Abstract
BACKGROUND
Clinical trials that have assessed the effect of revascularization in patients with stable coronary disease have routinely excluded those with advanced chronic kidney disease.
METHODS
We randomly assigned 777 patients with advanced kidney disease and moderate or severe ischemia on stress testing to be treated with an initial invasive strategy consisting of coronary angiography and revascularization (if appropriate) added to medical therapy or an initial conservative strategy consisting of medical therapy alone and angiography reserved for those in whom medical therapy had failed. The primary outcome was a composite of death or nonfatal myocardial infarction. A key secondary outcome was a composite of death, nonfatal myocardial infarction, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest.
RESULTS
At a median follow-up of 2.2 years, a primary outcome event had occurred in 123 patients in the invasive-strategy group and in 129 patients in the conservative-strategy group (estimated 3-year event rate, 36.4% vs. 36.7%; adjusted hazard ratio, 1.01; 95% confidence interval [CI], 0.79 to 1.29; P = 0.95). Results for the key secondary outcome were similar (38.5% vs. 39.7%; hazard ratio, 1.01; 95% CI, 0.79 to 1.29). The invasive strategy was associated with a higher incidence of stroke than the conservative strategy (hazard ratio, 3.76; 95% CI, 1.52 to 9.32; P = 0.004) and with a higher incidence of death or initiation of dialysis (hazard ratio, 1.48; 95% CI, 1.04 to 2.11; P = 0.03).
CONCLUSIONS
Among patients with stable coronary disease, advanced chronic kidney disease, and moderate or severe ischemia, we did not find evidence that an initial invasive strategy, as compared with an initial conservative strategy, reduced the risk of death or nonfatal myocardial infarction. (Funded by the National Heart, Lung, and Blood Institute and others; ISCHEMIA-CKD ClinicalTrials.gov number, NCT01985360.)
CARDIOVASCULAR DISEASE IS THE LEADING cause of death in patients with chronic kidney disease.1 The presence of kidney disease has been associated with an increased risk of procedural complications (including renal injury) from coronary angiography and revascularization, and it is uncertain whether these short-term risks result in longer-term benefits. Most trials involving patients with cardiovascular disease have either excluded patients with advanced kidney disease or included too few to permit a confident estimation of treatment benefits.2–6
In a randomized trial reported in 1992 involving 26 candidates for kidney transplantation, revascularization was associated with a lower risk of cardiovascular death or myocardial infarction than medical therapy (nifedipine and aspirin only).7 However, both coronary revascularization procedures and medical therapy have evolved dramatically during the subsequent three decades. We therefore conducted ISCHEMIA-CKD (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches–Chronic Kidney Disease) to test whether there is incremental benefit of an invasive strategy in patients with stable coronary disease and advanced chronic kidney disease.
METHODS
TRIAL DESIGN
In this investigator-initiated, international, randomized trial, we enrolled patients with advanced kidney disease (defined as an estimated glomerular filtration rate [eGFR] of <30 ml per minute per 1.73 m2 of body-surface area or the receipt of dialysis) and moderate or severe myocardial ischemia. The trial was designed to determine whether an initial invasive strategy added to medical therapy would be associated with a lower risk of cardiovascular events than an initial conservative strategy of medical therapy alone with angiography reserved for patients in whom medical therapy had failed. Enrollment in ISCHEMIA-CKD began approximately 2 years after the initiation of ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches),8 and the two trials ran in parallel and were conducted at most of the same sites. Details regarding the trial design,9 eligibility criteria, and differences between the two trials are briefly described in the Methods section and Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
The trial was funded by the National Heart, Lung, and Blood Institute, with donations of medical equipment and medications from the manufacturers (Table S2). Industry sponsors had no role in the design of the trial, collection or analysis of the data, interpretation of the results, or writing of the manuscript. The corresponding ethics committee or institutional review board at each participating center approved the trial. The statistical and data coordinating center at the Duke Clinical Research Institute monitored data collection and performed all statistical analyses. New York University Grossman School of Medicine was the clinical coordinating center. The first author had full access to the data, prepared the first draft of the manuscript, was responsible for editing and finalizing subsequent drafts, made the decision to submit the final manuscript for publication, and vouches for the accuracy and completeness of the trial data and for the fidelity of the trial to the protocol, available at NEJM.org.
ELIGIBILITY AND PROCEDURES
Eligible patients had advanced kidney disease and moderate or severe myocardial ischemia, as determined by the site investigators using trial-defined criteria (Table S3). In contrast with ISCHEMIA,8 in this trial the use of coronary computed tomographic (CT) angiography was not recommended as a screening test to exclude left main coronary artery disease or nonobstructive disease because of the risk of acute kidney injury. Also in contrast with ISCHEMIA,8 we did not perform core laboratory review of stress tests. Patients who met the eligibility criteria (Table S4) and provided written informed consent were randomly assigned in a 1:1 ratio to be treated with an initial invasive or conservative strategy; randomization was performed by means of a central interactive voice-response or Web-based response system with the use of randomly permuted blocks of varying sizes, with stratification according to the enrollment site.
The invasive strategy consisted of the use of coronary angiography within a target of 30 days after randomization, when feasible, with revascularization (percutaneous coronary intervention [PCI] or coronary-artery bypass grafting [CABG]) as soon thereafter as clinically appropriate. The selection of PCI versus CABG or medical therapy in cases in which revascularization would not be appropriate (e.g., nonobstructive coronary disease or diffuse small-vessel disease) was left to the discretion of the treating team. The inclusion on the team of an interventional cardiologist, a cardiac surgeon, a cardiologist, and a nephrologist was recommended (heart–kidney team). Strategies to reduce the risk of acute kidney injury included a customized hydration protocol10 and a contrast-volume threshold provided to the site on the basis of the patient’s eGFR and body weight, along with protocols for PCI techniques involving the use of ultralow contrast volume11 or no contrast agent.12 In the conservative-strategy group, coronary angiography was reserved for patients in whom medical therapy had failed, including those with an acute coronary syndrome, heart failure, resuscitated cardiac arrest, or angina refractory to medical therapy. The guidelines for revascularization — including recommendations with respect to the choice of PCI or CABG, an algorithm for determining fractional flow reserve, and strategies to minimize acute kidney injury — are outlined in the Methods section in the Supplementary Appendix.
Medical therapy consisted of intensive secondary prevention with lifestyle and pharmacologic interventions recommended equally to both groups on the basis of predetermined treat-to-target algorithms for the attainment of goals for reducing risk factors. The patients were followed at months 1.5, 3, 6, and 12 after randomization during the first year and every 6 months thereafter. Details regarding medical therapy are provided in the Supplementary Appendix.
TRIAL OUTCOMES
The primary outcome was a composite of death or nonfatal myocardial infarction. A key secondary outcome was a composite of death, nonfatal myocardial infarction, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest. We also used the Seattle Angina Questionnaire and the Canadian Cardiovascular Society angina class to evaluate angina-related quality of life. The safety outcomes were the initiation of dialysis in patients who were not receiving dialysis at baseline and a composite of newly initiated dialysis or death.
The definitions of the outcomes, including two separate definitions used for myocardial infarction, are outlined in the Supplementary Appendix.9 An independent clinical-events committee whose members were unaware of trial-group assignments adjudicated all deaths and episodes of myocardial infarction, hospitalization for unstable angina or heart failure, resuscitated cardiac arrest, and stroke or transient ischemic attack.
STATISTICAL ANALYSIS
The original planned sample size of approximately 1000 patients was revised to 650 patients (range, 500 to 700) because of slow recruitment.9 Power calculations that were performed in 2015 determined that the enrollment of 500 patients and a mean follow-up of 3 years would provide a power of more than 81% to detect an incidence of the primary outcome that was 23 to 27% lower in the invasive-strategy group than in the conservative-strategy group, based on the assumption that the cumulative 4-year event rate in the conservative-strategy group would be 60 to 75%. When we reestimated the power calculation using updated event-rate assumptions derived from blinded trial data obtained in 2018, we determined that the final sample size of 777 patients would provide a power of approximately 80% to detect an incidence of the primary outcome that was 22 to 24% lower in the invasive-strategy group than in the conservative-strategy group, assuming an aggregate 4-year event rate of 41 to 48% in the conservative-strategy group and an accrual of 240 to 270 primary outcome events.
Outcomes were analyzed according to the intention-to-treat principle. We used the Kaplan– Meier method to estimate event rates for outcomes that were not subject to competing risks (fatal outcomes and outcomes that included death in the composite) and a nonparametric estimator of the cumulative-incidence function for outcomes that were subject to competing risks (nonfatal outcomes and those including cause-specific deaths). We used a Cox proportional-hazards model to estimate average effect sizes for each treatment. Results are reported as hazard ratios and 95% confidence intervals. The confidence intervals have not been adjusted for multiple comparisons, so these intervals should not be used to infer definitive treatment effects.
The proportional-hazards assumption was assessed by visually inspecting Schoenfeld residuals and log-minus-log survival plots and by testing the null hypothesis of no interaction between time and treatment. The proportional-hazards assumption was met for all the models (Fig. S1). To account for heterogeneity among the trial patients, the Cox model was adjusted for prespecified baseline covariates, including age, sex, kidney function (dialysis status and eGFR in patients not receiving dialysis), left ventricular ejection fraction, and diabetes. We used the Cox model to assess the consistency of treatment effects in prespecified subgroups by estimating interactions between the treatment group and baseline covariates.
To supplement conventional confidence intervals, the Cox model was reexpressed in a Bayesian statistical framework. We implemented the Bayesian approach with a noninformative (neutral) prior distribution and used the resulting posterior distribution to evaluate hypotheses concerning the direction and magnitude of the unknown hazard ratio in the Cox model. All statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
RESULTS
BASELINE CHARACTERISTICS
Between April 29, 2014, and January 31, 2018, a total of 802 patients were enrolled in the trial. Of these patients, 777 (96.9%) underwent randomization (388 to the invasive-strategy group and 389 to the conservative-strategy group) at 118 sites in 30 countries (Fig. S2). The median age of the patients was 63 years; 57.1% had diabetes, and 53.4% were receiving dialysis. Among those who were not receiving dialysis, the median eGFR was 23 ml per minute per 1.73 m2. The patients had median scores for the frequency of angina that were consistent with occurrence several times per month. The qualifying stress test included various types of stress imaging in 81.5% of the patients and nonimaging exercise stress testing in 18.5%. Severe ischemia was present in 37.8% of the patients (Table 1).
Table 1.
Characteristics of the Patients at Baseline*.
| Characteristic | Invasive Strategy (N = 388) | Conservative Strategy (N = 389) | All Patients (N = 777) | |
|---|---|---|---|---|
| Median age (IQR) — yr | 62 (55–69) | 64 (56–70) | 63 (55–70) | |
| Male sex — no. (%) | 268 (69.1) | 267 (68.6) | 535 (68.9) | |
| Race — no./total no. (%)† | ||||
| White | 244/373 (65.4) | 237/374 (63.4) | 481/747 (64.4) | |
| Black | 33/373 (8.8) | 30/374 (8.0) | 63/747 (8.4) | |
| Asian | 91/373 (24.4) | 100/374 (26.7) | 191/747 (25.6) | |
| Multiple races | 5/373 (1.3) | 7/374 (1.9) | 12/747 (1.6) | |
| Severity of ischemia — no./total no. (%) | ||||
| Moderate | 242/387 (62.5) | 234/388 (60.3) | 476/775 (61.4) | |
| Severe | 141/387 (36.4) | 152/388 (39.2) | 293/775 (37.8) | |
| Risk factor — no./total no. (%) | ||||
| Hypertension | 349/386 (90.4) | 362/387 (93.5) | 711/773 (92.0) | |
| Diabetes | 226/388 (58.2) | 218/389 (56.0) | 444/777 (57.1) | |
| Current smoker | 46/388 (11.9) | 38/389 (9.8) | 84/777 (10.8) | |
| Prior myocardial infarction | 62/387 (16.0) | 71/389 (18.3) | 133/776 (17.1) | |
| Prior heart failure | 65/388 (16.8) | 70/389 (18.0) | 135/777 (17.4) | |
| Prior stroke | 36/388 (9.3) | 32/389 (8.2) | 68/777 (8.8) | |
| Peripheral-artery disease | 25/388 (6.4) | 23/389 (5.9) | 48/777 (6.2) | |
| Previous intervention — no. (%) | ||||
| PCI | 74 (19.1) | 72 (18.5) | 146 (18.8) | |
| CABG | 14 (3.6) | 14 (3.6) | 28 (3.6) | |
| Median left ventricular ejection fraction (IQR) — % | 58 (50–63) | 58 (50–64) | 58 (50–64) | |
| Renal transplantation — no./total no. (%) | ||||
| History of procedure | 9/388 (2.3) | 15/389 (3.9) | 24/777 (3.1) | |
| On waiting list | 37/358 (10.3) | 57/366 (15.6) | 94/724 (13.0) | |
| Dialysis | ||||
| With end-stage renal disease — no. (%) | 198 (51.0) | 217 (55.8) | 415 (53.4) | |
| Median duration (IQR) — yr | 3 (1–6) | 2 (1–4) | 2 (1–5) | |
| Type of dialysis — no./total no. (%) | ||||
| Hemodialysis | 162/196 (82.7) | 182/215 (84.7) | 344/411 (83.7) | |
| Peritoneal dialysis | 32/196 (16.3) | 28/215 (13.0) | 60/411 (14.6) | |
| Estimated GFR among those not receiving dialysis | ||||
| Median (IQR) — ml/min/1.73 m2 | 23 (16–27) | 23 (17–27) | 23 (17–27) | |
| Distribution — no./total no. (%) | ||||
| <15 | 28/190 (14.7) | 23/172 (13.4) | 51/362 (14.1) | |
| 15 to <30 | 162/190 (85.3) | 149/172 (86.6) | 311/362 (85.9) | |
| Seattle Angina Questionnaire‡ | ||||
| Median summary score (IQR) | 78 (60–94) | 79 (64–92) | 79 (63–94) | |
| Median angina frequency score (IQR) | 90 (80–100) | 90 (80–100) | 90 (80–100) | |
| Distribution — no./total no. (%) | ||||
| Daily or weekly angina | 43/358 (12.0) | 47/366 (12.8) | 90/724 (12.4) | |
| Monthly angina | 138/358 (38.5) | 145/366 (39.6) | 283/724 (39.1) | |
| No angina | 177/358 (49.4) | 174/366 (47.5) | 351/724 (48.5) | |
| Canadian Cardiovascular Society angina class — no./total no. (%)§ | ||||
| I | 72/388 (18.6) | 83/388 (21.4) | 155/776 (20.0) | |
| II | 147/388 (37.9) | 151/388 (38.9) | 298/776 (38.4) | |
| III | 15/388 (3.9) | 16/388 (4.1) | 31/776 (4.0) | |
| New York Heart Association class — no. (%) | ||||
| I | 65 (16.8) | 71 (18.3) | 136 (17.5) | |
| II | 139 (35.8) | 139 (35.7) | 278 (35.8) | |
| III | 0 | 1 (03) | 1 (01) | |
| Type of stress testing — no./total no. (%) | ||||
| Nuclear imaging | 234/387 (60.5) | 245/388 (63.1) | 479/775 (61.8) | |
| Echocardiography | 79/387 (20.4) | 73/388 (18.8) | 152/775 (19.6) | |
| Cardiac magnetic resonance imaging | 0 | 1/388 (0.3) | 1/775 (0.1) | |
| Exercise stress test | 74/387 (19.1) | 69/388 (17.8) | 143/775 (18.5) | |
Percentages may not total 100 because of rounding. CABG denotes coronary-artery bypass grafting, GFR glomerular filtration rate, IQR in terquartile range, and PCI percutaneous coronary intervention.
Race was reported by the patient.
On the Seattle Angina Questionnaire, a score of 0 to 60 indicates daily or weekly angina, a score of 61 to 99 monthly angina, and a score of 100 no angina. Data were excluded from four sites (42 patients) because of improper completion of forms. In addition, data were missing for 11 other patients.
Canadian Cardiovascular Society classes of angina are I (angina only with strenuous exertion), II (slight limitation of physical activity), III (marked limitation of physical activity), and IV (inability to perform any physical activity without angina); class IV angina was an exclusion criterion for the trial.
MEDICAL THERAPY AND ATTAINMENT OF RISK-FACTOR GOALS
The types of medications and attainment of risk-factor goals were similar in the two groups (Table S5). The median level of low-density lipoprotein cholesterol was 83 mg per deciliter (2.1 mmol per liter) at baseline and 70 mg per deciliter (1.8 mmol per liter) at the last visit. The median systolic blood pressure was 135 mm Hg at baseline and 130 mm Hg at the last visit. There was more use of antianginal medications in the conservative-strategy group and more use of dual antiplatelet therapy in the invasive-strategy group (Fig. S3).
INVASIVE PROCEDURES
In the invasive-strategy group, the 3-year cumulative incidences of coronary angiography and revascularization were 85.2% and 50.2%, respectively (85% with PCI and 15% with CABG) (Fig. S4). The most common reasons that coronary angiography was not performed in the invasive-strategy group were death and illness that occurred before the procedure (5%) and patient preference (6%). Multivessel coronary disease was present in 51.3% of the patients, with involvement of the left anterior descending artery in 57.2%. A total of 26.5% of the patients had no obstructive coronary disease. The most common reason that revascularization was not performed in the invasive-strategy group was a lack of obstructive coronary disease (Table S6).
In the conservative-strategy group, the 3-year cumulative incidences of coronary angiography and revascularization were 31.6% and 19.6%, respectively; the corresponding incidences of the two procedures for reasons other than a confirmed outcome event were 19.8% and 11.0%. The reasons for the use of coronary angiography and revascularization in the conservative-strategy group are shown in Figure S5.
FOLLOW-UP AND CLINICAL OUTCOMES
Overall, 4 patients (0.5%) withdrew from the trial, and 8 (1.0%) were lost to follow-up. More than 99% of expected patient-years of follow-up were completed. The median duration of follow-up was 2.2 years (interquartile range, 1.6 to 3.0).
A primary outcome event occurred in 123 patients in the invasive-strategy group and in 129 patients in the conservative-strategy group (adjusted hazard ratio, 1.01; 95% confidence interval [CI], 0.79 to 1.29; P = 0.95) (Fig. 1A and Table 2). The estimated 3-year cumulative incidence of the primary outcome was 36.4% in the invasive-strategy group and 36.7% in the conservative-strategy group (difference, −0.4%; 95% CI, −8.5 to 7.8). The key secondary outcome occurred in 132 patients in the invasive-strategy group and in 138 patients in the conservative-strategy group (adjusted hazard ratio, 1.01; 95% CI, 0.79 to 1.29) (Fig. 1B and Table 2).
Figure 1. Primary Outcome and Key Secondary Outcome.
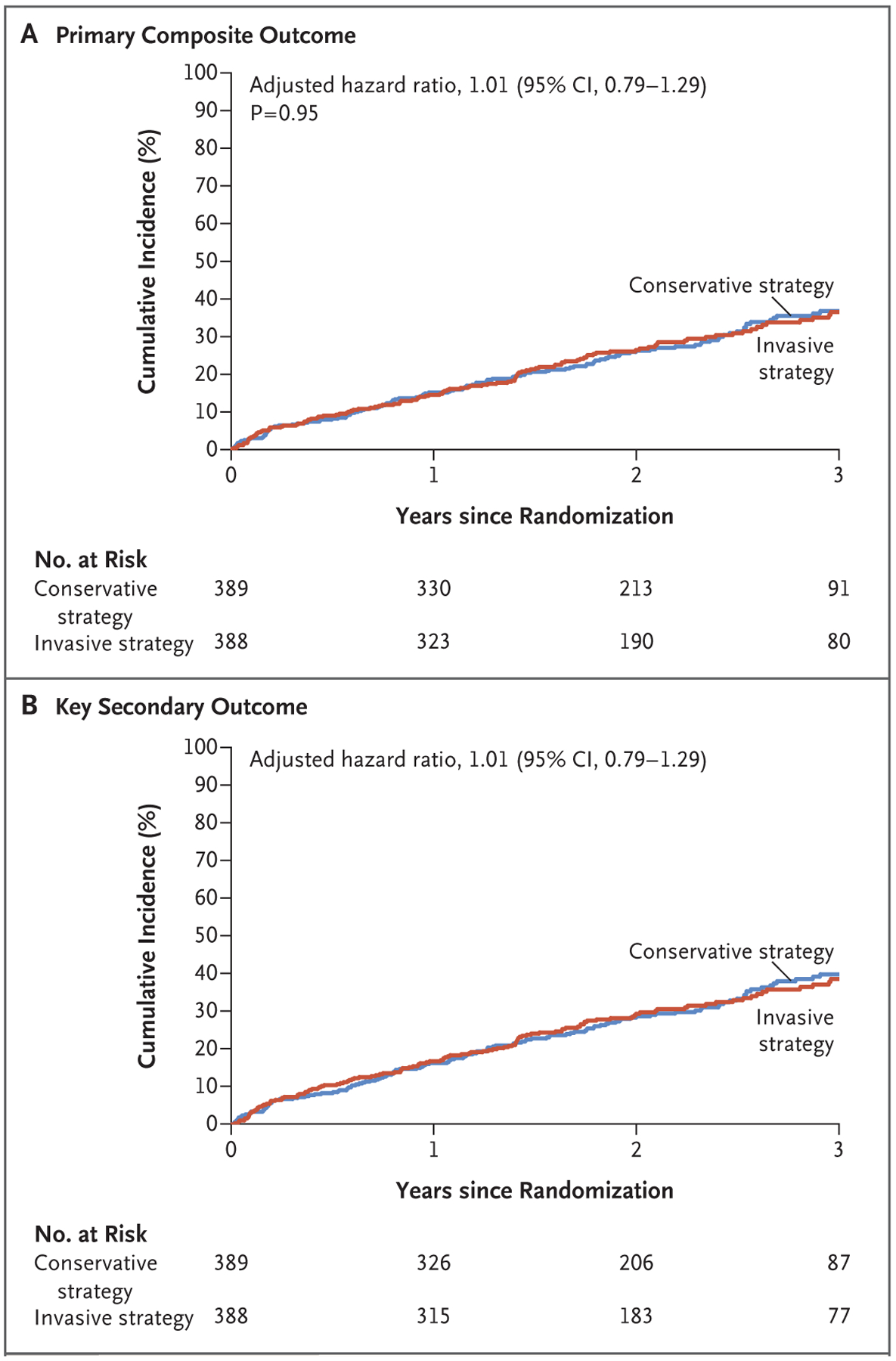
Shown are the results of the time-to-event analysis for the primary outcome (a composite of death or nonfatal myocardial infarction) (Panel A) and a key secondary outcome (a composite of death, nonfatal myocardial infarction, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest) (Panel B) among patients in the invasive-strategy group and the conservative-strategy group.
Table 2.
Primary and Secondary Outcomes*.
| Outcome | No. of Patients with Event | 3-Yr Cumulative Event Rate (95% CI) | Hazard Ratio (95% CI)† | P Value | |||
|---|---|---|---|---|---|---|---|
| Invasive Strategy (N = 388) | Conservative Strategy (N = 389) | Invasive Strategy | Conservative Strategy | Unadjusted | Adjusted | ||
| Primary outcome | |||||||
| Death from any cause or MI | 123 | 129 | 36.4 (30.5–42.2) | 36.7 (31.1–42.4) | 0.98 (0.77–1.26) | 1.01 (0.79–1.29) | 0.95 |
| Key secondary outcome | |||||||
| Death from any cause, MI, hospitalization for unstable angina or heart failure, or resuscitated cardiac arrest | 132 | 138 | 38.5 (32.6–44.3) | 39.7 (33.9–45.4) | 0.99 (0.78–1.26) | 1.01 (0.79–1.29) | |
| Net benefit outcome | |||||||
| Death from any cause, MI, or stroke | 133 | 131 | 39.5 (33.5–45.5) | 36.9 (31.3–42.6) | 1.08 (0.85–1.37) | 1.11 (0.87–1.41) | |
| Other clinical outcomes | |||||||
| Death | |||||||
| Any cause | 94 | 98 | 27.2 (22.0–32.6) | 27.8 (22.7–33.1) | 0.99 (0.75–1.32) | 1.02 (0.76–1.35) | |
| Cardiovascular cause | 76 | 82 | 22.9 (18.1–28.1) | 22.9 (18.2–27.8) | 0.96 (0.71–1.32) | 0.97 (0.71–1.33) | |
| Any MI‡ | 46 | 56 | 15.0 (10.9–19.8) | 15.9 (12.1–20.2) | 0.85 (0.58–1.26) | 0.84 (0.57–1.25) | |
| Procedural | 7 | 4 | 1.8 (0.8–3.6) | 1.1 (0.4–2.6) | 1.80 (0.53–6.16) | 2.03 (0.59v7.01) | |
| Nonprocedural | 37 | 52 | 12.7 (8.8–17.3) | 14.2 (10.6–18.3) | 0.73 (0.48–1.12) | 0.72 (0.47–1.09) | |
| Hospitalization | |||||||
| For unstable angina | 1 | 6 | 0.3 (0–14) | 1.7 (0.6–3.8) | 0.18 (0.02–1.48) | 0.15 (0.02–1.37) | |
| For heart failure | 17 | 12 | 4.7 (2.8–7.4) | 3.6 (1.9–6.1) | 1.49 (0.71–3.12) | 1.47 (0.69–3.12) | |
| Resuscitated cardiac arrest | 0 | 0 | |||||
| Stroke | |||||||
| Any | 22 | 6 | 6.4 (3.9–9.6) | 1.6 (0.6–3.5) | 3.97 (1.61–9.79) | 3.76 (1.52–9.32) | 0.004 |
| With TIA | 22 | 7 | 6.4 (3.9–9.6) | 1.8 (0.7–3.8) | 3.38 (1.45–7.93) | 3.25 (1.38–7.63) | |
| Cardiovascular death or MI | 107 | 114 | 32.9 (27.2–38.6) | 32.1 (26.8–37.6) | 0.97 (0.75–1.27) | 0.98 (0.75–1.28) | |
| Cardiovascular death, MI, hospitalization for unstable angina or heart failure, or resuscitated cardiac arrest | 116 | 124 | 35.1 (29.4–40.8) | 35.1 (29.6–40.6) | 0.97 (0.75–1.25) | 0.98 (0.76–1.27) | |
| Safety outcomes | |||||||
| Death from any cause or initiation of dialysis§ | 75 | 61 | 44.8 (35.9–53.2) | 42.4 (33.4–51.0) | 1.34 (0.95–1.88) | 1.48 (1.04–2.11) | 0.03 |
| Initiation of dialysis | 36 | 29 | 24.1 (16.8–32.2) | 25.0 (17.0–33.9) | 1.37 (0.84–2.23) | 1.47 (0.88–2.44) | 0.14 |
Confidence intervals have not been adjusted for multiple comparisons, so these intervals should not be used to infer definitive treatment effects. MI denotes myocardial infarction, and TIA transient ischemic attack.
The hazard ratio is for the invasive-strategy group as compared with the conservative-strategy group.
Not included in the two subcategories are patients who had silent myocardial infarction or type 3 myocardial infarction (a fatal event when biomarker values were unavailable).
This category consists of patients who were not receiving dialysis at baseline.
In the Bayesian analysis, the probability that the invasive strategy reduced the hazard ratio of the primary outcome by more than 10% (adjusted hazard ratio, <0.90) was 19%; the prob ability that the invasive strategy increased the hazard ratio for the primary outcome by more than 10% (adjusted hazard ratio, >1.10) was 24% (Fig. S6).
The incidences of death from any cause and cardiovascular death were high and similar in the two groups (Fig. 2A and Table 2). In addition, there were no significant between-group differences in the incidence of myocardial infarction (Fig. 2B), hospitalization for unstable angina (Fig. 2C), or hospitalization for heart failure (Fig. 2D). Patients in the invasive-strategy group had a higher incidence of stroke than those in the conservative-strategy group (adjusted hazard ratio, 3.76; 95% CI, 1.52 to 9.32; P = 0.004), a difference that was driven by a higher incidence of nonprocedural strokes (i.e., those that occurred more than 30 days after the procedure) (Fig. S8). Procedural strokes were uncommon, with only one such event in each group.
Figure 2. Death, Myocardial Infarction, and Hospitalization for Unstable Angina or Heart Failure.
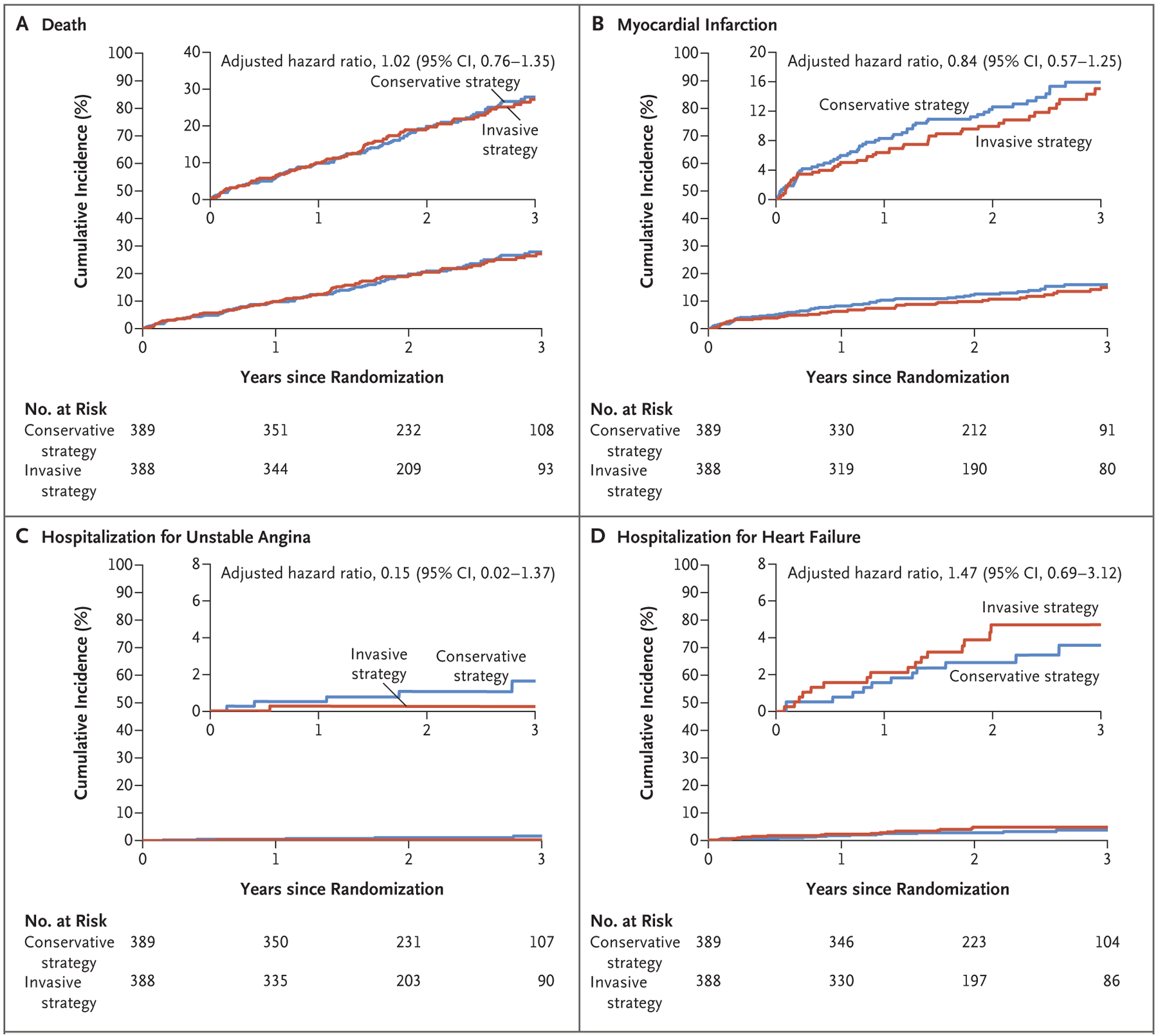
Shown are the results of time-to-event analyses of death (Panel A), myocardial infarction (Panel B), hospitalization for unstable angina (Panel C), and hospitalization for heart failure (Panel D) in the two trial groups. In each panel, the insets show the same data on an enlarged y axis.
The incidence of death or initiation of dialysis in patients who were not receiving dialysis at baseline was higher in the invasive-strategy group, a difference that was driven by a higher incidence of newly initiated dialysis (Figs. S9 and S10). Among the patients who were not receiving dialysis at baseline, the incidence of contrast-associated acute kidney injury in those who underwent coronary angiography or PCI that was not preceded by a clinical event was low (7.9% in the invasive-strategy group vs. 0% in the conservative-strategy group); the incidence of initiation of dialysis after CABG was 12.5% and 11.1%, respectively. Stent thrombosis was uncommon, with a 3-year cumulative incidence of 0.9% (95% CI, 0.3 to 2.0).
These results were consistent with analyses in which the secondary definition of myocardial infarction was used (Table S7). The findings were also consistent in most prespecified subgroups, including those based on the type of stress testing that was used at baseline (imaging or non-imaging). Possible heterogeneity of treatment effect was noted according to the degree of ischemia (severe or moderate), the ejection fraction (lower or higher), and the eGFR (lower or higher) (Fig. and Figs. S11, S12, and S13).
DISCUSSION
In this trial involving patients with stable coronary disease, advanced kidney disease, and moderate or severe ischemia, we found that an initial invasive strategy did not result in a lower incidence of death or nonfatal myocardial infarction than an initial conservative strategy. These results provide information that may assist in the treatment of such patients with stable coronary disease, since data from randomized trials involving patients with advanced kidney disease have been limited. The COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial13 enrolled only 16 patients with advanced kidney disease, the FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) 2 trial14 enrolled only 20 patients with a serum creatinine level of more than 2 mg per deciliter (177 μmol per liter), and the BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial5 excluded patients with a creatinine level of more than 2 mg per deciliter. Consequently, the clinical approach for such patients has been based on extrapolation of results from cohorts without advanced kidney disease. Given the high up-front risk of procedural complications, reduced long-term durability of revascularization, rapid progression of atherosclerotic disease, and high risk of death from nonatherosclerotic causes, it was not known whether such extrapolation was justifiable. We therefore designed the ISCHEMIACKD trial to answer this question in a cost-efficient manner by running the trial in parallel with ISCHEMIA, in which patients with advanced kidney disease were excluded.
The present trial used several strategies to reduce the up-front risk of acute kidney injury during angiography and revascularization. In the invasive-strategy group, the incidence of procedure-associated acute kidney injury was much lower than the incidence of 30 to 60% seen in prior studies involving patients with this degree of advanced kidney disease.15 Nonetheless, we observed an increased incidence of dialysis initiation in the invasive-strategy group, although we likewise noted that this incidence was similar in the two trial groups after 2 years of follow-up. It is possible that these events associated with progressive kidney disease were related to atheroembolic complications of coronary angiography and revascularization.
Coronary angiography was performed in approximately 85% of the patients who were assigned to be treated with the invasive strategy, whereas revascularization was performed in only 50%. The most common reason that revascularization was not performed was the absence of obstructive coronary disease, which was found in approximately one quarter of the patients despite the eligibility requirement of moderate or severe ischemia. This incidence of nonobstructive coronary disease was lower than that in unselected patients undergoing coronary angiography after noninvasive testing16 but much higher than that in ISCHEMIA.8 The higher incidence in the present trial may be due to reduced accuracy of stress testing and greater prevalence of micro- vascular disease in patients with advanced kidney disease.17 In addition, in ISCHEMIA, screening coronary CT angiography was used to exclude patients without obstructive disease before randomization; approximately 20% of coronary CT angiograms in ISCHEMIA showed no obstructive coronary disease.18 In prior trials in which patients were assigned to be treated with an invasive strategy as compared with a conservative strategy before the coronary anatomy had been determined on angiography, the observed frequency of revascularization was as low as 44% despite the enrollment of high-risk groups of patients with acute coronary syndromes (Table S8).
In ISCHEMIA, an initial invasive strategy reduced the incidence of nonprocedural myocar-dial infarction but increased the incidence of procedural myocardial infarction.8 A similar pattern was observed in the present trial (Fig. S7). In the FAME 2 trial, PCI that was guided by the fractional flow reserve was associated with a lower risk of the primary composite outcome than medical therapy alone, a difference that was driven by a reduction in urgent revascularization.14 We similarly observed a lower incidence of hospitalization for unstable angina with an invasive strategy, although the event rates were low. However, no trials involving patients with stable coronary disease, including ISCHEMIA-CKD, have shown differences in mortality between an invasive strategy and a conservative strategy.
In interpreting the findings of this trial, the following caveats should be considered. First, patients who were very symptomatic, had heart failure or recent acute coronary syndromes, or had an ejection fraction of less than 35% were excluded from the trial, so the findings do not extend to such patients. Second, the event rates were lower than projected, and together with a low incidence of revascularization in the invasive-strategy group (50%) and an 11% incidence of revascularization before a confirmed event in the conservative-strategy group, the trial had less power than anticipated to show a benefit for the invasive strategy. However, Bayesian analysis showed that the probability that assignment to the invasive strategy reduced or increased the risk of the primary outcome by more than 10% was low. Third, contrast-associated acute kidney injury was reported at each trial site and was not centrally adjudicated. Finally, we have not yet analyzed the effect of the completeness of revascularization on outcomes.
Among patients with stable coronary disease, advanced kidney disease, and moderate or severe ischemia, we did not find evidence that an initial invasive strategy, as compared with an initial conservative strategy, reduced the risk of death or nonfatal myocardial infarction.
The views expressed in this article are those of the authors and do not necessarily reflect the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the U.S. Department of Health and Human Services.
Supplementary Material
Figure 3. Subgroup Analysis of Treatment Effect for the Primary Outcome.
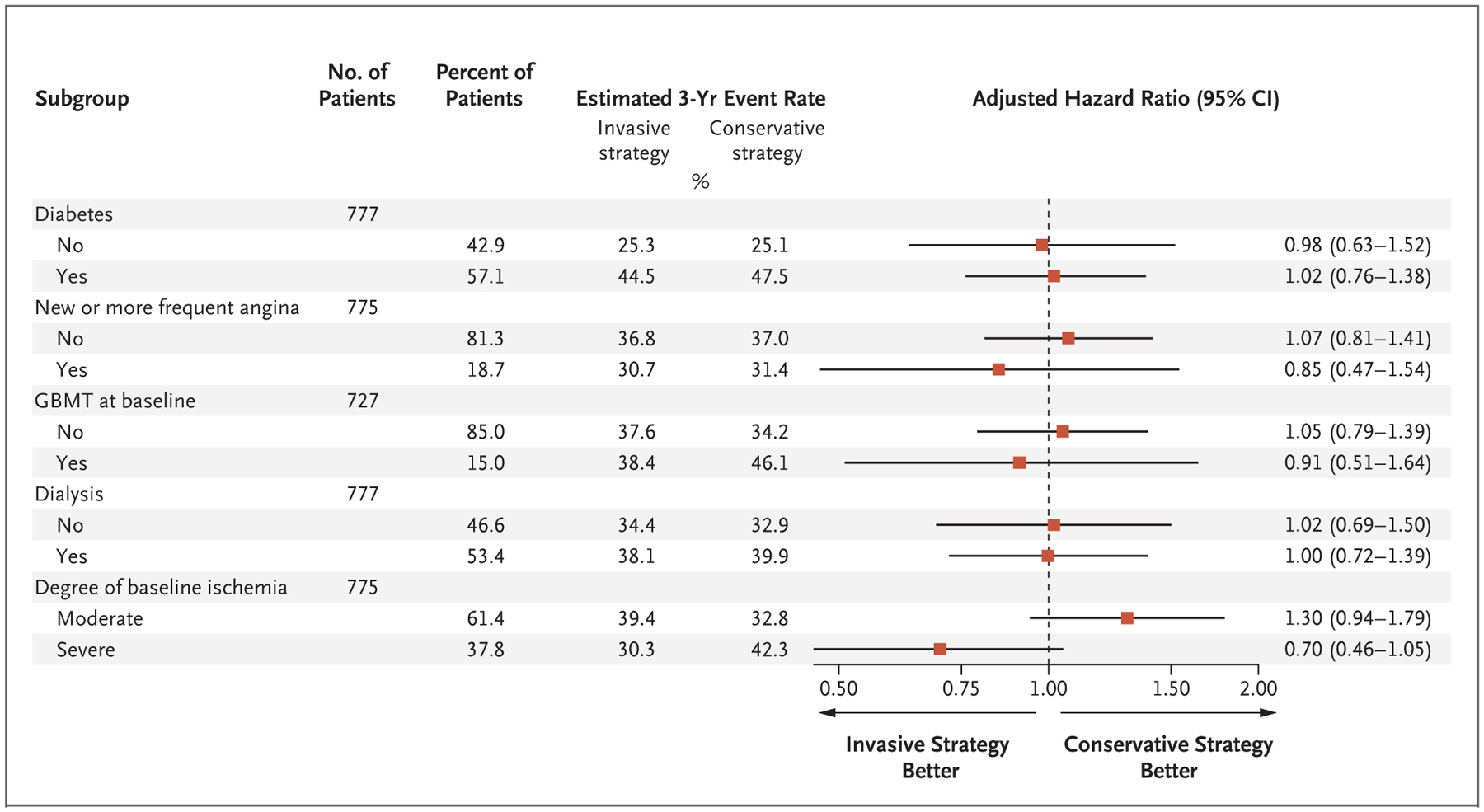
Shown are the adjusted hazard ratios for the primary outcome of death or nonfatal myocardial infarction according to prespecified subgroup. GBMT denotes guideline-based medical therapy.
Acknowledgments
Supported by grants (U01HL117904 and U01HL1179050) from the National Heart, Lung, and Blood Institute. Devices that were used in the trial were donated by Abbott Vascular, Medtronic, St. Jude Medical, Volcano, and Omron Healthcare; medications were provided by Arbor Pharmaceuticals, AstraZeneca Pharmaceuticals, and Merck Sharp & Dohme.
Footnotes
A list of the members of the ISCHEMIACKD Research Group is provided in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Contributor Information
Sripal Bangalore, New York University Grossman School of Medicine
David J. Maron, the Department of Medicine, Stanford University School of Medicine, Stanford, CA
Sean M. O’Brien, Duke Clinical Research Institute, Durham, NC
Jerome L. Fleg, the National, Heart, Lung and Blood Institute, Bethesda, MD
Evgeny I. Kretov, E.N. Meshalkin National Medical Research Center, Novosibirsk Bakulev National Medical Research Center for Cardiovascular Surgery, Moscow in Russia.
Carlo Briguori, Mediterranea Cardiocentro, Naples, Italy
Upendra Kaul, Batra Hospital and Medical Research Centre, New Delhi, India
Harmony R. Reynolds, New York University Grossman School of Medicine
Tomasz Mazurek, Medical University of Warsaw the Department of Coronary and Structural Heart Diseases, Institute of Cardiology Warsaw, Poland.
Mandeep S. Sidhu, New York, Albany Medical College and Albany Medical Center, Albany
Jeffrey S. Berger, New York University Grossman School of Medicine
Roy O. Mathew, Columbia Veterans Affairs (VA) Health Care System, Columbia, SC
Olga Bockeria, Bakulev National Medical Research Center for Cardiovascular Surgery, Moscow in Russia
Samuel Broderick, Duke Clinical Research Institute, Durham, NC
Radoslaw Pracon, the Department of Coronary and Structural Heart Diseases, Institute of Cardiology in Warsaw, Poland
Charles A. Herzog, Hennepin Healthcare, University of Minnesota, Minneapolis
Zhen Huang, Duke Clinical Research Institute, Durham, NC
Gregg W. Stone, Mount Sinai Hospital the Cardiovascular Research Foundation.
William E. Boden, VA New England Healthcare System and Boston University School of Medicine, Boston; Saint Luke’s Mid America Heart Institute/University of Missouri–Kansas City.
Jonathan D. Newman, New York University Grossman School of Medicine
Ziad A. Ali, the Cardiovascular Research Foundation Columbia University Irving Medical Center/New York Presbyterian Hospital; St. Francis Hospital, Roslyn in New York.
Daniel B. Mark, Duke Clinical Research Institute, Durham, NC
John A. Spertus, Saint Luke’s Mid America Heart Institute/University of Missouri–Kansas City
Karen P. Alexander, Duke Clinical Research Institute, Durham, NC
Bernard R. Chaitman, St. Louis University School of Medicine Center for Comprehensive Cardiovascular Care, St. Louis
Glenn M. Chertow, the Department of Medicine, Stanford University School of Medicine, Stanford, CA
Judith S. Hochman, New York University Grossman School of Medicine
REFERENCES
- 1.Sarnak MJ, Amann K, Bangalore S, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol 2019; 74: 1823–38. [DOI] [PubMed] [Google Scholar]
- 2.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356: 1503–16. [DOI] [PubMed] [Google Scholar]
- 3.Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int 2006; 70: 2021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zannad F, Rossignol P. Cardiovascular outcome trials in patients with advanced kidney disease: time for action. Circulation 2017; 135: 1769–71. [DOI] [PubMed] [Google Scholar]
- 5.Bari The 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360: 2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367: 991–1001. [DOI] [PubMed] [Google Scholar]
- 7.Manske CL, Wang Y, Rector T, Wilson RF, White CW. Coronary revascularisation in insulin-dependent diabetic patients with chronic renal failure. Lancet 1992; 340: 998–1002. [DOI] [PubMed] [Google Scholar]
- 8.Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bangalore S, Maron DJ, Fleg JL, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches–Chronic Kidney Disease (ISCHEMIA-CKD): rationale and design. Am Heart J 2018; 205: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet 2014; 383: 1814–23. [DOI] [PubMed] [Google Scholar]
- 11.Nayak KR, Mehta HS, Price MJ, et al. A novel technique for ultra-low contrast administration during angiography or intervention. Catheter Cardiovasc Interv 2010; 75: 1076–83. [DOI] [PubMed] [Google Scholar]
- 12.Ali ZA, Karimi Galougahi K, Nazif T, et al. Imaging- and physiology-guided percutaneous coronary intervention without contrast administration in advanced renal failure: a feasibility, safety, and outcome study. Eur Heart J 2016; 37: 3090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedlis SP, Jurkovitz CT, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention for patients with stable coronary artery disease and chronic kidney disease. Am J Cardiol 2009; 104: 1647–53. [DOI] [PubMed] [Google Scholar]
- 14.Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 2018; 379: 250–9. [DOI] [PubMed] [Google Scholar]
- 15.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol 2008; 51: 1419–28. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Dai D, Hernandez AF, et al. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J 2014; 167(6): 846–52.e2. [DOI] [PubMed] [Google Scholar]
- 17.Wang LW, Fahim MA, Hayen A, et al. Cardiac testing for coronary artery disease in potential kidney transplant recipients. Cochrane Database Syst Rev 2011; 12: CD008691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochman JS, Reynolds HR, Bangalore S, et al. Baseline characteristics and risk profiles of participants in the ISCHEMIA randomized clinical trial. JAMA Cardiol 2019; 4: 273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


