Abstract
Purpose
Osteoarthritis (OA) is associated with chronic low-grade inflammation. Resveratrol exerts protective effects on OA through its anti-inflammatory property; however, the mechanism of resveratrol on anti-inflammatory signaling pathways has not been fully elucidated yet. The aim of the present study was to investigate whether resveratrol-mediated PI3K/Akt expression is linked to TLR4/NF-κB pathway and the role of TLR4/Akt/FoxO1 axis in the anti-osteoarthritic effect of resveratrol.
Methods
SW1353 cells stimulated by IL-1β (10 ng/mL) were cultured in the presence or absence of resveratrol (50 μM) and then treated with TLR4 siRNA, PI3K inhibitor LY294002 or FoxO1 siRNA, respectively. The associated proteins of TLR4 signaling pathways and TLR4/Akt/FoxO1 axis were evaluated by Western blot. The level of IL-6 in the supernatant was detected by ELISA.
Results
IL-1β treatment increased the expression of TLR4/NF-κB and phosphorylation of PI3K/Akt and FoxO1, while additional resveratrol further upregulated the expression of PI3K/Akt and FoxO1 phosphorylation but downregulated TLR4 signals in SW1353 cells. Further analyses by the inhibition of TLR4, PI3K/Akt and FoxO1 signaling pathways, respectively, showed that the activation of TLR4 can induce PI3K/Akt phosphorylation, which increases the phosphorylation of FoxO1 and inactivates it. Next, inactivated-FoxO1 can reduce the expression of TLR4, which forms a self-limiting mechanism of inflammation. Resveratrol treatment can upregulate PI3K/Akt phosphorylation and inactivate FoxO1, thereby reducing TLR4 and inflammation.
Conclusion
This study reveals that TLR4/Akt/FoxO1 inflammatory self-limiting mechanism may exist in IL-1β-stimulated SW1353 cells. This study reveals a novel cross-talk mechanism which is between integrated PI3K/Akt/FoxO1 signaling network and TLR4-driven innate responses in IL-1β-stimulated SW1353 cells. Resveratrol may exert anti-OA effect by enhancing the self-limiting mechanism of inflammation through TLR4/Akt/FoxO1 axis.
Keywords: resveratrol, toll-like receptor 4, osteoarthritis, interleukin-1β, forkhead box O1, phosphoinositide-3-kinase/protein kinase B
Introduction
It is estimated that 10% of men and 18% of women over 60 years of age worldwide suffer from the osteoarthritis (OA).1 OA is caused by a variety of factors, such as trauma, joint fibrosis, obesity, or a disturbance of proinflammatory and anti-inflammatory signaling pathways.2 Although the exact pathogenesis of OA is not fully established, the studies have shown that OA may be associated with chronic low-grade inflammation, and the expression of cytokines and inflammatory mediators is significantly increased when OA occurs.3 Interleukin-1 beta (IL-1β) is a key pro-inflammatory cytokine which involved in the pathogenesis of OA.4 The level of IL-1β was significantly increased in the synovial fluid, synovial membrane, and cartilage in patients with OA.5,6 Studies have shown that IL-1β can promote catabolism and inhibit anabolism of cartilage by inducing the production of metalloproteinases (MMPs)7 and interfering with the synthesis of type-II collagen in chondrocytes.8
Toll-like receptor 4 (TLR4), a member of the family of innate immune receptors, has been studied and demonstrated to be closely related to the development of OA. The expression of TLR4 is elevated in the cartilage and the area of synovial injury of OA. Furthermore, TLR4-mediated signaling pathway is involved in the destruction of articular cartilage.9,10 In our previous study, we found that the expressions of TLR4 gene and protein in OA chondrocytes induced by IL-1β were significantly increased, while inhibiting the expression of TLR4 could alleviate the inflammation.11 In addition, the levels of TLR4 mRNA and protein expression in the cartilage tissue of the knees in animal OA models induced by a high-fat diet were significantly increased.12 Those studies demonstrated that TLR4-mediated signaling pathway may play an important role in the pathogenesis of OA.
Forkhead box O1 (FoxO1) is a transcriptional regulator of cell metabolism, proliferation and differentiation.13 In macrophages, FoxO1 potentiates TLR4 signaling by binding to multiple enhancer-like elements, while the activation of the TLR4 induces phosphoinositide-3-kinase/protein kinase B (PI3K/Akt) phosphorylation which inactivates FoxO1, establishing a self-limiting mechanism of inflammation.14 This TLR4/Akt/FoxO1 self-limiting axis presented in macrophages plays a critical role in chronic low inflammation associated with obesity. As it is known that OA is related to chronic low-degree inflammation, and obesity, in particular, is considered a major risk factor for OA. Therefore, it is of great significance to study whether TLR4/Akt/FoxO1 axis exists in chondrocytes of OA, which will contribute to reveal the pathogenesis of OA.
Current clinical treatments for OA are focused on relieving pain and improving joint function. However, most medications have quite side effects, such as increased risk of gastrointestinal or cardiovascular disease.15,16 Resveratrol, a natural polyphenolic compound, produced by plants in response to environmental stress and found in red grape skin, peanuts, a variety of berries and medical plants17,18 exerts anti-osteoarthritic effects by its anti-apoptotic, anti-inflammatory and anti-oxidative functions.19–23 Resveratrol may perform the anti-inflammatory effects by inhibiting the activation of the nuclear factor-кB (NF-кB) and then decreasing the expression of pro-inflammatory factors.24,25 Previously, we demonstrated that supplement with resveratrol (45mg/kg) by gastric for twelve weeks in C57BL/6J mice induced by a high-fat diet could improve OA symptoms, and TLR4 expression is decreased significantly in the knee joint cartilage in vivo.12,26 Moreover, we also found that resveratrol could exert the anti-osteoarthritic effect by inhibiting TLR4/MyD88 dependent and non-dependent signaling pathway on human osteoarthritic chondrocytes in vitro.27 In addition, our recent study demonstrated that resveratrol suppressed TLR4 expression by activating PI3K/Akt signaling in articular chondrocytes .28 However, cross-talk between TLR4/NF-κB and PI3K/Akt pathways is still obscure and the mechanisms of anti-osteoarthritic effect of resveratrol need to be further illustrated.
Thus, we hypothesized that TLR4/Akt/FoxO1 axis exists in OA and resveratrol may exert anti-osteoarthritic effect, at least in part by enhancing TLR4/Akt/FoxO1 axis, thereby decreasing TLR4 expression and subsequent TLR4/NF-κB signaling pathway suppression. To address this, in the present study, we mimic OA state by stimulating SW1353 cells with IL-1β29 to verify our hypothesis “in vitro”.
Materials and Methods
Cells, Reagents and Antibodies
The human chondrosarcoma cell line SW1353 was purchased from the Chinese academy of sciences cell bank (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM) was provided by HyClone (Logan UT, USA). Fetal bovine serum (FBS) was purchased from Clark Bioscience (Richmond, VA, USA). Resveratrol and LY294002 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Trypsin and Phosphatase inhibitors were provided by KeyGEN BioTECH (Nanjing, China). IL-1β was purchased from PeproTech (Rocky Hill, NJ, USA). Human IL-6 ELISA kits were purchased from Boster Biotechnology (Wuhan, China). The BCA kit, RIPA lysate and 6×SDS loading buffer were obtained from Beyotime (Shanghai, China). FoxO1 siRNA was obtained from Guangzhou RIBOBIO (Guangzhou, China). PMSF was provided by Dingguo Changsheng Biotechnology (Beijing, China). TLR4 siRNA (sc-40261), rabbit anti-β-actin (sc-477787), anti-TLR4 (sc-30002) and mouse anti-MyD88 (sc-74532) polyclonal antibodies were purchased from Santa Cruz Biotechnology (CA, USA). The rabbit antibody specific for anti-TRIF (No. 4596), p-NF-κB p65 (No. 3033), PI3K (No.4249), p-PI3K p85 (No.4228), Akt (No.4691), p-Akt (No.4060), FoxO1 (No.2880) and p-FoxO1 (No. 9461) were obtained from Cell Signal Technology (Beverly, MA, USA). Anti-mouse and anti-rabbit secondary antibodies were purchased from Zhongshan Biotechnology (Beijing, China), the enhanced chemiluminescence reagent was provided by Thermo Fisher Scientific (Rockford, IL, USA).
Cell Culture and Treatment
SW1353 cells cultures were maintained in DMEM supplemented with 10% fetal bovine serum (100 U/mL penicillin, and 100 μg/mL streptomycin). Cultures were kept in an incubator at 37°C with 5% CO2 and the medium was replaced every two or three days. The cells were passaged with 0.25% trypsin after reaching 90% confluence, then seeded into 6-well plates at a density of 1 × 105 cells/well with 2 mL DMEM media. Serum-starved (0.5% FBS) SW1353 cells were subjected to IL-1β (10 ng/mL) with or without resveratrol (50μM) treatments for the indicated time. PI3K inhibitor LY294002 was used as previously reported .28 Briefly, the serum-starved cells were incubated with 25 μg/mL LY294002. After 1 h, cells were exposed to 10 ng/mL IL-1β with or without 50 μM resveratrol for 30 min or 24 h.
Small Interfering RNA (siRNA)
A modified TLR4 siRNA transfection protocol was used. 1×105 cells/well in six-well culture microplates were transfected with 1.0 μg TLR4 siRNA or control siRNA sequences in 2 mL of antibiotic-free DMEM media (0.5% FBS). Six hours later, the medium was replaced with normal growth medium and the incubation was continued for 48 h at 37°C with 5% CO2. Then, the cells were exposed to 10 ng/mL IL-1β in the absence or presence of 50 μM resveratrol for 30 min or 24 h in serum-starved media (0.5% FBS). Cells and cultural supernatants were collected for the detection of Western blot and ELISA.
FoxO1 siRNA duplex was used as the following protocol. Cells were seeded in six-well plates at a density of 5×104 cells/well in antibiotic-free DMEM media (10% FBS) for 2 mL. The following day, cells were transfected with 50 nM siRNA duplex and 12 μL of siRNA transfection reagent mixed in antibiotic-free DMEM media (10% FBS). The transfected cells were incubated at 37°C with 5% CO2 for 48 h. Untreated cells and control siRNA were used as negative controls. Culture medium was then replaced with serum-starved media (0.5% FBS) and exposed to 10 ng/mL IL-1β with or without 50 μM resveratrol for 30 min or 24 h. Cells and cultural supernatants were collected for the subsequent experiments of Western blot and ELISA.
Western Blot Analysis
Western blot was performed with the same method as previously described .28 Briefly, the SW1353 cells were washed by cold PBS for three times, then RIPA buffer containing PMSF (1:100) and phosphatase inhibitors (1:100) was added. The sample was incubated on ice for 10 min and then centrifuged (12,000g, 15 min, 4 ºC). Total protein was estimated using BCA kit. Samples were stored at -80 ºC freezer until analysis. Protein samples were diluted to a concentration of 1μg/μL and protein denaturation heated at 95°C for 5 min in 6×SDS loading buffer. Extracted proteins (20μg/lane) were loaded onto 8% SDS-PAGE electrophoresis at a constant voltage of 100 V for 1.5 h and then the separated proteins were transferred onto PVDF membranes at a constant voltage of 85 V for 1.5 h at 4°C.
The membranes were then blocked nonspecific sites with 5% skim milk or 5% BSA for 1 h at room temperature, washed three times with TBS containing 0.1% Tween 20 (TBST) and then incubated with the following primary antibodies: β-actin (1:500), PI3K (1:1000), p-PI3K p85 (1:1000), Akt (1:1000), p-Akt (1:2000), FoxO1 (1:1000), p-FoxO1 (1:1000), TLR4 (1:500), MyD88 (1:500), TRIF (1:1000) and p-NF-κB p65 (1:1000) overnight at 4 ºC. The next day membranes were washed with TBST for three times and incubated with horseradish peroxidase-conjugated secondary antibody (1:5000 dilution) for 2 h at room temperature. After washing with TBST, blots were developed with an enhanced chemiluminescence reagent. Quantification of band was performed using the Scion Image 4.0 software (Scion Corporation, Frederick, MD, USA).
ELISA Assay
Cultural supernatants for 24 h were collected and centrifuged (12,000g, 5 min, 4 ºC), and then stored at -80 ºC until use. The amount of IL-6 in the culture medium was measured using ELISA kits. All experimental protocols were carried out according to the manufacture’s protocol.
Statistical Analysis
All data were expressed as means ± standard deviation (SD). Comparisons between groups were done by one-way analysis of variance (ANOVAs) and multiple comparisons post hoc tests were performed by Fisher’s least significant difference (LSD) using SPSS statistical software (SPSS 13.0 software, SPSS Inc, Chicago, IL, USA). Probability values <0.05 were considered statistically significant.
Results
Resveratrol Exerted Anti-Osteoarthritic Effect by Inhibiting TLR4/NF-КB Expression via MyD88-Dependent and -Independent Signaling Pathway in IL-1β-Stimulated SW1353 Cells
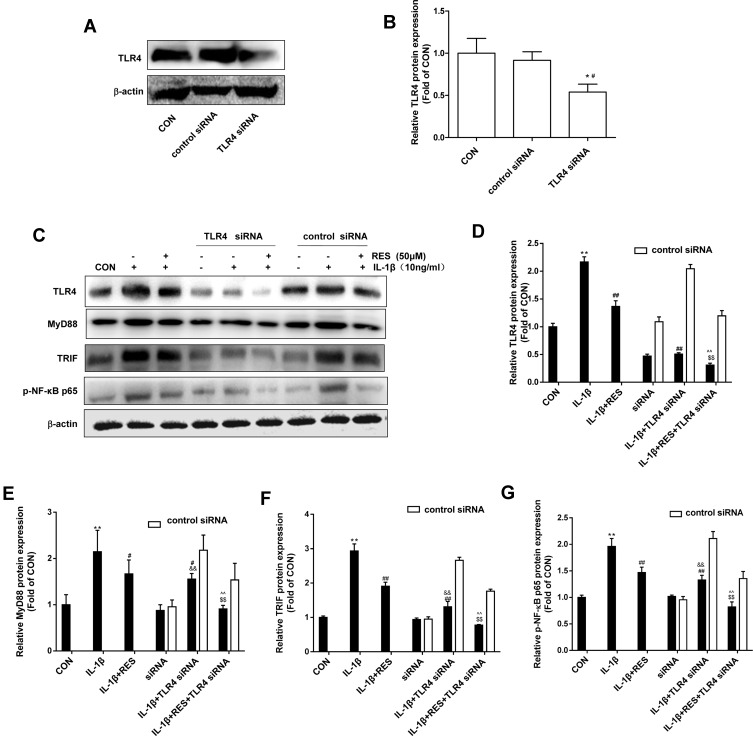
In order to confirm that the anti-osteoarthritic effect of resveratrol on IL-1β-induced SW1353 cells is involved in the TLR4/NF-кB signaling pathway, we used siRNA to block TLR4 expression, and detected related protein of MyD88-dependent and –independent signaling pathway. As shown in Figure 1A and B, TLR4 protein was significantly inhibited in siRNA transfected cells. Data presented in Figure 1C and D showed that a significant increase of TLR4 protein was observed in IL-1β-induced SW1353 cells, whereas additional resveratrol treatment reversed the up-regulation. Besides, TLR4-knockdown significantly attenuated the up-regulation of TLR4 levels in IL-1β-induced SW1353 cells, while the addition of resveratrol further reduced TLR4 expression. Consistently, MyD88, TRIF and NF-кB expression in IL-1β-stimulated SW1353 cells were activated significantly, whereas resveratrol treatment reduced their expression. Moreover, MyD88, TRIF and NF-κB activation were significantly down-regulated by TLR4 siRNA, but not abolished, the addition of resveratrol decreased the expression of these proteins in an even greater extent (Figure 1C, E–G). This result confirmed that TLR4 was activated by IL-1β treatment in SW1353 cells and resveratrol might exert anti-osteoarthritic effect partly by inhibiting TLR4/MyD88-dependent/- independent/NF-κB signaling pathways.
Figure 1.
Resveratrol down-regulated TLR4/NF-кB expression via MyD88-dependent and -independent signaling pathway in IL-1β-induced SW1353 cells. (A) SW1353 cells were transfected with TLR4 siRNA (100 nM) or control siRNA for 48 h, TLR4 expression was analyzed by Western blot. (B) The levels of TLR4 were normalized with β-actin. The results for Western blot were expressed as folds of CON. *P <0.05, versus the CON, #P <0.05 versus control siRNA. (C) SW1353 cells were transfected with TLR4 siRNA (100 nM) or control siRNA for 48 h and then exposed to 10 ng/mL IL-1β with or without 50 μM resveratrol (RES) for 24 h, TLR4, MyD88, TRIF, and p-NF-κB p65 expression were analyzed by Western blot. (D–G) The levels of TLR4, MyD88, TRIF and p-NF-κB p65 were normalized with β-actin. The results for Western blot were expressed as folds of CON. All data were expressed as the mean ± SD of three independent experiments. **P <0.01 versus the CON, #P <0.05, ##P <0.01 versus the IL-1β, $$P <0.01 versus IL-1β + RES, &&P <0.01 versus siRNA, ^^P <0.01 versus siRNA + IL-1β.
Either IL-1β or Resveratrol Treatment Activated PI3K/Akt but Inactivated FoxO1 in SW1353 Cells
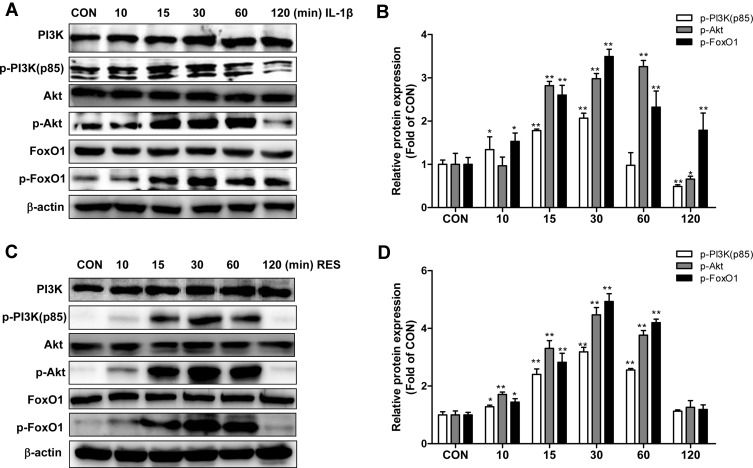
To investigate the effect of IL-1β or resveratrol on PI3K/Akt and FoxO1, SW1353 cells were treated with 10 ng/mL IL-1β or 50 μM resveratrol for indicated time. Data presented in Figure 2 showed that treatment with IL-1β (Figure 2A and B) or resveratrol (Figure 2C and D) elicited a rapid phosphorylation of PI3K, Akt and FoxO1. The peak levels of p-PI3K, p-FoxO1 appeared in 30 min in either IL-1β- or resveratrol-treated cells, while the peak levels of p-Akt appeared in 60 min in the cells with IL-1β stimulation, but presented in 30 min in cells with resveratrol treatment. These data demonstrated that both IL-1β and resveratrol activated the PI3K/Akt signaling pathways but inactivated FoxO1 in SW1353 cells.
Figure 2.
IL-1β or resveratrol treatment promoted activation of the PI3K/Akt signaling but led to inactivation of FoxO1 in SW1353 cells. (A), (C) Serum-starved (0.5% FBS) SW1353 cells were treated with 10 ng/mL IL-1β or resveratrol (50 μM) for 10, 15, 30, 60 and 120 min. p-PI3K, p-Akt, and p-FoxO1 expression were determined by Western blot analysis. (B), (D) The levels of p-PI3K, p-Akt, p-FoxO1 were normalized with their respective total PI3K, Akt, FoxO1 levels. The results for Western blot were expressed as folds of CON. Data were expressed as the mean ± SD of three independent experiments. *P <0.05, **P <0.01 versus the CON group.
TLR4-Knockdown Attenuated the Effect of Resveratrol on Activation of the PI3K/Akt and Inactivation of FoxO1 in IL-1β-Induced SW1353 Cells
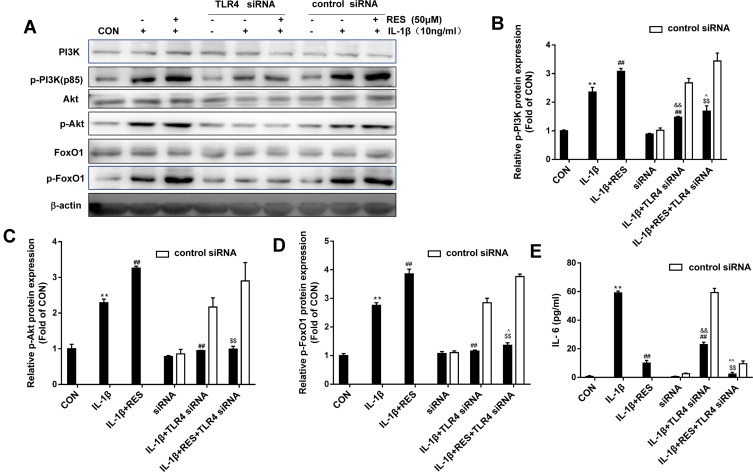
To investigate whether PI3K/Akt and FoxO1 are regulated by TLR4 in IL-1β-induced SW1353 cells and whether the anti-osteoarthritic effect of resveratrol is involved in the regulation. TLR4 siRNA was used to block TLR4 expression. As data presented in Figure 3A–D, IL-1β treatment increased the phosphorylation levels of PI3K/Akt and FoxO1 in SW1353 cells, while additional resveratrol further up-regulated their expression. Interestingly, the TLR4-specific siRNA significantly attenuated PI3K, Akt and FoxO1 phosphorylation in SW1353 cells treated with IL-1β, whereas the addition of resveratrol to cells treated with both TLR4 siRNA and IL-1β had an even greater increase in PI3K and FoxO1 phosphorylation levels. Compared to SW1353 cells cultured in the presence of IL-1β and resveratrol, cells pretreated with TLR4 siRNA presented with a significant alleviation in PI3K, Akt and FoxO1 phosphorylation. As shown in Figure 3E, IL-6 concentrations in the culture supernatants were obviously up-regulated in IL-1β-induced SW1353 cells, while marked reduction of IL-6 level was observed in the addition of resveratrol. Moreover, TLR4-knockdown decreased IL-6 production in the IL-1β-induced SW1353 cells, and additional resveratrol further reduced IL-6 level. These results indicated that PI3K/Akt and FoxO1 are regulated by TLR4, and the cross-talk of them may involve in the anti-inflammatory effect of resveratrol.
Figure 3.
Resveratrol activated PI3K/Akt and inactivated FoxO1 which were attenuated by TLR4-knockdown in IL-1β-induced SW1353 cells. (A) SW1353 cells were transfected with TLR4 siRNA for 48 h as described above, then stimulated with IL-1β (10 ng/mL) in the presence or absence of resveratrol (50 μM) for 30 min, p-PI3K, p-Akt and p-FoxO1 expression were determined by Western blot. (B–D) The levels of p-PI3K, p-Akt and p-FoxO1 were normalized with their respective total PI3K, Akt, FoxO1 levels. The results for Western blot were expressed as folds of CON. (E) IL-6 concentrations in the culture supernatants incubated for 24 h were determined by ELISA. All data above were expressed as the mean ± SD of three independent experiments. **P <0.01 versus the CON, ##P <0.01 versus the IL-1β, $$P <0.01 versus IL-1β + RES, &&P <0.01 versus siRNA, ^P <0.05, ^^P <0.01 versus siRNA + IL-1β.
Inhibition of PI3K Enhanced TLR4 Expression and Inflammatory Responses and Activation of FoxO1
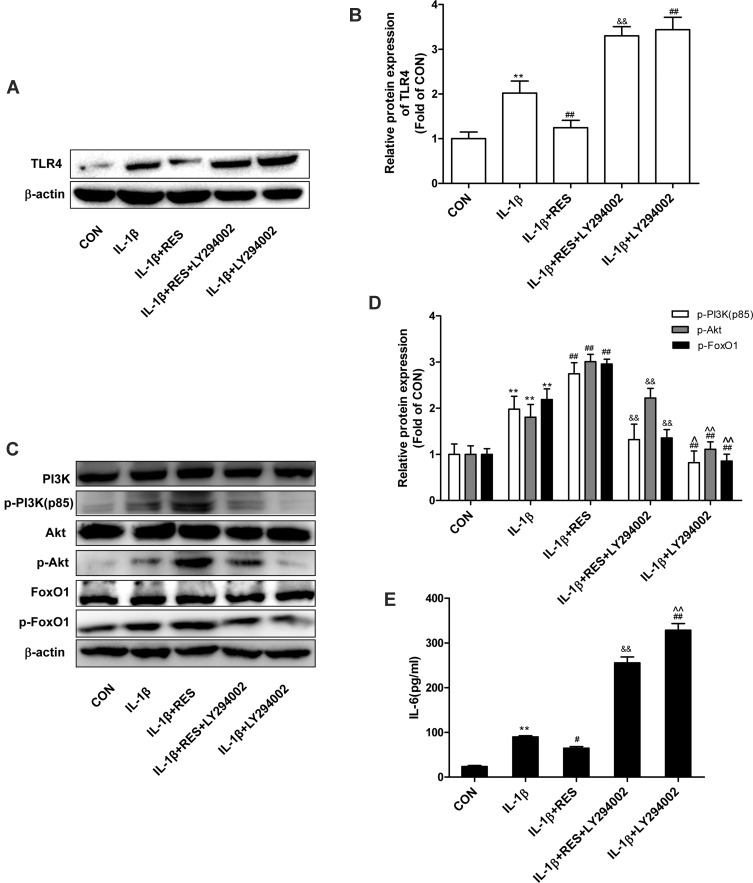
To further examine whether PI3K/Akt modulates TLR4 and FoxO1, we applied PI3K inhibitor LY294002 to block the PI3K/Akt pathway. The results presented in Figure 4A and B showed that TLR4 protein expression was significantly increased after blocking the PI3K/Akt pathway in IL-1β-induced SW1353 cells and in cells treated with IL-1β and resveratrol. As shown in Figure 4C and D, after the PI3K/Akt pathway was inhibited, p-PI3K, p-Akt and p-FoxO1 expression were attenuated in cells treated with IL-1β and a reduction of PI3K, Akt and FoxO1 phosphorylation levels was also observed in cells treated with IL-1β and resveratrol. In addition, compared with cells treated by IL-1β, IL-6 concentrations in the culture supernatants were obviously increased after blocking PI3K, while the addition of resveratrol could down-regulate IL-6 level. Compared cells exposed to IL-1β and resveratrol, LY294002 treatment led to a marked increase of IL-6 level (Figure 4E). These results indicated that the effects of resveratrol on the reduction of IL-1β-stimulated TLR4 expression and inactivation of FoxO1 were at least partly related to PI3K/Akt signaling pathway.
Figure 4.
PI3K/Akt regulated TLR4 and FoxO1 expression. (A) Serum-starved (0.5% FBS) SW1353 cells were pretreated with LY294002 (25 μg/mL) for 1 h and then exposed to IL-1β (10 ng/mL) with or without resveratrol (50 μM) for 24 h. TLR4 expression and were determined by Western blotting analysis. (B) The levels of TLR4 were normalized with β-actin. The results for Western blot were expressed as folds of CON. (C) After SW1353 cells pretreated with LY294002 were exposed to IL-1β (10 ng/mL) in the absence or presence of resveratrol (50 μM) for 30 min, p-PI3K, p-Akt, and p-FoxO1 expression were determined by Western blotting analysis. (D) The levels of p-PI3K, p-Akt and p-FoxO1 were normalized with their respective total PI3K, Akt, FoxO1 levels. The results for Western blot were expressed as folds of CON. (E) IL-6 concentrations in the culture supernatants incubated for 24 h were determined by ELISA. All data above were expressed as the mean ± SD of three independent experiments. **P <0.01 versus the CON group, #P <0.05, ##P <0.01 versus the IL-1β group, &&P <0.01 versus the IL-1β + RES group, ^P <0.05, ^^P <0.01 versus the IL-1β + RES + LY294002 group.
FoxO1-Knockdown Inhibited TLR4-Mediated PI3K/Akt Activation
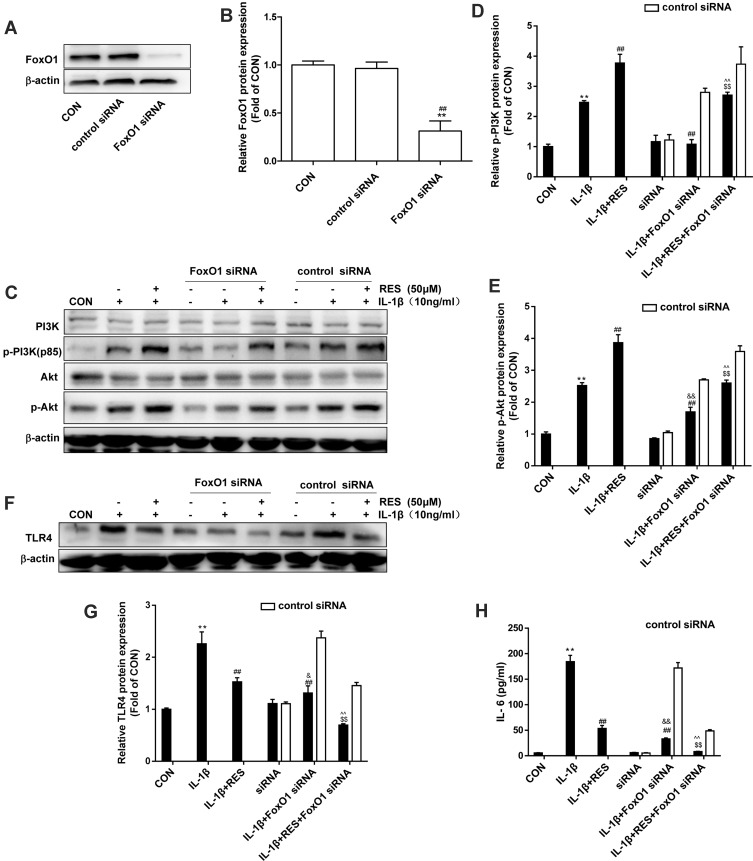
To explore whether FoxO1 is involved in the suppression of resveratrol on TLR4 expression and TLR4-mediated PI3K/Akt activation, SW1353 cells were transfected with FoxO1-specific siRNA. As shown in Figure 5A and B, FoxO1 protein was significantly inhibited by 70% in siRNA transfected cells. FoxO1-knockdown significantly attenuated p-PI3K, p-Akt and TLR4 protein expression in IL-1β-treated SW1353 cells and a reduction of p-PI3K, p-Akt and TLR4 expression also was observed in IL-1β-induced SW1353 cells in the presence of resveratrol. Compared to IL-1β-induced cells with FoxO1 siRNA treatment, the addition of resveratrol had an even greater reduction in TLR4 protein expression and activation in PI3K and Akt phosphorylation (Figure 5C–G). Consistently, IL-6 concentrations in the culture supernatants were also reduced by the FoxO1-specific siRNA, and the addition of resveratrol further decreased IL-6 level (Figure 5H). The results indicated that FoxO1 is involved in the inhibition of resveratrol on IL-1β-induced TLR4 expression and inflammatory response. Moreover, FoxO1-knockdown inhibited PI3K/Akt activation which may be mediated by TLR4.
Figure 5.
FoxO1-knockdown decreased p-PI3K, p-Akt and TLR4 protein expression in IL-1β-treated SW1353 cell. (A) SW1353 cells were transfected with FoxO1 siRNA (50 nM) or control siRNA for 48 h, FoxO1 expression was analyzed by Western blot. (B) The levels of FoxO1 were normalized with β-actin. The results for Western blot were expressed as folds of CON. **P <0.01, versus the CON, ##P <0.01 versus control siRNA. (C), (F) After SW1353 cells were transfected with FoxO1 siRNA for 48 h as described above, cells were exposed to 10 ng/mL IL-1β with or without 50 μM RES for 30 min or 24 h, p-PI3K, p-Akt, and TLR4 expression was analyzed by Western blot. (D–E), (G) The levels of p-PI3K, p-Akt, and TLR4 were normalized with their respective total PI3K, Akt or β-actin levels. The results for Western blot were expressed as folds of CON. (H) IL-6 concentrations in the culture supernatants were assessed by ELISA. All data above were expressed as the mean ± SD of three independent experiments. **P <0.01 versus the CON, ##P <0.01 versus the IL-1β, $$P <0.01 versus IL-1β + RES, &P <0.05, &&P <0.01 versus siRNA, ^^P <0.01 versus siRNA + IL-1β.
Discussion
The anti-inflammatory effects of resveratrol on OA have been largely demonstrated in vitro and in vivo studies,12,22,26 in which the inhibition of TLR4/NF-κB signaling pathway plays an important role.11,30 However, the mechanism of how resveratrol inhibits TLR4, an important gene in OA pathogenesis is not fully understood. In the present study, we demonstrated that resveratrol inhibits IL-1β-induced upregulation of TLR4 in SW1353 chondrocytes by activating the PI3K/Akt pathway and subsequent inactivation of FoxO1, and then downregulates TLR4 expression following inactivating NF-κB via the inhibition of TLR4/MyD88 - dependent and - independent signaling pathway, thereby improved the inflammatory state of chondrocytes. For our knowledge, it is the first time to reveal that TLR4/Akt/FoxO1 axis may exist in OA chondrocytes and the anti-osteoarthritic effect of resveratrol partly involved in enhancing this limiting axis which in turn inhibit TLR4/NF-κB signaling pathway.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) has two different structures: cis-and trans-resveratrol, and the cis-isomeric form is relatively stable.31 Oral administration of resveratrol is rapidly metabolized in the intestine and liver to form glucuronides, sulfates, or sulfoglucuronides conjugation.32,33 Several studies have demonstrated that resveratrol has potent anti-OA properties. It has been shown that resveratrol could prevent cartilage degeneration by inhibiting the synthesis of catabolic factors and pro-inflammatory mediators.34,35 In a rat model of OA, resveratrol alleviates inflammatory damage and exerts anti-OA effect by inhibiting NF-κB and activating HO-1/Nrf-2 signaling pathway.36
PI3K/Akt is the predominant signaling transduction pathway responsible for the cell proliferation, aging or some important physiological processes.37 Recent studies showed that the PI3K/Akt signaling pathway could be activated when inflammation is induced in vitro,38,39 while resveratrol could inhibit inflammatory responses by decreasing IL-6, TNF-α, IFN-γ, et al.22,40,41 FoxO1 is one of the earliest members of the FoxO family13 and is a key regulator of cell metabolism, cell cycle and death.42 The ability of FoxO is largely dependent on posttranscriptional modifications,43 such as phosphorylation44 and acetylation.45 Nuclear export or FoxO1 phosphorylation leads to FoxO1 inactivation which lost its capacity of binding to target regulatory elements.46 Resveratrol could exhibit different regulatory roles for FoxO1 under specific circumstances. It has been reported that resveratrol promoted wound healing in diabetic47 or increased glycolysis 48 by inhibiting FoxO1. However, the opposite results have also been reported which showed that resveratrol upregulated FoxO1 transcriptional activity in preventing osteoporosis49 or regulating oxidative stress and apoptosis of renal.50
In the present study, we found that either resveratrol or IL-1β treatment could activate PI3K/Akt signaling and inactivate FoxO1 in a time-dependent manner in SW1353 cells. Consistently, phosphorylation level of PI3K/Akt and FoxO1 was further enhanced by the addition of resveratrol and IL-1β together, but the effect was partly abolished by TLR4-knockdown, indicating that the activation of PI3K/Akt and the inactivation of FoxO1 were down-regulated by the inhibition of TLR4 expression. Our findings agreed with the report that PI3K/Akt is the downstream of TLR4,51 and its phosphorylation is dependent on the TLR4 activation.52 It has been reported that PI3K/Akt pathway could regulate the activity of FoxO1, and the activation of PI3K/Akt pathway leads to phosphorylation, cytoplasmic retention and inactivation of FoxO1.53 In this study, TLR4-knockdown inhibited PI3K/Akt pathway, which decreased the level of phosphorylation of FoxO1 in SW1353 cells treated with IL-1β, and the TLR4-specific siRNA also alleviated PI3K, Akt and FoxO1 phosphorylation in cells cultured in the presence of both IL-1β and resveratrol, suggesting that the activation of PI3K/Akt mediated by resveratrol is regulated by TLR4 signaling pathway, while FoxO1 is the downstream of PI3K/Akt.
Consistent with our previous results,28 we also demonstrated that the anti-osteoarthritic effect of resveratrol may exert by inhibiting TLR4 via the activation of PI3K/Akt signaling pathway in the present study. Furthermore, we found PI3K inhibitor down-regulated FoxO1 phosphorylation in IL-1β-induced SW1353 cells suggesting that the inhibition of PI3K/Akt pathway could activate FoxO1. Similarly, resveratrol suppressed FoxO1 activation in IL-1β-induced SW1353 cells, while blocking the PI3K/Akt pathway led to an activation of FoxO1, which demonstrated that resveratrol regulated FoxO1 by activating PI3K/Akt signaling.
Next, we investigated whether resveratrol regulates PI3K/Akt activation and TLR4 inhibition by FoxO1 in IL-1β-induced SW1353 cells. It was found that FoxO1-knockdown attenuated IL-1β-induced TLR4 expression indicating that FoxO1 could regulate TLR4 expression, which was agreed with previous report that FoxO1 triggers TLR4 activation in macrophage.14 Additionally, FoxO1-knockdown also repressed PI3K/Akt activation in IL-1β-induced SW1353 cells, implying that the inhibition of PI3K/Akt could be mediated by TLR4. While, the activation of resveratrol on PI3K/Akt was attenuated by FoxO1 siRNA, but not abolished, suggesting that the activation of PI3K/Akt mediated by resveratrol is affected by FoxO1, at least in part in IL-1β-stimulated SW1353 cells.
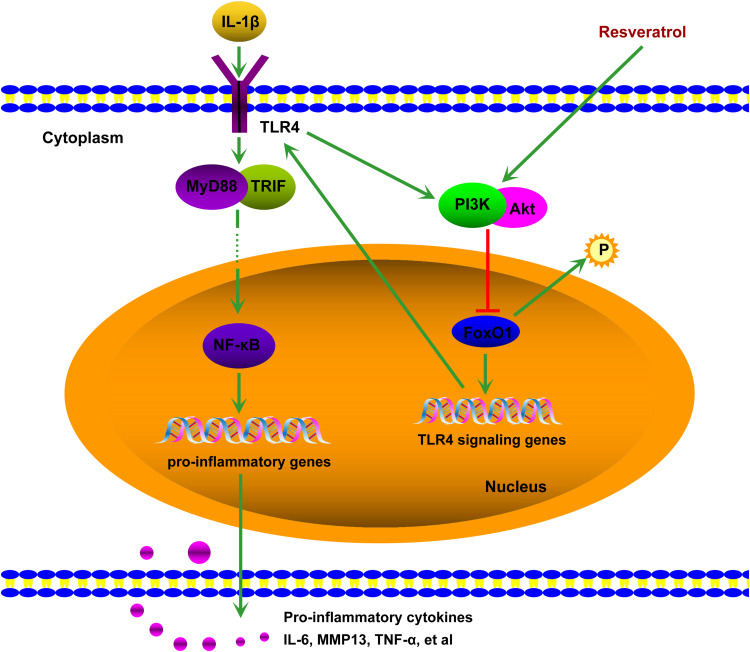
In summary, this study reveals a novel PI3K-dependent cross-talk between integrated PI3K/Akt/FoxO1 signaling network and TLR4-driven innate responses. IL-1β stimulated TLR4, which activate PI3K/Akt signaling and subsequently FoxO1 inactivation, establishing a self-limiting mechanism of inflammation, resveratrol could enhance this self-limiting mechanism of inflammation to reduce the level of TLR4/NF-кB and pro-inflammatory cytokines. A schematic of potential molecular mechanisms involved is described in Figure 6.
Figure 6.
Schematic description of resveratrol-mediated suppression of inflammation in IL-1β-induced SW1353 cells. IL-1β-upregulated TLR4 expression results in the activation of the TLR4/NF-кB signaling via MyD88-dependent and -independent pathways, which causes translocation of NF-кB into the nucleus resulting in the production of pro-inflammatory cytokines such as IL-6, MMP13, TNFα, etc. The activation of TLR4 by IL-1β can induce PI3K/Akt phosphorylation, which increases the phosphorylation of FoxO1 and inactivates it. Inactivated FoxO1 reduces the expression of TLR4, establishing a self-limiting mechanism of inflammation. Resveratrol treatment can upregulate PI3K/Akt phosphorylation and inactivate FoxO1, that is resveratrol highlights the self-limiting mechanism by promoting PI3K/Akt activation, thereby down-regulating TLR4/NF-кB pathway and inflammation. ➞, lead to/activate; ⊣, inhibit.
Conclusion
Our findings demonstrated that TLR4/Akt/FoxO1 self-limiting axis may exist in IL-1β-stimulated SW1353 cell, and resveratrol highlights this self-limiting axis to exert anti-osteoarthritic effect. Thus, this study reveals a novel cross-talk presented between integrated PI3K/Akt/Foxo1 signaling network and TLR4-driven innate responses. Taken together, our study may provide a potent therapeutic target for OA, and resveratrol may be of great value in the prevention and treatment of OA.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81372971), Shenyang Science and Technology Project (No. RC170476) and Natural funding of Liaoning Province (No. 2019JH3/10300415).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227. doi: 10.1186/ar2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580–592. doi: 10.1038/nrrheum.2016.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohn DH, Sokolove J, Sharpe O, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via toll-like receptor 4. Arthritis Res Ther. 2012;14(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massicotte F, Lajeunesse D, Benderdour M, et al. Can altered production of interleukin-1beta, interleukin-6, transforming growth factor-beta and prostaglandin E(2) by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis Cartilage. 2002;10(6):491–500. [DOI] [PubMed] [Google Scholar]
- 7.Wu TJ, Lin CY, Tsai CH, Huang YL, Tang CH. Glucose suppresses IL-1beta-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environ Toxicol. 2018;33(10):1061–1068. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Xu X, Kang Z, Zhang Z, Li Y. Paeonol prevents IL-1beta-induced inflammatory response and degradation of type II collagen in human primary chondrocytes. Artif Cells Nanomed Biotechnol. 2019;47(1):2139–2145. [DOI] [PubMed] [Google Scholar]
- 9.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20(5):565–572. [DOI] [PubMed] [Google Scholar]
- 10.Kim HA, Cho ML, Choi HY, et al. The catabolic pathway mediated by toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54(7):2152–2163. doi: 10.1002/art.21951 [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Gu H, Liu H, et al. Protective effect of resveratrol against IL-1beta-induced inflammatory response on human osteoarthritic chondrocytes partly via the TLR4/MyD88/NF-kappaB signaling pathway: an “in vitro study”. Int J Mol Sci. 2014;15(4):6925–6940. doi: 10.3390/ijms15046925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang M, Li X, Yu X, et al. Oral administration of resveratrol alleviates osteoarthritis pathology in C57BL/6J mice model induced by a high-fat diet. Mediators Inflamm. 2017;2017:7659023. doi: 10.1155/2017/7659023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galili N, Davis RJ, Fredericks WJ, et al. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5(3):230–235. doi: 10.1038/ng1193-230 [DOI] [PubMed] [Google Scholar]
- 14.Fan W, Morinaga H, Kim JJ, et al. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 2010;29(24):4223–4236. doi: 10.1038/emboj.2010.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunter BR, Butler KA, Wallace RL, Smith SM, Harirforoosh S. Non-steroidal anti-inflammatory drug-induced cardiovascular adverse events: a meta-analysis. J Clin Pharm Ther. 2017;42(1):27–38. doi: 10.1111/jcpt.12484 [DOI] [PubMed] [Google Scholar]
- 16.Abraham NS, El-Serag HB, Hartman C, Richardson P, Deswal A. Cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction and cerebrovascular accident. Aliment Pharmacol Ther. 2007;25(8):913–924. doi: 10.1111/j.1365-2036.2007.03292.x [DOI] [PubMed] [Google Scholar]
- 17.Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone? Clin Biochem. 1997;30(2):91–113. doi: 10.1016/S0009-9120(96)00155-5 [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Maulik G, Bagchi D, Das DK. Myocardial protection by protykin, a novel extract of trans-resveratrol and emodin. Free Radic Res. 2000;32(2):135–144. doi: 10.1080/10715760000300141 [DOI] [PubMed] [Google Scholar]
- 19.Gambini J, Ingles M, Olaso G, et al. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev. 2015;2015:837042. doi: 10.1155/2015/837042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eo SH, Cho H, Kim SJ. Resveratrol inhibits nitric oxide-induced apoptosis via the NF-Kappa B pathway in rabbit articular chondrocytes. Biomol Ther. 2013;21(5):364–370. doi: 10.4062/biomolther.2013.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin H, Liang Q, Chen T, Wang X. Resveratrol protects chondrocytes from apoptosis via altering the ultrastructural and biomechanical properties: an AFM study. PLoS One. 2014;9(3):e91611. doi: 10.1371/journal.pone.0091611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buhrmann C, Popper B, Aggarwal BB, Shakibaei M. Resveratrol downregulates inflammatory pathway activated by lymphotoxin alpha (TNF-beta) in articular chondrocytes: comparison with TNF-alpha. PLoS One. 2017;12(11):e0186993. doi: 10.1371/journal.pone.0186993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Im HJ, Li X, Chen D, et al. Biological effects of the plant-derived polyphenol resveratrol in human articular cartilage and chondrosarcoma cells. J Cell Physiol. 2012;227(10):3488–3497. doi: 10.1002/jcp.24049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11(6):R165. doi: 10.1186/ar2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliviero F, Scanu A, Zamudio-Cuevas Y, Punzi L, Spinella P. Anti-inflammatory effects of polyphenols in arthritis. J Sci Food Agric. 2018;98(5):1653–1659. doi: 10.1002/jsfa.8664 [DOI] [PubMed] [Google Scholar]
- 26.Gu H, Li K, Li X, et al. Oral resveratrol prevents osteoarthritis progression in C57BL/6J mice fed a high-fat diet. Nutrients. 2016;8(4):233. doi: 10.3390/nu8040233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu H, Jiao Y, Yu X, et al. Resveratrol inhibits the IL-1beta-induced expression of MMP-13 and IL-6 in human articular chondrocytes via TLR4/MyD88-dependent and -independent signaling cascades. Int J Mol Med. 2017;39(3):734–740. doi: 10.3892/ijmm.2017.2885 [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Liu X, Yang Y, et al. Resveratrol inhibits the development of obesity-related osteoarthritis via the TLR4 and PI3K/Akt signaling pathways. Connect Tissue Res. 2019;60(6):571–582. doi: 10.1080/03008207.2019.1601187 [DOI] [PubMed] [Google Scholar]
- 29.Hong GU, Lee JY, Kang H, et al. Inhibition of osteoarthritis-related molecules by isomucronulatol 7-O-beta-d-glucoside and ecliptasaponin A in IL-1beta-stimulated chondrosarcoma cell model. Molecules. 2018;23:11. doi: 10.3390/molecules23112807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estrov Z, Shishodia S, Faderl S, et al. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102(3):987–995. doi: 10.1182/blood-2002-11-3550 [DOI] [PubMed] [Google Scholar]
- 31.Borriello A, Cucciolla V, Della Ragione F, Galletti P. Dietary polyphenols: focus on resveratrol, a promising agent in the prevention of cardiovascular diseases and control of glucose homeostasis. NMCD. 2010;20(8):618–625. doi: 10.1016/j.numecd.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 32.Walle T, Hsieh F, DeLegge MH, Oatis JE Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–1382. doi: 10.1124/dmd.104.000885 [DOI] [PubMed] [Google Scholar]
- 33.Yu C, Shin YG, Chow A, et al. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19(12):1907–1914. doi: 10.1023/A:1021414129280 [DOI] [PubMed] [Google Scholar]
- 34.Dave M, Attur M, Palmer G, et al. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008;58(9):2786–2797. doi: 10.1002/art.23799 [DOI] [PubMed] [Google Scholar]
- 35.Shakibaei M, Csaki C, Nebrich S, Mobasheri A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol. 2008;76(11):1426–1439. doi: 10.1016/j.bcp.2008.05.029 [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Jia J, Jin X, Tong W, Tian H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol Med Rep. 2018;17(1):1493–1498. doi: 10.3892/mmr.2017.8036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT–a major therapeutic target. Biochim Biophys Acta. 2004;1697(1–2):3–16. doi: 10.1016/j.bbapap.2003.11.009 [DOI] [PubMed] [Google Scholar]
- 38.Tarassishin L, Suh HS, Lee SC. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J Neuroinflammation. 2011;8:187. doi: 10.1186/1742-2094-8-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou LT, Wang KJ, Li L, Li H, Geng M. Pinocembrin inhibits lipopolysaccharide-induced inflammatory mediators production in BV2 microglial cells through suppression of PI3K/Akt/NF-kappaB pathway. Eur J Pharmacol. 2015;761:211–216. doi: 10.1016/j.ejphar.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 40.Ahmad SF, Ansari MA, Nadeem A, et al. Resveratrol attenuates pro-inflammatory cytokines and activation of JAK1-STAT3 in BTBR T + Itpr3 tf /J autistic mice. Eur J Pharmacol. 2018;829:70–78. doi: 10.1016/j.ejphar.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Cao X, Cui Y, Zeng G, Chen J, Zhang G. Resveratrol alleviates lysophosphatidylcholine-induced damage and inflammation in vascular endothelial cells. Mol Med Rep. 2018;17(3):4011–4018. [DOI] [PubMed] [Google Scholar]
- 42.Xu M, Chen X, Chen D, Yu B, Huang Z. FoxO1: a novel insight into its molecular mechanisms in the regulation of skeletal muscle differentiation and fiber type specification. Oncotarget. 2017;8(6):10662–10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urbanek P, Klotz LO. Posttranscriptional regulation of FOXO expression: microRNAs and beyond. Br J Pharmacol. 2017;174(12):1514–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. [DOI] [PubMed] [Google Scholar]
- 45.Webb AE, Brunet A. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci. 2014;39(4):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kortylewski M, Feld F, Kruger KD, et al. Akt modulates STAT3-mediated gene expression through a FKHR (FOXO1a)-dependent mechanism. J Biol Chem. 2003;278(7):5242–5249. [DOI] [PubMed] [Google Scholar]
- 47.Huang X, Sun J, Chen G, et al. Resveratrol promotes diabetic wound healing via SIRT1-FOXO1-c-Myc signaling pathway-mediated angiogenesis. Front Pharmacol. 2019;10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sin TK, Yung BY, Siu PM. Modulation of SIRT1-Foxo1 signaling axis by resveratrol: implications in skeletal muscle aging and insulin resistance. Cell Physiol Biochem. 2015;35(2):541–552. [DOI] [PubMed] [Google Scholar]
- 49.Feng YL, Jiang XT, Ma FF, Han J, Tang XL. Resveratrol prevents osteoporosis by upregulating FoxO1 transcriptional activity. Int J Mol Med. 2018;41(1):202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong YA, Bae SY, Ahn SY, et al. Resveratrol ameliorates contrast induced nephropathy through the activation of SIRT1-PGC-1alpha-foxo1 signaling in mice. Kidney Blood Press Res. 2017;42(4):641–653. [DOI] [PubMed] [Google Scholar]
- 51.Lee JY, Ye J, Gao Z, et al. Reciprocal modulation of toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278(39):37041–37051. [DOI] [PubMed] [Google Scholar]
- 52.Saponaro C, Cianciulli A, Calvello R, Dragone T, Iacobazzi F, Panaro MA. The PI3K/Akt pathway is required for LPS activation of microglial cells. Immunopharmacol Immunotoxicol. 2012;34(5):858–865. [DOI] [PubMed] [Google Scholar]
- 53.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96(6):857–868. [DOI] [PubMed] [Google Scholar]