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol 24: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, and Forster MJ (2015) Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology (Berl) 232: 1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, and France CP (2016) Effects of Lorcaserin on Cocaine and Methamphetamine Self-Administration and Reinstatement of Responding Previously Maintained by Cocaine in Rhesus Monkeys. J Pharmacol Exp Ther 359: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, and Freeman KB (2017) Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology (Berl) 234: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE and Schuster CR (1981) A comparison of the behavioral effects of l- and dl-cathinone and d-amphetamine. J Pharmacol Exp Ther 219: 355–362. [PubMed] [Google Scholar]
- Johnson PS and Johnson MW (2014) Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs 46: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, and Patrick ME (2019) Monitoring the Future national survey results on drug use 1975-2018: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan. [Google Scholar]
- Lile JA, Charnigo RJ, and Nader MA (2013) The relative reinforcing strength of methamphetamine and D-amphetamine in monkeys self-administering cocaine. Behav Pharmacol 24: 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto K, Striebel J, Cho AK, and Wang C (2013) Clinical and pharmacological aspects of bath salt use: a review of the literature and case reports. Drug and Alcohol Dependence 132: 1–12. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, Wyatt N, Callaghan PD, Bowen MT, Cornish JL, and McGregor IS (2013) High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine. J Psychopharmacol 27: 823–36. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the care and use of laboratory animals, 8th ed, National Academies Press, Washington, DC. [Google Scholar]
- Palamar JJ, Rutherford C, and Keyes KM (2019) “Flakka” use among high school seniors in the United States. Drug Alcohol Depend 196: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser JM and Nelson LS (2012) The toxicology of bath salts: a review of synthetic cathinones. Journal of Medical Toxicology 8: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR and Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237: 1219–1223. [DOI] [PubMed] [Google Scholar]
- Schneir A, Ly BT, Casagrande K, Darracq M, Offerman SR, Thornton S, Smollin C, Vohra R, Rangun C, Tomaszewski C, and Gerona RR. (2014) Comprehensive analysis of “bath salts” purchased from California stores and the internet. Clin Toxicol (Phila) 52:651–658. [DOI] [PubMed] [Google Scholar]
- Schuster CR and Johanson CE (1979) Behavioral studies on cathinone in monkeys and rats. NIDA Res Monogr 27: 324–325. [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, and Liechti ME (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168: 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Negus SS, Poklis JL, Blough BE, and Banks ML (2017a) Cocaine-like discriminative stimulus effects of alpha-pyrrolidinovalerophenone, methcathinone and their 3,4-methylenedioxy or 4-methyl analogs in rhesus monkeys. Addict Biol 22: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Blough BE, and Banks ML (2017b) Cocaine-like discriminative stimulus effects of amphetamine, cathinone, methamphetamine, and their 3,4-methylenedioxy analogs in male rhesus monkeys. Psychopharmacology (Berl) 234: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, and Jansen J (2011) Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 49: 499–505. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2018. (United Nations publication, Sales No. E.18.XI.9).
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, and Olive MF (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, and Schifano F (2011) Mephedrone, new kid for the chop? Addiction 106: 154–161. [DOI] [PubMed] [Google Scholar]
- Woolverton WL and Johanson CE (1984) Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. J Exp Anal Behav 41: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita T (1979) Studies on cathinones: cardiovascular and behavioral effects in rats and self-administration experiment in rhesus monkeys. NIDA Res Monogr 27: 326–327. [PubMed] [Google Scholar]
- Yanagita T (1986) Intravenous self-administration of (−)-cathinone and 2-amino-1-(2,5-dimethoxy-4-methyl)phenylpropane in rhesus monkeys. Drug Alcohol Depend 17: 135–141. [DOI] [PubMed] [Google Scholar]


