Abstract
Shallow-sea hydrothermal systems, like their deep-sea and terrestrial counterparts, can serve as relatively accessible portals into the microbial ecology of subsurface environments. In this study, we determined the chemical composition of 47 sediment porewater samples along a transect from a diffuse shallow-sea hydrothermal vent to a non-thermal background area in Paleochori Bay, Milos Island, Greece. These geochemical data were combined with thermodynamic calculations to quantify potential sources of energy that may support in situ chemolithotrophy. The Gibbs energies (ΔGr) of 730 redox reactions involving 23 inorganic H-, O-, C-, N-, S-, Fe-, Mn-, and As-bearing compounds were calculated. Of these reactions, 379 were exergonic at one or more sampling locations. The greatest energy yields were from anaerobic CO oxidation with NO2- (-136 to -162 kJ/mol e-), followed by reactions in which the electron acceptor/donor pairs were O2/CO, NO3-/CO, and NO2-/H2S. When expressed as energy densities (where the concentration of the limiting reactant is taken into account), a different set of redox reactions are the most exergonic: in sediments affected by hydrothermal input, sulfide oxidation with a range of electron acceptors or nitrite reduction with different electron donors provide 85~245 J per kg of sediment, whereas in sediments less affected or unaffected by hydrothermal input, various S0 oxidation reactions and aerobic respiration reactions with several different electron donors are most energy-yielding (80~95 J per kg of sediment). A model that considers seawater mixing with hydrothermal fluids revealed that there is up to ~50 times more energy available for microorganisms that can use S0 or H2S as electron donors and NO2- or O2 as electron acceptors compared to other reactions. In addition to revealing likely metabolic pathways in the near-surface and subsurface mixing zones, thermodynamic calculations like these can help guide novel microbial cultivation efforts to isolate new species.
Introduction
Hydrothermal systems are prevalent in tectonically active settings, including plate boundaries and hot spots [1–5]. They are commonly categorized by location and water depth into (1) terrestrial, (2) deep-sea (water depth >200 m), and (3) shallow-sea (water depth <200 m) [4, 6, 7]. Due to their accessibility, terrestrial hydrothermal systems (often synonymous with geothermal springs) were the first to be explored [1, 3, 7]. Since the discovery in 1977 of the first deep-sea hydrothermal systems near the Galápagos Islands [8], ~700 hydrothermal vents (with 644 confirmed or inferred to be active) have been reported along the ~60,000 km-long ocean ridge system as well as in back-arc basins in every ocean basin (InterRidge Vents Database: https://vents-data.interridge.org/). Approximately 70 active shallow-sea hydrothermal vent systems have also been identified [6, 9, 10]. Compared to their deep-sea counterparts, they occur in more diverse tectonically active settings, including near submarine volcanoes, island and intra-oceanic arcs, ridge environments, intraplate oceanic volcanoes, continental margins, and rift basins. Corresponding to their setting, the source of water for these systems can be a mixture of meteoric, magmatic, groundwater, and/or seawater. Unlike deep-sea systems, their location in the euphotic zone allows for photosynthetic activity as well [6, 9, 10]. Perhaps because they are influenced by and transitional between terrestrial and off-shore geologic environments, shallow-sea hydrothermal vent systems are typically complex and dynamic, establishing unique microbial ecosystems.
The microbial ecology and physiology in and around hydrothermal systems—terrestrial and marine—have been studied for several decades, but the factors that control community composition and metabolic function remain elusive. What is known, however, is that these systems contain the necessary ingredients for life—carbon sources, chemical energy from thermodynamic disequilibrium, mineral surfaces, and compositional gradients. Because all biological processes, including anabolism, catabolism, growth, development, and reproduction, are dependent on energy transformations [11–17], quantifying the amounts of energy associated with biological processes guides our understanding of ecosystem dynamics. The amount of energy that microorganisms can gain by catalyzing catabolic reactions in their environment can be quantified by calculating the Gibbs energy of redox reactions (ΔGr), which depends on physicochemical variables, including temperature, pressure, pH, concentrations of products and reactants, and ionic strength. These physicochemical variables and consequently the redox reaction energy yields can vary considerably from one hydrothermal system to the next, with the structure and function of the resident microbial community closely tied to the geologic setting. We can build upon earlier studies that have shown that thermophilic archaea and bacteria in these environments can catalyze a tremendous array of redox reactions to gain energy. Many of these thermophiles are chemolithoautotrophic, i.e., they use metabolic strategies that rely only on inorganic compounds as sources of energy and carbon [14, 18–22]. In fact, a number of studies have quantified the energetic potentials in terrestrial geothermal springs [23–29], deep-sea hydrothermal systems [30–35], and shallow-sea hydrothermal systems [22, 36–42].
Several studies have described the geology, geochemistry and microbiology of the shallow-sea hydrothermal system at Milos [43–49], but the bioenergetic potential there has not been quantified. In this study, we quantify the energetics of 730 inorganic redox reactions in a shallow-sea hydrothermal system of Milos Island, Greece. The reactions include electron donors and acceptors of five major elements (H, C, N, O, S) and three trace elements (Fe, Mn, As) that are commonly enriched in hydrothermal fluids. The thermodynamic calculations can be used to link the energetic potential of microbial communities to molecular evidence of their identities and metabolic capacity.
Materials and methods
Field work and chemical analyses
Samples were collected in May 2014 from Paleochori Bay, Milos Island (Greece) under a permit from the Greek Ephorate of Underwater Antiquities. In this study, we investigated samples from the Saganaki diffuse vent (36°40’24N, 24°30’50E), located under ~12 m of water and ∼300 m offshore (Fig 1A and 1B). Milos Island is located in the South Aegean Sea and is part of the Hellenic Volcanic Arc [50, 51] (Fig 1A). The volcanic activity that is responsible for active gaseous hydrothermal venting on and around the island has occurred since the Pliocene. The field sampling, sample preservation, and analytical protocols used in this study were based on those described in detail elsewhere [36, 38, 47, 49, 52–54], with minor modifications for our specific field location.
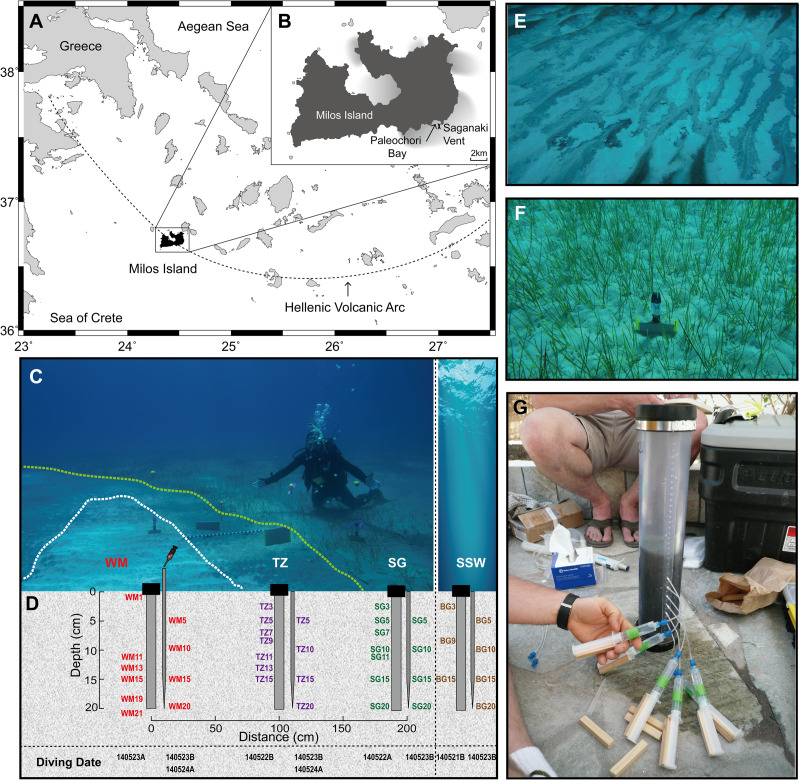
Fig 1. Site map.
(A) and (B) Location of the Saganaki diffuse vent in Paleochori Bay (~300 m offshore, 12 m water depth). (C) Photograph of Saganaki showing three of the biogeographic zones: white mat (WM), transition zone (TZ), and seagrass area (SG). (D) Schematic of sampling methods (push cores and long pipettes indicating the position and depth (in cm) of samples collected for geochemistry and sequencing analysis. (E) Photograph of white mat area. (F) Photograph of seagrass area. (G) Photograph of porewater sampling from sediment cores using rhizons.
A SCUBA diving team measured in situ temperatures and collected fluid (via long pipette) and gas samples from the vent system. Large volumes of fluids with coarse resolution were collected under water through long pipette tips inserted directly into the sediments (~5 cm depth interval, from 5 to 20 cm deep) and attached to 60-mL syringes (Fig 1C and 1D). Free gas samples were obtained with a stainless-steel funnel placed on sites with visible gas bubbles. Glass serum bottles with blue rubber stoppers were filled with seawater before the dive and then connected to the top of the funnel. Once the gas completely replaced the seawater and flushed through for approximately 5 minutes, the valves were closed. Sediment cores (in polycarbonate tubes) were collected and sealed underwater with rubber caps. Sites included the center of a white mat (WM), through a transition zone (TZ) and a sea grass-covered region (SG), ending in a background area (BG) (Fig 1C, 1D, 1E, and 1F).
On shore, waters were carefully transferred into pre-cleaned serum bottles and capped without trapping any air (for dissolved gas analyses) or filtered (0.2 μm) and stored as described below for other analyses. Rhizons (0.2 μm filter) were inserted into pre-drilled holes of the polycarbonate tubes at 2 cm intervals from 0 to 20 cm to obtain high-resolution samples while avoiding fluid reflux from different depths (Fig 1G). All porefluids were analyzed for pH and then subsampled and treated for later geochemical measurements. The subsamples were stored in acid-washed polypropylene bottles for organic acid and anion analyses; acidified with 2% ultrapure HNO3 in acid-washed plastic bottles for cation analyses; acidified with 2% HCl and kept in opaque acid-washed glass bottles for arsenic speciation; fixed with zinc acetate solution for sulfide analysis; and stored in acid-washed and combusted glass vials for dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) analyses.
Samples for major anions (F-, Cl-, Br-, SO42-, NO3-, NO2-, PO43-) were analyzed on a Metrohm 850 Professional ion chromatograph (IC). Major and minor cation (Li+, Na+, K+, Mg2+, Ca2+, Sr2+, Ba2+, Si2+, B3+, Mn2+, Fe2+) samples were measured on a Perkin-Elmer Optima inductively coupled plasma atomic emission spectrometer (ICP-OES). Samples set aside for arsenic (As3+, As5+, monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA)) concentrations were analyzed on a Dionex ion chromatograph coupled to a PSAnalytical atomic fluorescence spectrometer (IC-AFS). Sulfide samples were analyzed by spectrophotometry with the Cline method. Dissolved organic carbon (DOC) was determined using high-temperature combustion on a Shimadzu Total Organic Carbon Analyzer (TOC-V) at the DOM Analytical Lab, Marine Science Institute, University of California-Santa Barbara. The dissolved gases were extracted from water samples after equilibrium was attained between the water sample and a known volume of high purity argon, which was injected directly into the serum bottles. Both free and dissolved gases were measured with a Shimadzu GC-2014ATF headspace gas chromatograph equipped with TCD and FID detectors.
Thermodynamic modeling
The maximum amount of available energy from potential chemolithoautotrophic reactions at the temperature, pressure, and chemical composition of interest is given by the Gibbs energy (ΔGr). Values of ΔGr were calculated using the relation
| (1) |
where ΔGr0 denotes the standard state Gibbs energy of reaction, R designates the universal gas constant, T stands for the temperature in Kelvin, and Qr represents the reaction quotient. Values ΔGr0 were calculated at the temperatures and pressures of interest with the revised-Helgeson-Kirkham-Flowers (HKF) [55–57] equations of state using OrganoBioGeoTherm (OBIGT)—which is a user-friendly version of the SCUPCRT92 software package [58]—and thermodynamic data from several sources [56, 59–65].
Values of Qr were calculated with
| (2) |
where ai designates the activity of the ith species raised to its stoichiometric reaction coefficient vi,r, in the rth reaction, which is positive for products and negative for reactants. The activities of pure minerals (pyrite (FeS2), elemental sulfur (S0), magnetite (Fe3O4), hematite (Fe2O3), goethite (FeOOH), ferrihydrite (FeOOH), and pyrolusite (MnO2)) and water are taken to be unity (ai = 1). Molalities of the ith species in solution (mi) were obtained as noted above, and converted into activities using the individual activity coefficient of the ith species, γi:
| (3) |
Values of activity coefficients were calculated using the program SPEC8 (Geochemist’s Workbench Version 11, Aqueous Solutions LLC) employing the extended Debye-Hückel equation [66]. Aqueous activities of dissolved gases (H2, CH4, O2, CO, CO2, CH4) were calculated from free gas composition data assuming equilibrium. The reactions under consideration include numerous potential electron acceptors (O2, CO, CO2, HCO3-, N2, NO2-, NO3-, pyrite, elemental sulfur, magnetite, hematite, goethite, ferrihydrite, pyrolusite, H2AsO4-,) and donors (H2, CH4, CO, NH4+, N2, NO2-, H2S, pyrite, elemental sulfur, magnetite, Mn2+, H3AsO3,) (S1 Table). To permit us to evaluate the energetics of both the forward and reverse direction of every reaction, we also include H2O as a potential electron acceptor (where H in H2O can be reduced) and electron donor (where O in H2O can be oxidized).
To facilitate comparisons among reactions, values of ΔGr were normalized to the number of moles of electrons transferred in the redox process [14]. In order to scale energy availability to the limiting reactant, the Gibbs energies are also presented in terms of energy densities, Er [67]. To normalize the Er on a ‘per kg of venting fluid’ and on a ‘per kg of sediment’ basis, values of ΔGr were multiplied by the concentration of the limiting reactant in the fluid. The energy densities in fluid (Efluid) were calculated by
| (4) |
where [mi,fluid] refers to the molal concentration of the ith limiting electron donor or acceptor per kg of fluid, taking the stoichiometry of the reaction into account. The energy densities in sediment (Esediment) were calculated with
| (5) |
where [mi, sediment] is the molal concentration of the ith limiting electron donor or acceptor per kg of sediment, considering the porosity of sediments and the density of grains in them (Table 1). We did not evaluate the energy densities for reactions in which solid phases serve as both electron donor and acceptor (Reactions K66-68, L38-43, N17-19, O33-38, P33-38, Q33-38, R16-20, R25-27).
Table 1. Selected sediment properties and concentrations of Fe, Mg and S in solid phases in the Milos shallow-sea hydrothermal system.
| Parameter | Reference | |
|---|---|---|
| Porosity (%) | 36 | [68] |
| Grain density (g/cm3) | 2.66 | [68] |
| Wet density (g/cm3) | 2.07 | This study |
| Mean composition of clay pelites (%) | 6.53 | [69] |
| Mean Fe concentration in pelites (%) | 3.4 | [69] |
| Mean Mn concentration in pelites (ppm) | 1685 | [69] |
| Total Fe in sediment (%) | 0.22 | This study |
| Total Mn in sediment (%) | 0.011 | This study |
| Total S in 1g dry sediment | 10 μM | Unpublished data |
Mixing model
Values of ΔGr for all of the reactions listed in S1 Table were also calculated for different mixing ratios of end-member hydrothermal fluid (HF, also referred to as vent fluid) with seawater. The composition of the end-member HF was taken to be the average of white mat samples, and that for seawater was taken from Table 2. The activities of species that are very low in the end-member HF (e.g., oxygen) were taken to be 10−9. Although the compositions of the mixed fluids are simply proportional to the ratio of end-member fluid to seawater, the temperatures of the mixed fluids (Tmix) are not a linear combination of the source fluids due to their differing heat capacities. These values were calculated using
| (6) |
where mi, Cpi, and Ti refer to the mass, specific heat capacity, and temperature (K) of the ith fluid. Values of Cpi were calculated using the equations of state for water in SUPCRT92 [70, 71]. The temperature of the end-member HF was estimated by extrapolation using the [Mg] = 0 method [72, 73].
Table 2. Temperature, pH and composition of porefluids and seawater sampled at or near the Saganaki diffuse vent, Milos Island, Greece.
| Date-Dive-Type-Depth(cm) | T | pH | Na+ | K+ | Mg2+ | Ca2+ | Sr2+ | Ba2+ | Fe2+ | Mn2+ | As3+ | As5+ | Cl- | Br- | NO2- | NO3- | SO42- | SiO2 | H2S/HS- | DOC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| oC | mM | mM | mM | mM | μM | μM | μM | μM | μM | μM | mM | mM | mM | mM | mM | mM | μM | μM | ||
| White Mat | ||||||||||||||||||||
| 140523A-WM1 | 6.55 | 531.0 | 11.0 | 58.8 | 11.9 | 99.4 | 0.32 | 1.84 | 1.79 | 610.7 | 1.51 | b.d. | b.d. | 26.70 | 0.21 | 3.84 | ||||
| 140523A-WM5 | 5.37 | 498.9 | 12.4 | 53.8 | 11.9 | 91.3 | 0.75 | 1.91 | 28.31 | 573.7 | 0.72 | b.d. | b.d. | 25.97 | 2.72 | 324.67 | ||||
| 140523A-WM11 | 39.5 | 4.97 | 490.4 | 12.4 | 52.9 | 11.9 | 91.7 | 0.74 | 1.16 | 29.56 | 0.24 | b.d. | 564.0 | 0.72 | b.d. | b.d. | 24.29 | 2.95 | 1402.31 | |
| 140523A-WM13 | 4.89 | 496.6 | 13.5 | 53.0 | 12.6 | 96.6 | 0.67 | 0.34 | 30.75 | 0.23 | b.d. | 571.1 | 0.71 | b.d. | b.d. | 28.27 | 2.89 | 1115.29 | ||
| 140523A-WM15 | 47.5 | 4.86 | 499.3 | 13.2 | 53.7 | 12.5 | 95.7 | 0.69 | 0.28 | 28.42 | 0.17 | b.d. | 574.2 | 0.74 | b.d. | b.d. | 24.07 | 2.90 | 1366.07 | |
| 140523A-WM19 | 53.0 | 5.12 | 477.1 | 12.5 | 51.6 | 11.9 | 91.6 | 0.70 | 0.43 | 27.11 | 0.24 | b.d. | 548.7 | 0.71 | b.d. | b.d. | 28.04 | 2.77 | 711.94 | |
| 140523A-WM21 | 4.94 | 471.9 | 11.9 | 51.6 | 11.7 | 90.0 | 0.61 | 0.15 | 24.57 | 542.7 | 0.74 | b.d. | b.d. | 24.46 | 2.70 | 1169.07 | ||||
| 140523B-WM5 | 51.8 | 4.56 | 401.2 | 25.8 | 26.6 | 15.0 | 115.1 | 1.13 | 93.43 | 78.30 | 0.13 | 0.002 | 461.4 | 0.60 | 0.16 | b.d. | 14.35 | 3.73 | 960.40 | |
| 140523B-WM10 | 66.7 | 4.44 | 402.3 | 25.9 | 26.7 | 14.9 | 115.1 | 1.23 | 95.65 | 73.63 | 0.23 | 0.003 | 462.6 | 0.62 | 0.17 | b.d. | 13.73 | 3.70 | 1133.67 | |
| 140523B-WM15 | 72.6 | 4.66 | 412.1 | 22.7 | 31.9 | 13.4 | 107.1 | 1.10 | 69.98 | 51.06 | 0.44 | 0.010 | 473.9 | 0.66 | 0.18 | b.d. | 17.38 | 3.25 | 1043.87 | |
| 140523B-WM20 | 76.2 | 4.59 | 414.2 | 23.7 | 30.9 | 14.0 | 110.7 | 1.17 | 65.50 | 53.99 | 0.61 | 0.003 | 476.3 | 0.67 | 0.15 | b.d. | 13.39 | 3.37 | 1325.41 | |
| 140524A-WM5 | 38.9 | 4.71 | 411.3 | 10.4 | 44.6 | 8.1 | 66.0 | 0.60 | 6.99 | 44.80 | 0.32 | 0.002 | 473.0 | 0.60 | 0.16 | b.d. | 23.17 | 3.05 | 991.53 | 1036.98 |
| 140524A-WM10 | 57.6 | 4.70 | 410.7 | 10.1 | 44.5 | 8.1 | 66.2 | 0.68 | 6.09 | 43.14 | 0.28 | 0.001 | 472.3 | 0.62 | 0.16 | b.d. | 22.83 | 3.02 | 838.92 | 1086.84 |
| 140524A-WM15 | 66.5 | 4.68 | 409.6 | 10.2 | 44.4 | 8.1 | 65.9 | 0.71 | 5.80 | 42.97 | 0.27 | 0.001 | 471.0 | 0.66 | 0.18 | b.d. | 21.37 | 3.03 | 1037.74 | 1203.65 |
| 140524A-WM20 | 71.3 | 4.67 | 413.6 | 10.0 | 44.9 | 8.2 | 66.9 | 0.81 | 7.07 | 40.25 | 0.24 | 0.001 | 475.6 | 0.67 | 0.17 | b.d. | 20.81 | 2.94 | 1631.73 | 1198.96 |
| Transition Zone | ||||||||||||||||||||
| 140522B-TZ3 | 19.1 | 5.56 | 522.4 | 10.7 | 58.3 | 11.7 | 98.4 | 0.24 | 69.13 | 3.22 | 0.94 | b.d. | 600.8 | 0.90 | b.d. | b.d. | 33.00 | 0.72 | 11.02 | |
| 140522B-TZ5 | 23.0 | 5.64 | 520.5 | 10.6 | 58.1 | 11.7 | 98.1 | 0.30 | 50.58 | 3.32 | 0.37 | b.d. | 598.6 | 0.75 | b.d. | b.d. | 32.36 | 1.04 | 469.85 | |
| 140522B-TZ7 | 25.0 | 5.27 | 518.4 | 10.6 | 58.0 | 11.6 | 97.7 | 0.34 | 11.17 | 3.21 | 0.85 | b.d. | 596.2 | 0.79 | b.d. | b.d. | 34.45 | 1.21 | 823.59 | |
| 140522B-TZ9 | 27.0 | 5.21 | 521.1 | 10.6 | 58.4 | 11.7 | 99.2 | 0.36 | 2.77 | 3.32 | 0.66 | b.d. | 599.3 | 0.77 | b.d. | b.d. | 33.17 | 1.32 | 275.05 | |
| 140522B-TZ11 | 28.0 | 5.19 | 516.2 | 10.4 | 57.9 | 11.6 | 97.9 | 0.35 | 0.37 | 3.42 | 0.84 | b.d. | 593.6 | 0.77 | b.d. | b.d. | 33.00 | 1.36 | 729.99 | |
| 140522B-TZ13 | 30.0 | 5.12 | 526.1 | 10.6 | 59.0 | 11.8 | 99.8 | 0.44 | 0.99 | 3.01 | 1.40 | b.d. | 605.0 | 0.74 | b.d. | b.d. | 32.37 | 1.29 | 1228.93 | |
| 140522B-TZ15 | 31.0 | 5.12 | 524.3 | 10.6 | 58.8 | 11.8 | 99.5 | 0.38 | 0.22 | 3.73 | 0.47 | b.d. | 602.9 | 0.74 | b.d. | b.d. | 31.57 | 1.40 | 1162.86 | |
| 140523B-TZ5 | 23.1 | 5.15 | 518.4 | 10.5 | 57.6 | 12.1 | 96.6 | 0.39 | 8.10 | 7.91 | 0.06 | 0.001 | 596.2 | 0.77 | 0.22 | b.d. | 28.77 | 1.59 | 1122.10 | |
| 140523B-TZ10 | 27.2 | 4.88 | 528.0 | 10.8 | 58.4 | 12.4 | 99.2 | 0.41 | 0.28 | 9.46 | 0.04 | b.d. | 607.2 | 0.78 | 0.23 | b.d. | 28.55 | 1.88 | 1168.38 | |
| 140523B-TZ15 | 31.0 | 4.87 | 518.2 | 10.6 | 57.3 | 12.2 | 97.4 | 0.40 | b.d. | 9.25 | 0.02 | b.d. | 595.9 | 0.77 | 0.21 | b.d. | 25.97 | 1.86 | 1233.12 | |
| 140523B-TZ20 | 34.2 | 4.76 | 512.3 | 10.5 | 56.7 | 12.1 | 96.0 | 0.39 | b.d. | 9.32 | 0.03 | b.d. | 589.1 | 0.77 | 0.21 | b.d. | 29.50 | 1.90 | 1190.97 | |
| 140524A-TZ5 | 21.9 | 5.00 | 513.5 | 10.4 | 57.3 | 11.6 | 96.1 | 0.25 | 9.20 | 2.38 | 0.10 | 0.001 | 590.5 | 0.77 | 0.21 | b.d. | 27.99 | 0.78 | 896.83 | 1287.39 |
| 140524A-TZ10 | 23.9 | 4.77 | 512.2 | 10.5 | 57.0 | 11.6 | 96.8 | 0.33 | 2.36 | 2.67 | 0.08 | 0.001 | 589.0 | 0.78 | 0.21 | b.d. | 26.87 | 1.22 | 825.40 | 1133.40 |
| 140524A-TZ15 | 25.9 | 4.79 | 503.9 | 10.3 | 56.1 | 11.4 | 96.0 | 0.34 | 4.16 | 2.45 | 0.11 | 0.001 | 579.5 | 0.77 | 0.23 | b.d. | 28.66 | 1.26 | 1619.58 | 1063.70 |
| 140524A-TZ20 | 28.5 | 4.80 | 511.2 | 10.4 | 56.8 | 11.6 | 97.1 | 0.36 | 2.03 | 2.39 | 0.18 | 0.002 | 587.9 | 0.77 | 0.21 | b.d. | 24.74 | 1.30 | 1081.85 | 1188.49 |
| Seagrass | ||||||||||||||||||||
| 140522A-SG3 | 19.1 | 7.04 | 530.8 | 11.7 | 59.4 | 11.6 | 98.6 | 0.14 | 23.52 | 1.08 | 0.50 | 0.058 | 610.4 | 0.93 | b.d. | b.d. | 26.47 | 0.02 | 0.28 | |
| 140522A-SG5 | 19.1 | 7.12 | 525.7 | 11.6 | 58.9 | 11.5 | 98.1 | 0.20 | 11.21 | 0.82 | 604.6 | 0.87 | b.d. | b.d. | 25.97 | 0.02 | 1.27 | |||
| 140522A-SG7 | 19.1 | 538.3 | 12.1 | 59.4 | 11.7 | 99.5 | 0.16 | 11.05 | 1.12 | 0.66 | 0.053 | 619.0 | 0.91 | b.d. | b.d. | 25.41 | 0.02 | 0.21 | ||
| 140522A-SG11 | 19.1 | 7.13 | 538.1 | 12.1 | 59.5 | 11.7 | 99.0 | 0.18 | 12.01 | 0.91 | 0.41 | 0.077 | 618.8 | 0.94 | b.d. | b.d. | 26.31 | 0.03 | 0.42 | |
| 140522A-SG15 | 19.1 | 7.05 | 535.6 | 11.9 | 59.3 | 11.7 | 99.3 | 0.19 | 11.84 | 0.86 | 1.16 | 0.135 | 615.9 | 0.01 | b.d. | b.d. | 25.58 | 0.03 | 0.78 | |
| 140523B-SG5 | 19.8 | 6.80 | 534.3 | 11.8 | 59.1 | 11.6 | 98.7 | 0.13 | 14.38 | 0.83 | 0.49 | 0.001 | 614.4 | 0.87 | 0.21 | b.d. | 26.36 | 0.03 | 1.20 | 1219.35 |
| 140523B-SG10 | 20.3 | 6.54 | 534.9 | 11.8 | 59.3 | 11.6 | 98.5 | 0.15 | 17.02 | 1.17 | 0.41 | 0.001 | 615.1 | 0.98 | 0.23 | b.d. | 28.04 | 0.04 | 0.69 | 1210.81 |
| 140523B-SG15 | 20.8 | 6.84 | 530.1 | 11.7 | 58.7 | 11.5 | 98.2 | 0.16 | 20.56 | 1.36 | 0.41 | 0.017 | 609.6 | 0.86 | 0.23 | b.d. | 28.27 | 0.05 | 2.84 | 1160.12 |
| 140523B-SG20 | 21.3 | 6.93 | 536.4 | 11.9 | 59.3 | 11.7 | 98.8 | 0.18 | 21.20 | 1.51 | 0.45 | 0.011 | 616.9 | 0.90 | 0.20 | b.d. | 25.69 | 0.05 | 1.89 | 1323.48 |
| Background | ||||||||||||||||||||
| 140521B-BG3 | 19.1 | 6.34 | 524.8 | 11.4 | 58.7 | 11.4 | 97.1 | 0.08 | b.d. | b.d. | 0.10 | b.d. | 603.5 | 0.90 | b.d. | b.d. | 32.31 | b.d. | 0.21 | 481.33 |
| 140521B-BG9 | 19.1 | 6.75 | 527.4 | 11.5 | 59.1 | 11.5 | 98.2 | 0.13 | 2.28 | 0.07 | 0.01 | b.d. | 606.5 | 0.84 | b.d. | b.d. | 29.81 | 0.02 | b.d. | 773.76 |
| 140521B-BG15 | 19.2 | 6.64 | 528.4 | 11.5 | 59.1 | 11.5 | 98.6 | 0.15 | 14.74 | 0.39 | 0.17 | b.d. | 607.7 | 0.90 | b.d. | b.d. | 32.05 | 0.04 | 1.41 | 649.64 |
| 140523B-BG5 | 19.2 | 6.88 | 531.4 | 12.0 | 58.6 | 11.6 | 98.6 | 0.12 | 0.83 | 2.07 | 0.21 | 0.002 | 611.1 | 0.91 | 0.21 | b.d. | 23.39 | 0.05 | 0.19 | 1198.69 |
| 140523B-BG10 | 19.1 | 6.62 | 529.2 | 11.6 | 58.6 | 11.5 | 97.3 | 0.11 | b.d. | 0.18 | 0.04 | 0.001 | 608.6 | 0.82 | 0.23 | b.d. | 30.14 | 0.01 | 0.32 | 1134.78 |
| 140523B-BG15 | 19.1 | 7.09 | 527.7 | 11.6 | 58.5 | 11.5 | 97.6 | 0.13 | 0.34 | 0.05 | 0.06 | 0.002 | 606.9 | 0.81 | 0.20 | b.d. | 21.71 | 0.02 | 0.06 | 1019.07 |
| 140523B-BG20 | 19.0 | 7.14 | 539.2 | 11.9 | 59.6 | 11.7 | 99.2 | 0.15 | 1.33 | 0.03 | 0.09 | 0.003 | 620.1 | 0.92 | 0.21 | b.d. | 25.75 | 0.03 | 0.26 | 1089.60 |
| Seawater | ||||||||||||||||||||
| 140525A-SW | 19.0 | 7.4 | 528.5 | 11.6 | 59.2 | 11.6 | 98.5 | 0.06 | b.d. | 0.2 | 0.1 | b.d. | 607.8 | 1.01 | 0.23 | b.d. | 28.4 | 0.01 | 0.100 | 528.85 |
| Calculated Endmember | ||||||||||||||||||||
| Average | 199.41 | 4.12 | 119.98 | 29.56 | 0.00 | 11.57 | 61.04 | 3.75 | 121.82 | 204.17 | 0.79 | 0.01 | 18.09 | 0 | 0 | 0 | 0 | 14.73 | 5180.57 | - |
b.d. = below detection.
Results and discussion
Sample location and geochemistry
The 47 fluid samples from the Saganaki vent area (Fig 1B and Table 2) represent different mixing ratios and physicochemical properties of venting fluids in this system. The hottest area (up to 76.2°C) was covered by a ~1 cm thick, fluffy, white mat (WM) (Figs 1C, 1E, 2 and Table 2). A ~1 m wide transition zone (TZ) separates a flourishing seagrass (SG) area from the diffuse venting site (Fig 1C). Fifteen samples were taken from WM, 15 from TZ, 9 from SG, 7 from background sediment (BG) and 1 from surface seawater (SW).
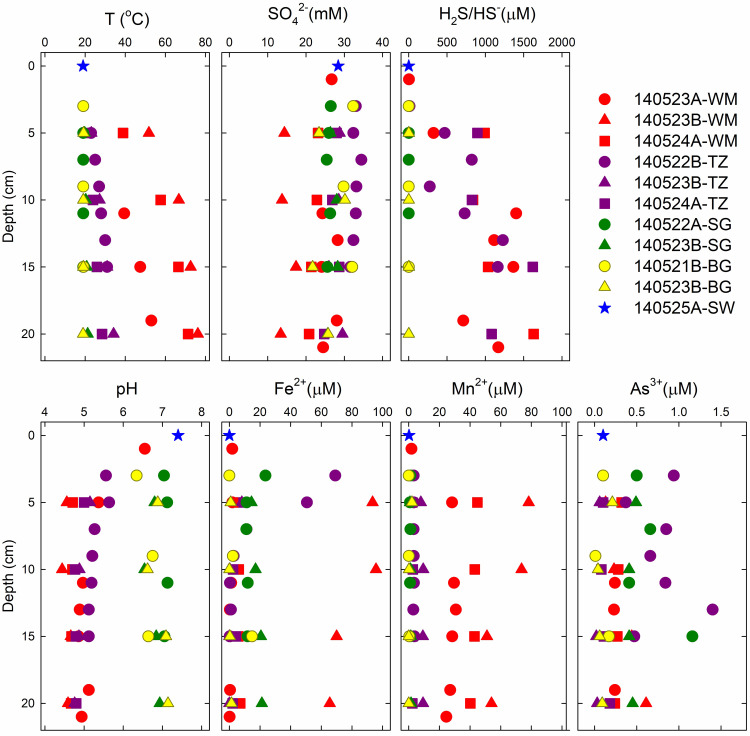
Fig 2. Geochemical profiles of temperature, pH, and selected ions.
Red symbols refer to white mat (WM), purple to transition zone (TZ), green to seagrass (SG), yellow to background (BG) and blue to surface seawater (SW). Different symbols of the same color indicate different date/time of the sampling dives.
Fluid measurements (Fig 2 and Table 2) reveal a wide range of temperature (19.1–76.2°C) and pH (4.4–7.4), as well as sharp differences in geochemistry for the five sampling regions. For example, concentrations of SO42- (13.4–34.5 mM), Na+ (401.2–539.2 mM), and Mg2+ (26.6–59.6 mM) increase as a function of distance from the diffuse vent in the WM area. In contrast, K+ levels were almost twice as high (22.7–25.9 mM) at WM than at other sites (10.3–12.1 mM). NO3- concentrations were below detection (b.d., 8 μM) in all samples, but NO2- levels were relatively high (0.15–0.23 mM) in several samples. We note that very low nitrate levels (< 0.5–3.5 μM) have previously been reported in Mediterranean surface waters, especially around Greece [74], and near mM concentrations of nitrite are known to occur in marine sediments [75]. Concentrations of reduced species, such as H2S, Fe2+, and Mn2+ range from b.d. (1 μM, 0.1 μM, and 0.04 μM, respectively) to relatively high levels (93 μM, 78 μM, and 1631 μM, respectively). Consistent with the stable and conservative nature of salinity in seawater (i.e., the principle of constant proportions), and to maintain required charge balance, concentrations of Cl- in Table 2 were calculated from a well-established Cl-:Na+ ratio of 1.15 (e.g., [76]).
Porefluid energetic potential
Values of ΔGr for the 730 reactions listed in S1 Table were calculated with Eqs (1–4) using the fluid compositions and environmental conditions given in Tables 2 and 3. Values of ΔGr in kJ per mole of electron transferred are depicted in Fig 3A for the 379 reactions that are exergonic (ΔGr<0) in at least one sampling location. The reactions are plotted from top (-162.3 kJ/mole e-) to bottom (near 0 kJ/mole e-) in order of the average energy yield at all 47 sampling sites. It can clearly be seen that for most reactions, the energy yields vary by 20–50 kJ/mol e- across the different locations and sample depths; in a number of examples, especially reactions with iron (e.g. L34: Fe2+/S0, and N16: H2S/Fe3O4), the range approaches and even exceeds 80 kJ/mol e-. Reactions with nitrite (purple bar) and oxygen (light grey bar) as electron acceptors and with CO as electron donor are the most exergonic, with the top 10 reactions (electron donor/acceptor) being CO/NO2- (reaction H13, -162.3 kJ/mole e-), CO/NO2-(H14, -151.4 kJ/mole e-), CO/O2 (B5, -147.9 kJ/mole e-), CO/NO3- (I18, -139.9 kJ/mole e-), CO/O2 (B6, -142.6 kJ/mole e-), CO/MnO2 (R5, -140.9 kJ/mole e-), H2S/NO2- (H19, -136.0 kJ/mole e-), CO/NO3- (I19, -131.0 kJ/mole e-), CO/MnO2 (R6, -130.7 kJ/mole e-), CO/FeOOHFer (Q13, -124.9 kJ/mole e-) (Fig 3A).
Table 3. Dissolved and free gas composition in white mat (WM), transition zone (TZ) and background (BG) areas.
| Sample | H2 | O2 | N2 | CO2 | CH4 | CO |
|---|---|---|---|---|---|---|
| WM Free Gas (%) | b.d. | 2.72 | 18.00 | 61.36 | 0.299 | 0.025 |
| WM Dissolved Gas at 10cm (μM) | b.d. | 0.496 | 38.5 | 11.93 | 0.038 | 0.076 |
| TZ Dissolved Gas at 5cm (μM) | b.d. | 1.001 | 76.6 | 2.725 | 0.089 | b.d. |
| BG (μM) | b.d. | 220 | 456.00 | 2.638 | b.d. | b.d. |
| End-member (average) (μM) | 0 | 0 | 0 | 47.304 | 0.183 | 0.365 |
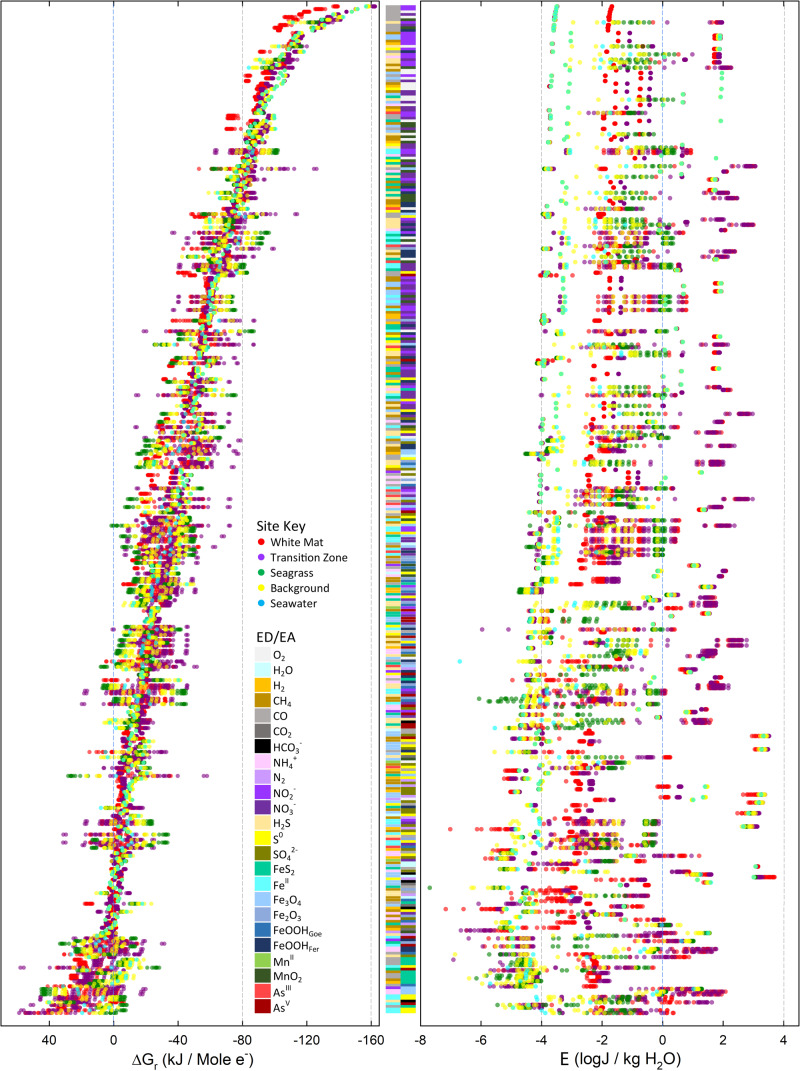
Fig 3. Overall Gibbs energy yields of the catabolic reactions considered in this study.
Circles refer to values of ΔGr of the 379 exergonic reactions shown in S2 Table at individual sample sites in units of kJ/mol e- (A) and J/kg fluid (B). The colors of the circles encode for the five biogeographic regions (see key). The colored bars in the middle of the two panels refer to the identities of electron acceptors and donors in the reactions as noted in the key. The reactions are ordered from most exergonic at the top to least exergonic at the bottom, based on the averages of ΔGr values from all samples.
In Fig 3B, energy yields are plotted as energy densities (Efluid). Again, the range of energy yields across sites and sample depths vary tremendously, often exceeding 6 orders of magnitude in J/kg H2O. This broad range is due to the rapid dilution of hydrothermal fluid with seawater, whereby the concentrations of some key redox species change by 2–3 orders of magnitude over short distances (Table 2). For example, the concentration of Mn in WM (28.31–78.30 μM) is 150–400 times that in seawater, and therefore Efluid of Mn-redox reactions point to a much larger energy potential than the corresponding value of ΔGr would indicate. In addition, one of the consequences of setting the activities of pure minerals to 1.0 means that energy densities of reactions involving hematite, goethite, ferrihydrite, pyrolusite, pyrite, elemental sulfur, and magnetite are high (100–10,000 J/kg H2O). Finally, we note that the most exergonic reactions in Fig 3B are not the same as those in Fig 3A. In terms of energy density, the oxidation of sulfide, sulfur, and ammonia are thermodynamically most favorable. A phylogenetic analysis (16S rRNA) of hydrothermal sediments at Milos showed that heterotrophs and sulfur oxidizers were among the most abundant [77]. In fact, several aerobic and anaerobic sulfur oxidizers have been isolated from Milos, including Halothiobacillus kellyi [78], Stetteria hydrogenophila [79], Thiomicrospira sp. Milos-T1 [80], and Gamma Proteobacteria Milos strain ODI4G, OBII5, ODIII6, OBII5 [81].Values of ΔGr and Efluid for the exergonic reactions shown in Fig 3 are re-plotted as colored circles in Fig 4, but with average values for each of the five regions and classified by electron donors (Fig 4A and 4C) and electron acceptors (Fig 4B and 4D). In addition, the total average energy yields for all 47 samples are also plotted (black squares). In terms of ΔGr, reactions with O2 as the electron acceptor supplied the most energy, followed by reactions with NO3-, NO2-, and MnO2, and then reactions with iron minerals, S0, AsV, SO42-, and inorganic carbon. In units of energy density (Efluid), reactions with ammonia and sulfide as electron donors are the most exergonic, especially in the WM and TZ regions (Fig 4C). When color-coded by electron acceptor (Fig 4D), we note that values of Efluid are generally highest (with some exceptions) in the WM region, followed by the TZ, SG, BG, and SW. See LaRowe and Amend (2019) for a discussion on reaction energetics in molal versus density units [82]. This difference can best be seen in reactions with CO, where energy values are -132 to -162 kJ/mol e-, but only 1.7 to 3.5 J/kg H2O (e.g. reaction H13). Similarly, for the oxidation of methane, carbon monoxide, and ammonium, values of the energy densities are more exergonic than the per-electron counterparts reveal, particularly in the WM and TZ regions. Because the energy densities of sulfide oxidation are so variable, it is likely that the importance of this process is highly localized, with notable potential in the more hydrothermally influenced areas (WM, TZ). Energy densities for iron, manganese, and arsenite oxidation reactions are also scattered, while those for the oxidation of elemental sulfur, pyrite and magnetite show no clear trends. Energy densities for the WM and TZ regions appearing on the right hand (i.e., more exergonic) side of Fig 4D indicates that chemolithotrophic primary production has a positive correlation with temperature, or hydrothermal source.
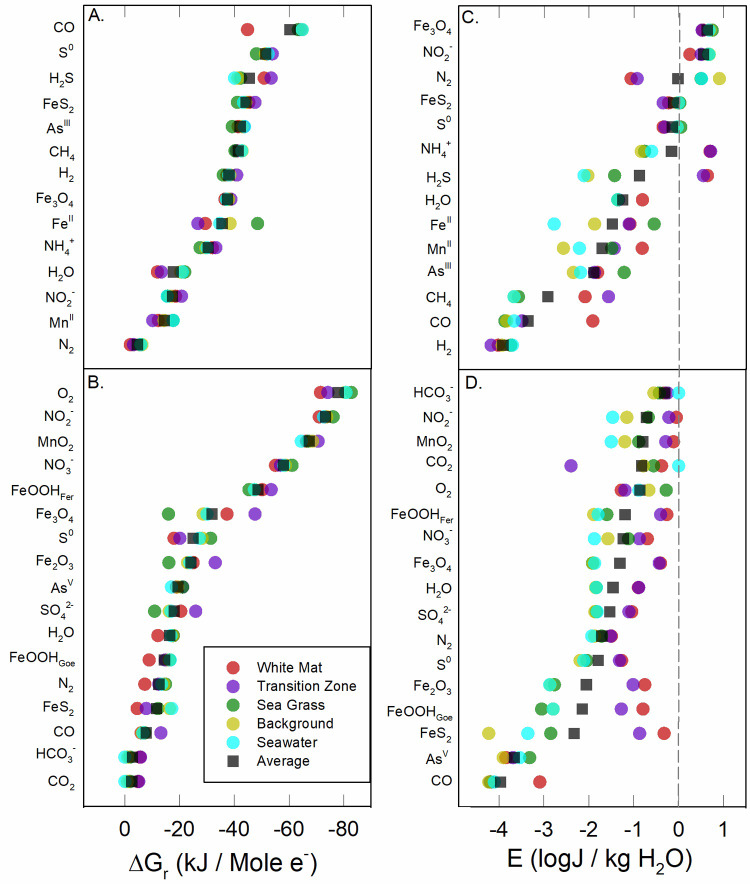
Fig 4. Average Gibbs energy yields of exergonic reactions.
Average values of ΔGr (panels A and B, in kJ/mol e-) and Efluid (panels C and D, in J/kg H2O) grouped by electron donors (panels A and C) and electron acceptors (panels B and D) for the five biogeographic regions considered in this study.
Similar studies of redox reaction energetics for putative chemotrophic metabolisms have been carried out for geochemically diverse hot springs in Yellowstone National Park [26], shallow-sea hydrothermal systems in the Aeolian Islands (Italy) [22, 38] and the continental subsurface at the Sanford Underground Research Facility (SURF) in South Dakota (USA) [83]. Analogous to the present study at Milos, those communications concluded that reactions with energy yields >100 kJ/mol e- are rare and involve O2 or NO3-/NO2- as electron acceptors. It should be noted that the maximum yields in the Milos system are at ~160 kJ/mol e-, while those in the Aeolian Islands are ~120 kJ/mol e-, in Yellowstone hot springs are ~110 kJ/mol e-, and at SURF are ~100 kJ/mol e-. Nine of the most exergonic reactions at Milos are aerobic or anaerobic carbon monoxide oxidation. We note two reasons for the higher ΔGr yields at Milos than at other sites: First, CO and NO2- have large and opposite redox potentials, which tends to lead to large Gibbs energies of reaction. Second, we measured and considered a larger range of redox sensitive species than most other studies, which rarely include CO and NO2- because they decay quickly, or their concentrations are below detection limits.
This set of investigations also demonstrated that in a number of examples, changes in chemical composition—with pH being a major driver—can ‘flip’ a reaction from exergonic to endergonic. In other words, the forward reaction may serve as a putative metabolism in some environments, while the reverse direction could do so at very different geochemical conditions. Similarities among these different studies are also observed when energy densities are considered. (Note that studies focused on the shallow-sea vents at Vulcano, Aeolian Islands [22] and Yellowstone hot springs [26] did not provide such results. In each case, the most exergonic reactions in terms of energy density are different from those labeled as most exergonic in ΔGr space.
Bulk sediment energetic potential
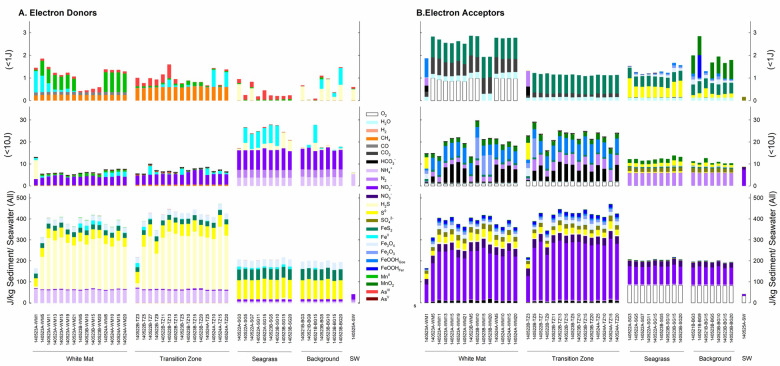
The calculations summarized above only considered porefluids and not the solid phase minerals that can be used as energy sources. Following the same color scheme as in Fig 3, the amount of energy available per kg of sediment (Esediment, J/kg sediment) is shown in Fig 5A (by electron donor) and 4B (by electron acceptor). The bottom panels reveal total Esediment for all reactions, while the middle and top panels show values of Esediment <1 J/kg sediment and <10 J/kg sediment, respectively. It can be seen in the bottom panel in Fig 5A that reactions with ammonia, sulfide, sulfur, magnetite, and pyrite as electron donors are the most exergonic; the bottom panel in Fig 5B shows that reactions with nitrate, nitrite, sulfur and sulfate as electron acceptors provide the most energy. These panels reveal three energy-based habitats: (1) hydrothermal-influenced sediments in the WM and TZ regions, (2) seawater-influenced sediments in the SG and BG areas and (3) surface seawater. Energy yields are similar in the WM and TZ regions, increasing with depth at both locations. Conversely, depth is not a correlating factor in the SG and BG areas. Reactions that provide relatively small amounts of energy are shown in the middle and top panels in Fig 5, with patterns of energy yields changing with the distance from the diffuse vent. Both the WM and TZ settings show increasing energy yield with sediment depth, and the dominance of aerobic respiration in seawater-influenced sediments and seawater.
Fig 5. Energy densities at all sample locations and depths.
Colored bars refer to identities of different electron donors (A) and electron acceptors (B). The upper and middle panels zoom in on reactions with low energy yields (<1 J and <10 J, respectively). The energy densities refer to either those in 1 kg sediment or 1 kg seawater. See S1 Table for details on the reactions.
From an energetics perspective, oxygen/nitrate/nitrite and sulfur/sulfide/ammonia should be the main electron acceptors and donors, respectively, to be used by chemolithotrophs at Milos (Fig 5). Based on values of Gibbs energy and elevated levels of abundance, sulfide oxidation coupled to nitrite reduction could support much of the chemolithotrophic primary production. Some evidence, though limited, is available to support this claim: three chemolithotrophic nitrate-reducing sulfate-oxidizing bacteria have been isolated from Milos [81] and isotopic evidence of microbial sulfate reduction in the TZ has been reported [84].
Mixed fluid (SW:HF) energetic potential
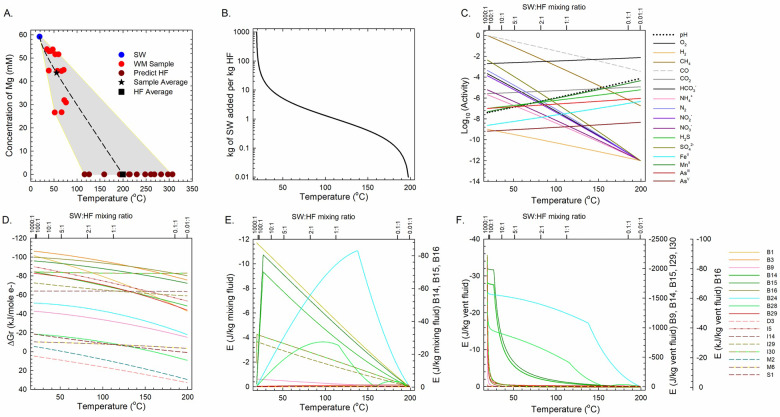
The estimated, weighted average temperature of the end-member HF, based on Mg content in 13 WM samples, is 199.4°C (Fig 6A). The curve shown in Fig 6B depicts the calculated temperature of a fluid that results from titrating cold seawater (SW) into 1 kg of this end-member HF, and Fig 6C presents how the activities of major redox-active species change with the mass ratios of SW:HF from 103:1 to 10−2:1, as well as a temperature range from 19.0 to 199.4°C. The remaining panels in Fig 6 show how much energy is available from the 17 reactions listed in Table 4 normalized to kJ per mole electron transferred (Fig 6D), J per kg mixed fluid (Fig 6E), and J per kg vent fluid (Fig 6F), where solid lines refer to aerobic processes, dashed lines represent anaerobic processes, and the different colors identify different reactions.
Fig 6. Temperature, composition and energetics resulting from the hydrothermal fluid-seawater mixing model.
(A) Extrapolated temperature of end-member HF at [Mg] = 0 (brown circles) based on values of [Mg] in WM samples (red circles) and SW (blue circle). The temperature, composition and energetics of redox reactions resulting from fluid mixing were calculated using the average WM composition (black star) and average HF composition (black square). (B) Fluid temperature as a function of SW:HF mixing ratio. (C) Activities of redox-sensitive species as a function of temperature and SW:HF mixing ratio. Values of ΔGr (D) and Efluid (E, F) of exergonic reactions listed in Table 4. Because the results span several orders of magnitude, reactions B14, B15 and B16 have a different Y-axis scale on right side in (E); and reactions B9, B14, B15, I29, I30, and B16 refer to the two Y-axis scales on the right side in (F).
Table 4. Catabolic reactions used to define metabolic groups in the analysis shown in Fig 5.
| Metabolic Group | # | Reaction |
|---|---|---|
| Hydrogen Oxidizers | B1 | 2H2 + O2 →2 H2O |
| I5 | 5H2 + 2NO3- + 2H+ → N2 + 6H2O | |
| Methane Oxidizers | B3 | CH4 + 2O2 → CO2 + 2H2O |
| I14 | CH4 + 4NO3- → 4NO2- + CO2 + 2H2O | |
| M6 | CH4 + SO42- + 2H+ → H2S + CO2 + 2H2O | |
| Ammonium Oxidizers | B9 | NH4+ + 2 O2 → NO3- + 2 H+ + H2O |
| Sulfur Oxidizers | B14 | 2 H2S + O2 → 2 S + 2 H2O |
| B15 | H2S + 2 O2 → SO42- + 2 H+ | |
| B16 | 2 S + 3 O2 + 2 H2O → 2 SO42- + 4 H+ | |
| I29 | 5 H2S + 2 NO3- + 2 H+ → 5 S + N2 + 6 H2O | |
| I30 | 5 H2S + 8 NO3- → 5 SO42- + 4 N2 + 2 H+ + 4 H2O | |
| Iron Oxidizers | B24 | 4 Fe2+ + O2 + 6 H2O → 4 FeOOH Ferrihydrite + 8 H+ |
| Manganese Oxidizer | B28 | 2 Mn2+ + O2 + 2 H2O → 2 MnO2 Pyrolusite + 4 H+ |
| Arsenite Oxidizers | B29 | 2 H3AsO3 + O2 → 2 H2AsO4- + 2 H+ |
| Methanogens | D3 | 4H2 + CO2 → CH4 + 2H2O |
| Sulfate Reducers | M2 | 4H2 + SO42- + 2H+ → H2S + 4H2O |
| Arsenate Reducers | S1 | H2 + H2AsO4- + H+ → H3AsO3 + H2O |
Thermodynamic predictions of redox reaction energetics can change substantially when different normalization schemes are used [85, 86] For instance, when normalized to kJ per mole electron transferred (ΔGr/e-) for the SW:HF mixing calculations, the energy yields of all reactions decreased with increasing proportion of HF (Fig 6D). When normalized per kg of mixed fluid (Efluid), however, a very different picture emerges (Fig 6E); here, the reactions fall into two groups: three reactions (B14-B16) that can provide >60 J/kg mixed fluid at optimal SW:HF ratios, and the other 14 reactions where energy yields maximize at ~11 J/kg mixed fluid for one example (B24) and ~4 J/kg mixed fluid for the rest. Finally, the potential energy per kg of pure vent fluid is given in Fig 6F. In this normalization, the reactions are most exergonic at a SW:HF mixing ratio of ~100:1, corresponding to a temperature of ~20°C. At these conditions, the energy yields from sulfide oxidation with O2 or NO3- (B14, B15, I29, I30) and aerobic ammonia oxidation (B9) exceed by several orders of magnitude those of the other reactions. A similar story emerges when normalizing values of ΔGr per kg mixed fluid and per kg vent fluid for aerobic and anaerobic sulfur/sulfide oxidation in the WM area (Fig 6E and 6F). This can be seen most clearly at temperatures <40°C, where these reactions are 20–50 times as exergonic as the other reactions. As noted above, isotopic data also point to a dynamic sulfur cycle at Milos with microbial sulfate reduction, sulfide oxidation, and rapid recycling of sulfur intermediates that vary with location and time [47, 84].
The curves in Fig 6E (J/kg mixed fluid) and 6F (J/kg hydrothermal fluid) show how the energy densities of the reactions in Table 4 change with HF:SW ratio. The multiple y-axes are used in Fig 6E and 6F because the results span several orders of magnitude. It can clearly be seen that as the proportion of HF decreases, the energy yields for most reactions also decrease, especially when these are plotted per kg of vent fluid (Fig 6F). The kinks and inflection points in some of the curves in Fig 6E and 6F are a result of calculating the reaction energies using the concentration of the limiting electron donor or acceptor. These abrupt changes in slope correspond to points where the concentrations of the electron donors and acceptors are equal. The inflection points in Fig 6E also illustrate the temperatures at which the reactions are most exergonic. For example, the sulfur and sulfide oxidation reactions B14 and B15 have inflection points at 27°C, above which their potential energy yields decrease. Similarly, the limiting reactant for reactions B24 and B28 changes from iron and manganese, respectively, to oxygen as temperature increases (Fig 6E). Our mixing model suggests that microbial metabolic strategies often shift with mixing ratio, and therefore temperature, a notion that is supported by other lines of evidence. Note that cell abundances of sulfur oxidizing bacteria, sulfate reducing bacteria and dissimilatory iron-reducing bacteria at Milos decrease with increasing depth and temperature [87]. Also at Milos, microbial cell numbers are highest in the shallowest sediment [77].
Conclusions
Seawater and porewater chemistries, a fluid mixing model, and thermodynamic calculations were combined to determine the energy yields of more than 700 redox reactions in fluids and sediments of a Milos Island shallow-sea hydrothermal environment. These yields were reported in several normalization schemes—in kJ per mole electrons transferred and in J per kg water or sediment—revealing potential chemolithotrophic microbial metabolisms as a function of depth and distance from a diffuse vent area. We demonstrated that in this system at Milos, analogous to other shallow-sea hydrothermal systems, hot spring environments, and the deep continental subsurface, a large number of inorganic redox reactions can be exergonic, suggesting that diverse chemolithotrophic metabolisms may be occurring simultaneously. Based on modeling of SW:HF mixed solutions in shallow sediments, we also showed that energy yields can change dramatically along the posited steep gradients in temperature, pH, and composition of redox-sensitive aqueous solutes, together with consideration of redox-sensitive minerals. In the Milos hydrothermal system, this applies to a transect from the White Mat area across the Transition Zone to the Seagrass and Background zones and into seawater. An environmental energy framework of the type provided here can help interpret biodiversity data and ecosystem function, and also guide efforts to cultivate dominant as well as important minor members of a chemolithotrophic microbial community.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors thank Pratixaben Savalia, Laura Zinke, and Jayme Feyhl-Buska, for insightful discussions and analytical support, and Laura Wehrmann for help with cation analyses.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial assistance was provided by the NSF through grant awards MGG-1061476 (to DAF, JPA, GKD) and OCE-0939564 (to JPA) as well as the USC Zumberge Fund Individual Grant, the NASA Astrobiology Institute grant NNA13AA92A (to DEL and JPA), the NASA-NSF Origins of Life Ideas Lab program under grant NNN13D466T (to DEL) and grant 80NSSC20K0228 (to DEL and REP), the Alfred P. Sloan Foundation through the Deep Carbon Observatory (all to DEL), and the C-DEBI contribution number 529 to JPA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brock TD. High temperature systems. Annual review of ecology and systematics. 1970;1(1):191–220. [Google Scholar]
- 2.Renner J, White D, Williams D. Hydrothermal convection systems. Assessment of geothermal resources of the United States. 7261975.
- 3.Rinehart JS. Geysers and geothermal energy: Springer; 1980. [Google Scholar]
- 4.Baker ET, German CR. On the global distribution of hydrothermal vent fields. Mid-ocean ridges. 2004:245–66. [Google Scholar]
- 5.Beaulieu SE. InterRidge Global Database of Active Submarine Hydrothermal Vent Fields: prepared for InterRidge, Version 3.3. World Wide Web electronic publication. Version 3.4 accessed 2018-04-28: InterRidge program; 2015.
- 6.Prol-Ledesma RM, Dando PR, de Ronde CEJ. Special issue on “shallow-water hydrothermal venting”. Chem Geol. 2005;224(1–3):1–4. 10.1016/j.chemgeo.2005.07.012 [DOI] [Google Scholar]
- 7.Waring GA, Blankenship RR. Thermal Springs of the United States and Other Countries: A Summary: US Government Printing Office; 1965. [Google Scholar]
- 8.Corliss JB, Dymond J, Gordon LI, Edmond JM, Herzen RPV, Ballard RD, et al. Submarine thermal springs on the galapagos rift. Science. 1979;203(4385):1073–83. 10.1126/science.203.4385.1073 PubMed PMID: WOS:A1979GM37500005. [DOI] [PubMed] [Google Scholar]
- 9.Tarasov VG, Gebruk AV, Mironov AN, Moskalev LI. Deep-sea and shallow-water hydrothermal vent communities: Two different phenomena? Chem Geol. 2005;224(1–3):5–39. 10.1016/j.chemgeo.2005.07.021 [DOI] [Google Scholar]
- 10.Price RE, Giovannelli D. A Review of the Geochemistry and Microbiology of Marine Shallow-water Hydrothermal Vents. Reference Module in Earth Systems and Environmental Sciences. 2017. [Google Scholar]
- 11.Madigan MT, Bender KS, Buckley DH, Sattley WM, Stahl DA. Brock Biology of microorganisms 15th edn: Pearson; 2017. 10.3390/microorganisms5020034 [DOI] [Google Scholar]
- 12.Branch GM. The biology of limpets: physical factors, energy flow, and ecological interactions. 1981.
- 13.Hill T. Free energy transduction in biology: the steady-state kinetic and thermodynamic formalism: Elsevier; 2012. [Google Scholar]
- 14.Amend JP, Shock EL. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. Fems Microbiology Reviews. 2001;25(2):175–243. 10.1111/j.1574-6976.2001.tb00576.x PubMed PMID: WOS:000167599700002. [DOI] [PubMed] [Google Scholar]
- 15.Reece JB, Urry LA, Cain ML, Wasserman SA, Minorsky PV, Jackson RB. Campbell biology: Pearson Higher Ed; 2013. [Google Scholar]
- 16.Larowe DE, Helgeson HC. Quantifying the energetics of metabolic reactions in diverse biogeochemical systems: electron flow and ATP synthesis. Geobiology. 2007;5(2):153–68. 10.1111/j.1472-4669.2007.00099.x PubMed PMID: WOS:000247529000006. [DOI] [Google Scholar]
- 17.Amend JP, LaRowe DE, McCollom TM, Shock EL. The energetics of organic synthesis inside and outside the cell. Philosophical Transactions of the Royal Society B-Biological Sciences. 2013;368(1622). 10.1098/rstb.2012.0255 PubMed PMID: WOS:000320105200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jannasch H. Review lecture: The chemosynthetic support of life and the microbial diversity at deep-sea hydrothermal vents. Proceedings of the Royal Society of London B: Biological Sciences. 1985;225(1240):277–97. [Google Scholar]
- 19.Daniel RM. Modern life at high temperatures. Origins of life and evolution of the biosphere. 1992;22(1):33–42. 10.1007/bf01808017 [DOI] [Google Scholar]
- 20.Lutz RA, Kennish MJ. Ecology of deep‐sea hydrothermal vent communities: A review. Reviews of Geophysics. 1993;31(3):211–42. [Google Scholar]
- 21.Segerer AH, Burggraf S, Fiala G, Huber G, Huber R, Pley U, et al. Life in hot springs and hydrothermal vents. Origins of life and evolution of the biosphere. 1993;23(1):77–90. 10.1007/BF01581992 [DOI] [PubMed] [Google Scholar]
- 22.Amend JP, Rogers KL, Shock EL, Gurrieri S, Inguaggiato S. Energetics of chemolithoautotrophy in the hydrothermal system of Vulcano Island, southern Italy. Geobiology. 2003;1(1):37–58. 10.1046/j.1472-4669.2003.00006.x PubMed PMID: WOS:000207171100005. [DOI] [Google Scholar]
- 23.Berenguer J. Thermophile In: Gargaud M, Amils R, Quintanilla JC, Cleaves HJ, Irvine WM, Pinti DL, et al. , editors. Encyclopedia of Astrobiology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. p. 1666–7. [Google Scholar]
- 24.Inskeep WP, Ackerman GG, Taylor WP, Kozubal M, Korf S, Macur RE. On the energetics of chemolithotrophy in nonequilibrium systems: case studies of geothermal springs in Yellowstone National Park. Geobiology. 2005;3(4):297–317. 10.1111/j.1472-4669.2006.00059.x PubMed PMID: WOS:000207172000006. [DOI] [Google Scholar]
- 25.Shock EL, Holland M, Meyer-Dombard D, Amend JP. Geochemical sources of energy for microbial metabolism in hydrothermal ecosystems: Obsidian Pool, Yellowstone National Park. Geothermal biology and geochemistry in Yellowstone National Park. 2005;1:95–112. [Google Scholar]
- 26.Shock EL, Holland M, Meyer-Dombard D, Amend JP, Osburn GR, Fischer TP. Quantifying inorganic sources of geochemical energy in hydrothermal ecosystems, Yellowstone National Park, USA. Geochim Cosmochim Ac. 2010;74(14):4005–43. 10.1016/j.gca.2009.08.036 PubMed PMID: WOS:000278977100011. [DOI] [Google Scholar]
- 27.Spear JR, Walker JJ, McCollom TM, Pace NR. Hydrogen and bioenergetics in the Yellowstone geothermal ecosystem. PNAS. 2005;102(7):2555–60. 10.1073/pnas.0409574102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spear JR, Walker JJ, Pace NR. Hydrogen and primary productivity: inference of biogeochemistry from phylogeny in a geothermal ecosystem. Geothermal Biology and Geochemistry in Yellowstone National Park. 2005:113–28. [Google Scholar]
- 29.Windman T, Zolotova N, Schwandner F, Shock EL. Formate as an energy source for microbial metabolism in chemosynthetic zones of hydrothermal ecosystems. Astrobiology. 2007;7(6):873–90. 10.1089/ast.2007.0127 [DOI] [PubMed] [Google Scholar]
- 30.Dahle H, Okland I, Thorseth IH, Pederesen RB, Steen IH. Energy landscapes shape microbial communities in hydrothermal systems on the Arctic Mid-Ocean Ridge. ISME J. 2015;9(7):1593–606. 10.1038/ismej.2014.247 PubMed PMID: WOS:000356778000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCollom TM, Shock EL. Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim Cosmochim Ac. 1997;61(20):4375–91. 10.1016/s0016-7037(97)00241-x PubMed PMID: WOS:A1997YH27900012. [DOI] [PubMed] [Google Scholar]
- 32.Shibuya T, Russell MJ, Takai K. Free energy distribution and hydrothermal mineral precipitation in Hadean submarine alkaline vent systems: Importance of iron redox reactions under anoxic conditions. Geochim Cosmochim Ac. 2016;175:1–19. 10.1016/j.gca.2015.11.021 PubMed PMID: WOS:000369070000001. [DOI] [Google Scholar]
- 33.Eecke HCV, Akerman NH, Huber JA, Butterfield DA, Holden JF. Growth kinetics and energetics of a deep-sea hyperthermophilic methanogen under varying environmental conditions. Env Microbiol Rep. 2013;5(5):665–71. 10.1111/1758-2229.12065 PubMed PMID: WOS:000325142700005. [DOI] [PubMed] [Google Scholar]
- 34.Hentscher M, Bach W. Geochemically induced shifts in catabolic energy yields explain past ecological changes of diffuse vents in the East Pacific Rise 9° 50'N area. Geochem T. 2012;13(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKay L, Klokman VW, Mendlovitz HP, LaRowe DE, Hoer DR, Albert D, et al. Thermal and geochemical influences on microbial biogeography in the hydrothermal sediments of Guaymas Basin, Gulf of California. Env Microbiol Rep. 2016;8(1):150–61. 10.1111/1758-2229.12365 PubMed PMID: WOS:000371481100019. [DOI] [PubMed] [Google Scholar]
- 36.Akerman NH, Price RE, Pichler T, Amend JP. Energy sources for chemolithotrophs in an arsenic- and iron-rich shallow-sea hydrothermal system. Geobiology. 2011;9(5):436–45. 10.1111/j.1472-4669.2011.00291.x PubMed PMID: WOS:000294172100005. [DOI] [PubMed] [Google Scholar]
- 37.Han Y, Perner M. The globally widespread genus Sulfurimonas: versatile energy metabolisms and adaptations to redox clines. Front Microbiol. 2015;6 10.3389/fmicb.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price RE, LaRowe DE, Italiano F, Savov I, Pichler T, Amend JP. Subsurface hydrothermal processes and the bioenergetics of chemolithoautotrophy at the shallow-sea vents off Panarea Island (Italy). Chem Geol. 2015;407:21–45. 10.1016/j.chemgeo.2015.04.011 PubMed PMID: WOS:000356234100004. [DOI] [Google Scholar]
- 39.Rogers KL, Amend JP. Archaeal diversity and geochemical energy yields in a geothermal well on Vulcano Island, Italy. Geobiology. 2005;3(4):319–32. 10.1111/j.1472-4669.2006.00064.x PubMed PMID: WOS:000207172000007. [DOI] [Google Scholar]
- 40.Rogers KL, Amend JP. Energetics of potential heterotrophic metabolisms in the marine hydrothermal system of Vulcano Island, Italy. Geochim Cosmochim Ac. 2006;70(24):6180–200. 10.1016/j.gca.2006.08.046 PubMed PMID: WOS:000243166200017. [DOI] [Google Scholar]
- 41.Amend JP, McCollom TM, Hentscher M, Bach W. Catabolic and anabolic energy for chemolithoautotrophs in deep-sea hydrothermal systems hosted in different rock types. Geochim Cosmochim Ac. 2011;75(19):5736–48. 10.1016/j.gca.2011.07.041 PubMed PMID: WOS:000294479900022. [DOI] [Google Scholar]
- 42.Boettger J, Lin HT, Cowen JP, Hentscher M, Amend JP. Energy yields from chemolithotrophic metabolisms in igneous basement of the Juan de Fuca ridge flank system. Chem Geol. 2013;337:11–9. 10.1016/j.chemgeo.2012.10.053 PubMed PMID: WOS:000314738600002. [DOI] [Google Scholar]
- 43.Dando PR, Aliani S, Arab H, Bianchi CN, Brehmer M, Cocito S, et al. Hydrothermal studies in the Aegean Sea. Phys Chem Earth PT B. 2000;25(1):1–8. 10.1016/s1464-1909(99)00112-4 PubMed PMID: WOS:000084911000001. [DOI] [Google Scholar]
- 44.Dando PR, Hughes JA, Leahy Y, Niven SJ, Taylor LJ, Smith C. Gas venting rates from submarine hydrothermal areas around the island of Milos, Hellenic Volcanic Arc. Cont Shelf Res. 1995;15(8):913–29. 10.1016/0278-4343(95)80002-U PubMed PMID: WOS:A1995QM09100002. [DOI] [Google Scholar]
- 45.Valsami-Jones E, Baltatzis E, Bailey EH, Boyce AJ, Alexander JL, Magganas A, et al. The geochemistry of fluids from an active shallow submarine hydrothermal system: Milos island, Hellenic Volcanic Arc. J Volcanol Geoth Res. 2005;148(1–2):130–51. 10.1016/j.jvolgeores.2005.03.018 PubMed PMID: WOS:000233951500010. [DOI] [Google Scholar]
- 46.Dando PR, Thomm M, Arab H, Brehmer M, Hooper LE, Jochimsen B, et al. Microbiology of shallow hydrothermal sites off Palaeochori Bay, Milos (Hellenic Volcanic Arc). Cah Biol Mar. 1998;39(3–4):369–72. PubMed PMID: WOS:000078751400040. [Google Scholar]
- 47.Gilhooly WP III, Fike DA, Druschel GK, Kafantaris F-CA, Price RE, Amend JP. Sulfur and oxygen isotope insights into sulfur cycling in shallow-sea hydrothermal vents, Milos, Greece. Geochem T. 2014;15(12). 10.1186/s12932-014-0012-y PubMed PMID: WOS:000341039700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godelitsas A, Price RE, Pichler T, Amend J, Gamaletsos P, Gottlicher J. Amorphous As-sulfide precipitates from the shallow-water hydrothermal vents off Milos Island (Greece). Mar Chem. 2015;177:687–96. 10.1016/j.marchem.2015.09.004 PubMed PMID: WOS:000366789100001. [DOI] [Google Scholar]
- 49.Price RE, Savov I, Planer-Friedrich B, Buhring SI, Amend JP, Pichler T. Processes influencing extreme As enrichment in shallow-sea hydrothermal fluids of Milos Island, Greece. Chem Geol. 2013;348:15–26. 10.1016/j.chemgeo.2012.06.007 PubMed PMID: WOS:000321604600003. [DOI] [Google Scholar]
- 50.Jolivet L, Faccenna C, Huet B, Labrousse L, Le Pourhiet L, Lacombe O, et al. Aegean tectonics: Strain localisation, slab tearing and trench retreat. Tectonophysics. 2013;597:1–33. 10.1016/j.tecto.2012.06.011 PubMed PMID: WOS:000320896500001. [DOI] [Google Scholar]
- 51.Varnavas SP, Cronan DS. Submarine hydrothermal activity off Santorini and Milos in the Central Hellenic Volcanic Arc: A synthesis. Chem Geol. 2005;224(1–3):40–54. 10.1016/j.chemgeo.2005.07.013 PubMed PMID: WOS:000234118800003. [DOI] [Google Scholar]
- 52.Price RE, Lesniewski R, Nitzsche KS, Meyerdierks A, Saltikov C, Pichler T, et al. Archaeal and bacterial diversity in an arsenic-rich shallow-sea hydrothermal system undergoing phase separation. Front Microbiol. 2013;4 10.3389/fmicb.2013.00158 PubMed PMID: WOS:000331247100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price RE, Pichler T. Distribution, speciation and bioavailability of arsenic in a shallow-water submarine hydrothermal system, Tutum Bay, Ambitle Island, PNG. Chem Geol. 2005;224(1–3):122–35. 10.1016/j.chemgeo.2005.07.017 [DOI] [Google Scholar]
- 54.Bayraktarov E, Price RE, Ferdelman TG, Finster K. The pH and pCO2 dependence of sulfate reduction in shallow-sea hydrothermal CO2—venting sediments (Milos Island, Greece). Front Microbiol. 2013;4 10.3389/fmicb.2013.00111 PubMed PMID: WOS:000331102100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helgeson HC, Kirkham DH, Flowers GC. Theoretical prediction of thermodynamic behavior of aqueous electrolytes at high pressures and temperatures: 4.Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 600°C and 5 kb. American Journal of Science. 1981;281(10):1249–516. 10.2475/ajs.281.10.1249 PubMed PMID: WOS:A1981MZ34200001. [DOI] [Google Scholar]
- 56.Shock EL, Oelkers EH, Johnson JW, Sverjensky DA, Helgeson HC. Calculation of the thermodynamic properties of aqueous species at high pressures and temperatures—effective electrostatic radii, dissociation constants and standard partial molal properties to 1000°C and 5 kbar. Journal of the Chemical Society-Faraday Transactions. 1992;88(6):803–26. 10.1039/ft9928800803 PubMed PMID: WOS:A1992HK65300006. [DOI] [Google Scholar]
- 57.Tanger JC, Helgeson HC. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures—revised equations of state for the standard partial molal properties of ions and electrolytes. American Journal of Science. 1988;288(1):19–98. 10.2475/ajs.288.1.19. PubMed PMID: WOS:A1988L632800002. [DOI] [Google Scholar]
- 58.Johnson JW, Oelkers EH, Helgeson HC. SUPCRT92—a software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 bar to 5000 bar and 0°C to 1000°C. Computers & Geosciences. 1992;18(7):899–947. 10.1016/0098-3004(92)90029-q PubMed PMID: WOS:A1992JR49100006. [DOI] [Google Scholar]
- 59.Schulte MD, Shock EL, Wood RH. The temperature dependence of the standard-state thermodynamic properties of aqueous nonelectrolytes. Geochim Cosmochim Ac. 2001;65(21):3919–30. 10.1016/s0016-7037(01)00717-7 PubMed PMID: WOS:000172225600017. [DOI] [Google Scholar]
- 60.Shock EL, Helgeson HC. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures—correlation algorithms for ionic species and equation of state predictions to 5 kb and 1000°C. Geochim Cosmochim Ac. 1988;52(8):2009–36. 10.1016/0016-7037(88)90181-0 PubMed PMID: WOS:A1988P889900006. [DOI] [Google Scholar]
- 61.Shock EL, Helgeson HC. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures—standard partial molal properties of organic species. Geochim Cosmochim Ac. 1990;54(4):915–45. 10.1016/0016-7037(90)90429-o PubMed PMID: WOS:A1990DB65700001. [DOI] [Google Scholar]
- 62.Shock EL, Helgeson HC, Sverjensky DA. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures—standard partial molal properties of inorganic neutral species. Geochim Cosmochim Ac. 1989;53(9):2157–83. 10.1016/0016-7037(89)90341-4 PubMed PMID: WOS:A1989AP91800002. [DOI] [Google Scholar]
- 63.Sverjensky DA, Shock EL, Helgeson HC. Prediction of the thermodynamic properties of aqueous metal complexes to 1000 degrees C and 5 kb. Geochim Cosmochim Ac. 1997;61(7):1359–412. 10.1016/s0016-7037(97)00009-4 PubMed PMID: WOS:A1997WV63100003. [DOI] [PubMed] [Google Scholar]
- 64.Bricker O. Some stability relations in the system Mn-O2-H2O at 25° and one atmosphere total pressure. American Mineralogist. 1965;50(9):1296-&. PubMed PMID: WOS:A19656996000009. [Google Scholar]
- 65.Hem JD, Roberson CE, Fournier RB. Stability of BETA-MnOOH and manganese oxide deposition from spring water. Water Resources Research. 1982;18(3):563–70. 10.1029/WR018i003p00563 PubMed PMID: WOS:A1982NT27000013. [DOI] [Google Scholar]
- 66.Helgeson HC. Thermodynamics of hydrothermal systems at elevated temperatures and pressures. American Journal of Science. 1969;267(7):729-&. 10.2475/ajs.267.7.729 PubMed PMID: WOS:A1969D857000001. [DOI] [Google Scholar]
- 67.LaRowe D, Amend J. Energetic constraints on life in marine deep sediments In: Kallmeyer J, Wagner D, editors. Microbial Life of the Deep Biosphere. Life in Extreme Environments. 12014. p. 279–302. [Google Scholar]
- 68.Pehlivanoglou K. Lithology and mineralogy of surface sediments in the vicinity of the Kafireas Strait (Aegean Sea). Geo-Marine Letters. 2001;21(2):75–85. [Google Scholar]
- 69.Karageorgis A, Anagnostou C, Sioulas A, Chronis G, Papathanassiou E. Sediment geochemistry and mineralogy in Milos bay, SW Kyklades, Aegean Sea, Greece. J Marine Syst. 1998;16(3–4):269–81. 10.1016/s0924-7963(97)00020-1 PubMed PMID: WOS:000076604200006. [DOI] [Google Scholar]
- 70.Sengers JL, Kamgar‐Parsi B, Balfour F, Sengers J. Thermodynamic properties of steam in the critical region. Journal of Physical and Chemical Reference Data. 1983;12(1):1–28. [Google Scholar]
- 71.Haar L. NBS/NRC steam tables: CRC Press; 1984. [Google Scholar]
- 72.Palmer M. Controls over the chloride concentration of submarine hydrothermal vent fluids: evidence from Sr/Ca and87Sr/86Sr ratios. Earth and Planetary Science Letters. 1992;109(1–2):37–46. [Google Scholar]
- 73.Albarede F, Michard A, Minster J, Michard G. 87Sr/86Sr ratios in hydrothermal waters and deposits from the East Pacific Rise at 21 N. Earth and Planetary Science Letters. 1981;55(2):229–36. [Google Scholar]
- 74.de Fommervault OP, d'Ortenzio F, Mangin A, Serra R, Migon C, Claustre H, et al. Seasonal variability of nutrient concentrations in the Mediterranean Sea: Contribution of Bio‐Argo floats. Journal of Geophysical Research: Oceans. 2015;120(12):8528–50. [Google Scholar]
- 75.Philips S, Laanbroek HJ, Verstraete W. Origin, causes and effects of increased nitrite concentrations in aquatic environments. Reviews in environmental science and biotechnology. 2002;1(2):115–41. [Google Scholar]
- 76.Garrison TS, Ellis R. Essentials of oceanography. Boston, MA: Cengage Learning; 2018. 71–3 p. [Google Scholar]
- 77.Giovannelli D, d'Errico G, Manini E, Yakimov M, Vetriani C. Diversity and phylogenetic analyses of bacteria from a shallow-water hydrothermal vent in Milos island (Greece). Front Microbiol. 2013;4 10.3389/fmicb.2013.00184 PubMed PMID: WOS:000331246100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sievert SM, Heidorn T, Kuever J. Halothiobacillus kellyi sp. nov., a mesophilic, obligately chemolithoautotrophic, sulfur-oxidizing bacterium isolated from a shallow-water hydrothermal vent in the Aegean Sea, and emended description of the genus Halothiobacillus. Int J Syst Evol Micr. 2000;50(3):1229–37. [DOI] [PubMed] [Google Scholar]
- 79.Jochimsen B, Peinemann-Simon S, Völker H, Stüben D, Botz R, Stoffers P, et al. Stetteria hydrogenophila, gen. nov. and sp. nov., a novel mixotrophic sulfur-dependent crenarchaeote isolated from Milos, Greece. Extremophiles. 1997;1(2):67–73. 10.1007/s007920050016 [DOI] [PubMed] [Google Scholar]
- 80.Brinkhoff T, Sievert SM, Kuever J, Muyzer G. Distribution and Diversity of Sulfur-OxidizingThiomicrospira spp. at a Shallow-Water Hydrothermal Vent in the Aegean Sea (Milos, Greece). Appl Environ Microb. 1999;65(9):3843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuever J, Sievert SM, Stevens H, Brinkhoff T, Muyzer G. Microorganisms of the oxidative and reductive part of the sulphur cycle at a shallow-water hydrothermal vent in the Aegean Sea (Milos, Greece). Cah Biol Mar. 2002;43(3/4):413–6. [Google Scholar]
- 82.Amend JP, LaRowe DE. Mini‐Review: Demystifying Microbial Reaction Energetics. Environ Microbiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Osburn MR, LaRowe DE, Momper LM, Amend JP. Chemolithotrophy in the continental deep subsurface: Sanford Underground Research Facility (SURF), USA. Front Microbiol. 2014;5 10.3389/fmicb.2014.00610 PubMed PMID: WOS:000345698500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Houghton JL, Gilhooly WP III, Kafantaris F-CA, Druschel GK, Lu G-S, Amend JP, et al. Spatially and temporally variable sulfur cycling in shallow-sea hydrothermal vents, Milos, Greece. Mar Chem. 2019;208:83–94. [Google Scholar]
- 85.LaRowe D, Amend J. Energy limits for life in the subsurface: Cambridge University Press; 2020. [Google Scholar]
- 86.LaRowe DE, Dale AW, Aguilera DR, L'Heureux I, Amend JP, Regnier P. Modeling microbial reaction rates in a submarine hydrothermal vent chimney wall. Geochim Cosmochim Ac. 2014;124:72–97. 10.1016/j.gca.2013.09.005 PubMed PMID: WOS:000327394100005. [DOI] [Google Scholar]
- 87.Sievert SM, Brinkhoff T, Muyzer G, Ziebis V, Kuever J. Spatial heterogeneity of bacterial populations along an environmental gradient at a shallow submarine hydrothermal vent near Milos Island (Greece). Appl Environ Microb. 1999;65(9):3834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.