Abstract
Background
Hand, foot, and mouth disease (HFMD) has become one of the most important infectious diseases recent years. Qingdao City has suffered from serious HFMD epidemic. This study aimed to describe epidemiological characteristics and investigate spatial-temporal distribution at town level in Qingdao City.
Method
The surveillance data of HFMD during 2013–2018 were collected from the National Notifiable Disease Surveillance System. The global Moran’s I statistic was used to detect the spatial autocorrelation of HFMD cases by ArcGis 10.0 software. Purely spatial and spatial-temporal analysis was used to detect epidemic clusters by SatScanTM v9.6 software.
Results
The annual average incidence of HFMD cases in Qingdao City from 2013 to 2018 was 123.16 per 100000, while the incidence rate of children≤5years old was 2879.80 per 100000. The majority (88.97%) of HFMD cases were aged within 0–5 years old and the males were 60.20%. Other enterovirus (EV), enteriovirus 71(EV71), and Coxsackievirus A16 (CA16) accounted for 48.75%, 30.91% and 20.34%. The seasonal peak was between May and October. HFMD had positive spatial autocorrelation at town level with global Moran’s I from 0.19 to 0.31(P<0.001). Spatial-temporal cluster analysis detected six most likely clusters and three secondary clusters from 2013 to 2018. The most likely cluster was located in urban and urban-rural fringe areas.
Conclusions
Urban and urban-rural fringe areas were the major locations of the clusters with other EV as the dominant pathogen between May and October. The findings suggested that the prevention and control of HFMD in Qingdao City should be focus on these high-risk periods and locations which had important public health significance for the allocation of public health resources.
Introduction
Hand, foot, and mouth disease (HFMD) is a common intestinal infectious disease caused by viruses that belong to the Enterovirus group principally including Enterovirus 71(EV71) and Coxsackievirus A16 (CA16) [1, 2]. The clinical symptoms of HFMD are characterized by fever, oral ulcer, vesicular exanthema on hands, feet, and mouths. The transmission of HFMD is mainly from person to person through direct contact with saliva, respiratory droplets, faeces, vesicular fluid of patient and indirectly by contaminated articles [3]. Symptomatic cases mostly affect children aged 0–5 years, but also can affect older children and adults. Most cases are mild and self-limiting, but few cases may develop severe complications involving neurological symptoms such as encephalitis, meningitis and even death [4, 5].
In the last decade, there are a large number of HFMD outbreaks reported in East and Southeast Asia, including China [6–8]. Large-scale HFMD outbreaks were reported in Shandong and Anhui Province in 2007 and 2008, which resulted in a large number of server cases and deaths [9, 10]. Many spatial analysis had been carried out to describe spatial patterns and cluster locations in many provinces in China, such as Shandong, Beijing, Sichuan, and so on. These studies mainly focused on the analysis of the epidemiology characteristics, global or local spatial autocorrelations and spatiotemporal clusters. In order to better deal with the outbreaks of HFMD, the Ministry of Health of China listed HFMD as a Class C statutory infectious disease on May 2, 2008 [11, 12]. In addition, the inactivated monovalent EV71 vaccines were licensed in China in 2016 [13], which provide effective measures to prevent HFMD caused by EV71, especially severe cases.
The HFMD epidemic in Qingdao City exhibited an upward tendency with the incidence rates ranged from 31 in 2008 to 138 in 2018 per million person-years, which was much higher than that of Shandong Province with the average rate of 10.44 per million person-years (1.87~32.84 per million person) during 2008 to 2012. However, previous studies about HFMD in Qingdao City mainly concentrated on descriptive studies and forecasting analysis [14, 15]. There are no clear analysis on the spatiotemporal characteristic at town level, which is not conductive to take targeted preventive and control measures. Thus, based on the data from the surveillance system, we performed the scan statistics analysis to explore the town-level epidemiological characteristics and spatial-temporal distribution of HFMD in Qingdao City, which could provide reference for the accurate prevention and control of HFMD and guide the optimal allocation of social health resources.
Material and methods
Study area
Qingdao City is one of the largest tourist port cities in China with a population of 9.20 million people. The city covers an area of 11282 km2, which is divided into 10 districts including 130 streets/townships. According to the geographical characteristics, the 10 districts were divided into three regions including urban areas (Shinan District, Shibei District and Licang District), urban-rural fringe areas (Chengyang District, Laoshan District, Jimo District, and Huangdao Distirct) and rural areas (Pingdu District, Laixi District and Jiaozhou District).
Data collection
The surveillance data of HFMD from 2013 to 2018 were obtained from the National Notifiable Disease Surveillance System. The information included gender, age, occupation, address, onset date, diagnosis date and the pathogen type (CoxA16, EV71 and other EV). The clinical criteria for diagnosis of HFMD cases was provided in the HFMD Control and Prevention Guide published by the Chinese Ministry of Health [11].The range of time was from January 1, 2013 to December 31, 2018. The cases were diagnosed and reported to the surveillance system by professional doctors. Throat swabs were collected from at least 5 cases in each district. All swab samples were stored at 4°C immediately after collection and quickly sent to the national network laboratory in Qingdao for aetiological indentification. EV71, CA16, and other EV were tested by real-time PCR. In this study, we focused on the HFMD cases (0 to 5 years) which accounted for about 90% of the total cases. The corresponding demographic data of each town were obtained from Qingdao Statistical Yearbook.
Statistical analysis
Descriptive statistics were used to describe the epidemiological characteristics of HFMD. Demographic characteristics of HFMD cases and the pathogen types of some cases were analyzed by year and seasonal pattern by month. The town-level polygon map at 1:100000 scale was obtained to perform the spatial distribution by ArcGIS 10.0 (http://www.arcgis.com).
The autocorrelation statistic (Moran’s I) [16] was used to detect the global spatial autocorrelation of HFMD cases in the study area by year. ArcGIS 10.0 was used to compute Moran’s I test statistic [17,18].
The spatial and space-time scan statistics were used to identify high-incidence clusters of HFMD in Qingdao City during 2013 to 2018 [19]. The analysis was performed by SatScanTM v9.6 software (http://www.satscan.org/), using the kulldorff method of retrospective space-time scan statistic based on a discrete Possion model [20]. Spatial scan statistic was used to identify purely spatial clusters of HFMD cases. The purely spatial scan statistic applied a circular window that centered on each geographical area. According to the population range (specified by user), the radius of the window varied continuously in size. The space-time scan statistic was defined by a cylindrical window with a circular geographic base and with height corresponding to time [21, 22]. The base and the height of the windows are in dynamic changes in order to detect possible spatial-temporal clusters. The scan statistic was based on the null hypothesis that the rate or the independence of cases in space and time was the same within and outside the scanning window. The Log Likelihood Ratio(LLR) and relative risk(RR) were calculated to test the hypothesis. The P value(P<0.05) was obtained with Monte Carlo hypothesis testing with 999 simulations [23]. The window with the maximum LLR was considered as most likely cluster, the others were defined as secondary clusters.
For this study, we analyzed 130 towns of Qingdao City in 72 months from January 2013 to December 2018. In order to find possible clusters, the maximum radius and height were all set to 50% of the total population at risk in the total study period. The results of scan statistic analysis were visualized by ArcGis 10.0.
Results
Demographic characteristics
A total of 69646 HFMD cases were reported in Qingdao City from 2013 to 2018 including 1635 severe cases and no fatal cases. The average annual incidence rate was 123.16 (ranged from 68.05 in 2013 to 146.90 in 2015) per 100000 inhabitants.
During the study period, the number of children ≤5 years old accounted for the largest proportion (from 86.50% to 90.67%) among these cases, with the annual average incidence rate 2879.80 (ranged from 1725.45 in 2013 to 3667.06 in 2014) per 100000 inhabitants (Table 1). Thus, we mainly conducted the following spatial analysis on children≤5 years old.
Table 1. Demographic characteristic of HFMD cases and the pathogen types of some cases in Qingdao City, 2013–2018.
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Total | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| 0–5 year | 5606 | 11224 | 12448 | 10116 | 11048 | 11471 | 61913 |
| >5 year | 636 | 1199 | 1281 | 1262 | 1565 | 1790 | 7733 |
| Sex(0–5 year) | |||||||
| Male | 3493 | 6799 | 7552 | 5989 | 6520 | 6918 | 37271 |
| Female | 2113 | 4425 | 4896 | 4127 | 4528 | 4553 | 24642 |
| Occupation(0–5 year) | |||||||
| Scattered children | 4217 | 7721 | 9856 | 6569 | 7742 | 9090 | 45195 |
| Nursery children | 1386 | 3494 | 2583 | 3538 | 3287 | 2370 | 16658 |
| Other | 3 | 9 | 9 | 9 | 19 | 11 | 60 |
| Pathogen(0–5 year) | |||||||
| CoxA16 | 42 | 241 | 92 | 214 | 57 | 111 | 757 |
| EV71 | 222 | 315 | 87 | 82 | 429 | 15 | 1150 |
| Other EV | 254 | 341 | 258 | 250 | 249 | 462 | 1814 |
Of 61913 HFMD cases, 37271 were males and 24642 were females, with an average male-to-female sex ratio 1.51 (1.65:1 in 2013,1.53:1 in 2014,1.54:1 in 2015,1.44:1 in 2016, 1.44:1 in 2017, and 1.52:1 in 2018 respectively). Table 1 showed that the majority (99.90%) of HFMD cases were preschoolers including 73.00% scattered children and 26.91% nursery children. 6072 specimens were collected from 61913 HFMD cases with the positive rate 61.28%. Among these positive specimens, other EV was the major pathogens accounting for 48.75%, followed by EV71 and CoxA16 accounting for 30.91% and 20.34% respectively (Table 1).
Seasonal pattern
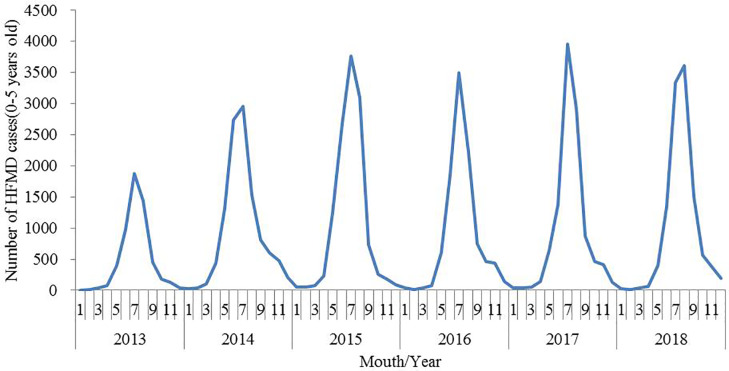
Fig 1 illustrated that the occurrence of HFMD in Qingdao City during 2013–2018 presented significant seasonality. The incidence of HFMD began to rise in May, and reached the peak in July each year. The cases reported during the high-incidence period (from May to October) accounted for 92.74% of the total number of cases.
Fig 1. Monthly number of HFMD cases in Qingdao City, 2013–2018.
Geographic distribution and spatial autocorrelation of HFMD cases
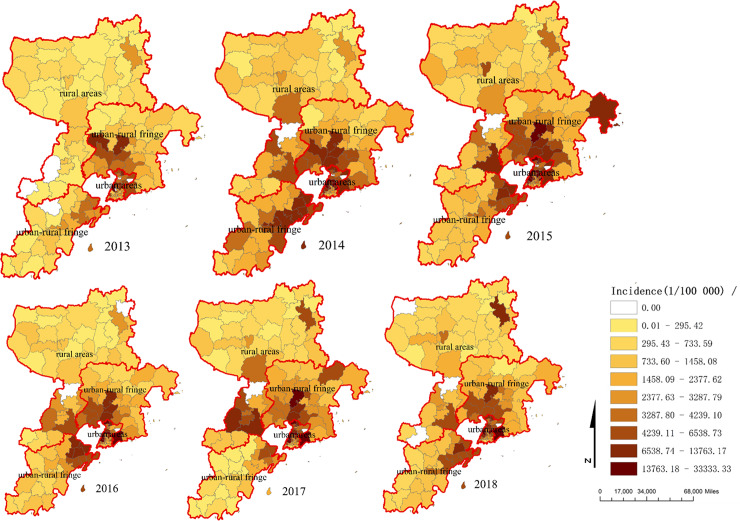
The geographic distribution of HFMD in children 0–5 year old at town level was shown in Fig 2. It clearly indicated that the distribution of HFMD was heterogeneous at town level. There were 47 towns of which the average incidence rate higher than that of the whole city. The locations of high-incidence town mainly concentrated on densely populated areas which included urban and urban-rural fringe areas with the average incidence rates for these areas 5409.60 per 100000 inhabitants and 3300.27 per 100000 inhabitants respectively. The rural areas had lower incidence rate with the average incidence rate 1154.54 per 100000 inhabitants. The difference of incidence in the three regions was statistically significant though statistical analysis (χ2 = 12427.40, P<0.05). Table 2 showed the results of the spatial autocorrelation test. The results indicated that the distribution of HFMD was nonrandom with the global Moran′I from 0.19 to 0.31 between 2013 and 2018.
Fig 2. The incidence rate of HFMD among children aged 0–5 year old at street/township level in Qingdao City, 2013–2018.
Table 2. The results of the spatial autocorrelation test on HFMD cases in Qingdao Province, 2013–2018.
| Year | Moran′s I | Z Score | P-value |
|---|---|---|---|
| 2013 | 0.2574 | 8.9579 | <0.001 |
| 2014 | 0.3135 | 10.2977 | <0.001 |
| 2015 | 0.2764 | 9.1146 | <0.001 |
| 2016 | 0.1851 | 6.3516 | <0.001 |
| 2017 | 0.2659 | 8.7284 | <0.001 |
| 2018 | 0.2510 | 8.4413 | <0.001 |
Purely spatial analysis
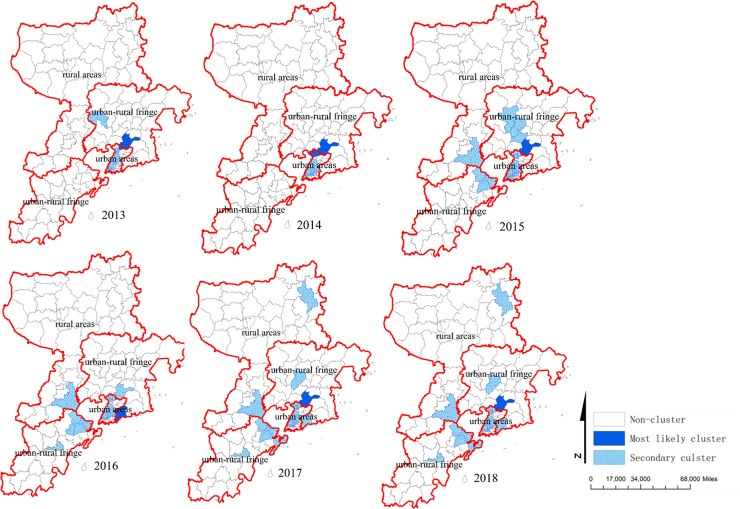
Using purely spatial scan statistic based on discrete Poisson model, the clusters which the sizes and locations varied each year were detected from 2013 to 2018. The most likely clusters were in Xiazhuang Street (2013, 2015, 2017, 2018), Xiangtan Street (2013, 2017), Xingrong Road, Jiaxing Road, Hailun Road(2014), Zhonghan Road (2016). The indexes of most likely clusters and the distribution of hotspots were showed in Table 3 and Fig 3.
Table 3. The most likely high risk clusters of HFMD cases detected using the purely spatial analysis.
| Years | Clusters areas(n) | Radius(km) | Observed cases | Expected cases | Relative Risk | P-value |
|---|---|---|---|---|---|---|
| 2013 | 2 | 7.19 | 258 | 77.46 | 3.45 | <0.001 |
| 2014 | 24 | 6.98 | 1961 | 1156.05 | 1.86 | <0.001 |
| 2015 | 1 | 0 | 381 | 72.77 | 5.38 | <0.001 |
| 2016 | 1 | 0 | 444 | 119.24 | 3.86 | <0.001 |
| 2017 | 2 | 7.19 | 570 | 149.79 | 3.97 | <0.001 |
| 2018 | 1 | 0 | 363 | 61.66 | 6.06 | <0.001 |
Fig 3. Spatial clusters of HFMD in Qingdao City, 2013–2018.
Spatial-temporal clusters analysis
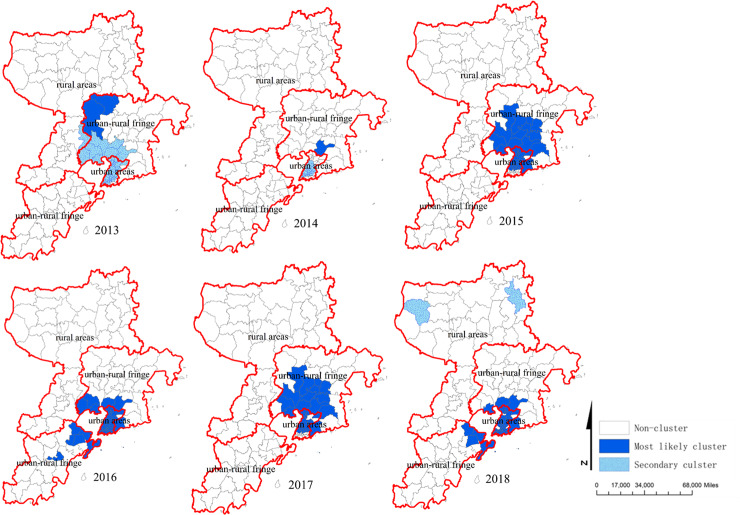
The spatial-temporal clusters were analyzed by space-time scan statistics method. 6 most likely cluster and 3 secondary clusters were found during the study period (Table 4). The most likely clusters in 2013 were located in Shibei, Licang, Shinan, Chengyang, Jimo District including 47 towns. The cluster time was from 1 Jun to 30 Sep 2013 and the radius was 18.97km with the RR 1.61. In 2014, the most likely clusters were located in Shibei, Shinan District including 24 towns. The cluster time was from 1 May to 31 Oct and the radius was 6.98km with the RR 1.88. The most likely clusters in 2015 and 2017 were Shibei, Licang, Chengyang District including 40 and 39 towns. The cluster times were all from 1 Jun to 30 Aug and the radius were all 19.66km with the RR were 1.87, 2.09. Similarly, in 2016 and 2018, the most likely clusters were in Shibei, Shinan, and Licang District including 53 and 49 towns. The cluster times were also from 1 Jun to 30 Aug and the radius were 25.03 and 22.92km with the RR were 1.82, 1.92. All the spatial-temporal clusters analysis of HFMD were shown in Table 4 and Fig 4.
Table 4. Spatial-temporal clusters analysis of HFMD in Qingdao City, 2013–2018.
| Year | Cluster type | Cluster time | Radius(km)/towns(n) | Observed cases | Expected cases | RR | LLR | P-value |
|---|---|---|---|---|---|---|---|---|
| 2013 | Most-likely | Jun 1-Sep 30 | 18.97(47) | 2478 | 1874.12 | 1.61 | 145.24 | <0.001 |
| Secondary | May 1-Aug 31 | 12.53(4) | 171 | 103.73 | 1.67 | 18.65 | <0.001 | |
| 2014 | Most-likely | May 1-Oct 31 | 6.98(24) | 1700 | 978.79 | 1.88 | 246.10 | <0.001 |
| Secondary | May 1-Oct 31 | 0.00(1) | 236 | 45.15 | 5.33 | 201.25 | <0.001 | |
| 2015 | Most-likely | Jun 1-Aug 31 | 19.66(40) | 4973 | 3323.46 | 1.87 | 529.81 | <0.001 |
| 2016 | Most-likely | Jun 1-Aug 31 | 25.03(53) | 4222 | 2913.58 | 1.82 | 399.85 | <0.001 |
| 2017 | Most-likely | Jul 1- Aug 31 | 19.66(39) | 3853 | 2285.85 | 2.09 | 607.18 | <0.001 |
| 2018 | Most-likely | Jul 1- Aug 31 | 22.92(49) | 3844 | 2425.37 | 1.92 | 482.61 | <0.001 |
| Secondary | Jun 1-Sep 30 | 10.41(2) | 95 | 46.00 | 2.07 | 20.01 | <0.001 |
Fig 4. The spatial-temporal clusters of HFMD in Qingdao City, 2013–2018.
Discussion
In our study, we described the epidemiology of HFMD in Qingdao City from 2013 to 2018. We found that the incidence of HFMD presented an increasing trend and had been at a high level between 2015 and 2018, which was much higher than that of Shandong Province (93.70 per 100000) [24]. The result indicated that great pressures were still to be dealt with the prevention and control of HFMD in Qingdao City.
We observed that the children under 5 years old accounted for 88.90% of all cases, comparing well with other studies [25–28]. This could attribute to differences in antibodies level in different age groups. A seroepidemiological studies had shown that seropositivity to EV71 decreased rapidly from 1 month to 2 years old, increased gradually from 2 to 5 years old, and reached a stable state after 5 years old [29]. Another study in Zhejiang Province also found that the EV71 seroprevanlence in 6–10 years group and 11–20 years group (54.60% and 61.80% respectively) were significantly higher than that in 0–5 years group (29.10%) [30]. In addition, we found that scattered children (73.00%) accounted for a much larger proportion than nursery children (26.91%). Similarly, Liu et al showed that about half of HFMD cases in Nanchang, China, were scattered children [31]. And a number of studies also noted that a higher percentage of HFMD cases occurred among children who did not attend a nursery or preschool [32, 33]. One possible reason was that some prevention and control measures, such as hand-washing intervention, morning check, case isolation system, and school closure, were implemented in institutional settings in recent years. Previous studies of HFMD in other regions had shown that males were more susceptible to enterovirus than females, and the same was true of our findings [34–36]. This could be attributed to the fact that males were more active and more contacted favoring the spread of HFMD.
Although EV71 and CA16 were the most common etiological pathogens in many HFMD cases around the world [37, 38], our study showed that other EV was the major pathogens of HFMD in Qingdao during 2013–2018 except 2017. Notably, some other studies showed that other EV, such as CA6 and CA10, was going to become the important causative agents for HFMD in China, Finland, France, and Japan [39–41]. This suggested that other EV should be further classified to prevent HFMD cases associated with other new enteroviruses.
As in other reports [36, 37], the study showed that HFMD presented distinctly seasonality. The single seasonal peaks could be found between May and October. Some studies found that there were two kinds of peak patterns of HFMD epidemic in China: one peak in summer in northern China and two peaks in spring and autumn in southern China [42, 43]. These differences might be partly attributed to climatic factors, such as temperature and humidity [44].
Our study also found that HFMD cases in Qingdao City were mainly concentrated in urban and urban-rural fringe areas. Liao J et al [45] also had reported that the incidence of HFMD in urban areas with high population density was much higher than rural areas. By spatial autocorrelation analysis, the value of global Moran′I was from 0.19 to 0.31 between 2013 and 2018, indicating that the spatial distribution was not random at town level. This result was similar to the spatial autocorrelation patterns in China at city spatial scale level [24,37,45]. In order to better explore the spatial epidemic trend of HFMD, further spatial autocorrelation analysis should be done at village or community level.
Using purely spatial scan statistic based on discrete Poisson model, we found that the most likely clusters were in Xiazhuang Street (2013, 2015, 2017, 2018), Xiangtan Street (2013, 2017), Xingrong Road, Jiaxing Road, Hailun Road (2014), and Zhonghan Road (2016). These areas were respectively belonged to urban and urban-rural fringe areas with highly developed economy, high population density and mobility. The space-time scanning results found that the clusters almost occurred in May to October, which was basically the same as the seasonal peaks of HFMD in Qingdao City. Furthermore, the most likely cluster was located in urban and urban-rural fringe areas in Qingdao City during the whole study period, consistent with previous studies [45]. The reason for the urban areas as most likely cluster areas might be related with high-density populations, high-level medical diagnosis and cases report timely. It was worth noting that there were many “villages within cities” in urban-rural fringe areas with large number of migrant workers, backward municipal facilities, weak health awareness and poor health environment in China. Some studies indicated that many migrant workers lived with their children, most of whom were under 5 years old and not at school, which leaded to high incidence of HFMD in urban-rural fringe areas [38]. Moreover, the space-time scanning results indicated that the tendency of HFMD in Qingdao City spreaded from urban area to rural area. Therefore, this result reminded us that prevention and control measures should be implemented not only in urban area, but also in rural area which should be particularly strengthened.
Owning to adequately utilize the time and spatial information of data, the spatial-temporal clusters analysis could simultaneously locate the time and space of high incidence disease, and identify the high-risk time and areas of disease. To explore the dynamic characteristics of disease in temporal and spatial dimensions, the Scan software was better than the traditional descriptive mathematical statistical methods. Therefore, this method which was widely used to detect clusters of infectious disease could facilitate to adopt preventive and control measures in key areas.
Our study was the first time to analyze the spatial-temporal clusters of HFMD at town level in Qingdao City. In this study, we obtained more accurate high-incidence areas of HFMD, which could help to carry out prevention and control of HFMD purposefully and provide certain guiding significance on saving social resource. Despite insights gained from our study, there were still several limitations. First, we were only able to include HFMD cases reported to surveillance system. As a self-limiting illness, some patients may not attend hospital, which were not included in the surveillance system. Second, the dominant enteroviruses (other EV) were not further classified to identify new enteroviruses.
In summary, our study illuminated the spatial-temporal epidemiological characteristics of HFMD at town scale level from 2013 to 2018 in Qingdao City. The results revealed that urban and urban-rural fringe areas were the major locations of the clusters with other EV as the dominant pathogen. With the help of the finding, the prevention and control of HFMD in Qingdao City should be focus on the high-risk periods and locations which had important public health significance for the allocation of public health resources.
Supporting information
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, et al. (2010) Virololgy, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 10:778–790. 10.1016/S1473-3099(10)70194-8 [DOI] [PubMed] [Google Scholar]
- 2.Xing W, Liao Q, Viboud C, Zhang J, Sun J, et al. (2014) Hand, foot, and mouth disease in China, 2008–12: an apidemiological study. Lancet Infect Dis 14:308–318. 10.1016/S1473-3099(13)70342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng FC, Huang HC, Chi CY, Lin TL, Liu CC, et al. (2007) Epidemiological survey of enterovirus infections occurring in Taiwan between 2000 and 2005: analysis of sentinel physician surveillance data. J Med Virol 12:1850–1860. [DOI] [PubMed] [Google Scholar]
- 4.Weng KF, Chen LL, Huang PN, Shi SR.(2010) Neural pathogenesis of enterovirus 71 infection. Microbes Infect 12:505–510. 10.1016/j.micinf.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 5.Hosoya M, Kawasaki Y, Sato M, Honzumi K, Kato A, et al. (2006) Genetic diversity of enterovirus 71 associated with hand, foot, and mouth disease epidemics in Japan from 1983 to 2003. Pediatr Infect Dis J 25:691–694. 10.1097/01.inf.0000227959.89339.c3 [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Yeo A, Poon MC, Tan EL, Poh CL, et al. (2010) The largest outbreak of hand, foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis 14: e1076–1081. 10.1016/j.ijid.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 7.Van TP, Thao NTT, Perera D, Truong KH, Tien NTK, et al. (2005) Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam. Emerg Infect Dis 13: 1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto T, Chikahira M, Yoshida S, Ebira H, Hasegawa A, et al. (2002) Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol Immunol 46: 621–627. 10.1111/j.1348-0421.2002.tb02743.x [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhu Z, Yang W, Ren J, Tan X, et al. (2010) An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand, foot and mouth disease in Fuyang city of China. Virol J 12: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Tan XJ, Wang HY, Yan DM, Zhu SL, et al. (2009) An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong. J Clin Virol 44:262–267. 10.1016/j.jcv.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 11.The Ministry of Health of The People’s Republic of China.Guide for the preparedness and control measures of hand, foot, and mouth disease in China.(2008 version)(in Chinese). Cap J Public Health:146–148. [Google Scholar]
- 12.Chinese Center for Disease Control and Prevention(China CDC). National incidence and death cases of notifiable class A or class B infectious disease(2008,2009,2010,2011).(http://www.chinacdc.cn)
- 13.Announcement on licensed drugs approved by China Food and Drug Administration in China Food and Drug Administration;2016.
- 14.Jiang FC, Yang F, Chen L, Jia J, Han YL, et al. (2016)Meteorological factors affect the hand,foot,and mouth disease epidemic in Qingdao,China,2007–2014. Epidemiol Infect, 144:2354–2362. 10.1017/S0950268816000601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Ding G, Zhang Y, Liu Q, Jiang B, et al. (2018)Assessmen on the burden of bacillary dysentery associated with floods during 2005–2009 in Zhengzhou. J Infect Public Health 11:500–507. 10.1016/j.jiph.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 16.Pfeiffer DU, Robinson TP, Stevenson M, Stevens KB, Rogers DJ, et al. Spatial analysis in epidemiology New York: Oxford University Press;2008 [Google Scholar]
- 17.How Spatial Autocorrelation: Moran’s I (Spatial Statistics) Works [http://edndoc.Esri.com/arcobjects/9.2/net/shared/geoprocessing/spatial_statistics_tools/how_spatial_autocorrelation_colon_moran’sIspatialstatisticsworks.htm]
- 18.Waldhor T.(1996)The spatial autocorrelation coefficient Moran’s I under heteroscedasticity. Stat Med 15:887–892. [DOI] [PubMed] [Google Scholar]
- 19.Kulldorff M. A spatial scan statistic. Communiactions in Statics Theory and Methods 26:1481–1496. [Google Scholar]
- 20.Kulldorff M, Feuer EJ, Miller BA, Freedman LS, et al. (1997) Breast cancer clusters in the northeast United States: a geographic analysis. American Jouranl of Epidemiology 146:161–170. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Wang L, Fang L, Ma J, Xu Y, et al. (2008) Spatial nanlysis of malaria in Anhui province, China. Malar J 7:206 10.1186/1475-2875-7-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Q, Hao Y, Ma J, Yu S, Wang Y. (2011) Surveillance of hand, foot, and mouth disease in mainland China(2008–2009). Biomed Environ Sci 24:349–356. 10.3967/0895-3988.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 23.Kulldorff M. SaTScan user guide for version 9.0 Department of Ambulatory Care and Prevention, Harvard Medical School, Boston, MA: 2010. [Google Scholar]
- 24.Liu Y, Wang X, Liu Y, Sun D, Ding S, et al. (2013) Detecting Spatial-Temporal Clusters of HFMD from 2007 to 2011 in Shandong Province, China. Plos one 8:e63447 10.1371/journal.pone.0063447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YR, Sun LL, Xiao WL, Chen LY, Wang XF,et al. (2013) Epidemiology and clinical characteristics of hand, foot, and mouth disease in a Shenzhen sentinel hospital from 2009 to 2011. BMC Infect Dis 13:539 10.1186/1471-2334-13-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samphuttbanon R, Tripathi NK, Ninsawat S, Duboz R.(2013) Spatio-temporal distribution and hotspots of hand, foot and mouth disease(HFMD) in northern Thailand. Int J Environ Res Public Health 11:312–336. 10.3390/ijerph110100312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kar BR, Dwibedi B, Kar SK.(2013) An outbreak of hand, foot and mouth disease in Bhubaneswar, Odisha. Indian Pediatr 50:139–142. 10.1007/s13312-013-0033-0 [DOI] [PubMed] [Google Scholar]
- 28.Park SK, Park B, Ki M, Kim H, Lee K, et al. (2010) Transmission of seasonal outbreak of childhood enteroviral aseptic meningitis and hand-foot-mouth disease. J Korean Med Sci 25:677–683. 10.3346/jkms.2010.25.5.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu FC, Liang ZL, Meng FY, Zeng Y, Mao QY, et al. (2012)Retrospective study of the incidence of HFMD and seroepidemiology of antibodies against EV71 and CoxA16 in prenatal women and their infants. Plos One 7:e37206 10.1371/journal.pone.0037206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gui J, Liu Z, Zhang T, Hua Q, Jiang Z, et al. (2015) Epidemiological characteristics and spatial-temporal clusters of hand, foot, and mouth disease in Zhejiang Province, China,2008–2012. Plose One 10:e0139109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu MY, Liu W, Luo J, Liu Y, Zhu Y, et al. (2011) Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010.Plos One 6:e25287 10.1371/journal.pone.0025287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Cao Z, Zeng DD, Wang Q, Wang X, et al. (2014) Epidemiological analysis, detection, and comparison of space-time patterns of Beijing hand-foot-mouth disease(2008–2012). Plos One 9:e92745 10.1371/journal.pone.0092745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao LX, Wu B, Bao WX, Han FA, Xu L, et al. (2010)Epidemiology of hand, foot, and mouth disease and genotype characterization of enterovirus 71 in Jiangsu, China. J Clin Virol 49:100–104. 10.1016/j.jcv.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 34.Sun LM, Zheng HY, Zheng HZ, Guo X, He JF, et al. (2011)An enterovirus 71 epidemic in Guangdong Province of China,2008: epidemiological, clinical, and virogenic manifestations. Jpn J Infect Dis 64:13–18. [PubMed] [Google Scholar]
- 35.Qiaoyun F, Xiongfei J, Lihuan L, Angao X.(2013) Epidemiology and etiological characteristics of hand, foot and mouth disease in Huizhou city between 2008 and 2011. Arch Virol 158:895–899. 10.1007/s00705-012-1566-6 [DOI] [PubMed] [Google Scholar]
- 36.Li J, Fu Y, Xu A.(2014) A spatial-temporal ARMA model of the incidence of hand, foot, and mouth disease in Wenzhou, China. Abstr Appl Anal 14:1–9. [Google Scholar]
- 37.Liu L, Zhao X, Yin F, Lv Q.(2015) Spatio-temporal clustering of hand, foot and mouth disease at the county level in Sichuan province, China, 2008–2013. Epidemiology & Infection 143:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu L, Shi Y, Rainey JJ, Zhang Z, Zhang H, et al. (2018) Epidemiological features and spatial clusters of hand, foot, and mouth disease in Qinghai Province, China, 2009–2015. BMC Infect Dis 18:624 10.1186/s12879-018-3509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flett K, Youngster I, Huang J.(2012) Hand, foot and mouth disease caused by coxsackievirus A6. Emerg Infect Dis 18:1702–1704. 10.3201/eid1810.120813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blomqvist S, Klimola P, Kaijalainen S, Paaananen A, Simonen ML, et al. (2010) Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol 48:49–54. 10.1016/j.jcv.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 41.Li JL, Yuan J, Yang F, Wu ZQ, Hu YF, et al. (2014)Epidemic characteristics of hand, foot, and mouth disease in southern China,2013:Coxsackievirus A6 has emerged as the predominat causative agent. J Infect 69:299–303. 10.1016/j.jinf.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Hu T, Sun D, Ding S, Carr MJ, et al. (2017). Epidemiological characteristics of hand, foot, and mouth disease in Shandong, China, 2009–2016. Sci Rep 7: 8900 10.1038/s41598-017-09196-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xing W, Liao Q, Viboud C. (2014). Hand, foot, and mouth disease in China, 2008–2012: an epidemiological study. Lancet Infect Dis 14: 308–318. 10.1016/S1473-3099(13)70342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang XF, Lu J, Liu XX, Dai T. (2018). Epidemiological features of hand, foot and mouth disease outbreaks among Chinese Preschool Children: a Meta-analysis. Iran J Pubic Health 47: 1235–1244. [PMC free article] [PubMed] [Google Scholar]
- 45.Liu W, Ji H, Shan J, Bao J, Sun Y, et al. (2015) Spatiotemporal dynamics of hand-foot-mouth disease and its relationship with meteorological factors in Jiangsu Province, China. Plos One 10:e0131311 10.1371/journal.pone.0131311 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.