Abstract
The murine innate immune response against Toxoplasma gondii is predominated by the interaction of TLR11/12 with Toxoplasma profilin. However, mice lacking Tlr11 or humans, who do not have functional TLR11 or TLR12, still elicit a strong innate immune response upon Toxoplasma infection. The parasite factors that determine this immune response are largely unknown. Herein, we investigated two dense granule proteins (GRAs) secreted by Toxoplasma, GRA15 and GRA24, for their role in stimulating the innate immune response in Tlr11-/- mice and in human cells, which naturally lack TLR11/TLR12. Our results show that GRA15 and GRA24 synergistically shape the early immune response and parasite virulence in Tlr11-/- mice, with GRA15 as the predominant effector. Nevertheless, acute virulence in Tlr11-/- mice is still dominated by allelic combinations of ROP18 and ROP5, which are effectors that determine evasion of the immunity-related GTPases. In human macrophages, GRA15 and GRA24 play a major role in the induction of IL12, IL18 and IL1β secretion. We further show that GRA15/GRA24-mediated IL12, IL18 and IL1β secretion activates IFNγ secretion by peripheral blood mononuclear cells (PBMCs), which controls Toxoplasma proliferation. Taken together, our study demonstrates the important role of GRA15 and GRA24 in activating the innate immune response in hosts lacking TLR11.
Author summary
In mice, the early immune response against Toxoplasma is dominated by TLR11-mediated release of IL12, which subsequently induces protective IFNγ. Here we show that in Tlr11-/- mice and in human cells, which do not have TLR11, the Toxoplasma GRA15 and GRA24 effectors play an important role in induction of IL12, IL18 and IL1β, and thus in the subsequent protective IFNγ secretion.
Introduction
Toxoplasma gondii is an obligate intracellular parasite capable of infecting any nucleated cell of any warm-blooded animal, including humans. It can cause lifelong persistent infections by forming semi-dormant cysts in muscles and the brain [1–3]. Toxoplasma resides within a non-fusogenic vacuole called the parasitophorous vacuole (PV), which is separated from the host cell cytosol by the PV membrane (PVM), preventing the parasite from being recognized by the host innate immune system. However, the cytokine interferon-gamma (IFNγ) activates effector mechanisms that can mediate the elimination of Toxoplasma. Inflammatory cytokines produced by macrophages and dendritic cells (DCs) in response to Toll-like receptor (TLR) recognition of conserved pathogen associated molecular patterns (PAMPs) are important for subsequent production of IFNγ. For example, in mice the Toxoplasma actin-binding protein profilin is recognized by a heterodimer of TLR11/12 that is located in the endosome, inducing a signaling cascade leading to the production of interleukin (IL)12 by DCs [4–6]. IL12 in turn activates Natural Killer (NK) and T cells to secrete IFNγ, which can trigger a variety of toxoplasmacidal mechanisms [7, 8]. In mice, IFNγ-induced immunity related GTPases (IRGs) that can coat and vesiculate the PVM, and ultimately destroy the parasite inside, play a dominant role in resistance to Toxoplasma [9–11].
Innate immunity can also be activated by specific cytosolic receptors (often nucleotide-binding domain and leucine-rich repeat-containing receptors or NLRs) as a part of a multi-protein complex called the inflammasome [12]. In mice, Toxoplasma can activate the NLRP1 and NLRP3 inflammasomes [13], leading to IL1β/IL18 production, which together with IL12 can enhance IFNγ secretion and thereby contribute to host resistance against Toxoplasma [14, 15]. However, inflammasome activation in Tlr11-/- mice can also induce a pathological inflammatory response [16].
Human sensing and killing of Toxoplasma differs from mice, as humans lack functional TLR11/12 and do not have IRGs [17]. It was recently shown that the alarmin S100A11 secreted from infected monocytes or fibroblasts can shape the human immune response through secretion of the chemokine ligand 2 (CCL2) [18]. In addition, cytosolic recognition of Toxoplasma in human monocytes was shown to partly rely on the NLRP1 and NLRP3 inflammasome, resulting in secretion of IL1β [19, 20]. Furthermore, guanylate binding protein (GBP)1 facilitates disruption of the PV in IFNγ-stimulated human macrophages, which causes release of parasite nucleic acids that can activate cytosolic absent in melanoma 2 (AIM2) and caspase 8-dependent apoptosis [21]. This could be another potential route of immune activation as recognition of cytosolic nucleic acids induces the type I interferon pathway.
Toxoplasma can counteract the host immune response by secreting effector proteins, ROPs and GRAs, into the host cell from specialized secretory organelles called rhoptries and dense granules, respectively [22, 23]. In Europe and North America, strains belonging to four different Toxoplasma clonal lineages (types I, II, III and XII) are commonly isolated in animals and humans, although most infections are caused by type II strains [24–26]. In mice, strain differences in virulence and modulation of host cell signaling are largely due to polymorphisms in ROPs and GRAs. For example, ROP18 (a secreted kinase) and ROP5 (a pseudokinase) determine strain differences in virulence in mice by cooperatively blocking the IFNγ-induced IRGs [10, 27–29]. Several GRA proteins are localized on the PVM and can modulate the host immune response [30–32]. For instance, GRA15 from type II strains activates the NFκB pathway, leading to macrophage production of inflammatory cytokines such as IL12 and IL1β [31, 33]. Other GRAs are secreted beyond the PVM, where they can modulate host cell signaling pathways [22, 34–36]. For example, GRA24 binds to p38α MAPK leading to its autophosphorylation and constitutive activation [35]. Together, GRA15 and GRA24 drive the classical activation of macrophages (M1) via the activation of NFκB and p38 MAPK [35, 37, 38]. By contrast, the polymorphic kinase ROP16 from type I and type III strains drives the alternative activation of macrophages (M2) via the phosphorylation of the STAT6 and STAT3 transcription factors [37, 39, 40]. It is likely that the deliberate activation of the immune response by Toxoplasma effectors is a strategy to limit its virulence thereby promoting the survival of its host and the formation of tissue cysts, which are the only stages in the intermediate host that are orally infectious.
Given the large impact of GRA15 and GRA24 on macrophage gene expression and production of IL12 and IL1β, it seems surprising that their in vivo effect on parasite virulence is relatively minor [31, 35]. Mice infected with type II Δgra15 or Δgra24 parasites had elevated parasite numbers early after infection, but as the infection progressed, parasite burden and host susceptibility were no different from those following wild-type type II strain infections [31, 35, 41]. Increased type II Δgra15 early parasite burdens were associated with decreased IL12 and IFNγ levels 2 days after infection [31]. Similar results were obtained after infection of mice with the Δgra24 strain [35]. Possibly, the effects of GRA15 and GRA24 in these studies were masked by profilin: as the infection progresses and parasites lyse out of host cells, PAMPS, such as profilin, are released and activate TLR11/12. At this stage, IL12/IL1β and subsequent IFNγ production are probably no longer dependent on GRA15 and GRA24. However, humans and many animals do not have functional TLR11/12 or IFNγ-inducible IRGs [17], and therefore Toxoplasma virulence of a particular strain in mice might not correlate with virulence in other species. In Tlr11-/- mice, neutrophils are the main producers of IFNγ with a minor role of NK and T cells [42]. The production of IFNγ by neutrophils is dependent on IL1β and TNFα, but not on IL12 [42]. In addition, IL18 secreted upon inflammasome activation plays a key role in the IFNγ response from CD4+ T cells and the subsequent disease outcome in Tlr11-/- mice [43]. Thus, GRA15 and GRA24, by inducing IL1β, IL18, TNFα and IL12, might play an important role in the production of IFNγ in hosts lacking TLR11. Herein, we tested this hypothesis by infecting Tlr11-/- mice with wild-type, Δgra15, Δgra24 and Δgra15/24 parasites. Our data indicate that although parasites that do not express GRA15 and/or GRA24 induced significantly less inflammatory cytokines, a significant increase in virulence compared to wild-type was only observed after subcutaneous infection, likely because Tlr11-/- mice were already extremely susceptible to wild-type Toxoplasma infections. We further show that in Tlr11-/- mice IRG-mediated killing of Toxoplasma is likely still the major mechanism of resistance, as parasites that express avirulent ROP5 and ROP18 were completely avirulent in these mice. In human THP1-derived macrophages and PBMCs, GRA15 and GRA24 determined the induction of inflammatory cytokines and thereby had a large effect on parasite proliferation. Thus, in the absence of TLR11, GRA15 and GRA24 are the major parasite effectors that activate the innate immune response and it is likely that in humans they determine parasite virulence.
Results
GRA15 and GRA24 regulate murine macrophage function in vitro
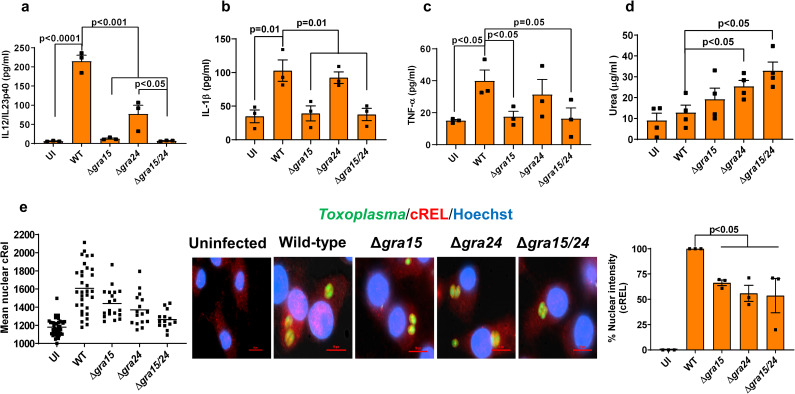
Synthesis of IL12 by Toxoplasma-infected macrophages was previously shown to be dependent on p38 MAPK and NFκB activation [44, 45]. Indeed, deletion of either GRA15 or GRA24 significantly reduced macrophage IL12/IL23p40 production, consistent with their activation of the NFκB and p38 MAPK pathway, respectively [31, 35]. It was previously shown that GRA24 from both type I RH and type II Pru strains activates the p38 MAPK pathway. However, the GRA24-dependent transcriptional changes in murine macrophages are much more pronounced after infection with type II strains compared to type I strains [35]. For example, without GRA24 the Pru induction of inflammatory cytokines, including IL12/IL23p40, is significantly affected, while GRA24 only has a minor effect on the modulation of these cytokines by RH. It is possible that many of the GRA24-mediated transcriptional changes are dependent on GRA15, as RH does not express a functional GRA15 [31]. To more directly test whether GRA15 and GRA24 have any synergistic or additive effect on macrophages, we generated gra24 single knockout and gra15/gra24 double knockout parasites in the type II Pru strain (S1 Fig). We infected murine bone marrow derived macrophages (BMDMs) with Pru wild-type, Δgra15 [31], Δgra24 and Δgra15/24 parasites for 24 h and measured the release of IL12/IL23p40, IL1β and TNFα, which are cytokines often used as M1 macrophage polarization markers [38]. BMDMs infected with Δgra15 or Δgra15/24 parasites secreted significantly less IL12/IL23p40, IL1β and TNFα (Fig 1A–1C) compared to wild-type infected BMDMs. Deletion of GRA24 significantly impaired the release of IL12/IL23p40 but had no effect on IL1β and TNFα (Fig 1A–1C). We also measured arginase activity (a marker for M2 macrophages) in macrophages infected with Δgra15, Δgra24 or Δgra15/24 parasites. Δgra24 and Δgra15/24, but not wild-type and Δgra15 parasites, induced significant arginase activity in macrophages (Fig 1D). The transcription of the p40 subunit of IL12 in mice is primarily dependent on cREL and moderately on the NFκB p65 subunit [46]. Macrophages infected with wild-type parasites contained significantly more nuclear (activated) cREL compared to macrophages infected with Δgra15, Δgra24 or Δgra15/24 parasites (Fig 1E). Similar results were obtained in human foreskin fibroblasts (HFFs) (S3 Fig). Nuclear translocation of the NFκB p65 subunit in macrophages and HFFs was significantly reduced after infection with Δgra15 or Δgra15/24 parasites but not after infection with Δgra24 parasites (S1E and S1F Fig). Thus, our results show that while GRA15 is required for the secretion of IL1β and TNFα from BMDM, both GRA15 and GRA24 are required for the secretion of IL12/IL23p40.
Fig 1. GRA15 and GRA24 activate the in vitro macrophage response.
BMDMs were infected with indicated Toxoplasma strains for 24 h and IL12/IL23p40 (a), IL1β (b) and TNFα (c) were measured in the supernatant. Experiments were done 3 times. Arginase activity was measured (d) from RAW 264.7 macrophages infected with indicated strains 24 h p.i. Nuclear translocation of the cREL subunit of NFκB (e) was quantified from infected RAW 264.7 macrophages 18 h p.i. with indicated strains. In each experiment at least 15 cells were quantified as shown in the graph (left) and experiment was done 3 times (right panel graph). A representative image for each group is shown in the middle panel. Scale bar is 10 μm. Each dot represents the mean of 3 technical replicates from individual experiments, except for the scatter diagram in (e). Statistical analysis was done by two sample student’s t test for figures a-e. Data are represented as mean ± standard error of the mean (SEM).
Deletion of GRA15 and GRA24 affects in vivo parasite growth and cytokine production in Tlr11-/- mice
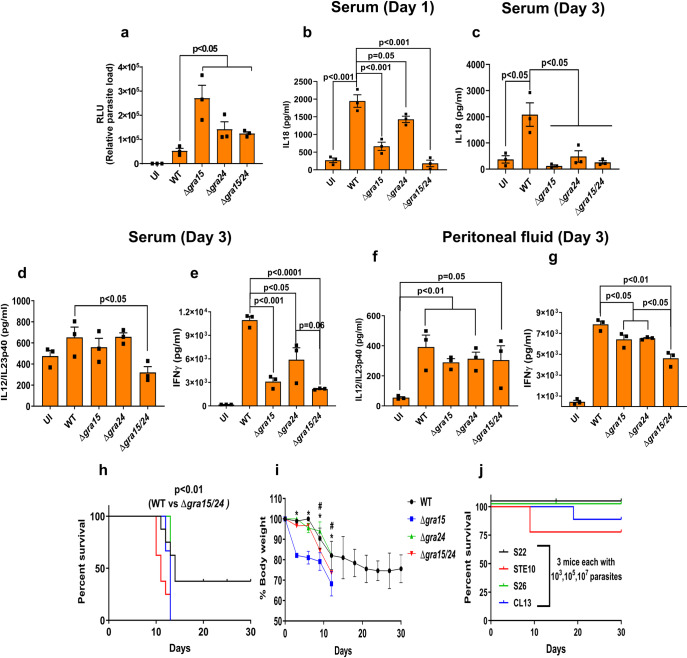
It was previously shown that the intraperitoneal parasite load in C57BL/6 mice intraperitoneally (i.p.) infected with either Δgra15 or Δgra24 was higher compared to wild-type parasites, but the mortality of the mice was not significantly different [31, 35]. To determine if Toxoplasma profilin-mediated activation of TLR11 might have masked the effect of GRA15 and GRA24 we investigated parasite burden and mortality in the Tlr11-/- mice. Tlr11-/- mice i.p. infected with Δgra15, Δgra24 or Δgra15/24 parasites had a significantly larger peritoneal parasite load 3 days p.i. compared to wild-type infected mice, with Δgra15-infected mice having the highest parasite load (Fig 2A). On day 1 p.i., the level of IL12/IL23p40, IFNγ, IL1β or TNFα in the serum was not higher than in uninfected mice. It was recently shown that in Tlr11-/- mice IL18 is necessary and sufficient for induction of IFNγ production [43]. The IL18 level was significantly increased in mice infected with wild-type parasites (Fig 2B) while mice infected with Δgra15 or Δgra24 parasites had significantly lower serum IL18 levels on day 1 p.i. compared to wild-type parasite infected mice, which was even further reduced in mice infected with Δgra15/24 parasites. On day 1 p.i. IFNγ, IL18, IL1β or TNFα were not detected in the peritoneal fluid but the IL12/IL23p40 levels were significantly higher in mice infected with wild-type parasite compared to uninfected mice (S4A Fig). Mice infected with Δgra15/24 parasites contained significantly lower IL12/IL23p40 levels compared to wild-type parasite infected mice (S4A Fig). On day 3 p.i., the IL18 level in serum still remained significantly higher in wild-type parasite infected mice, while Δgra15, Δgra24 or Δgra15/24 parasites elicited significantly lower IL18 levels (Fig 2C). Although IL12/IL23p40 levels were significantly lower at day 3 p.i. in sera of mice infected with Δgra15/24 parasites compared to wild-type infected mice (Fig 2D), this difference was not detected in the peritoneal fluid (Fig 2F). On day 3 p.i., there was a large increase in IFNγ in both serum and peritoneal fluid of wild-type parasite infected mice (Fig 2E and 2G) which was significantly decreased in mice infected with Δgra15 and Δgra24 parasites, and even further decreased in mice infected with Δgra15/24 parasites (Fig 2E and 2G). All Tlr11-/- mice i.p. infected with wild-type or Δgra15/24 parasites died by day 10 p.i. with similar severe reduction in body weight (S4B and S4C Fig). Thus, GRA15 and GRA24 both contribute to induction of IFNγ from Tlr11-/- mice by inducing IL18 and IL12, which impacts the early intraperitoneal parasite load.
Fig 2. Deletion of GRA15 and GRA24 leads to enhanced parasite virulence in Tlr11-/- mice.
Tlr11-/- mice were i.p. infected with 5,000 tachyzoites of indicated Toxoplasma strains expressing luciferase, and serum and peritoneal fluid were collected from each mouse (N = 3 mice per group) at different time points. (a) Three days p.i. peritoneal cells were isolated, plated in tissue culture plates for 24 h and the following day luciferase reading was taken to measure the parasite burden. (b) Serum IL18 levels at 1 day p.i. Serum IL18 (c), IL12/IL23p40 (d) and IFNγ (e) levels 3 days p.i. IL12/IL23p40 (f) and IFNγ (g) levels in the peritoneal fluid 3 days p.i. All data represent mean ± SEM. Statistical analysis was done with two sample Student’s t tests. Tlr11-/- mice were s.c. infected with 5,000 tachyzoites of indicated strains and (h) survival and (i) weight (plotted as an average of the change in body weight for each cohort, where 100% body weight corresponds to the day of infection) of the mice were monitored for 30 days. Statistical analysis was done using a log-rank test. N = 8 each for wild-type and Δgra15/24 whereas N = 5 for Δgra15 and Δgra24. Tlr11-/- mice were i.p injected with indicated doses of tachyzoites of different Toxoplasma strains derived from F1 progenies of a type II X type III cross (51) containing avirulent ROP18 and ROP5 alleles and survival was monitored (j). Each dot represents a mean of 3 technical replicates from an experiment. Statistical analysis was done by two sample student’s t test for figures a-g. Data are represented as mean ± standard error of the mean (SEM).
It was previously described that Tlr11-/- mice can survive i.p. infection with tissue cysts (20–25 cysts) [5, 6, 42]. In our hands, however, all Tlr11-/- mice i.p. infected with 10 cysts of wild-type or Δgra15/24 parasites succumbed by day 13 p.i. with similar body weight reduction, although the Δgra15/24 parasites caused significantly earlier mortality (S4D and S4E Fig). Because of the unexpected extreme susceptibility of the Tlr11-/- mice after i.p infection with tachyzoites or with tissue cysts, we performed s.c. infections to curb rapid Toxoplasma dissemination. Compared to wild-type, Δgra15/24 parasites caused significantly more mortality in s.c. infected Tlr11-/- mice (Fig 2H), whereas Δgra15 and Δgra15/24 parasites caused a significantly larger body weight reduction (Fig 2I). Thus, GRA15 and GRA24 have a significant effect on the innate immune response in Tlr11-/- mice. However, these mice are already extremely susceptible to wild-type parasite infection and an increased virulence of parasites without GRA15 and/or GRA24 can therefore not be detected after i.p. infection.
The ROP18 and ROP5 allelic combinations determines survival of Tlr11-/- mice
Although there was a significant difference in cytokine induction in Tlr11-/- mice i.p. infected with Δgra15, Δgra24 or Δgra15/24 parasites (Fig 2B–2G), all mice succumbed within 10–13 days p.i. In mice, acute parasite virulence is determined by the exact ROP18 and ROP5 allele [10, 47–50]. To determine the role of ROP18 and ROP5 in Tlr11-/- mice we i.p. infected Tlr11-/- mice with 4 F1 progeny (S22, S26, STE10 and CL13) from a type IIxIII cross [51] that have the avirulent alleles of ROP18 and ROP5. While 100% mortality was observed after infection with 100 Pru wild-type or Δgra15/24 parasites (S4B Fig), mice infected with these F1 progeny strains survived doses up to 105 parasites (Fig 2H and 2I). Only 1 mouse infected with CL13 and 2 mice infected with STE10 parasites (out of 5) died at the 107 dose (Fig 2J), accompanied with significant body weight reduction (7–10%) only in mice infected with the STE10 strain (S4F–S4H Fig). Thus, although in Tlr11-/- mice the cytokine response is significantly influenced by GRA15 and GRA24, survival after i.p. infection is almost entirely dependent on ROP18 and ROP5. It therefore appears that Tlr11-/- mice are not a good model for the human immune response to Toxoplasma as humans do not have IRGs and ROP18 and ROP5 do not affect Toxoplasma resistance to IFNγ in human cells [10, 17, 52].
GRA15 and GRA24 induce cytokine secretion in human macrophages through activation of p38 MAPK and NFκB
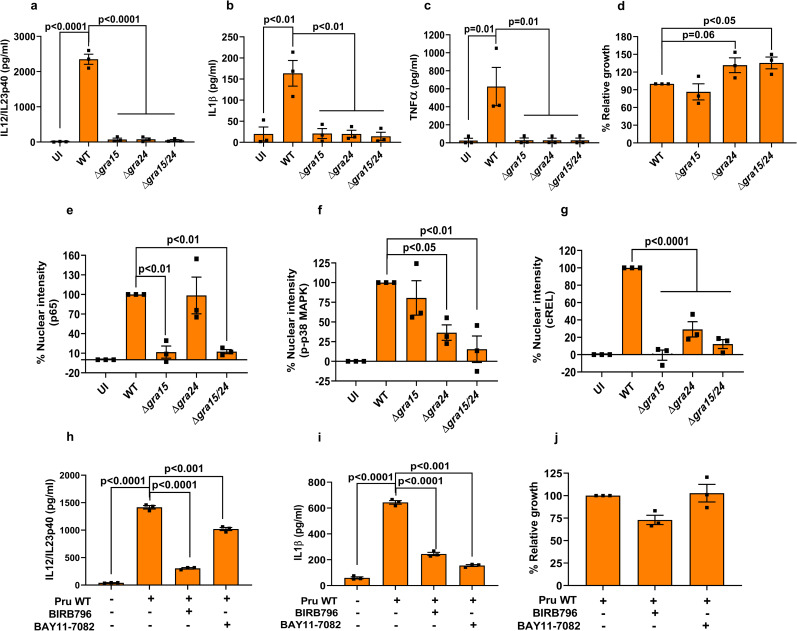
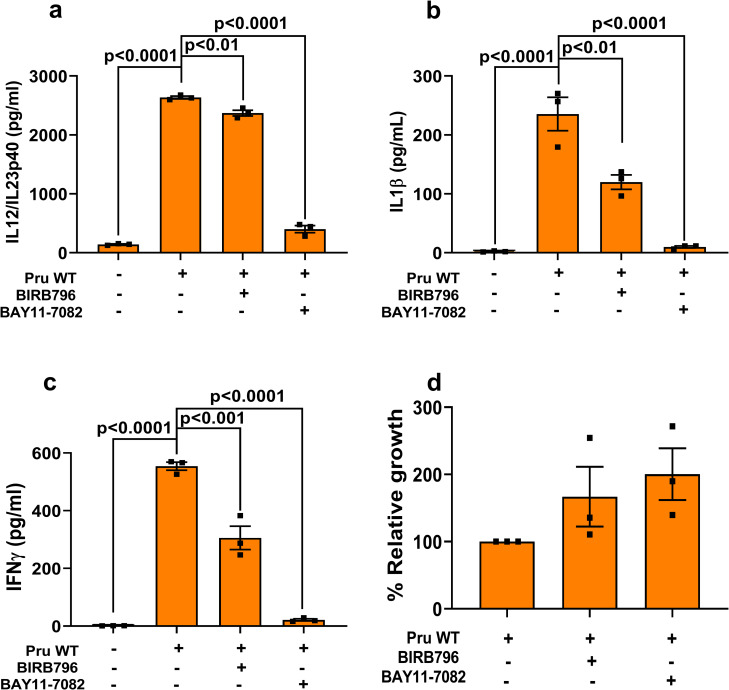
The innate immune response against Toxoplasma in human monocytes is affected by the infecting strain type. For example, type II, but not type I strains, are major inducers of IL1β [18, 33], which is primarily dependent on type II GRA15 [33]. However, the role of GRA24 in the induction of pro-inflammatory cytokines by human macrophages is not known. We measured IL12/IL23p40, IL1β and TNFα from THP1-derived macrophages infected with wild-type, Δgra15, Δgra24 and Δgra15/24 parasites and observed that, akin to murine macrophages, GRA15 and GRA24 were both important for generation of IL12/IL23p40 (Fig 3A). However, in contrast to murine macrophages, both GRA15 and GRA24 determined IL1β and TNFα production by THP1-derived macrophages (Fig 3B and 3C). This difference between human and murine macrophages could be due to species-specific transcription factor dependence as often seen specifically for macrophages [53]. We also measured the growth of the different parasite lines in THP1 macrophages and detected a growth advantage in parasites lacking GRA24 compared to wild-type parasites (Fig 3D). Furthermore, similar to murine macrophages, while GRA15 and GRA24 specifically activated nuclear translocation of p65 NFκB and p38 MAPK, respectively, in THP1 macrophages (Fig 3E and 3F, S5A and S5B Fig), both GRA15 and GRA24 were required for nuclear translocation of cREL (Fig 3G and S5C Fig). To confirm the role of NFκB and p38 MAPK on cytokine secretion from THP1 macrophages, we inhibited the two pathways using BAY11-7082, an irreversible inhibitor of IκB phosphorylation important for NFκB activation [54] and BIRB796, a potent inhibitor of p38 MAPKα [55], respectively. Inhibition of either the NFκB or p38 MAPK pathway significantly inhibited the secretion of IL12/IL23p40 and IL1β from THP1 macrophages infected with wild-type parasites (Fig 3H–3I), while the inhibitors did not affect parasite growth (Fig 3J). Thus, GRA15 and GRA24 regulate pro-inflammatory cytokine production from THP1-derived macrophages through NFκB- and p38 MAPK-dependent pathways.
Fig 3. GRA15 and GRA24 activate pro-inflammatory cytokine secretion by human macrophages.
THP1 monocyte-derived macrophages were infected with indicated Toxoplasma strains for 24 h, after which IL12/IL23p40 (a), IL1β (b) and TNFα were measured. Relative parasite growth was measured by luciferase growth assay (d). Nuclear translocation of the NFκB p65 (e), p-p38 MAPK (f), and NFκB cREL (g) subunits were quantified from infected THP1 macrophages 18 h p.i with indicated strains. In each experiment at least 15 cells were quantified. THP1 macrophages were treated with indicated inhibitors 2 h prior to infection and subsequently infected for an additional 20 h. IL12/IL23p40 (h), IL1β (i) and growth (j) were measured. Each dot represents a technical mean value from a single experiment, and each experiment was done 3 times. Statistical analysis was done by One-way ANOVA followed with Tukey’s multiple comparison test. Data are represented as mean ± standard error of the mean (SEM).
IL12 and NLRP3 inflammasome-derived IL18 induce the secretion of IFNγ from human PBMCs
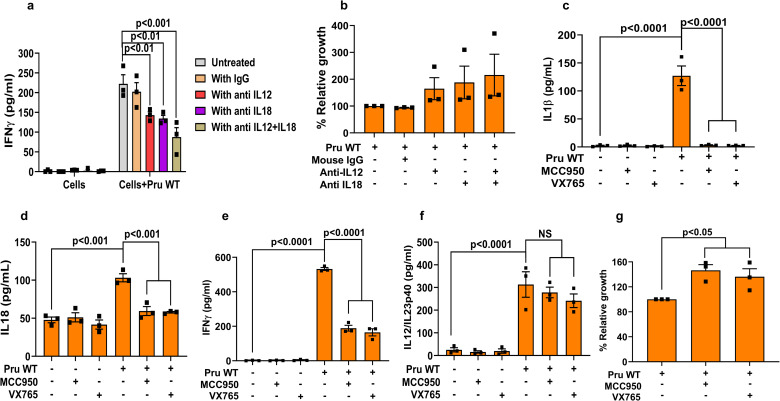
In mice, IFNγ is known to be induced by IL12, which can be further enhanced by IL18 and IL1β [14, 15]. To determine the role of IL12 and IL18 in the induction of IFNγ in human cells we used PBMCs as a model [56]. When PBMCs were infected with Pru wild-type there was a significant decrease in IFNγ secretion upon blocking IL12 or IL18 compared to either untreated or isotype antibody-treated cells (Fig 4A), which was more pronounced when cells were treated with blocking antibodies against both IL12 and IL18. These results suggest that both IL12 and IL18 are required for optimal IFNγ production by human PBMC (Fig 4A). This was further corroborated by the increased parasite growth detected in PBMCs treated with blocking antibodies against IL12, IL18, or both (Fig 4B). IL18 and IL1β are secreted as a result of inflammasome activation [12]. In Toxoplasma-infected human PBMCs, IL1β secretion is mediated via NLRP3 inflammasome activation [20]. Indeed, inhibition of the NLRP3 inflammasome with MCC950 or inhibition of CASP1 with VX765 led to a significant decrease in IL1β, IL18, and IFNγ secretion (Fig 4C–4E) accompanied by increased parasite growth (Fig 4G). These effects on IFNγ by the inhibitors were not dependent on IL12/IL23p40 secretion, as the inhibitors did not alter IL12/IL23p40 levels (Fig 4F). Thus, IFNγ secretion from human PBMCs infected with the type II Pru strain, is dependent on IL12 and NLRP3 inflammasome-derived IL18 and IL1β.
Fig 4. Secretion of IFNγ from human PBMCs is dependent on IL12 and NLRP3 inflammasome-derived IL18 and IL1β.
PBMCs were infected with Pru wild-type parasites and treated with either anti-IL12, anti-IL18, isotype specific antibody, or anti-IL12+anti-IL18 1 h p.i. Supernatants were harvested for quantification of IFNγ (a) and parasite growth (b). PBMCs were treated with the caspase 1/4 inhibitor VX765 or NLRP3 inhibitor MCC950 2 h pre-infection followed by infection for another 20 h. After harvesting the culture supernatant, IL1β (c), IL18 (d), IFNγ (e) and IL12/IL23p40 (f) were measured. Parasite growth was measured from the cell lysate using luciferase assay (g). Each dot represents the mean of 3 technical replicates from a single experiment. Statistical analysis was done with Two-way ANOVA followed by Tukey’s multiple comparison test (a), two sample Student’s t test (b and g), and One-way ANOVA followed by Tukey’s multiple comparison test (c-f). Data are represented as mean ± standard error of the mean (SEM).
Cytokine secretion from human PBMCs is mediated through activation of NFκB and p38 MAPK by GRA15 and GRA24
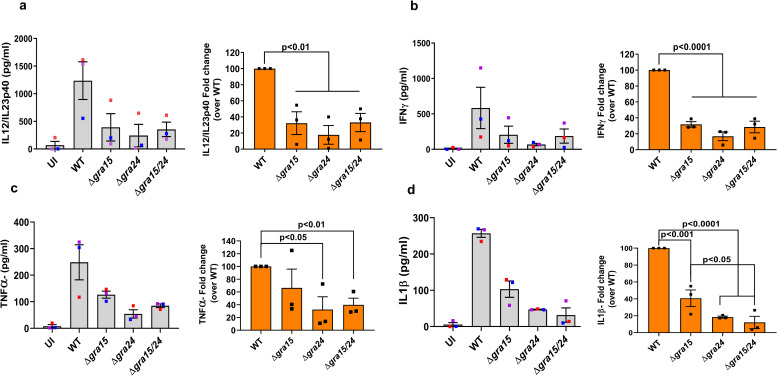
To test whether p38 MAPK and NFκB signaling are involved in cytokine production from PBMCs infected with Pru wild-type, we used the inhibitors BIRB796 (inhibits p38 MAPK) and BAY11-7082 (inhibits IκB phosphorylation). Both inhibitors inhibited secretion of IL12/IL23p40 (Fig 5A), IL1β (Fig 5B) and IFNγ (Fig 5C), with BAY11-7082 having a much greater effect (Fig 5A–5C). Furthermore, compared to untreated PBMCs, those treated with either of these inhibitors supported more parasite growth (Fig 5D). As we observed that GRA15 and GRA24 induced inflammatory cytokine generation in THP1-derived macrophages, we also tested their effect on human PBMCs. PBMCs infected with Δgra15, Δgra24, or Δgra15/gra24 parasites secreted significantly less IL12/IL23p40 compared to PBMCs infected with wild-type parasites (Fig 6A). Furthermore, parasites lacking GRA15, GRA24, or both grew more in PBMCs compared to wild type parasites probably due to lack of anti-parasitic IFNγ secretion from PBMCs infected with the knockout parasites (S6A Fig). Likewise, PBMCs infected with Δgra15, Δgra24, or Δgra15/gra24 parasites secreted significantly less IFNγ, TNFα and IL1β compared to PBMCs infected with wild-type (Fig 6B–6D). Thus, GRA15 and GRA24 together determine the secretion of proinflammatory cytokines IL12/23p40, IFNγ, TNFα and IL1β from infected human PBMCs.
Fig 5. Cytokine secretion from human PBMCs is mediated by activation of NFκB and p38 MAPK.
PBMCs were treated with indicated inhibitors 2 h prior to infection and subsequently infected for 20 h, after which IL12/IL23p40 (a), IL1β (b) and IFNγ were measured. The relative parasite growth was measured by luciferase growth assay (d). Each dot represents the mean of 3 technical replicates from an experiment. Statistical analysis was done with One-way ANOVA followed by Tukey’s multiple comparison test. Data are represented as mean ± standard error of the mean (SEM).
Fig 6. GRA15 and GRA24 induce cytokine secretion by human PBMCs.
PBMCs were infected with indicated Toxoplasma strains for 24 h, after which IL12/IL23p40 (a), IFNγ (b), TNFα (c), IL1β (d) were measured. Each dot represents the mean of 3 technical replicates from an experiment. Statistical analysis was done with One-way ANOVA followed by Tukey’s multiple comparison test. Data are represented as mean ± standard error of the mean (SEM).
Recently, it was shown that in humans, alarmin S100A11 is released from Toxoplasma-infected fibroblasts and sensed by THP1 monocytes, which upregulated CCL2 production to induce recruitment of additional monocytes [18]. However, the secretion of alarmin S100A11 did not differ between uninfected and parasite infected PBMCs (S6B Fig). On the other hand, akin to a previous study [18], CCL2 secretion was observed from PBMCs upon Toxoplasma infection independent of either GRA15 or GRA24 (S6C Fig). Similarly, in accordance with Safronova et al. [18], HFFs infected with wild-type parasites secreted significantly more S100A11 compared to uninfected cells, which could be due to increased activity of inflammatory caspases 1 and 4 (S6C and S6D Fig). Secretion of S100A11 is dependent on permeabilization mediated cell lysis [18] and fibroblasts treated with Triton X100 released very high levels of S100A11 (S6B Fig). Our data indicate that inflammatory cytokine secretion from human PBMCs infected with type II Toxoplasma is regulated by both GRA15 and GRA24.
Discussion
Innate recognition of Toxoplasma gondii DNA/RNA and profilin by nucleic acid sensing TLRs or TLR11/12 heterodimers is critical for robust IL12 production and subsequent activation of host protective IFNγ [7, 57, 58]. Nevertheless, hosts lacking TLR11/12 can still produce IL12 from monocytes and dendritic cells while IFNγ is produced from T cells, NK cells and neutrophils [18, 42, 43, 56]. Our study showed that, compared to wild-type parasite infected mice, Tlr11-/- mice infected with parasites lacking GRA15 and/or GRA24 have an increased parasite load which was correlated with significantly lower IL18, IL12 and IFNγ levels. However, even in the Tlr11-/- mouse model, parasite virulence is primarily determined by ROP18 and ROP5. ROP18 and ROP5 counteract the IRGs, which are not present in humans, and which likely explains why ROP18 and ROP5 do not determine parasite susceptibility to human IFNγ [10, 17, 52]. Thus Tlr11-/- mice do not appear to be a good model for the human immune response to Toxoplasma. We show that in human THP1-derived macrophages GRA15 and GRA24 induced IL12, TNFα and IL1β through their ability to activate the NFκB and p38 MAPK pathways. In PBMCs we show that IFNγ secretion is dependent on IL12 and NLRP3 inflammasome-derived IL18 and IL1β, which are also induced by GRA15 and GRA24. Thus, GRA15 and GRA24 are major activators of the human immune response.
In the murine model of toxoplasmosis, IL12 production is largely dependent on dendritic cells (DCs) and macrophages [4, 44, 59, 60]. However, the mechanism of IL12 production by these two cell types is different [8], as in DCs it is primarily determined by TLR11, chemokine receptor 5 (CCR5) and the myeloid differentiation factor 88 (MyD88) pathway, with an additional role of G protein coupled receptor signaling (GPCR) [4, 61, 62]. On the other hand, generation of IL12 from macrophages is independent of TLR11 and is induced primarily by cREL NFκB driven transcription [8, 63]. Additionally, compared to cREL NFκB, p65 NFκB plays a moderate role in induction of IL12/IL23p40 [46]. The larger impact on IL12/IL23p40 production by GRA15 compared to GRA24 might be due to the activation of both p65 and cREL by GRA15, while GRA24 only activates cREL. It has been shown that p38 MAPK can phosphorylate acetyltransferase coactivator p300, which can acetylate NFκB p65 and thereby regulate its transcriptional activity [64]. Acetylated NFkB p65 can stimulate or repress the expression of specific genes [65]. Therefore, optimal activation of cREL could be mediated by both upregulation of its transcription and its nuclear translocation. This is consistent with the data from a recent study that reported GRA24-mediated mRNA induction of IL12/IL23p40 in bone marrow derived dendritic cells (BMDCs) [66]. TNFα and IL1β are regulated by the NFκB p65-p50 subunit in murine macrophages [46] which explains why these cytokines were not affected by GRA24.
Compared to an in vitro cell culture system, the immune response in vivo is much more complex, as multiple cell types interact and can exert a considerable influence on disease outcome. For instance, although Tlr11-/- mice lack the increased level of IL12 compared to wild-type mice upon Toxoplasma infection [4, 5, 57] the IFNγ response in these mice is intact and even higher than in wild-type mice, possibly due to the higher parasite load [42, 43, 57]. One important gap in these studies is the determination of what parasitic factors are responsible for the residual IL12 or IL18 that activate NK and T cells to produce IFNγ. Although, GRA15 and GRA24 control cytokine induction in Tlr11-/- mice, a significant level of IFNγ was still detected in Tlr11-/- mice infected with Δgra15/24 parasites. This is probably due to the release of parasite derived nucleic acids after IRG and GBP-mediated destruction of the PVM, which can induce interferon production through nucleic acid sensing TLRs [57, 67]. Mice lacking either Tlr11-/- or Tlr3/7/9-/- still produce IL12 and IFNγ upon Toxoplasma infection, whereas in 3d mice (lacking the endosomal chaperone UNC93B1 for TLR 3/7/9/11/12) or Tlr3/7/9/11-/- quadruple knockout mice, the IL12 and IFNγ response was completely abrogated [57]. This could explain why a significant level of IFNγ in Tlr11-/- mice infected with Δgra15/24 parasites was still detected.
It was previously shown that Tlr11-/-mice infected with ME49 tissue cysts were not more susceptible compared to wild-type mice [42, 57]. By contrast, in our study acute susceptibility of Tlr11-/- mice was observed when infected with either a low number of Pru cysts or tachyzoites which was accompanied by a steady reduction of body weights. Similarly, a recent study showed an increased susceptibility of Tlr11-/- mice after ME49 infection [43]. This variable susceptibility of Tlr11-/- mice could be due to the parasite strains used for infection, i.e ME49 versus Pru, which can elicit a different immune response [68]. Alternatively, the different susceptibility of Tlr11-/- mice could be explained by variations in the microbiota in these mice, as they were housed in different colonies [5, 42, 43, 57, 69]. Acute mortality of mice upon Toxoplasma infection depends on the route of infection and virulence of the parasite strain [70]. For virulent parasites a difference between knockout and wild-type parasites is more likely to be detected after s.c. infections, as this results in a slower dissemination of parasites from the site of infection [32]. Similarly, in our study significantly enhanced virulence of Δgra15/24 parasites compared to Pru wild type was only observed after s.c. infection.
The human immune response to Toxoplasma has been studied in THP1 monocytes [18, 33], isolated PBMCs [18], or elutriated monocytes [18, 20, 56]. Previous studies have shown that although THP1 monocytes infected with type II strains secrete IL1β [18, 33], they do not secrete IL12/IL23p40 [18]. However, we observed that PMA-differentiated THP1 macrophages secreted both IL12/IL23p40 and IL1β in a GRA15- and GRA24-dependent way. This could be attributed to differences between monocytes used in those studies [18, 33] versus macrophages in our study as primary human monocytes and macrophages from the same donor differ in their cytokine secretion pattern [20, 56]. In the present work, we detected a large amount of IL12/IL23p40 secretion from Toxoplasma infected PBMCs. This is in accordance with Tosh et al. [56], where they showed that elutriated monocytes or column purified monocytes were equally efficient in secreting IL12/IL23p40 secretion upon Toxoplasma infection regardless of the strain type. On the other hand, Safronova et al. [18] did not observe any IL12/IL23p40 secretion from PBMCs infected with type II Toxoplasma. This discrepancy could be due to differences in the isolation and purification of monocytes, time point of the assay and, most importantly, the multiplicity of infection (MOI) [71].
THP1 cells are a homogenous monocytic cell population, whereas human PBMCs contain a mixture of cell types comprising a small proportion (approx. 10%) of monocytes [72]. T cells constitute the largest fraction of PBMCs (roughly ¾ th), and IFNγ production by these cells is primarily determined by IL12 and IL18-mediated activation of STAT4, p38 MAPK, NFκB, and activator protein 1 (AP-1) family of transcription factors, respectively [73]. It has been shown that within human PBMCs Toxoplasma preferentially infects monocytes but lymphocytes were also infected at a lower level [74, 75]. Furthermore, a recent study showed that another Toxoplasma secreted factor, ‘Toxoplasma E2F4-associated EZH2-inducing gene regulator’ or TEEGR, negatively regulates the NFκB pathway and selectively suppresses the cytokines regulated by NFκB [76]. The fact that TEEGR suppresses IL1β induction but has no role in IL12 induction could indicate that TEEGR inhibits the NFκB but not the cREL pathway. Based on these facts and the results observed in our study, we hypothesize that in human PBMCs the parasite-infected monocytes produce IL12 through cREL activation and IL18 by inflammasome activation, which together activate T cells to produce anti-parasitic IFNγ which could destroy some PVs. Subsequently, PAMPs get released inside the host cytosol and could be sensed by cytosolic nucleic acid sensing TLRs such as TLR3/7/9, in turn inducing IL12. Indeed, it has been shown that IFNγ-primed human PBMCs produce IL12, IL1β and TNFα when treated with type II parasite derived DNA and RNA [57]. Taken together, we determined that the immune response against Toxoplasma in TLR11 deficient mice or human cells is largely dependent on GRA15/GRA24-induced inflammasome-mediated secretion of IL18/IL1β which together with IL12 activate NK and T cells to secrete IFNγ that kills the parasite. Our study advances our understanding of the human immune response against Toxoplasma.
Materials and methods
Ethics statement
Animal experiments were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the Animal Welfare Act, approved by the Institutional Animal Care and Use Committee at UC Davis (assurance number A-3433-01).
Culture of cells and parasites
Human foreskin fibroblasts (HFFs) and RAW 264.7 macrophages were cultured as described previously [31, 77]. All parasite lines were maintained in vitro by serial passage on HFFs monolayers and cultured in the same medium as HFFs but with 1% fetal bovine serum FBS). A Toxoplasma gondii Pru strain expressing firefly luciferase and GFP (PruΔhpt, PruA7) was used as representative of type II [78].
Generation of bone marrow-derived macrophages and THP1 macrophages
Bone marrow-derived macrophages (BMDMs) were isolated from C57BL/6 mice and cultured as described previously [13]. THP1 monocytes were cultured in RPMI-1640 supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin/streptomycin and 10 mg/mL gentamicin. For differentiation into macrophages, THP1 monocytes were stimulated with 100 nM phorbol 12-myristate 13-acetate (PMA) for 3 days and then rested for 1 day with replacement of the PMA containing medium with complete medium without PMA before performing experiments. All the experiments involving THP1 monocytes were performed with passage numbers <10.
Generation of knockout parasites
It was previously shown that type II Toxoplasma strains lacking hypoxanthine-guanine phosphoribosyl transferase (HXGPRT or HPT) are more virulent than strains having HPT [79]. To remove the HPT gene from the Δgra15 strain, we mutated the HPT locus (TGME49_200320) by using a clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 based system. The sgRNA sequence against HPT (Table 1) was cloned into the pSS013-Cas9 vector (pU6 plasmid, Addgene plasmid # 52694) using the BsaI specific sites. To generate PruΔgra15Δhpt, the circular pSS013-Cas9 vector containing the sgRNA against HPT was transfected (10 μg) as described elsewhere [80]. For the selection of Δhpt parasites, single clones were grown in parallel with Mycophenolic acid (MPA)-Xanthine (25 μg/mL) and 6-Thioxanthine (177 μg/mL) containing media. Parasites that were able to grow in 6-Thioxanthine but not in MPA-xanthine media were selected and further confirmed by PCR and sequencing (Table 1). To disrupt GRA24 (TgME49_230180) in PruΔhpt and PruΔhptΔgra15 strains, the pSS013-Cas9 vector containing a sgRNA against GRA24 was transfected along with NotI (New England Biolabs) linearized pLoxP-DHFR-mCherry-LoxP (Addgene Plasmid #70147), at a 5:1 molar ratio, as described previously [81]. This plasmid contains a pyrimethamine resistance cassette tagged with the fluorescence marker mCherry and flanked by two LoxP sites. After three rounds of pyrimethamine selection (1 μM) and limiting dilution cloning, GRA24 knockout parasites were assessed by PCR and confirmed by sequencing (Table 1). To flox out DHFR-mCherry, Δgra24 and Δgra15/24 parasites were transfected with a plasmid [78] expressing Cre recombinase (50 μg) as described above. Single clones were checked for their inability to grow in the presence of pyrimethamine and absence of mCherry.
Table 1. Primer and sgRNA sequences.
| Name | Sequence |
|---|---|
| TGME49_200320_gRNA1_Fwd | 5’ GACAAAATCCTCCTCCCTGG 3’ |
| TGME49_200320_gRNA1_Rev | 5’ CCAGGGAGGAGGATTTTGTC 3’ |
| TGME49_200320_gRNA2_Fwd | 5’ GGACATAGTGCTCGAAGAAG 3’ |
| TGME49_200320_gRNA2_Rev | 5’ CTTCTTCGAGCACTATGTCC 3 |
| TGME49_230180_gRNA1_Fwd | 5’ GTACCAGGCTACAAATAGAGA 3’ |
| TGME49_230180_gRNA1_Rev | 5’ TCTCTATTTGTAGCCTGGTAC 3’ |
| TGME49_230180_gRNA2_Fwd | 5’ GGGACCGAAATGCCGAATCA 3’ |
| TGME49_230180_gRNA2_Rev | 5’ TGATTCGGCATTTCGGTCCC 3 |
| HPT_Fwd | 5’ ATGGCGTCCAAACCCATTGA 3’ |
| HPT_Rev | 5’ TCGTTGAAGTCGTAGCAGCA 3’ |
| GRA24_Fwd | 5’ ATGCTCCAGATGGCACGATATACCG 3’ |
| GRA24_Rev | 5’ CTGTCGTCTGCTGGTGGTAGC 3’ |
| DHFR_Rev | 5’ ATAGTCCTGTCGGGTTTCGCCAC 3’ |
| GRA15_Fwd | 5’ AACACGACGAGGCAGGAGAATTAC 3’ |
| GRA15_Rev | 5’ GACGACTGTAGCCTGAGCATCC 3’ |
| Neo74 | 5’ GTGGGATTAGATAAATGCCTGCTC 3’ |
| 5ARMF2 | 5’ AACACAGGCTCAGAAGAGAAGAGG 3’ |
| 2285 | 5’ TTGATGTATTCGTGTCCCACTGC 3’ |
| +3AMR | 5’ GCGGACACCTTCCATCTCTCAGTT 3’ |
Nucleotides highlighted in grey are part of the BsaI cloning site of pSS013 and nucleotides in bold are the actual sgDNA sequence.
Generation and maintenance of Tlr11-/- mice
To generate the Tlr11-/- mouse colony, two 4-week-old Tlr11-/- male mice [42, 69] were bred with wild-type female C57BL/6 mice (Jackson laboratories). The Tlr11+/- F1 progeny mice were subsequently crossed to obtain Tlr11-/- mice. Genotyping of F2 individuals was performed by PCR from DNA isolated from tail clips to identify the hybrid or homozygous variants (S2 Fig). Once the homozygous mice were confirmed by PCR genotyping (S2 Fig), they were bred to get the entire colony of Tlr11-/- mice. Mice were maintained at the University of California, Davis (UC Davis) mouse housing facility, where water and feed were provided ad libitum.
In vivo infection, parasite burden and cytokine measurement
Male and female 6–10-week-old Tlr11-/- mice were used in the experiments. For infection, tachyzoites were cultured in HFFs and extracted from host cells by passage through 27- and 30-gauge needles, washed two times in PBS, and quantified with a hemocytometer. Parasites were diluted in PBS, and mice were inoculated either i.p. or s.c. with tachyzoites of each strain (100–5,000 tachyzoites in 200 μl) using a 29-gauge needle. Body weights were recorded every day and for survival analysis mice were kept for 30 days. To obtain brain cysts, 5,000 tachyzoites of wild-type and Δgra15/24 parasites were injected i.p. into CD1 mice (Charles River) and 4 weeks later these mice were sacrificed, brains aseptically collected and cysts isolated for both strains [41]. Subsequently, 10 cysts of each parasite strain were infected i.p. into Tlr11-/- mice to determine survival and body weight reduction.
In vivo parasite burden was measured by i.p. infecting 5,000 tachyzoites into Tlr11-/-mice for 3 days. Subsequently, peritoneal fluids were collected and cells isolated by centrifugation. A total of 1×105 cells were plated in 96-well plates in triplicate for each group for 24 h. Following incubation, supernatants were removed and lysis buffer was added prior to measure relative parasite growth by luciferase assay [77].
To quantify cytokines in vivo, mice were sacrificed on day 1 or 3 p.i. to collect blood and peritoneal fluid. Serum was diluted 1:10 for IL12/IL23p40 and IL18, and 1:20 for IFNγ and TNFα measurement. IFNγ, IL12/IL23p40, TNFα and IL18 levels were determined using commercially available matched pair ELISA kits (Invitrogen, Thermo Fisher Scientific and Sino Biologicals for IL18), following the manufacturer’s instructions.
In vitro parasite growth determination
Freshly confluent HFFs 24-well plates were used to determine relative parasite growth by plaque assay. On the day of infection, the media was replaced, and 250 freshly harvested parasites were added to each well. Plates were then left undisturbed for 6 days at 37°C 5% CO2, after which plaque areas were imaged and measured. Plaque areas were captured and analyzed using a Nikon TE2000 inverted microscope equipped with Hamamatsu ORCA-ER digital camera and NIS Elements Imaging Software, respectively. For all experiments, at least 20–25 plaques from technical duplicate wells were imaged. For measurement of total parasite growth, a luciferase-based assay was performed [77].
Immunofluorescence detection of p65 (NFκB), p-p38 MAPK and c-REL (NFκB) nuclear translocation
Immunofluorescence to detect nuclear translocation of p65 (NFκB), p-p38 MAPK and c-REL was done in HFFs, MEFs, RAW 264.7 macrophages and THP1-derived macrophages using the following antibodies: rabbit anti p65 (1:200 dilution, sc-109, Santacruz Biotechnology, CA, USA), rabbit anti p-p38 MAPK (1:800 dilution, #4511, Cell Signaling Technology, MA, USA), rabbit anti cREL (1:500 dilution, #4727, Cell Signaling Technology, MA, USA) and mouse anti cREL (1:200 dilution, NBP2-37593, Novus Biologicals, CO, USA for RAW 264.7 macrophages). Briefly, cells were plated on coverslips in 24-well plates (1×105 cells/well) and subsequently infected with Toxoplasma with a MOI of 3 for 24 h. Following incubation, cells were fixed with 3% formaldehyde, permeabilized and blocked with buffer containing 0.2% Triton X-100 along with 3% BSA and 5% goat serum. Cells were incubated with primary antibodies overnight at 4°C, after which each well was washed 3 times with 1×PBS, followed by incubation with goat anti rabbit Alexa fluor 594 (1:1,000 dilution, Invitrogen) and Hoechst 33258 (1:500 dilution, Invitrogen, Thermo Fisher Scientific) for 1 h. Finally, coverslips were washed 5 times with 1×PBS and mounted with VECTASHIELD antifade mounting medium (Vector Laboratories, CA, USA). Nuclear intensity of at least 15 infected cells was measured for each experiment and coverslip. Pictures were taken using NIS-Elements software (Nikon) and a digital camera (Cool SNAP EZ; Roper Scientific) connected to a fluorescence microscope (model eclipse Ti-S; Nikon). Mean fluorescence intensity quantification of nuclear signal was performed using the NIS-Elements software and Hoechst dye to define nuclei.
Isolation of human peripheral blood mononuclear cells (PBMCs)
PBMCs were isolated from leukocyte reduction chambers (LRS) from individual donors, which were purchased from BloodSource (CA, USA) and tested seronegative for Toxoplasma. After collecting the blood from the LRS according to the manufacturer's protocol using sterile needles and blades, PBMCs were isolated using ficoll-Paque premium 1.077 gm/dL (GE Healthcare, PA, USA) as described previously [82]. Isolated PBMCs were subsequently used for experiments using RPMI-1640 supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin/streptomycin and 10 mg/mL gentamicin or kept frozen in 90% FBS and 10% dimethyl sulfoxide (DMSO) for later use.
In vitro cytokine ELISA
C57BL/6 BMDMs, Raw 264.7 macrophages, THP1 macrophages or PBMCs were seeded (1×105 cells per well) in 96-well plates at 37°C in 5% CO2. Cells were infected with freshly lysed tachyzoites of the different parasite strains at MOI = 3, 5 and 7, and supernatants (200 μl) were collected 24 h p.i. and used to determine IL12/IL23p40, IL1β, TNFα, IL18 and IFNγ levels. To verify that cells were infected with equal numbers of viable parasites, plaque assays were performed as described above. All the cytokine levels were measured using commercially available matched pair ELISA kits for both mouse and human (Invitrogen, Thermo Fisher Scientific), following the manufacturer’s instructions.
In some assays, THP1 cells or PBMCs were first treated 2 h prior to infection with different inhibitors: BAY 11–7082 at 5 μM, (APExBIO, TX, USA), BIRB796 at 10 μM (Tocris Bioscience, MN, USA), VX765 at 50 μM (Sellelckchem, TX, USA) and MCC950 at 10 μM (Adipogen Life Sciences, CA, USA), and culture supernatants collected after 20 h. A total of 30 μL of cell lysis buffer was added per well and a luciferase-based growth assay was performed as mentioned above.
For IFNγ determination in IL12 and/or IL18 neutralized conditions, PBMCs were first infected with Pru wild-type parasites and 1 h p.i. cells were treated with either 2 μg/mL of IL12, IL18 or isotype specific antibodies (MBL International, Japan), or both IL12 and IL18 together for another 20 h before harvesting the culture supernatant. Parasite growth was measured by luciferase assay as described above.
Measurement of alarmin S100A11 by ELISA
Alarmin S100A11 was measured by using a commercially available pre-coated kit from RayBiotech (GA, USA). Briefly, PBMCs (1×105 cells/well) or HFFs (2×104 cells/well) were seeded in 96-well plates at 37°C in 5% CO2 and subsequently infected with freshly lysed tachyzoites of the different parasite strains at MOI = 3, 5 and 7, and supernatants (100 μl) were collected 24 h p.i. As a positive control for S100A11 secretion, 2% Triton X-100 was used in complete medium. To compare the level of S100A11 secretion between different strains, parallel plaque assays were performed.
Arginase assay from macrophages
Arginase activity was measured from lysates of RAW 264.7 macrophages infected with different strains of Toxoplasma 24 h p.i in a 96-well plate with three different MOIs each time as described previously [77]. To determine that cells were infected with equal numbers of viable parasites, plaque assays were performed as described above and values relativized.
Caspase 1/4/5 activity assay
Activity of the inflammatory caspases 1/4/5 was measured using a commercially available Caspase-Glo 1 Inflammasome Assay (Promega, WI, USA) from HFFs seeded in 96-well plates (2×104 cells per well). HFFs were pre-treated with an inhibitor of caspases 1/4/5 (VX765 at 50 μM) 2 h before infection and subsequently infected with Pru wild-type parasites for another 20 h. Caspase activity was measured from the lysate and data were recorded from a single channel luminometer with a 10 s delay program.
Supporting information
Sequence of the Hypoxanthine-guanine phosphoribosyl transferase (HPT) gene showing the sgDNA sequence in red for Cas9-mediated disruption and primer sequence in yellow (a). Disruption of the HPT gene was determined by using specific primers designed to amplify the region shown in the bottom figure, while GRA27 was used as a housekeeping PCR control (at the top) (b). Schematic diagram of the strategy followed to delete GRA24 (top) and PCR to screen the clones confirming the disruption of the gene (P1+P2) (bottom) is shown in (c). wild-type (left) and the presence of the insertion of the repair template in the locus (P1+P3) (right). Identification of Δgra24 and Δgra15/24 double knockout using specific primer sets for GRA24 (top panel), GRA15 (middle panel) and GRA27 as a control for quality of the input DNA (lower panel) (d). Nuclear translocation of the p65 subunit of NFκB was quantified from infected RAW 264.7 macrophages 18 h p.i. with indicated strains. At least 15 cells were quantified as shown in the graph (left) and representative images are shown on the right (e). Phenotypic confirmation of single clones of wild-type, Δgra15, Δgra24 and Δgra15/24 parasites by their ability to activate NFκB (f,g) and p38 MAPK (h). Each dot represents the mean value of at least 15 host cell nuclei (f and h) or 3 technical replicates (g) from a single experiment. Statistical analysis was done by One-way ANOVA followed by Tukey’s multiple comparison test. Data are represented as mean ± standard error of the mean (SEM).
(TIF)
Mouse breeding scheme to generate in house TLR11 knockout mice by cross-breeding homozygous Tlr11-/- male with homozygous Tlr11+/+ female mice (a). Primers in the TLR11 locus (top) and PCR of the F1 progeny (bottom) where the homozygous Tlr11+/+ yields a single band around 700 bp, homozygous Tlr11-/- generates a single band around 900 bp and all heterozygous mice generate both the bands at 700 bp and 900 bp (b).
(TIF)
Indicated parasite strains were added (MOI of 3) to confluent monolayers of HFFs grown on coverslips in 24-well plates. 16 h p.i. cells were fixed and stained with cREL antibody. Each dot represents the mean value of at least 15 host cell nuclei from a single experiment. A representative image for each group is shown on the right. Scale bar represents 10 μm. All the data are shown as mean ± SEM. Statistical analysis was done by two sample Student’s t test.
(TIF)
Tlr11-/- mice were i.p infected with 5,000 tachyzoites of indicated Toxoplasma strains and 1-day p.i. serum was collected from each of the groups to measure IL12/IL23p40 (a). Survival and body weight measurements of Tlr11-/- mice (N = 8 mice per group) that were i.p infected with 100–1000 tachyzoites (b-c) or 10 tissue cysts (d-e) of the indicated strains. Tlr11-/- mice were i.p injected with indicated doses of tachyzoites of different Toxoplasma strains derived from F1 progenies of type II X type III crosses (51) and body weight was measured daily throughout the infection (f-h). All the data are represented as mean ± SEM. Statistical analysis was done by two sample Student’s t test and log rank test for survival curve.
(TIF)
PMA differentiated THP1 macrophages were infected with indicated strains for 24 h and immunofluorescence assay was performed to quantify nuclear translocation of the NFκB p65 subunit (a), p-p38 MAPK (b) and NFκB cREL subunit (c). Scale bar represents 10 μm.
(TIF)
PBMCs or HFFs were infected with indicated Toxoplasma strains at three different MOIs for 24 h, after which supernatants were collected to measure S100A11 in PBMCs (a) and the PBMC lysates were used to measure parasite growth (b). CCL2 was measured from culture supernatants of PBMCs infected with indicated strains as described above (c). S100A11 was measured in HFFs (d). Caspase 1/4 activity assay was measured from HFFs as described in materials and methods (e). Each dot represents the mean value of 3 technical replicates performed for each experiment. Statistical analysis was performed by One-way ANOVA followed by Tukey’s multiple comparison test. Data are represented as mean ± standard error of the mean (SEM).
(TIF)
Acknowledgments
Authors are thankful to Professor Andreas J. Bäumler and Professor Felix O. Yarovinsky for providing the pair of male Tlr11-/- mice and all the members of Saeij lab for providing meaningful insights.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the National Institutes of Health (R01-AI080621) awarded to J.P.J.S. DM was supported by the American Heart Association Post-doctoral fellowship (18POST34030036). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sullivan WJ Jr, Jeffers V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev. 2012;36: 717–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11: 267–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363: 1965–1976. [DOI] [PubMed] [Google Scholar]
- 4.Yarovinsky F. 2005. TLR11 Activation of Dendritic Cells by a Protozoan Profilin-Like Protein. Science. 2005;308: 1626–1629. [DOI] [PubMed] [Google Scholar]
- 5.Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, Mathur R, et al. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity. 2013;38: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raetz M, Kibardin A, Sturge CR, Pifer R, Li H, Burstein E, et al. Cooperation of TLR12 and TLR11 in the IRF8-Dependent IL-12 Response to Toxoplasma gondii Profilin. J Immunol. 2013;191: 4818 LP–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol. 2014;14: 109–121. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003; 3: 133–146. [DOI] [PubMed] [Google Scholar]
- 9.Howard JC, Hunn JP, Steinfeldt T. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr Opin Microbiol. 2011;14: 414–421. [DOI] [PubMed] [Google Scholar]
- 10.Niedelman W, Gold DA, Rosowski EE, Sprokholt JK, Lim D, Arenas AF, et al. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 2012;8(6):e1002784… [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNγ-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009;5(2):e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broz P, Dixit VM. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16: 407–420. [DOI] [PubMed] [Google Scholar]
- 13.Gorfu G, Cirelli KM, Melo MB, Mayer-Barber K, Crown D, Koller BH, et al. Dual Role for Inflammasome Sensors NLRP1 and NLRP3 in Murine Resistance to Toxoplasma gondii. mBio. 2014;5(1). pii: e01117–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai G, Kastelein R, Hunter CA. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect Immun. 2000;68: 6932–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter CA, Chizzonite R, Remington JS. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155: 4347–4354. [PubMed] [Google Scholar]
- 16.Yarovinsky F, Hieny S, Sher A. Recognition of Toxoplasma gondii by TLR11 prevents parasite-induced immunopathology. J Immunol. 2008;181: 8478–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazzinelli RT, Mendonça-Neto R, Lilue J, Howard J, Sher A. Innate resistance against Toxoplasma gondii: an evolutionary tale of mice, cats, and men. Cell Host Microbe 2014;15: 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safronova A, Araujo A, Camanzo ET, Moon TJ, Elliott MR, Beiting DP, et al. Alarmin S100A11 initiates a chemokine response to the human pathogen Toxoplasma gondii. Nat Immunol. 2018;20: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witola WH, Mui E, Hargrave A, Liu S, Hypolite M, Montpetit A, et al. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun. 2011;79: 756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gov L, Schneider CA, Lima TS, Pandori W, Lodoen MB. NLRP3 and Potassium Efflux Drive Rapid IL-1β Release from Primary Human Monocytes during Toxoplasma gondii Infection. J Immunol. 2017;199: 2855 LP–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisch D, Bando H, Clough B, Hornung V, Yamamoto M, Shenoy AR, et al. Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO J. 2019; 38(13):e100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakimi M-A, Olias P, Sibley LD. Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin Microbiol Rev. 2017;30: 615–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10: 766–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzi H, Khan A, Behnke MS, Namasivayam S, Swapna LS, Hadjithomas M, et al. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat Commun. 2016;7:10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeij JPJ, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005;21: 476–481. [DOI] [PubMed] [Google Scholar]
- 26.Ajzenberg D, Cogné N, Paris L, Bessières M, Thulliez P, Filisetti D, et al. Genotype of 86Toxoplasma gondiiIsolates Associated with Human Congenital Toxoplasmosis, and Correlation with Clinical Findings. J Inf Dis. 2002;186: 684–689. [DOI] [PubMed] [Google Scholar]
- 27.Behnke MS, Khan A, Lauron EJ, Jimah JR, Wang Q, Tolia NH, et al. Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of Toxoplasma gondii. PLoS Genet. 2005;11:e1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleckenstein MC, Reese ML, Konen-Waisman S, Boothroyd JC, Howard JC, Steinfeldt T. A Toxoplasma gondii pseudokinase inhibits host IRG resistance proteins. PLoS Biol. 2012;10:e1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinfeldt T, Konen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, et al. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 2010;8:e1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alaganan A, Fentress SJ, Tang K, Wang Q, Sibley LD. Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse. Proc Natl Acad Sci U S A. 2014;111: 1126–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KDC, et al. Strain-specific activation of the NF-κB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med. 2011;208: 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma JS, Sasai M, Ohshima J, Lee Y, Bando H, Takeda K, et al. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J Exp Med. 2014;211: 2013–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gov L, Karimzadeh A, Ueno N, Lodoen MB. Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. mBio. 2013;4:e00255–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, Kieffer S, et al. Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe. 2013;13: 489–500. [DOI] [PubMed] [Google Scholar]
- 35.Braun L, Brenier-Pinchart M-P, Yogavel M, Curt-Varesano A, Curt-Bertini R-L, Hussain T, et al. A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J Exp Med. 2013;210: 2071–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gay G, Braun L, Brenier-Pinchart M-P, Vollaire J, Josserand V, Bertini R-L, et al. Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-γ–mediated host defenses. J Exp Med. 2016;213: 1779–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen KDC, Wang Y, Wojno EDT, Shastri AJ, Hu K, Cornel L, et al. Toxoplasma Polymorphic Effectors Determine Macrophage Polarization and Intestinal Inflammation. Cell Host Microbe. 2011;9: 472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, Christian DA, Kochanowsky JA, Phan AT, Clark JT, Wang S, et al. The Toxoplasma gondii virulence factor ROP16 acts in cis and trans, and suppresses T cell responses. J Exp Med. 2020; 217(3). pii: e20181757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butcher BA, Fox BA, Rommereim LM, Kim SG, Maurer KJ, Yarovinsky F, et al. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 2011; 7(9):e1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen KDC, Hu K, Whitmarsh RJ, Hassan MA, Julien L, Lu D, et al. Toxoplasma gondii rhoptry 16 kinase promotes host resistance to oral infection and intestinal inflammation only in the context of the dense granule protein GRA15. Infect Immun. 2013;81: 2156–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sturge CR, Benson A, Raetz M, Wilhelm CL, Mirpuri J, Vitetta ES, et al. TLR-independent neutrophil-derived IFN- is important for host resistance to intracellular pathogens. Proc Natl Acad Sci U S A. 2013;110: 10711–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López-Yglesias AH, Camanzo E, Martin AT, Araujo AM, Yarovinsky F. TLR11-independent inflammasome activation is critical for CD4+ T cell-derived IFN-γ production and host resistance to Toxoplasma gondii. PLoS Pathog. 2019; 15(6):e1007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by Macrophages Infected with Toxoplasma gondii Depends on the Parasite Genotype. J Immunol. 2004;172: 3686–3694. [DOI] [PubMed] [Google Scholar]
- 45.Kim L, Rio LD, Butcher BA, Mogensen TH, Paludan SR, Flavell RA, et al. p38 MAPK Autophosphorylation Drives Macrophage IL-12 Production during Intracellular Infection. J Immunol. 2005;174: 4178–4184. [DOI] [PubMed] [Google Scholar]
- 46.Sanjabi S, Hoffmann A, Liou H-C, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci U S A. 2000;97: 12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reese ML, Zeiner GM, Saeij JPJ, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A. 2011;108: 9625–9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saeij JPJ, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314: 1780–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314: 1776–1780. [DOI] [PubMed] [Google Scholar]
- 50.Behnke MS, Fentress SJ, Mashayekhi M, Li LX, Taylor GA, Sibley LD. The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog. 2012;8(11):e1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sibley LD, LeBlanc AJ, Pfefferkorn ER, Boothroyd JC. Generation of a restriction fragment length polymorphism linkage map for Toxoplasma gondii. Genetics. 1992;132: 1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selleck EM, Orchard RC, Lassen KG, Beatty WL, Xavier RJ, Levine B, et al. A Noncanonical Autophagy Pathway Restricts Toxoplasma gondii Growth in a Strain-Specific Manner in IFN-gamma-Activated Human Cells. mBio. 2015;6:e01157–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiller KL, Wrona EA, Romero-Torres S, Pallotta I, Graney PL, Witherel CE, et al. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp Cell Res. 2016;347: 1–13. [DOI] [PubMed] [Google Scholar]
- 54.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, et al. Novel Inhibitors of Cytokine-induced IκBα Phosphorylation and Endothelial Cell Adhesion Molecule Expression Show Anti-inflammatory Effects in Vivo. J Biol Chem. 1997;272: 21096–21103. [DOI] [PubMed] [Google Scholar]
- 55.Pargellis C, Tong L, Churchill L, Cirillo PF, Gilmore T, Graham AG, et al. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat Struct Biol. 2002;9: 268–272. [DOI] [PubMed] [Google Scholar]
- 56.Tosh KW, Mittereder L, Bonne-Annee S, Hieny S, Nutman TB, Singer SM, et al. The IL-12 Response of Primary Human Dendritic Cells and Monocytes to Toxoplasma gondii Is Stimulated by Phagocytosis of Live Parasites Rather Than Host Cell Invasion. J Immunol. 2016;196: 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrade WA, Souza M do C, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, et al. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe. 2013;13: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sturge CR, Yarovinsky F. Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection. Infect Immun. 2014;82: 3090–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, et al. CD8α+ Dendritic Cells Are the Critical Source of Interleukin-12 that Controls Acute Infection by Toxoplasma gondii Tachyzoites. Immunity. 2011;35: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schade B, Fischer HG. Toxoplasma gondii induction of interleukin-12 is associated with acute virulence in mice and depends on the host genotype. Vet Parasitol. 2001;100: 63–74. [DOI] [PubMed] [Google Scholar]
- 61.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, et al. MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168: 5997–6001. [DOI] [PubMed] [Google Scholar]
- 62.Aliberti J, Reis e Sousa C, Schito M, Hieny S, Wells T, Huffnagle GB, et al. CCR5 provides a signal for microbial induced production of IL-12 by CD8α+ dendritic cells. Nat Immunol. 2000;1: 83–87. [DOI] [PubMed] [Google Scholar]
- 63.Grumont R, Hochrein H, O’Keeffe M, Gugasyan R, White C, Caminschi I, et al. c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J Exp Med. 2001;194: 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saha RN, Jana M, Pahan K. MAPK p38 Regulates Transcriptional Activity of NF-κB in Primary Human Astrocytes via Acetylation of p65. J Immunol. 2007;179: 7101–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buerki C, Rothgiesser KM, Valovka T, Owen HR, Rehrauer H, Fey M, et al. Functional relevance of novel p300-mediated lysine 314 and 315 acetylation of RelA/p65. Nucleic Acids Res. 2008;36: 1665–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ten Hoeve AL, Hakimi MA, Barragan A. Sustained Egr-1 Response via p38 MAP Kinase Signaling Modulates Early Immune Responses of Dendritic Cells Parasitized by Toxoplasma gondii. Front Cell Infect Microbiol. 2019;9: 349 10.3389/fcimb.2019.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melo MB, Nguyen QP, Cordeiro C, Hassan MA, Yang N, McKell R, et al. Transcriptional Analysis of Murine Macrophages Infected with Different Toxoplasma Strains Identifies Novel Regulation of Host Signaling Pathways. PLoS Pathog. 2013;9(12):e1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgado P, Sudarshana DM, Gov L, Harker KS, Lam T, Casali P, et al. Type II Toxoplasma gondii induction of CD40 on infected macrophages enhances interleukin-12 responses. Infect Immun. 2014;82: 4047–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song J, Wilhelm CL, Wangdi T, Maira-Litran T, Lee S-J, Raetz M, et al. Absence of TLR11 in Mice Does Not Confer Susceptibility to Salmonella Typhi. Cell. 2016;164: 827–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson AM. Strain-dependent, route of challenge-dependent, murine susceptibility to toxoplasmosis. Z Parasitenkd. 1984;70: 303–309. [DOI] [PubMed] [Google Scholar]
- 71.Acosta Davila JA, Hernandez De Los Rios A. An Overview of Peripheral Blood Mononuclear Cells as a Model for Immunological Research of Toxoplasma gondii and Other Apicomplexan Parasites. Front Cell Infect Microbiol. 2019;9:24 10.3389/fcimb.2019.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Autissier P, Soulas C, Burdo TH, Williams KC. Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytometry A. 2010;77: 410–419. [DOI] [PubMed] [Google Scholar]
- 73.Berenson LS, Yang J, Sleckman BP, Murphy TL, Murphy KM. Selective Requirement of p38α MAPK in Cytokine-Dependent, but Not Antigen Receptor-Dependent, Th1 Responses. J Immunol. 2006;176: 4616–4621. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Y, Marple AH, Ferguson DJP, Bzik DJ, Yap GS. Avirulent strains of Toxoplasma gondii infect macrophages by active invasion from the phagosome. Procs Natl Acad Sci USA. 2014;111: 6437–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Channon JY, Seguin RM, Kasper LH. Differential Infectivity and Division of Toxoplasma gondii in Human Peripheral Blood Leukocytes. Infect Immun. 2000;68: 4822–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braun L, Brenier-Pinchart MP, Hammoudi PM, Cannella D, Kieffer-Jaquinod S, Vollaire J, et al. The Toxoplasma effector TEEGR promotes parasite persistence by modulating NF-κB signalling via EZH2. Nat Microbiol. 2019;4: 1208–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mukhopadhyay D, Saeij JPJ. Assays to Evaluate Toxoplasma-Macrophage Interactions. Methods Mol Biol. 2020;2071: 347–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim S-K, Karasov A, Boothroyd JC. Bradyzoite-Specific Surface Antigen SRS9 Plays a Role in Maintaining Toxoplasma gondii Persistence in the Brain and in Host Control of Parasite Replication in the Intestine. Infect Immun. 2007;75: 1626 LP–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fox BA, Falla A, Rommereim LM, Tomita T, Gigley JP, Mercier C, et al. Type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for cyst development and latent infection. Eukaryot Cell. 2011;10: 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Cirelli KM, Barros PDC, Sangaré LO, Butty V, Hassan MA, et al. Three Toxoplasma gondii Dense Granule Proteins Are Required for Induction of Lewis Rat Macrophage Pyroptosis. mBio. 2019;10:e02388–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heaslip AT, Nishi M, Stein B, Hu K. The Motility of a Human Parasite, Toxoplasma gondii, Is Regulated by a Novel Lysine Methyltransferase. PLoS Pathog. 2011;7:e1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mukhopadhyay D, Mukherjee S, Roy S, Dalton JE, Kundu S, Sarkar A, et al. M2 Polarization of Monocytes-Macrophages Is a Hallmark of Indian Post Kala-Azar Dermal Leishmaniasis. PLoS Negl Trop Dis. 2015;9:e0004145. [DOI] [PMC free article] [PubMed] [Google Scholar]