Abstract
Background
In the context of the COVID-19 pandemic the risk of misdiagnosis of other causes of respiratory infection is likely. In this work we aim to describe the clinical characteristics, treatment and outcome of pneumococcal infection in COVID-19 patients.
Patients and methods
Every COVID-19 patient presenting with concomitant pneumococcal pneumonia during March 2020 in a tertiary teaching Hospital In Barcelona, Spain.
Results
Five patients with PCR confirmed COVID19 or clinical and radiological suspicion were diagnosed of pneumococcal infection. In all cases chest X-ray were abnormal, with unilateral or bilateral infiltrates. Procalcitonin showed to be not sensitive enough to detect pneumococcal infection. Antibiotherapy was promptly started in all five cases with subsequent satisfactory evolution.
Conclusion
International guidelines do not include the universal screening for bacterial co-infection. Radiological pattern of COVID-19 can be indistinguishable from that of pneumococcus pneumonia and frequency of co-infection is not well stablished, therefore clinicians should be aware of the possible SARS-CoV-2-pneumococcus association to avoid misdiagnosis and delay antibiotic therapy.
Keywords: COVID-19, S. pneumoniae, co-infection, Diagnosis, Treatment
Abstract
Introducción
En el contexto de la pandemia por COVID-19 el riesgo de errores en el diagnóstico de otras causas de infección respiratoria es elevado. En este trabajo describimos las características clínicas, el tratamiento y la evolución de los pacientes con coinfección por COVID-19 y neumococo.
Pacientes y métodos
Todos los pacientes con COVID-19 que presentaron neumonía neumocócica durante marzo 2020 en un hospital universitario de Barcelona, España.
Resultados
Cinco pacientes con COVID-19 confirmada por PCR o sospecha radiológica fueron diagnosticados de infección por neumococo. En todos los casos la radiografía de tórax era patológica con infiltrado unilateral o bilateral. La procalcitonina demostró no ser suficientemente sensible para detectar la infección neumocócica. La antibioterapia fue iniciada de manera precoz en los 5 casos con evolución satisfactoria.
Conclusiones
Las guías internacionales no incluyen el cribado universal para coinfección bacteriana. El patrón radiológico del COVID-19 puede ser indistinguible de la neumonía neumocócica, y la frecuencia de la coinfección no ha sido establecida. Los clínicos deben de ser conscientes de la posible asociación de SARS-CoV-2 y neumococo para evitar errores diagnósticos y retrasos en el tratamiento antibiótico.
Palabras clave: COVID-19, S. pneumoniae, Coinfección, Diagnóstico, Tratamiento
Introduction
The current SARS-CoV-2 pandemic is overwhelming health-care systems worldwide. Resource constraints have large undermined the effective implementation of preventive and therapeutic measures and protection of healthcare workers with proper equipment in some settings. Regarding diagnostic tests, the shortage of real-time polymerase chain reaction (PCR) specific reagents has led many centers to perform diagnosis of suspected COVID-19 disease based on clinical and radiological data, also considering the low-to-moderate rate of false negative tests.1 Even though this may seem practical in the current context, it might lead to over-diagnosis of SARS-CoV-2 infection and to overlook other causes of respiratory infections that require specific treatment.
Streptococcus pneumoniae is the first cause of community-acquired pneumonia (CAP). The burden of pneumococcal disease in the community is reflected by the high mortality rate associated with the invasive form of infection that is up to 10–36% and affects predominantly elderly, chronic and immunocompromised patients.2 Diagnosis of pneumococcal infection is usually suspected on clinical grounds and confirmed by isolation of the bacteria in cultures (blood or sputum) or detection of the C-polysaccharide antigen in urine. The radiological appearance is usually that of a consolidative lobar pneumonia. However, it can also present with bilateral infiltrates, including an interstitial pattern. COVID-19 most common radiological patterns include interstitial infiltrates, either unilateral or bilateral, but it can also present as a consolidative pneumonia.3 This can lead to misdiagnosis of pneumococcal pneumonia if not suspected in the context of COVID-19 pandemic.
Nonetheless, concomitant or secondary bacterial infection has been scarcely and irregularly reported (4.8–15%) in the larger series of COVID-19 published to date.4, 5 Meanwhile, although clinical guidelines recommend empirical antibacterial therapy in critically ill patients with elevated inflammatory parameters,6 there is no clear guidance on the screening of S. pneumoniae and other common bacterial causative agents of respiratory infection during the pandemic.
We report 5 cases of COVID-19 in whom urinary antigen screening revealed a posteriori the coexistence of pneumococcal infection.
Case series
The characteristics of the 5 patients are shown in Table 1 . Median age was 56 (IQR 44–79), and 3 (60%) were females. Hypertension and diabetes was present in 40% of cases respectively, while there were no cases of diabetes or former/current smoking habit. The main symptoms leading to consultation were fever, cough and dyspnea, with diarrhea, arthromyalgia and dysgeusia being less common. SARS-CoV-2 infection was confirmed in 3 cases by means of PCR-positive swab, while in 2 cases was presumed due to typical clinical presentation, radiological appearance and epidemiological criteria (contact with a positive person). The pneumococcal infection was diagnosed by positive detection of the pneumococcal C-polysaccharide antigen in urine sample in all cases.
Table 1.
Clinical, laboratory and radiological characteristics of patients coinfected by SARS-2-CoV and Streptococcus pneumoniae.
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age | 86 | 38 | 65 | 79 | 44 |
| Sex | Male | Female | Male | Female | Female |
| Days from symptoms onset | |||||
| to hospital admission | 5 | 4 | 13 | 14 | 5 |
| to SARS-CoV-2 swab | 5 | N/A | 13 | N/A | 4 |
| to S. pneumoniae detection | 6 | 5 | 16 | 15 | 5 |
| Diabetes | Yes | No | No | No | Yes |
| Hypertension (treatment) | Yes (ARBs) |
No | No | Yes (ARBs, diuretic) |
No |
| Comorbidities | Ischemic Heart Disease Aortic Stenosis CKD |
None | Depression | CKD Chronic anemia |
Obesity Asthma |
| Symptoms | Fever Cough Dyspnea |
Fever Cough Dyspnea Arthromyalgia |
Fever Cough Dyspnea Diarrhea |
Fever Cough Diarrhea Arthromyalgia |
Fever Cough Hyposmia Disgeusia Arthromyalgia |
| Chest X-ray at presentation | Unilateral consolidative infiltrate | Bilateral interstitial infiltrates | Bilateral interstitial infiltrates | Bilateral consolidative infiltrates | Bilateral interstitial infiltrates |
| C-Reactive Protein (mg/dl) (Baseline/Peak) | 4.37/15,31 | 6.41 (peak) | 16.51 (30.24) | 9.28 (15.34) | 6.76 (peak) |
| Lymphocytes (cells/mm3) (Baseline/Nadir) | 600/400 | 2100 (nadir) | 1300/500 | 400 (nadir) | 1300 (nadir) |
| Procalcitonin (ng/ml) (Baseline/Peak) | 0.9/2.29 | 0.06 (peak) | 0.1/0.14 | 0.14/0.16 | N/A |
| LDH (UI/L) (Baseline/Peak) | 276/558 | 251 (nadir) | 378/322 | 284/319 | 345/362 |
| Creatinine (mg/dl) (Baseline/Peak) | 1.43 (2.02) | 0.57 (0.66) | 0.63/0.68 | 1.21 (1.34) | 0.61 (0.68) |
| Ferritin (ng/ml) (Baseline/Peak) | 315/1699 | 51 (peak) | 94/214 | 1599 (peak) | 186 (peak) |
| D-dimer (ng/ml) (Baseline/Peak) | 900/5400 | 800 (peak) | 500/1000 | 300/1000 | 300/400 |
| Use of O2(max FiO2) | Yes (0.24) | No | Yes (0.28) | Yes (0.28) | Yes (0.24) |
| Treatment COVID-19 | |||||
| Lopinavir/Ritonavir | Yes (2) | Yes (2) | Yes (2) | Yes (7) | Yes (2) |
| Hydroxycloroquine | Yes (2) | Yes (6) | Yes (13) | Yes (5) | Yes (10) |
| Azithromycin | Yes (4) | Yes (4) | Yes (5) | Yes (5) | Yes (5) |
| Other | Tocilizumab Anakinra |
None | None | Tocilizumab | None |
| Treatment of S. pneumoniae | Ceftriaxone Ceftaroline |
Ceftriaxone Cefixime |
Ceftriaxone Levofloxacin |
Ceftriaxone Teicoplanin |
Ceftriaxone Cefixime |
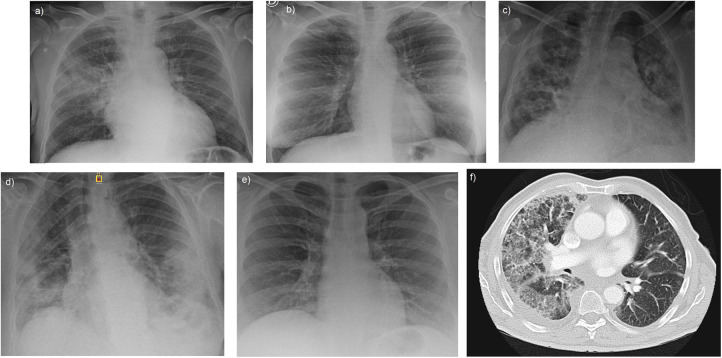
Chest X-ray revealed bilateral interstitial infiltrates in 3 cases, and bilateral consolidative infiltrate and unilateral consolidative infiltrate in one case each (Fig. 1 ). All but one case received low-flux oxygen during the hospital stay and none presented circulatory shock, acute respiratory distress syndrome or pulmonary embolism.
Fig. 1.
Chest X-ray in our series show bilateral infiltrates with an interstitial pattern in patients 2, 3 and 5 (image b, c, e) and a more consolidative pattern in patient 4 (image d). In patient 1, the appearance was that of a lobar consolidative pneumonia (image a). Subsequent CT scan of this patient (image f) also revealed ground-glass opacities suggesting organizing pneumonia.
The local clinical protocol for treatment of COVID-infection is based on lopinavir/ritonavir 200/50 mg two pills bid for one week, hydroxycloroquine 200 mg bid for six days after a loading dose of 400 mg bid for 1 day and azithromycin 250 mg daily for 4 days after a single loading dose of 500 mg. Empirical antibiotic treatment was started when procalcitonin levels were elevated according to the local protocol. Treatment for S. pneumoniae included ceftriaxone 1 g daily. In patient 1 it was upgraded to ceftaroline 600 mg bid and in the case of patient 4 was associated with teicoplanine 6 mg/kg for three doses followed by 6 mg/kg daily. Upon discharge, patients 2 and 5 were switched to oral cefixime 400 mg daily and patient 4 to oral levofloxacin 500 mg daily.
In three cases, clinical course was complicated by late inflammatory response consisting in worsening dyspnea, increase in oxygen requirement, radiological progression and increase in CRP. Patients 1, 3 and 4 received a single dose of tocilizumab 400 mg and patient number 1 also received 2 doses of 200 mg of anakinra. In that case, a thorax CT-scan was also performed, revealing bilateral ground glass opacities along with a pattern suggestive of organizing pneumonia (Fig. 1f), thus a 30-day course of oral prednisone 30 mg was started. Subsequent evolution was satisfactory and all patients were discharged from the hospital, two of them at home and three at a medicalized hotel for recovery. Remarkably, patient 1 and 4 were vaccinated against S. pneumoniae with 23-valent vaccine in 2007 and 2008.
All five patients provided either written or oral informed consent following the procedure established by our institution Ethical Board during the state of infectious disease exceptionality.
Discussion
S. pneumoniae is the leading cause of CAP and affects predominantly frail populations, being the major risk factors age, diabetes, toxic habits (smoking and alcoholism), chronic pulmonary disease and immunodeficiency states. Pneumococcal infection has seasonal and latitude variations, being more common in winter and in countries with temperate-to-cold climate.7 The radiological appearance is classically thought to be unilateral and consolidative. However, in many cases, it appears to be bilateral and assumes an interstitial pattern8; this may generate doubts when performing differential diagnosis with other causes of pneumoniae, such as the actual COVID-19 pandemic. Thus, radiological criteria per se are not sufficient to confirm or rule out pneumococcal infection and there can be a substantial overlap with COVID-19 clinical presentation, therefore highlighting the relevance of microbiological confirmation of SARS-CoV-2 infection in these cases. PCR for SARS-2-CoV infection is overall highly sensitive, even though several pre-analytical issues and during early phases of the infection can yield false-negative results.9 Regarding confirmation of S. pneumoniae infection, sputum culture is limited by very low sensitivity. On the contrary, detection of the pneumococcal C-polysaccharide antigen in urine is a fast mean for screening and diagnosis with a sensitivity and specificity as high as 75 and 95%, respectively.10
In our series of 5 patients, 3 proved to be positive at swab PCR test for SARS-CoV-2 infection, while in 2 cases the test was omitted due to suggestive radiological appearance (Fig. 1) along with typical laboratory findings and epidemiological criteria. According to the local protocol, these cases were promptly isolated and treatment for COVID-19 was started with lopinavir/ritonavir, hydroxycloroquine and azithromycin. However, subsequent testing proved that patients were also infected with S. pneumoniae by detection of the C-polysaccharide antigen in urine, and thus antibiotic treatment was started. Remarkably, procalcitonin was not elevated in all cases, thus suggesting that this should not be the only parameter used to decide further microbiological test to rule out bacterial infection.
With the present report we would like to highlight that common causes of respiratory infection should not be overlooked and that coinfection of SARS-2-CoV with other pathogens should be bear in mind. In case of coinfection with S. pneumoniae, diagnostic and therapeutic challenges may arise, especially when anti-inflammatory drugs are used to control the excessive inflammatory response associated with SARS-2-CoV. Given the expected persistence of the current pandemic in the next months, vaccination against S. pneumoniae infection seems a reasonable option especially for high-risk patients. Meanwhile, specific antibiotic coverage and hospitalization besides antiviral treatment are advisable in patients with suspected or confirmed COVID-19 and pneumococcal co-infection.
Authors’ contributions
DC, JMP and DN designed the study; DC, JR, systematically collected the data; DC drafted the first version of the manuscript, and JMP and DN initially reviewed it; all other authors contributed by gathering data, critically revising the manuscript and providing final approval.
Conflict of interest
None of the authors declare any conflict of interest.
Appendix. Members of the H4H team
Andrea Arenas, Aleix Agelet, Pol Maymó, Eugenia Butori, Carmen Aranda, Marta Sala, Ana Fernández, Cristina Escobar, Laura Moreno, Adolfo Suarez, Susana Cano, Maribel Avalos, Anna Carbonell, Regina Garcia, Nuria Subirana, Jose Vicente Picón, Magali Rodriguez, Eduarda Alves, Maria Martinez, Alba Martinez, Elisabeth Rosero, Carme Hernandez, Nuria Seijas, Maria Asenjo (Hospital at Home Unit, Medical and Nurse Direction); Almudena Sánchez, Aida Alejaldre, Sara Llufriu, Daniela Lopera, Patricia Buendia, Guadalupe Fernandez, Maria Navarro (Neurology Service, Institut Clinic de Neurociències); Miguel Ángel Torrente, Andrea Rivero, Marta Cervera, Desiré Vigo Conde, Alberto Fernández (Hematology Service, Institut Clinic de Hematologia i Oncologia); Francis Espósito (Oncology Service, Institut Clinic de Hematología i Dermatología, Hospital Clinic de Barcelona), Daniela Barreto (Radiation Oncology Service, Institut Clinic de Hematologia I Oncologia); Agustí Toll, Daniel Morgado, Constanza Riquelme, Andrea Combalía (Dermatology Service, Institut de Medicina i Dermatologia); Josep M Nicolás, Alex Soriano, Alfons Lopez-Soto, Marta Bodro, Celia Cardozo, Ramón Estruch, Joaquim Fernàndez-Solà, Marta Farré (Institut Clínic de Medicina i Dermatologia); Elena Guillen, Ana Santamaria, Lidia Gomez, Mònica Sorroche (Nephrology Service, Institut Clinic de Nefrologia i Urologia); Monica Peradejordi, Alberto Tello, Juan M López, Antonio Alcaraz (Urology Service, Institut Clinic de Nefrologia i Urologia); Belén Massó (Reumathology Service, Institut Clinic d’Especialitats Médico-Quirùrgiques), Carolina Montoya (Traumatology and Orthopedics Service, Institut Clinic d’Especialitats Mèdico-Quirúrgiques), Josep Miranda, Elena Salas, Carlos Garcia, (AGC); Gemma Martinez, Antoni Castells (Nursing and Medical Direction); Laura Perelló, Raquel Crespo, Ariadna Patricia Mejía (CDI); Roser Cadena, Maria Galisteo (DIR.Qualitat); Natalia Charines, Mª Carmen Hernández, Julia Prieto, Laia Sarto, Marta Jimenez, Maria Jesús Sánchez (ICGON); Immaculada Sebastián, Silvia Vidorreta (CDB); Anna Campreciós, Olga Hernando, Carmen Tares (A.QUIR); Ana Mancebo (ICMDM); Gemma Mercade (ICOF); Darwin Barboza, Emilia Abad (ICR); Anna Planell (CDB); Ana Labarta, Jaume Gas, Andrea Ocaña, and Eva Martinez (CAPSBE); all from Hospital Clinic de Barcelona, Barcelona, Spain.
References
- 1.Hao W., Li M. Clinical diagnostic value of CT imaging in COVID-19 with multiple negative RT-PCR testing. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman C., Anderson R. The role of Streptococcus pneumoniae in community-acquired pneumonia. Semin Respir Crit Care Med. 2016 doi: 10.1055/s-0036-1592074. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X., Cai H., Hu J., Lian J., Gu J., Zhang S. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Cellès M.D., Arduin H., Lévy-Bruhl D., Georges S., Souty C., Guillemot D. Unraveling the seasonal epidemiology of pneumococcus. Proc Natl Acad Sci U S A. 2019 doi: 10.1073/pnas.1812388116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haroon A., Higa F., Fujita J., Watanabe A., Aoki N., Niki Y. Pulmonary computed tomography findings in 39 cases of Streptococcus pneumoniae pneumonia. Intern Med. 2012 doi: 10.2169/internalmedicine.51.7326. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G., Simundic A.M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0285. [DOI] [PubMed] [Google Scholar]
- 10.Horita N., Miyazawa N., Kojima R., Kimura N., Inoue M., Ishigatsubo Y. Sensitivity and specificity of the Streptococcus pneumoniae urinary antigen test for unconcentrated urine from adult patients with pneumonia: a meta-analysis. Respirology. 2013 doi: 10.1111/resp.12163. [DOI] [PubMed] [Google Scholar]