Abstract
An 18-year-old male without prior medical history developed fulminant myocarditis concomitant to severe COVID-19 pneumonia, which was confirmed using serial cardiac magnetic resonance. This may have important diagnostic, monitoring, and pathogenic implications. (Level of Difficulty: Intermediate.)
Key Words: cardiac magnetic resonance, coronavirus disease 2019, myocarditis
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; EF, ejection fraction; EGE, early gadolinium enhancement; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; SARS-CoV-19, severe acute respiratory syndrome-coronavirus-2019
Graphical abstract
An 18-year-old man without prior medical history developed fulminant myocarditis concomitant to severe COVID-19 pneumonia, which was confirmed…
An 18-year-old male without prior medical history was admitted for cough, fever (38.5°C), fatigue, and myalgias. From his vital signs, blood pressure was 120/70 mm Hg, heart rate 110 beats/min, he had tachypnoea (22/min), and oxygen saturation was 94% in room air. He had no neurological symptoms. He did not have any past medical history.
Learning Objectives
-
•
To make a diagnosis of fulminant myocarditis concomitant with COVID-19 pneumonia.
-
•
To understand the value of serial cardiac magnetic resonance after myocarditis due to COVID-19.
Differential Diagnosis
Primary differential included community acquired pneumonia, atypical pneumonia, and severe acute respiratory syndrome-coronavirus-2019 (SARS-CoV-19).
Investigations
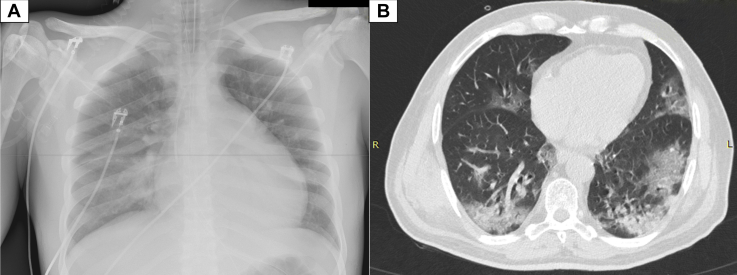
The reverse transcriptase–polymerase chain reaction assay was positive for COVID-19 on the nasopharyngeal swab whereas chest computed tomography demonstrated diffuse peripheral opacity (“crazy paving”) compatible with COVID-19–related lesions (Figure 1).
Figure 1.
Chest Radiograph and Axial Unenhanced Chest Computed Tomography Scan Obtained on Day 2 After the Onset of Symptoms
(A) Chest X-ray and (B) axial unenhanced chest computed tomography scan. Presence of diffuse typical COVID-19–related lesions (typical peripheral opacity, “crazy paving”). COVID-19 = coronavirus disease 2019.
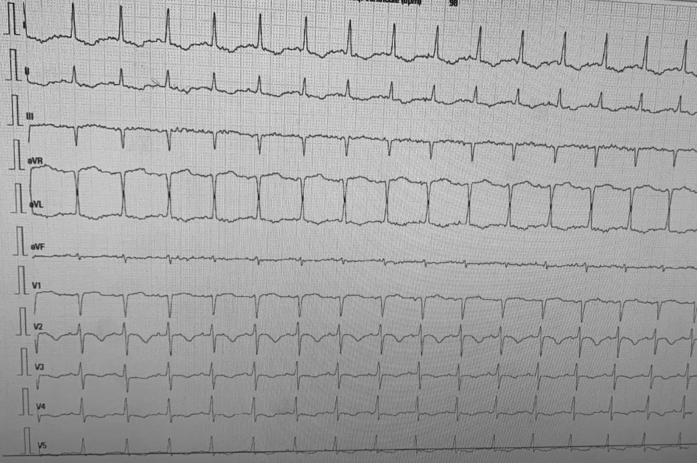
On day 3 of his admission, he presented acute respiratory distress with severe hypoxemia and his oxygen saturations decreased to 82%. The patient was agitated and demonstrated neurological signs, which is why he was intubated under general anesthesia. Prior to intubation, arterial gas analysis showed pH of 7.27, oxygen partial pressure of 51 mm Hg, carbon dioxide partial pressure of 46 mm Hg, and high lactate (14 mmol/l; 0.4 to 2.2 mmol/l). Initially, the patient required 100% oxygen concentration and 15 cm H2O positive expiratory pressure without being in the prone position. Laboratory tests showed very high levels of troponin (11,716 IU/ml), N-terminal pro-type natriuretic peptide (11,719 pg/ml), fibrinogen of 9.5 g/l, peak creatine kinase 2216 IU/l, and C-reactive protein 351 mg/l (N = <5). Electrocardiogram demonstrated sinus tachycardia (120 beats/min) with negative T waves from V2 to V4 (Figure 2). The left ventricle (LV) was mildly enlarged (32 mm/m2 end-diastolic diameter) on echocardiography with increased LV wall thickness (interventricular septum and posterior walls of 14 mm) and marked diffuse hypokinesis (ejection fraction [EF] 30% using biplane Simpson’s method).
Figure 2.
Electrocardiogram on Day 1
Sinus tachycardia (100 beats/min) with negative T waves from V2 to V4.
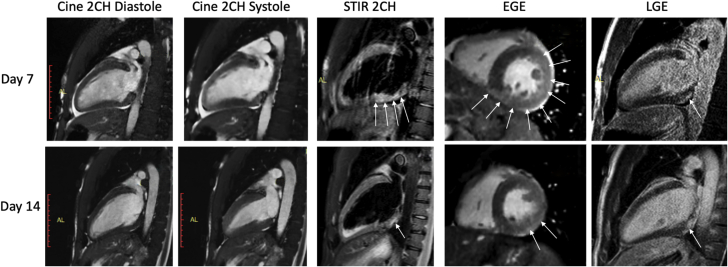
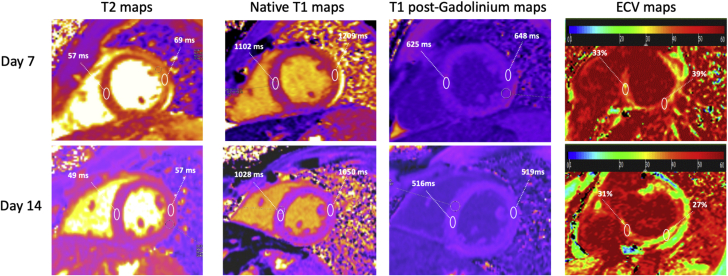
Following the intubation, there was a rapid and significant respiratory and hemodynamic improvement along with adequate diuresis, allowing spontaneous breathing and extubation on day 5. After extubation, echocardiography demonstrated E/e’ ratio suggestive of elevated LV filling pressures. Cardiovascular magnetic resonance was performed on Day 7 and Day 14 with the same protocol. On the first scan, standard cine steady-state free precession showed increased LV wall thickness (14 mm), increased LV volumes (LV tele-diastolic index 127 ml/m2; N = <100) with marked diffuse hypokinesis (left ventricular ejection fraction [LVEF] 33% from the stack of short-axis LV views), and mild pericardial effusion (Videos 1A, 1B, and 1C). Short-tau inversion recovery images indicated marked extensive hypersignal of the LV basal posterolateral wall (Figure 3) indicative of myocardial edema. Dynamic myocardial perfusion images acquired immediately after the injection of 0.1 mmol/l gadolinium chelates showed no perfusion defects. SSFP cines acquired within 2 min after contrast indicated early gadolinium enhancement (EGE) of the LV basal posterolateral wall indicating hyperemia (Figure 3). Late gadolinium enhancement (LGE) images demonstrated nodular subepicardial enhancement of the LV basal posterolateral wall (Figure 3). The diagnosis of acute myocarditis was confirmed by the presence of the 3 major Lake Louise criteria (1). Native T1 and T2 maps of the LV myocardium were acquired in short-axis views through the modified Look-Locker inversion-recovery sequence (Figure 4). Native T1 of the LV myocardium was 1,102 ms in the anteroseptal and 1,209 ms in the posterolateral walls (57 ms and 69 ms for T2, respectively). The mean extracellular volume fraction of the LV myocardium was 33% in the anteroseptal and 39% in the posterolateral walls (N = 20 to 23).
Online Video 1A.
Cardiac Magnetic Resonance Day 7
Compressed-sensing cine SSFP in the 2-CH view (A), 3-CH view (B), and 4-CH view (C) showing increased LV wall thickness (14 mm), increased LV volumes (LV tele-diastolic index 127 ml/m2; N < 100), and marked diffuse hypokinesis (LVEF 33%).
Online Video 1B.
Cardiac Magnetic Resonance Day 7
Compressed-sensing cine SSFP in the 2-CH view (A), 3-CH view (B), and 4-CH view (C) showing increased LV wall thickness (14 mm), increased LV volumes (LV tele-diastolic index 127 ml/m2; N < 100), and marked diffuse hypokinesis (LVEF 33%).
Online Video 1C.
Cardiac Magnetic Resonance Day 7
Compressed-sensing cine SSFP in the 2-CH view (A), 3-CH view (B), and 4-CH view (C) showing increased LV wall thickness (14 mm), increased LV volumes (LV tele-diastolic index 127 ml/m2; N < 100), and marked diffuse hypokinesis (LVEF 33%).
Figure 3.
Cardiac Magnetic Resonance: Evolution Between Day 7 and Day 14
Improvement of LV function is shown on cines in the 2CH view, along with a decrease of myocardial edema on T2-weighted STIR (arrows), a decrease of EGE (arrows), and only minor and stable tissue damage on LGE (arrows). 2CH = 2-chamber; EGE = early gadolinium enhancement; LGE = late gadolinium enhancement; LV = left ventricular; STIR = short-tau inversion recovery.
Figure 4.
Cardiac Magnetic Resonance: Evolution of T1, T2, and ECVs Between Day 7 and Day 14
Myocardial T2 was increased in the posterolateral wall on Day 7, indicating the presence of edema and it decreased on Day 14. T1 times were higher on Day 7 because of edema. ECV maps depict the dramatic decrease of ECV in the posterolateral wall on Day 14 from 39% to 27%. ECV = extracellular volume.
Management
The patient was treated with paracetamol (3 g/day), hydroxychloroquine (400 mg daily), and 2 l/min nasal oxygen. Then, he was treated with 1 mg/h noradrenalin because of severe hypotension (75/45 mm Hg) and antibiotics (intravenous cefotaxime and rovamycine).
Discussion
Clinical and diagnostic features associated with COVID-19 are mostly respiratory infections, and the most frequent complications are severe pneumonia and acute respiratory distress syndrome (2, 3, 4). We report the case of a young male without comorbidity who presented fulminant myocarditis very early during the course of COVID-19 pneumonia. COVID-19 SARS has been associated with tachyarrhythmia or even heart failure (5). During the current global pandemic, acute myocarditis has been reported in a patient infected by COVID-19 with moderate heart failure and no signs of upper respiratory tract infection (6). This case provided evidence of cardiac involvement as a possible late complication of the respiratory infection and suggested that a secondary exaggerated inflammatory response could be responsible for acute myocarditis (6). We describe fulminant myocarditis occurring early during the course of a COVID-19 pneumonia. The rapid and spectacular improvement of the respiratory function observed is very uncommon and has not been reported with SARS–CoV-2. The elevated LV filling pressures and rapid improvement of respiratory and hemodynamic conditions suggest that acute respiratory and circulatory failures might be caused by acute LV dysfunction and cardiogenic pulmonary edema. This simultaneous presentation of fulminant myocarditis and COVID-19 pneumonia favors an alternative pathogenetic pathway with possible acute replication and direct dissemination of the virus through the blood or the lymphatic system from the respiratory tract to the myocardium (7). In support, a potential binding to a viral receptor of the myocyte could facilitate the internalization and replication of the capsid proteins and the viral genome (8,9). Because of the rapid clinical recovery of the patient, endomyocardial biopsy was not performed and the presence of the coronavirus in the myocardium was not demonstrated.
Follow-Up
Cardiac magnetic resonance on Day 14 showed a significant LV reverse remodeling (wall thickness 11 mm, LV telediastolic index 88 ml/m2, and LVEF 54%) (Videos 2A, 2B, and 2C), a clear decrease of focal myocardial edema and EGE in the posterolateral wall, and stable LGE lesions in the subepicardium of the posterolateral wall (Figure 3). The evolution of native T1 to T2 relaxation times and extracellular volume are reported in Table 1. The patient had complete clinical recovery with normal respiratory function and hemodynamics, and was discharged on Day 15 with bisoprolol and angiotensin-converting enzyme inhibitors.
Online Video 2A.
Cardiac Magnetic Resonance Day 14
Compressed-sensing cine SSFP in the 2-CH view (A), 3-CH view (B), and 4-CH view (C) showing a significant LV reverse remodelling (wall thickness 11 mm, LV telediastolic index 88 ml/m2, LVEF 54%).
Online Video 2B.
Cardiac Magnetic Resonance Day 14
Compressed-sensing cine SSFP in the 2-CH view (A), 3-CH view (B), and 4-CH view (C) showing a significant LV reverse remodelling (wall thickness 11 mm, LV telediastolic index 88 ml/m2, LVEF 54%).
Online Video 2C.
Cardiac Magnetic Resonance Day 14
Compressed-sensing cine SSFP in the 2-CH view (A), 3-CH view (B), and 4-CH view (C) showing a significant LV reverse remodelling (wall thickness 11 mm, LV telediastolic index 88 ml/m2, LVEF 54%).
Table 1.
Evolution of T1 and T2 Relaxation Times of the Myocardium and ECV
| Native T1 (ms) |
T1 Post-Gadolinium (ms) |
T2 (ms) |
ECV (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| AS | PL | AS | PL | AS | PL | AS | PL | |
| Day 7 | 1,102 | 1,209 | 625 | 648 | 57 | 69 | 33 | 39 |
| Day 14 | 1,028 | 1,050 | 516 | 519 | 49 | 57 | 31 | 27 |
AS = anteroseptal wall; ECV = extracellular volume fraction; PL = posterolateral wall.
Conclusions
This report demonstrates that fulminant myocarditis may occur during the acute phase of COVID-19 pneumonia and suggests a direct pathogenic role of the virus on the myocyte, although this has not been proven using histopathology. We believe this finding may have important implications for diagnostic and monitoring purposes, but also for the evaluation of future treatment strategies of acute myocarditis related to SARS-CoV infection.
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Friedrich M.G., Sechtem U., Schulz-Menger J. International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Pneumonia of unknown cause—China. https://www.who.int/csr/don/05-january-2020pneumonia-of-unkown-cause-china/en/ Available at:
- 3.World Health Organization Novel coronavirus—China. https://www.who.int/csr/don/12-january-2020novel-coronavirus-china/en/ Available at:
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu C.M., Wong R.S., Wu E.B. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esfandiarei M., McManus B.M. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 8.Liu P.P., Mason J.W. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 9.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]