Abstract
Objective
To determine whether neighbourhood socioeconomic status (SES) was associated with large for gestational age (LGA) while considering key sociodemographic and clinical confounding factors.
Setting and patient
All singleton infants whose parents were living in the city of Marseilles, France, between 2013 and 2016.
Method
Population-based study based on new-born hospital birth admission charts from the French National Uniform Hospital Discharge Data Set Database. LGA infants were compared to appropriate-for-gestational-age (AGA) infants. Multiple generalized logistic model analysis was used to examine factors associated with LGA.
Results
A total of 43,309 singleton infants were included, and 4,747 (11%) were born LGA. LGA infants were more likely to have metabolic and respiratory diseases and to be admitted to the neonatal intensive care unit. Multiparity, advanced maternal age, obesity and diabetes were associated with an increased risk of LGA. Lower neighbourhood SES was associated with LGA (aOR = 1.24, 95% CI: 1.14; 1.36; p<0.0001) independent of age, diabetes, obesity, maternal smoking and multiparity. The strength of this association increased with maternal age, reaching an aOR of 1.50 (95% CI: 1.26; 1.78; p<0.0001) for women > 35 years old.
Conclusion
Neighbourhood SES could be considered an important factor for clinicians to better identify mothers at risk of having LGA births in addition to well-known risk factors such as maternal diabetes, obesity and age. The intensification of the association between SES and LGA with increasing maternal age suggests that neighbourhood disadvantage may act on LGA cumulatively over time.
Background
Large for gestational age (LGA) is a growing public health problem in developed countries [1–3], and the prevalence of infants born LGA varies from 5 to 20% in these countries [4]. Recent studies suggest that the number of LGA infants has increased over the last two decades [2, 5]. The number of LGA births is also expected to rise due to the current epidemic context of obesity and diabetes, two major LGA risk factors [6]. Among women with these metabolic complications, the risk of LGA is increased twofold to threefold [7, 8]. LGA increases the risk of numerous adverse perinatal and birth outcomes, including caesarean delivery, postpartum haemorrhage, shoulder dystocia, perinatal asphyxia, neonatal hypoglycaemia, hyperbilirubinemia and neonatal respiratory distress disorders [9–11]. LGA also affects long-term health outcomes by increasing the risk for cancer [12]; metabolic disorders including overweight, obesity and type 2 diabetes in children and adults [13]; and cardiovascular disease [14]. A better way to identify pregnant women who are at risk of delivering a LGA infant is of particular concern for improving neonatal health outcomes, interrupting the intergenerational chain of metabolic diseases and preserving offspring health and well-being in adulthood [15].
Several factors, including maternal overweight/obesity, excessive gestational weight gain and gestational diabetes mellitus, are known to induce LGA [16, 17]. LGA may also be influenced by maternal age: advanced maternal age (i.e., over 35 years) has been suggested to increase the risk of LGA [18]. Births to women of advanced maternal age have increased over the past few decades, reaching rates of approximately 10% to 20% of births in high-income countries [19, 20]. This age group of women has increased rates of various complications, such as preeclampsia, gestational diabetes mellitus, low birth weight and perinatal mortality, during pregnancy [21–23]. A large body of literature has reported the impact of neighbourhood socioeconomic status (SES) on perinatal and birth outcomes, including low birth weight, small for gestational age (SGA) and preterm birth [24–26]. However, the influence of neighbourhood SES on LGA and combined effects with other LGA factors, including maternal age, has received little attention. Neighbourhood SES may affect LGA through the neighbourhood’s effects on maternal behaviours, such as the lack of access to healthy food or opportunities for exercise to combat obesity and diabetes and the low access to high-quality maternity care [27]. To our knowledge, only a few studies in the United States and Canada have addressed this issue with conflicting results, e.g., two studies showing an increased risk of LGA and one study showing a decreased risk of LGA for lower neighbourhood SES [28–30]. Further studies are thus needed to confirm the influence of neighbourhood SES on LGA. Explorations of this issue in other countries and health care systems may provide additional information. France may offer an interesting view, as the French Health system offers universal health coverage guaranteeing access to care for pregnant women regardless of cost [31]. The standards of maternity care are considered high, with a total of 7 recommended prenatal examinations. According to the World Health Organization, prenatal examinations provide an important opportunity to prevent and manage concurrent diseases through integrated service delivery [32], and France is one of the European countries with the lowest percentages of late prenatal examinations [33, 34]. Medical costs during the perinatal period are covered at a rate of 100% by the French social security system. Investigating the effect of SES on LGA at the whole population level with universal maternity coverage would therefore be a significant step in the identification of LGA risk factors.
The objective was to determine whether neighbourhood SES was associated with LGA while considering key sociodemographic and clinical confounding factors in a large population-based study using data from the French National Hospital Database system.
Methodology
Study design and data source
This population-based cohort study was based on new-born hospital birth admission charts from the French National Uniform Hospital Discharge Database (Programme de Médicalisation du Système d’Information, PMSI), which systematically collects administrative and medical information, including obstetrical characteristics. This database is compiled based on diagnosis-related groups, and each diagnosis (both maternal and new-born infant) is coded according to the International Classification of Diseases, Tenth Revision (ICD-10) [35]. Infants were classified using diagnosis-related groups related to births. Only singleton births were included in the analysis. The charts of infants with errors on individual linkage or without a maternal address code were excluded. All singleton infants whose parents were living in the city of Marseilles, France, between 2013 and 2016 were eligible for the study regardless of the maternity ward address. This study was part of a regional project aimed at identifying factors influencing perinatal health outcomes. Marseille is the second-largest city of France, with almost 860,000 inhabitants and 13,000 births per year and is one of the French cities with the highest levels of social inequality [36]. The size of the city was sufficient to use a geographic socioeconomic index based on zip codes. Previous studies in Marseilles reported that social inequalities were well captured by zip codes, i.e., inhabitants shared resembling socioeconomic characteristics in the same zip code [37], which has not been demonstrated in other French cities or in studies associating urban and rural populations.
From the PMSI database, we selected all in-hospital stays corresponding to a neonatal diagnosis-related group (DRG n°15 in France and with an age equal to 0 days) and with address codes localized to Marseilles. We excluded in-hospital stays with linking problems, transfers from hospitals in another geographical area, infants from multiple pregnancies or when the number of infants was not specified, and infants who were small for gestational age (birth weights lower than the 10th percentile). Then, we defined two populations: infants who were LGA (birth weights lower than the 10th percentile) and controls who were appropriate for gestational age (AGA).
Neighbourhood SES
For each birth, maternal SES was evaluated using the available neighbourhood deprivation index (NDI) developed in France (French Deprivation index, FDep09) based on the residential zip code [38]. The NDI was developed from variables included in the 2009 national census data published by the French National Institute of Statistics and Economic Studies (INSEE, Institut National de la Statistique et des Etudes Economiques) and was calculated for each district in Marseille (n = 16). The NDI was used as a proxy for the social environment and was the first component used in a principal component analysis involving four socioeconomic variables: median household income, percentage of the population aged 15 years or older that graduated from high school, percentage of blue-collar workers and the unemployment rate of the population aged between 15 and 64 years. The NDI was categorized according to quartiles, from the least (NDI level 1) to the most deprived area (NDI level 4). This index was adjusted to the study period and validated by variables from the national 2015 Census data (S1 Table).
Maternal and neonatal data collection
Maternal and neonatal characteristics were derived from the infant in-hospital stay. Obstetrical data were included in the new-born hospital birth admission chart. Gestational age, birth weight, admissions to the neonatal intensive care unit (NICU) and data on some neonatal morbidities, including metabolic disorders (ICD-10 codes P700, 701, 711, 709, and 719), jaundice (ICD-10 codes P599, P58, and P590), respiratory diseases (ICD-10 codes P22, P24, and P293), perinatal asphyxia with neonatal encephalopathy (ICD-10 codes P21 and P916) and neonatal death up to day 28, were collected. Preterm birth was defined as gestational age less than 37 weeks. SGA, AGA and LGA were defined as birth weights lower than the 10th percentile, within the 10th and 90th or higher than the 90th percentile, respectively, based on the national French growth chart(19).
Data on the following maternal comorbidities were also searched in the infant in-hospital stay: maternal hypertension or preeclampsia (ICD-P000); gestational diabetes mellitus, including maternal diabetes and gestational diabetes mellitus codes (ICD-P700 and P701); obesity (ICD-10 code E66*: body mass index greater than 30); multiparity; mode of delivery (ICD-10 code P034 for caesarean section); and maternal smoking, through the proxy ICD 10 code P042, which is ‘New-born affected by maternal use of tobacco.’ Due to coding instructions, parity is only available for vaginal deliveries. Data on maternal age were collected after linkage with maternal hospital admission charts. For infants with unavailable linkage, we replaced the corresponding maternal age with the mean age of the mothers who delivered in the same maternity ward in the same year.
Statistical analysis
Descriptive data for sociodemographic and clinical characteristics are presented as frequencies and percentages. First, we described and compared the characteristics of the LGA vs AGA infants as well as the characteristics according to the NDI (Table 1). Univariate analyses were performed using a generalized logistic model with the hospital or maternity ward as a random effect to take into account correlations of data due to hospital clustering (Table 2). To determine whether the NDI was an independent factor associated with LGA, we performed stepwise logistic regression analyses. Because data on maternal age was absent for 10% of women, we performed an analysis with imputation to the mean (i.e., replacement of maternal age by the mean age of the mothers who delivered in the same maternity ward in the same year) and an analysis excluding mothers with missing data. Interaction effects were tested between the different variables, particularly the interaction between maternal age and NDI levels presented in Fig 1. Gestational diabetes mellitus and obesity were not included in the same model because of multicollinearity. Because multiparity data were only available for vaginal deliveries, another model for sensitivity analysis was computed only on vaginal delivery data to include multiparity in the analysis. All multivariate analyses are presented in Table 3. Odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated. Statistical significance was defined as p<0.05. The statistical analysis was performed with SAS 9·4 (SAS Institute), and a univariate/multivariate generalized logistic regression model was performed using PROC GLIMMIX in SAS®.
Table 1. Maternal and neonatal characteristics of appropriate-for-gestational-age (AGA) and large-for-gestational-age (LGA) infants.
| Perinatal characteristics | Total population | LGA | AGA | p |
|---|---|---|---|---|
| (n = 43,309) | (n = 4747) | (n = 38,562) | ||
| Obstetrical data | ||||
| Maternal age, n (%) | < 0.0001 | |||
| < 25 y | 10,781 (24.9) | 1054 (22.2) | 9727 (25.2) | |
| 25–30 y | 11,362 (26.2) | 1175 (24.7) | 10,187 (26.4) | |
| 30–35 y | 12,449 (28.7) | 1392 (29.3) | 11,057 (28.7) | |
| ≥ 35 y | 8717 (20.1) | 1126 (23.7) | 7591 (19.7) | |
| Obesity, n (%) | 2108 (4.9) | 385 (8.1) | 1723 (4.5) | <0.0001 |
| Multiparity*, n (%) | 18,672/31,858 | 2183/2983 | 16,489/28,875 | <0.0001 |
| (58.6) | (73.2) | (57.1) | ||
| GDM**, n (%) | 2794 (6.4) | 643 (13.5) | 2151 (5.6) | <0.0001 |
| Preeclampsia, n (%) | 777 (1.8) | 99 (2.1) | 678 (1.8) | 0.11 |
| Maternal smoking, n (%) | 629 (1.4) | 29 (0.6) | 600 (1.5) | <0.0001 |
| Caesarean delivery, n (%) | 8967 (20.7) | 1513 (31.8) | 7454 (19.3) | <0.0001 |
| Neonatal data | ||||
| GA, mean (SD) | 39.1 (1.6) | 39.1 (1.7) | 39.1 (1.6) | 0.81 |
| Preterm birth, n(%) | 2156 (4.9) | 1909 (4.9) | 207 (5.2) | 0.45 |
| BW, mean (SD) | 3374 (467) | 4062 (399) | 3289 (400) | < 0.001 |
| Sex (female), n (%) | 20,864 (48.2) | 2340 (49.3) | 18,524 (48) | 0.10 |
| Congenital malformations, n(%) | 2705 (6.2) | 330 (6.9) | 2375 (6.1) | 0.07 |
| NICU admissions, n (%) | 1993 (6.4) | 304 (6.4) | 1689 (4.4) | <0.001 |
| Metabolic disorders***, n (%) | 3021 (7) | 691 (14.5) | 2330 (6) | <0.001 |
| Jaundice, n (%) | 3086 (7.1) | 341 (7.2) | 2745 (7.1) | 0.92 |
| Respiratory disease, n (%) | 3045 (7) | 450 (9.5) | 2595 (6.7) | <0.001 |
| Perinatal asphyxia, n (%) | 1675 (3.9) | 189 (4) | 1489 (3.7) | 0.42 |
| Neonatal deaths, n (‰) | 45 (1.03) | 7 (1.47) | 38 (0.98) | 0.32 |
| Maternal SES | ||||
| NDI, n (%) | <0.001 | |||
| 1 least deprived | 11,845 (27.3) | 1181 (24.9) | 10,664 (27.6) | |
| 2 | 9702 (22.4) | 1045 (22) | 8657 (22.4) | |
| 3 | 9218 (21.3) | 975 (20.5) | 8243 (21.4) | |
| 4 most deprived | 12,544 (28.9) | 1546 (32.6) | 10,998 (28.5) | |
BW: birth weight (g); GA: gestational age (weeks); GDM: gestational diabetes mellitus; metabolic disorders: hypoglycaemia and hypocalcaemia; NICU: neonatal intensive care unit; NDI: neighbourhood deprivation index.
*Multiparity was calculated for infants delivered vaginally (data not available for caesarean delivery).
**GDM: gestational diabetes mellitus.
***metabolic disorders: hypoglycaemia and hypocalcaemia
Table 2. Perinatal characteristics of the population according to the neighbourhood deprivation index.
| Neighbourhood deprivation index | |||||
|---|---|---|---|---|---|
| Perinatal characteristics | Q1 | Q2 | Q3 | Q4 | p |
| Least deprived | Most deprived | ||||
| (n = 11,845) | (n = 9702) | (n = 9218) | (n = 12,544) | ||
| Maternal data | |||||
| Maternal age, n (%) | <0.001 | ||||
| < 25 y | 2174 (18.3) | 2222 (22.9) | 2653 (28.8) | 3732 (29.7) | |
| 25–30 y | 2892 (24.4) | 2720 (28) | 2293 (24.9) | 3457 (27.5) | |
| 30–35 y | 4036 (34.1) | 2867 (29.5) | 2491 (27) | 3055 (24.3) | |
| ≥ 35 y | 2743 (23.1) | 1893 (19.5) | 1781 (19.3) | 2300 (18.3) | |
| Obesity, n (%) | 205 (1.7) | 444 (4.6) | 570 (6.2) | 889 (7.1) | <0.001 |
| Multiparity*, n (%) | 4857/9147 | 4034/7241 | 3877/6527 (59.4) | 5904/8943 (66) | <0.001 |
| (53.1) | (55.1) | ||||
| Gestational diabetes mellitus, n (%) | 475 (4) | 573 (5.9) | 633 (6.9) | 1113 (8.9) | <0.001 |
| Preeclampsia, n (%) | 170 (1.4) | 192 (1.9) | 168 (1.8) | 247 (1.9) | 0.005 |
| Maternal smoking, n (%) | 92 (0.8) | 188 (1.9) | 175 (1.9) | 174 (1.4) | <0.001 |
| Caesarean delivery, n (%) | 2146 (18.1) | 1957 (20.2) | 1893 (20.5) | 2639 (21) | <0.001 |
| Neonatal data | |||||
| GA, mean (SD) | 39.20 (1.5) | 39.13 (1.6) | 39.16 (1.6) | 39.08 (1.7) | <0.001 |
| BW, mean (SD) | 3372 (449) | 3365 (467) | 3378 (458) | 3379 (489) | 0.33 |
| Preterm birth, n (%) | 546 (4.6) | 501 (5.1) | 425 (4.6) | 684 (5.4) | < 0.01 |
| Sex (female), n (%) | 5666 (47.8) | 4672 (48.1) | 4453 (48.3) | 6073 (48.4) | 0.82 |
| LGA, n (%) | 1181 (9.9) | 1045 (10.8) | 975 (10.6) | 1546 (12.3) | <0.001 |
| LGA with diabetes mellitus/GDM, n (%) | 110 (0.9) | 136 (1.4) | 137 (1.5) | 260 (2.1) | <0.001 |
| Congenital malformations, n (%) | 755 (6.4) | 655 (6.7) | 600 (6.5) | 695 (5.5) | 0.001 |
| NICU admissions, n (%) | 433 (3.7) | 509 (5.2) | 425 (4.6) | 626 (5) | <0.001 |
| Neonatal death, n (‰) | 9 (0.76) | 9 (0.97) | 9 (0.97) | 18 (1.43) | 0.3 |
BW: birth weight (g); GA: gestational age (weeks); GDM: gestational diabetes mellitus; NICU: neonatal intensive care unit; Neonatal death: death during the first 28 days of life.
*Multiparity: calculated for infants delivered vaginally.
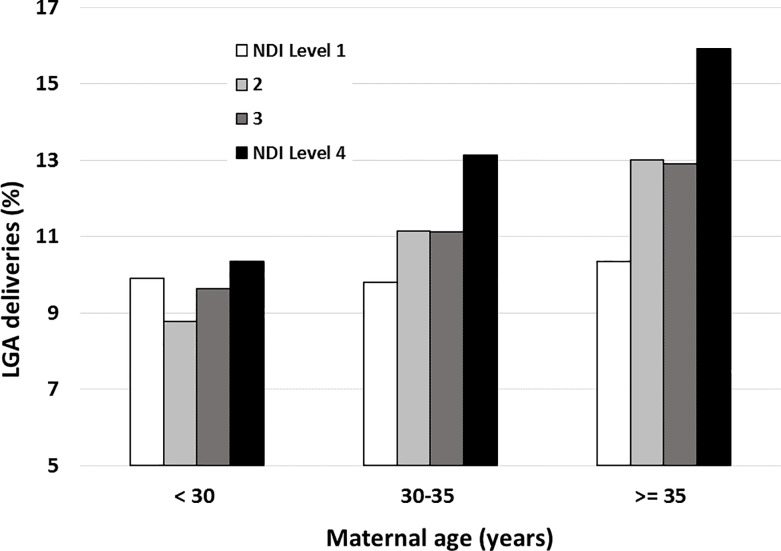
Fig 1. LGA, neighbourhood deprivation index (NDI) and maternal age.
The rate of infants born LGA increased gradually with advancing maternal age, and the NDI reached the highest level among infants born to mothers aged 35 years and living in the most deprived neighbourhoods.
Table 3. Factors associated with LGA delivery.
Odds ratios (95% CI) from the stepwise logistic regression models.
| Model with imputation of maternal age* N = 43,309 | Model without imputation of maternal age N = 38,547 | Model with obesity instead of GDM N = 38,547 | Model including multiparity only for vaginally delivered infants N = 31,858 | |||||
|---|---|---|---|---|---|---|---|---|
| aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | |
| Maternal age | <0.001 | <0.001 | <0.001 | <0.01 | ||||
| < 25 y | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||||
| 25–30 y | 1.06 (0.97 - 1.16) | 0.17 | 1.40 (1.25 - 1.57) | <0.001 | 1.43 (1.27 - 1.60) | <0.001 | 1.26 (1.10 – 1.44) | <0.001 |
| 30–35 y | 1.15 (1.05 - 1.26) | 0.002 | 1.52 (1.36 -1.70) | <0.001 | 1.59 (1.42 -1.77) | <0.001 | 1.20 (1.05 – 1.37) | <0.01 |
| > 35 y | 1.28 (1.17 - 1.41) | <0.001 | 1.70 (1.51 -1.91) | <0.001 | 1.83 (1.63 -2.05) | <0.001 | 1.24 (1.07 - 1.42) | <0.01 |
| Gestational diabetes mellitus | 2.61 (2.36 - 2.88) | <0.001 | 2.49 (2.24 - 2.76) | <0.001 | - | - | 1.92 (1.67 - 2.20) | <0.001 |
| Obesity | - | - | - | - | 1.87 (1.66 - 2.12) | <0.001 | - | - |
| Multiparity | - | - | - | - | - | - | 1.95 (1.79 - 2.14) | <0.001 |
| Maternal smoking | 0.38 (0.26 - 0.55) | <0.001 | 0.37 (0.25 - 0.55) | <0.001 | 0.37 (0.25 - 0.54) | <0.001 | 0.38 (0.23 - 0.61) | <0.001 |
| NDI | <0.001 | <0.001 | <0.001 | 0.047 | ||||
| 1 least deprived | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||||
| 2 | 1.08 (0.99 - 1.18) | 0.08 | 1.12 (1.02 - 1.23) | 0.03 | 1.13 (1.02 - 1.24) | 0.015 | 1.12 (1.00 - 1.18) | 0.04 |
| 3 | 1.08 (0.96 - 1.16) | 0.24 | 1.11 (1.00 - 1.23) | 0.05 | 1.12 (1.01 - 1.23) | 0.036 | 1.05 (0.93 - 1.18) | 0.39 |
| 4 most deprived | 1.24 (1.14 - 1.36) | <0.001 | 1.28 (1.16 - 1.41) | <0.001 | 1.28 (1.16 - 1.41) | <0.001 | 1.15 (1.03 - 1.29) | 0.01 |
| Goodness of fit (AIC) | 29.606 | 25.864 | 26.040 | 19.364 | ||||
NDI: neighbourhood deprivation index; aOR: adjusted OR.
*A significant interaction was found between maternal age and NDI: The strength of the association between SES and LGA increased with maternal age, reaching an aOR of 1.50 (95% CI: 1.26; 1.78; p<0.0001) for women older than 35 years in ND4.
Ethics
Our institution (Assistance Publique—Hôpitaux de Marseille) was granted access to the French National Uniform Hospital Discharge Data Set Database by the ATIH (Agence Technique d’Information sur l’Hospitalisation https://www.atih.sante.fr/), the organization in charge of this database in France, in compliance with French law after a deliberation of the French Commission for Data Protection and Liberties (CNIL). In addition, research on retrospective data is excluded from the framework of the French Law Number 2012–300 of 5 March 2012 relating to the research involving human participants, as modified by the Order Number 2016–800 of 16 June 2016. Neither French competent authority (Agence Nationale de Sécurité du Médicament et des Produits de Santé, ANSM) approval nor French ethics committee (Comités de Protection des Personnes, CPP) approval is required in this context.
Results
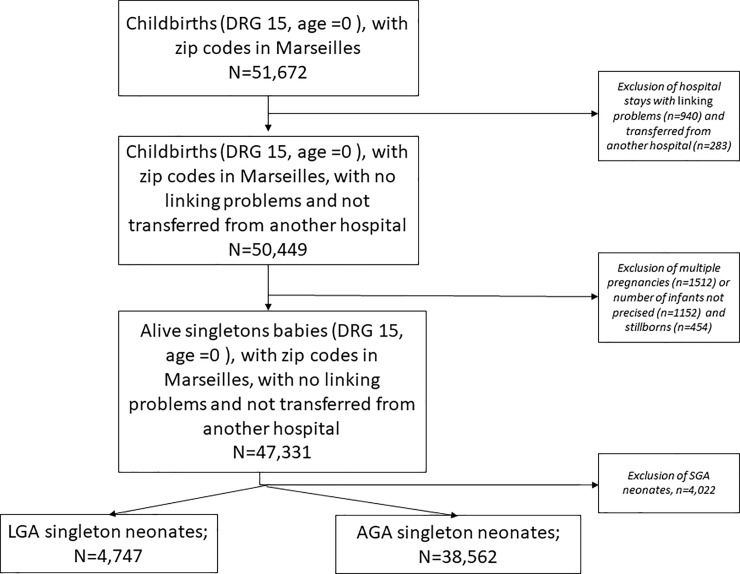
The study period included 47,331 singleton infants after exclusion of 4,341 infants’ charts due to errors or missing data, the absence of the mother’s address code, and multiple pregnancies. After the exclusion of 4,022 infants for SGA, 43,309 were included in this study, of which 4,747 (10.9%) were LGA and 38,562 were AGA (Fig 2). The linkage between mother and infants was available for 90% of the infants. The obstetrical and neonatal characteristics of the study population are detailed in Table 1. The rates of infants born to mothers of advanced age (i.e., over 35 years), gestational diabetes mellitus and preeclampsia were 20.1%, 6.4% and 1.8%, respectively. Approximately 5% of the infants in the study population were born preterm (gestational age less than 37 weeks).
Fig 2. Flowchart.
LGA population characteristics
The comparison of AGA and LGA infants is presented in Table 1. In comparison with AGA infants, LGA infants were more likely to be born to older (maternal age above 35 years) and multiparous mothers (p<0.0001). The rate of gestational diabetes mellitus was 2.5-fold higher (p<0.0001) among LGA infants than among AGA infants. In contrast, the rate of infants born to tobacco-smoking mothers was 3-fold lower (p<0.0001) in the LGA group. While the mean GA and preterm birth rate were comparable between groups, NICU admissions were significantly higher for LGA infants than for AGA infants (p<0.0001), with higher rates of hypoglycaemia, hypocalcaemia and respiratory disorders (p< 0.0001).
Neighbourhood deprivation and perinatal outcomes
Perinatal outcomes according to the NDI are detailed in Table 2. Approximately 30% of the population lived in the most deprived neighbourhoods (NDI level 4). The proportion of LGA varied by NDI level (12.3% in NDI level 4 vs 9.9% in NDI level 1, p <0.0001). Infants born to mothers living in the most deprived areas were more likely to be born preterm (p<0.001). Infants from NDI levels 2, 3 and 4 were more frequently exposed to maternal smoking than those from NDI level 1 (p < 0.001). In contrast, in less deprived neighbourhoods, infants were more likely to be born to older (p<0.0001) and nulliparous (p<0.0001) mothers. The mean GA decreased with NDI, and the mean birth weight was unchanged due to a higher rate of LGA. Similarly, gestational diabetes mellitus (p<0.0001) and LGA associated with gestational diabetes mellitus (p<0.01) were 2-fold higher in NDI level 4 than in NDI level 1.
Factors associated with LGA
Multivariate analyses are detailed in Table 3. NDI, advanced maternal age (maternal age ≥ 35 years), gestational diabetes mellitus and obesity were significantly associated with LGA (p<0.001). Infants born to mothers living in deprived neighbourhoods (NDI level 4) had 25% higher odds of being LGA (aOR = 1.24, 95% CI: 1.14; 1.36; p<0.0001) after adjustment for maternal age, gestational diabetes mellitus, obesity, maternal smoking and multiparity. The strength of the association between SES and LGA increased with maternal age, reaching an aOR of 1.50 (95% CI: 1.26; 1.78; p<0.0001) for women older than 35 years in NDI level 4 (significant interaction). Fig 1 illustrates how LGA varies according to maternal age and the NDI. The differences in the rate of LGA between NDI levels 1 and 4 increased with advanced maternal age, with differences ranging from 4% to 54% in mothers under the age of 30 years relative to those aged 35 years and older (p < 0.001). SES was still significant after the inclusion of multiparity in the sensitivity analysis performed only on vaginal deliveries (n = 31,858), with an aOR = 1.15 (95% CI: 1.03–1.29, p < 0.001); multiparity was associated with LGA with an aOR = 1.19 (95% CI: 1.79–2.14). Maternal smoking was associated with lower odds of LGA (p<0.001).
Discussion
Main findings
The present findings can be summarized as follows. Between 2013 and 2016, 47,331 singleton infants whose parents were living in the city of Marseille were identified in the French National Hospital Database system. The prevalence of LGA varied according to neighbourhood SES, reaching 12% in the most deprived neighbourhoods. Lower neighbourhood SES was associated with LGA independent of maternal age, gestational diabetes mellitus, obesity, maternal smoking and multiparity. The strength of this association increased with maternal age, reaching an aOR of 1.50 for women older than 35 years of age.
Interpretation
The first important finding is the significant association between neighbourhood SES and LGA in a large population-based study, complementing the previous results on the effect of SES on other perinatal outcomes such as low birth weight, SGA and preterm birth [16, 39, 40]. To date, the association between SES and LGA has rarely been investigated, especially in industrialized countries with developed health care systems. Our findings confirm the results of two previous studies [28,30] in another socioeconomic and cultural contexts and with a large population but contradict the results of Shankardass et al [29], which did not adjust their findings to potential confounding characteristics, such as in our study. Importantly, this association remained significant after adjustment for metabolic disturbances, suggesting that neighbourhood SES could be considered relevant information for clinicians to better identify mothers at risk of having LGA births in addition to well-known risk factors such as gestational diabetes mellitus, maternal obesity and advanced maternal age. The association between neighbourhood SES and LGA may be explained by mechanisms such as poor health behaviours, endocrine disruptors and race/ethnicity characteristics. Future studies should explore the association between neighbourhood SES and inadequate diets (e.g., micronutrient and vitamin deficiencies [41]), endocrine disruptor exposure and ethnic characteristics. For ethical concerns, it is not possible to record ethnicity in the French databases. However, some previous studies have suggested that gypsy, Romanian and sub-Saharan migrant children may be at higher risk of LGA [42].
The LGA rate in deprived neighbourhoods was 25% higher than that in less deprived neighbourhoods. This finding confirms that LGA remains an important health disparity and that universal health coverage is not sufficient to counter the multiple factors that contribute to health and health care disparities [43], namely, the levels and distribution of income, education, housing, nutrition and safety [43, 44, 45]. In accordance with this finding, a previous French study performed in Paris reported insufficient healthcare follow-up among women with low SES [46]. Future studies should better explore economic, social, or environmental disadvantages, and targeted interventions should be proposed to reduce health disparities in these deprived neighbourhoods [47].
Finally, we found a significant interaction between maternal age and neighbourhood SES on the risk of LGA. Advanced maternal age is known to adversely affect birth and neonatal outcomes, but its effects on LGA remain poorly explored [21–23]. Kenny LC et al. reported an increase in LGA births among older women (> 30 years), and as we observed in our study, they found larger effects among women living in deprived neighbourhoods [18]. The underlying mechanism is unclear. Although we do not have information on the duration of residence, it may be reasonably hypothesized that LGA risk may increase with the duration of exposure to the abovementioned SES-associated factor. This hypothesis could be supported by previous studies on the life-course accumulation of neighbourhood disadvantage and its impact on health [48]. It can be speculated that older mothers with low SES have a cumulative disease risk over time, including repeated adverse health events that are stressful, diabetes, obesity and multiparity, which in turn promote LGA [3]. An association has been reported between older mothers and low SES and several risky behavioural factors, such as psychosocial issues, smoking practices, alcohol exposure, and stress, which should be specifically targeted early in pregnancy [49–51]. If this cumulative risk hypothesis were to be confirmed, our results would suggest that preventive efforts in current and individual conditions are not sufficient and that early health and social support (individual and/or collective) interventions are also necessary to improve perinatal and birth outcomes.
Strengths and limitations
The French National Uniform Hospital Discharge Data Set Database does not include some relevant individual factors, including educational status, ethnicity, maternal BMI before or in early pregnancy, maternal body weight gain, hyperglycaemic oral test, maternal nutritional status and data regarding foetal growth. Thus, we were unable to identify how these factors and their severity may mediate the effects of neighbourhood deprivation on LGA. Some comorbidities and clinical characteristics derived from ICD codes could be underreported, such as maternal smoking or obesity. In addition, we did not analyse congenital defects that could affect pregnancy and infant size at birth. Specific studies should explore this issue. Such limitations may nevertheless be mitigated by the fact that our study is the first large population-based study performed in industrialized countries. Our data were extracted from the French national database, which accurately registers hospitalized new-borns, maternal health data and the domicile postal code of the mother’s address. All consecutive deliveries in private or public hospitals providing different levels of health care were collected in this registered database system, which strengthens the findings of the study. Last, the accuracy of geographical methods based on the residential postal code can nevertheless be put into question in studies including urban and rural populations with nonhomogeneous social neighbourhood characteristics. This study avoided such a bias by limiting investigations to a single large city. However, conducting a study in a single French city may limit the generalizability of our findings. Future studies should thus confirm our findings in other geographical areas.
Conclusions
This study showed that infants born to mothers living in deprived socioeconomic areas had a higher risk of being LGA than those who were born to mothers living in less socioeconomically deprived areas. Neighbourhood disadvantages should be taken into account in routine follow-up care of pregnant women as well as other risk factors to prevent LGA deliveries. The intensification of the association between SES and LGA with increasing maternal age also suggests that neighbourhood disadvantage may act cumulatively on LGA. This finding argues for the integration of early health and social support interventions in the most deprived neighbourhoods to reduce LGA.
Supporting information
(DOCX)
Data Availability
Data cannot be shared publicly because of this data are issued from French national medicoadministrative database. Data are available from the ATIH Institutional Data Access (contact via https://www.atih.sante.fr/) for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from ATIH (https://www.atih.sante.fr/).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Ananth CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Semin Perinatol. 2002;26(4):260–7. 10.1053/sper.2002.34772 . [DOI] [PubMed] [Google Scholar]
- 2.Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104(4):720–6. 10.1097/01.AOG.0000141442.59573.cd . [DOI] [PubMed] [Google Scholar]
- 3.Bowers K, Laughon SK, Kiely M, Brite J, Chen Z, Zhang C. Gestational diabetes, pre-pregnancy obesity and pregnancy weight gain in relation to excess fetal growth: variations by race/ethnicity. Diabetologia. 2013;56(6):1263–71. 10.1007/s00125-013-2881-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet Gynecol Scand. 2008;87(2):134–45. 10.1080/00016340801899289 . [DOI] [PubMed] [Google Scholar]
- 5.Chiavaroli V, Castorani V, Guidone P, Derraik JG, Liberati M, Chiarelli F, et al. Incidence of infants born small- and large-for-gestational-age in an Italian cohort over a 20-year period and associated risk factors. Ital J Pediatr. 2016;42:42 10.1186/s13052-016-0254-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang LF, Wang HJ, Ao D, Liu Z, Wang Y, Yang HX. Influence of pre-pregnancy obesity on the development of macrosomia and large for gestational age in women with or without gestational diabetes mellitus in Chinese population. J Perinatol. 2015;35(12):985–90. 10.1038/jp.2015.119 . [DOI] [PubMed] [Google Scholar]
- 7.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964–8. 10.1016/j.ajog.2004.05.052 . [DOI] [PubMed] [Google Scholar]
- 8.Black MH, Sacks DA, Xiang AH, Lawrence JM. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care. 2013;36(1):56–62. 10.2337/dc12-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672 e1–4. 10.1016/j.ajog.2009.02.035 . [DOI] [PubMed] [Google Scholar]
- 10.Weissmann-Brenner A, Simchen MJ, Zilberberg E, Kalter A, Weisz B, Achiron R, et al. Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstet Gynecol Scand. 2012;91(7):844–9. 10.1111/j.1600-0412.2012.01412.x . [DOI] [PubMed] [Google Scholar]
- 11.Scifres CM, Feghali M, Dumont T, Althouse AD, Speer P, Caritis SN, et al. Large-for-Gestational-Age Ultrasound Diagnosis and Risk for Cesarean Delivery in Women With Gestational Diabetes Mellitus. Obstet Gynecol. 2015;126(5):978–86. 10.1097/AOG.0000000000001097 . [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Yang L, Pu F, Lin H, Wang B, Liu J, et al. High Birth Weight Increases the Risk for Bone Tumor: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2015;12(9):11178–95. 10.3390/ijerph120911178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 2015;10(2):77–83. 10.1111/ijpo.230 . [DOI] [PubMed] [Google Scholar]
- 14.Mullett MD, Cottrell L, Lilly C, Gadikota K, Dong L, Hobbs G, et al. Association between birth characteristics and coronary disease risk factors among fifth graders. J Pediatr. 2014;164(1):78–82. 10.1016/j.jpeds.2013.08.064 . [DOI] [PubMed] [Google Scholar]
- 15.Orskou J, Kesmodel U, Henriksen TB, Secher NJ. An increasing proportion of infants weigh more than 4000 grams at birth. Acta Obstet Gynecol Scand. 2001;80(10):931–6. 10.1034/j.1600-0412.2001.801010.x . [DOI] [PubMed] [Google Scholar]
- 16.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA. 2017;317(21):2207–25. 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitanchez D, Yzydorczyk C, Siddeek B, Boubred F, Benahmed M, Simeoni U. The offspring of the diabetic mother—short- and long-term implications. Best Pract Res Clin Obstet Gynaecol. 2015;29(2):256–69. 10.1016/j.bpobgyn.2014.08.004 . [DOI] [PubMed] [Google Scholar]
- 18.Kenny LC, Lavender T, McNamee R, O'Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One. 2013;8(2):e56583 10.1371/journal.pone.0056583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breart G, Barros H, Wagener Y, Prati S. Characteristics of the childbearing population in Europe. Eur J Obstet Gynecol Reprod Biol. 2003;111 Suppl 1:S45–52. 10.1016/j.ejogrb.2003.09.005 . [DOI] [PubMed] [Google Scholar]
- 20.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2017. Natl Vital Stat Rep. 2018;67(8):1–50. . [PubMed] [Google Scholar]
- 21.Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377(9774):1331–40. 10.1016/S0140-6736(10)62233-7 . [DOI] [PubMed] [Google Scholar]
- 22.Hoffman MC, Jeffers S, Carter J, Duthely L, Cotter A, Gonzalez-Quintero VH. Pregnancy at or beyond age 40 years is associated with an increased risk of fetal death and other adverse outcomes. Am J Obstet Gynecol. 2007;196(5):e11–3. 10.1016/j.ajog.2006.10.862 . [DOI] [PubMed] [Google Scholar]
- 23.Schimmel MS, Bromiker R, Hammerman C, Chertman L, Ioscovich A, Granovsky-Grisaru S, et al. The effects of maternal age and parity on maternal and neonatal outcome. Arch Gynecol Obstet. 2015;291(4):793–8. 10.1007/s00404-014-3469-0 . [DOI] [PubMed] [Google Scholar]
- 24.Metcalfe A, Lail P, Ghali WA, Sauve RS. The association between neighbourhoods and adverse birth outcomes: a systematic review and meta-analysis of multi-level studies. Paediatr Perinat Epidemiol. 2011;25(3):236–45. 10.1111/j.1365-3016.2011.01192.x . [DOI] [PubMed] [Google Scholar]
- 25.Ncube CN, Enquobahrie DA, Albert SM, Herrick AL, Burke JG. Association of neighborhood context with offspring risk of preterm birth and low birthweight: A systematic review and meta-analysis of population-based studies. Soc Sci Med. 2016;153:156–64. 10.1016/j.socscimed.2016.02.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundquist J, Sundquist K, Johansson SE, Li X, Winkleby M. Mothers, places and small for gestational age births: a cohort study. Arch Dis Child. 2011;96(4):380–5. 10.1136/adc.2009.180042 . [DOI] [PubMed] [Google Scholar]
- 27.Joseph KS, Liston RM, Dodds L, Dahlgren L, Allen AC. Socioeconomic status and perinatal outcomes in a setting with universal access to essential health care services. CMAJ. 2007;177(6):583–90. 10.1503/cmaj.061198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayborne ZM, Giesbrecht GF, Bell RC, Tomfohr-Madsen LM. Relations between neighbourhood socioeconomic status and birth outcomes are mediated by maternal weight. Soc Sci Med. 2017;175:143–51. 10.1016/j.socscimed.2016.12.041 . [DOI] [PubMed] [Google Scholar]
- 29.Shankardass K, O'Campo P, Dodds L, Fahey J, Joseph K, Morinis J, et al. Magnitude of income-related disparities in adverse perinatal outcomes. BMC Pregnancy Childbirth. 2014;14:96 10.1186/1471-2393-14-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wentz AE, Messer LC, Nguyen T, Boone-Heinonen J. Small and large size for gestational age and neighborhood deprivation measured within increasing proximity to homes. Health Place. 2014;30:98–106. 10.1016/j.healthplace.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodwin VG. The health care system under French national health insurance: lessons for health reform in the United States. Am J Public Health. 2003;93(1):31–7. 10.2105/ajph.93.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organization WH. Integrated Management of Pregnancy and Childbirth (IMPAC) In: Maternal, newborn, child and adolescent health [WHO web page]. Geneva: World Health Organization; (http://www.who.int/maternal_child_adolescent/topics/maternal/impac/en/, accessed 17 October 2016). [Google Scholar]
- 33.Eurostat. EUROPEAN PERINATAL HEALTH REPORT Health and Care of Pregnant Women and Babies in Europe in 2010. https://www.europeristat.com/images/doc/Peristat%202013%20V2.pdf. 2010. [DOI] [PubMed]
- 34.Wildman K, Blondel B, Nijhuis J, Defoort P, Bakoula C. European indicators of health care during pregnancy, delivery and the postpartum period. Eur J Obstet Gynecol Reprod Biol. 2003;111 Suppl 1:S53–65. 10.1016/j.ejogrb.2003.09.006 . [DOI] [PubMed] [Google Scholar]
- 35.Boudemaghe T, Belhadj I. Data Resource Profile: The French National Uniform Hospital Discharge Data Set Database (PMSI). Int J Epidemiol. 2017;46(2):392-d 10.1093/ije/dyw359 . [DOI] [PubMed] [Google Scholar]
- 36.OCDE. Towards more inclusive growth in the metropolitan area of Aix-Marseille: International insights. 2013. https://www.oecd.org/cfe/regional-policy/Summary-Aix-Marseille.pdf. 2013.
- 37.Bocquier A, Cortaredona S, Boutin C, David A, Bigot A, Chaix B, et al. Small-area analysis of social inequalities in residential exposure to road traffic noise in Marseilles, France. Eur J Public Health. 2013;23(4):540–6. 10.1093/eurpub/cks059 . [DOI] [PubMed] [Google Scholar]
- 38.Rey G, Jougla E, Fouillet A, Hemon D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:33 10.1186/1471-2458-9-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haugen M, Brantsaeter AL, Winkvist A, Lissner L, Alexander J, Oftedal B, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth. 2014;14:201 10.1186/1471-2393-14-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nohr EA, Timpson NJ, Andersen CS, Davey Smith G, Olsen J, Sorensen TI. Severe obesity in young women and reproductive health: the Danish National Birth Cohort. PLoS One. 2009;4(12):e8444 10.1371/journal.pone.0008444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michels N, Vynckier L, Moreno LA, Beghin L, de la OA, Forsner M, et al. Mediation of psychosocial determinants in the relation between socio-economic status and adolescents' diet quality. Eur J Nutr. 2018;57(3):951–63. 10.1007/s00394-017-1380-8 . [DOI] [PubMed] [Google Scholar]
- 42.Tutlam NT, Liu Y, Nelson EJ, Flick LH, Chang JJ. The Effects of Race and Ethnicity on the Risk of Large-for-Gestational-Age Newborns in Women Without Gestational Diabetes by Prepregnancy Body Mass Index Categories. Matern Child Health J. 2017;21(8):1643–54. 10.1007/s10995-016-2256-x . [DOI] [PubMed] [Google Scholar]
- 43.Howell EA. Reducing Disparities in Severe Maternal Morbidity and Mortality. Clin Obstet Gynecol. 2018;61(2):387–99. 10.1097/GRF.0000000000000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–15. 10.2105/AJPH.2006.102525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kullgren JT, McLaughlin CG. Beyond affordability: the impact of nonfinancial barriers on access for uninsured adults in three diverse communities. J Community Health. 2010;35(3):240–8. 10.1007/s10900-010-9230-0 . [DOI] [PubMed] [Google Scholar]
- 46.Gonthier C, Estellat C, Deneux-Tharaux C, Blondel B, Alfaiate T, Schmitz T, et al. Association between maternal social deprivation and prenatal care utilization: the PreCARE cohort study. BMC Pregnancy Childbirth. 2017;17(1):126 10.1186/s12884-017-1310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones NL, Gilman SE, Cheng TL, Drury SS, Hill CV, Geronimus AT. Life Course Approaches to the Causes of Health Disparities. Am J Public Health. 2019;109(S1):S48–S55. 10.2105/AJPH.2018.304738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustafsson PE, San Sebastian M, Janlert U, Theorell T, Westerlund H, Hammarstrom A. Life-course accumulation of neighborhood disadvantage and allostatic load: empirical integration of three social determinants of health frameworks. Am J Public Health. 2014;104(5):904–10. 10.2105/AJPH.2013.301707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delpisheh A, Brabin L, Attia E, Brabin BJ. Pregnancy late in life: a hospital-based study of birth outcomes. J Womens Health (Larchmt). 2008;17(6):965–70. 10.1089/jwh.2008.051410.1089/jwh.2007.0511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delpisheh A, Brabin L, Brabin BJ. Pregnancy, smoking and birth outcomes. Womens Health (Lond). 2006;2(3):389–403. 10.2217/17455057.2.3.389 . [DOI] [PubMed] [Google Scholar]
- 51.Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104(4):727–33. 10.1097/01.AOG.0000140682.63746.be . [DOI] [PubMed] [Google Scholar]