Abstract
Objective
Tailored services for adolescents and young adults (AYA) living with HIV may improve treatment outcomes. We surveyed HIV clinics throughout Kenya to determine AYA clinic practices, disclosure and transition services.
Methods
We deployed a mobile team to conduct surveys in a random sample of 102 public HIV clinics with> 300 total clients. Data were collected from healthcare workers offering AYA services who had >6 months of experience delivering AYA care.
results
Of 102 surveyed HIV clinics, almost all (101/102) had the same staff to provide services to all age groups. AYA-specific services included dedicated clinic days (91%), the majority being on weekends (57%) and designated clinic spaces (20%). Activities to support AYA retention and adherence were common (support groups [97%] and HIV literacy meetings [93%]). Fewer clinics offered more holistic care, including psychosocial support (16%) and career education (2%), posted additional staff during the AYA day (17%), provided food (17%) or had sporting activities (10%) as incentives. Tracking of disclosure of HIV status to AYA was common (87%). In 40% of clinics, disclosure discussions with caregivers or AYA occurred a median of 2 years later in practice than stated in clinic policy. Transition was not routinely tracked, and definitions were heterogeneous. Median age at transition was reported as 20 years (range: 14–30 years).
conclusion
HIV programmes have implemented varied approaches to enhance AYA services that could be leveraged to support transition to adult services. Research on the impact of these services on health outcomes is needed.
Keywords: HIV, retention in care, adherence, adolescents and young adults, specific support, Kenya, cross-sectional survey
Keywords: Sustainable Development Goals (SDGs): SDG 3 (good health and well-being), SDG 17 (partnerships for the goals)
Introduction
Adolescents and young adults (AYA) living with HIV are a high-priority population due to poor health outcomes. Poor adherence and unsuppressed viral loads[1–3], high rates of mortality[4] and loss to follow-up[5,6] remain highest in AYA compared to other age groups. Loss to follow-up is particularly high among 15- to 24-year-olds [5,6], an age group that experiences healthcare transition to adult services. Supporting AYA during the vulnerable period of transition is crucial to optimising health outcomes.
Despite being a critical process, transition from paediatric to adult HIV services in sub-Saharan Africa (SSA) is poorly defined and often unstructured[7]. Data on transition services for chronic non-infectious diseases are similarly lacking in this setting, making it difficult to adapt transition practices for AYA living with HIV. In resource-rich countries, transition programmes have been developed for HIV[8] and chronic non-infectious diseases [9–12]. In these settings, structured transition programmes are associated with improved clinical outcomes [13,14]. These programmes are often multidisciplinary, are individually tailored and support AYA to gain the knowledge and skills they need in adult care systems. Toolkits to support development of transition programmes are also available[15,16]. Key elements considered in these toolkits include defining transition policies, developing systems for individual tracking and monitoring, assessing readiness to transition, planning transition and actual transfer, and monitoring outcomes post-transition[17,18].
There are few data on current transition practices in SSA[7,19]. Adolescent-friendly services are recommended [20], but clinic practices vary[7]. Practices and tools around disclosure of HIV status to adolescents, a prereq-uisite to transition, are also poorly described. Late age at disclosure, partly due to lack of healthcare worker (HCW) guidelines and tools to support disclosure, is one of the common challenges[21–23]. Tools for disclosure are available[24] but may be underutilised.
In Kenya, an estimated 7% of the 1.5 million people living with HIV are adolescents aged 10–19, and AYA contributed to almost 40% of new HIV infections in 2018 [25]. The Kenyan Ministry of Health (MoH) introduced the adolescent package of care (APOC) in 2015[26]. This package includes HCW training on AYA services and a checklist to prompt and capture services offered. The checklist includes assessment of body mass index, Tanner staging, disclosure, adherence to medication, mental status, school attendance, participation in support groups, transition preparation, and for AYA girls, pregnancy testing, contraception and HPV vaccination. Use of the checklist may be associated with improved viral suppression and better uptake of family planning services among AYA aged 10–19[27]. While the checklist reminds health workers to prepare AYA for transition, and a job aid with a transition algorithm for guidance on age-based transition milestones is available, consensus national definitions of transition, measures of successful transition and tools to track individual progress are lacking. With the growing population of perinatally infected children ageing into adolescence and high numbers of newly infected AYA in SSA, there is a need to better understand current AYA services, models of care and disclosure and transition practices in order to build effective support strategies.
This study aimed to characterise AYA services, HIV disclosure and transition services in a sample of 102 HIV clinics providing services to AYA aged 10–24 years in Kenya.
Methods
Study design and setting
We conducted a cross-sectional study as part of pre-trial clinic assessments to understand transition practices in Kenya in the Adolescent Transition to Adult Care for HIV-infected Adolescents (ATTACH) study (NCT03574129). ATTACH is a cluster-randomised controlled trial (RCT) that aims to test the effectiveness of an adolescent transition package to improve rates of disclosure, transition readiness and successful transition among AYA living with HIV in Kenya.
Site selection
Of 2449 HIV clinics, 590 had electronic medical records (EMRs) in 2016. Of these, 300 clinics that had >300 clients in care were selected. The 300 clinics were divided into tertiles based on the total number of clients ever enrolled, and 34 clinics were randomly selected from each tertile for a total of 102 clinics (Figure 1), allowing equal representation of clinics with different patient volumes. Inclusion of clinics with >300 clients assumed that approximately 10% of the population would be adolescents (age 10–19)[25]; therefore, it was expected that each surveyed clinic would have at least 30 adolescents enrolled in care.
Figure 1.
Distribution of study sites and county HIV prevalence. [Colour figure can be viewed at wileyonlinelibrary.com]
Ethical considerations
The study received approval from the University of Washington (UW) Institutional Review Board (IRB) and the Kenyatta National Hospital (KNH)/University of Nairobi (UoN) Ethical Review Committee (ERC). In addition, the study received approval from the Kenya Ministry of Health National AIDS and STI Control Program (NASCOP), county departments of health in counties of participating clinics, and clinic managers. All surveyed participants provided written informed consent.
Data collection and analysis
Data collection began in December 2017 and was completed in January 2019. Survey respondents were HCWs aged >18 years currently working with AYA in the HIV clinic, who had worked in the clinic for >6 months. Surveys were conducted in-person or by phone, and questions focused on identifying AYA services, models of care, disclosure services and transition services. Respondents completed a paper-based version of the questionnaire prior to the interview, and reviewed responses with study staff during the interview. Data were then entered into a REDCap[28] database. We used descriptive statistics to describe clinic characteristics, models of care and disclosure and transition services, and chi-square tests and t-tests to compare clinic practices. All data analysis was done using Stata version 14.
Results
The 102 selected clinics were from 27 of the 47 counties in Kenya. The majority of staff interviewed were clinical officers (58%) or nurses (25%). The median duration of time they had worked in AYA HIV care and in their current clinic was 2.7 (interquartile range [IQR]: 1.4, 4.0) and 2 years (IQR: 1.3, 3.4), respectively.
Clinic demographics: clinic volume and staffing
The majority of clinics (n = 51) were in health centres or dispensaries, 12 were in county hospitals, 37 were in subcounty hospitals and 1 each was in mission and foundation hospitals. Clinics served a median of 51 (IQR: 34, 115) AYA (Table 1). The total number of staff in the HIV clinics was 1745, with a median of 15 staff (IQR: 10, 21).
Table 1.
Clinic characteristics and AYA services available
| Clinic characteristics (N = 102) | n (%)/Median (IQR) |
|---|---|
| Facility characteristics | |
| Facility type | |
| County | 12 (12) |
| Subcounty | 37 (36) |
| Health centre/dispensary | 51 (50) |
| Mission/foundation | 2 (2) |
| AYA in active follow-up (N = 90) | 51 (34, 115) |
| County | 260 (120, 365) |
| Subcounty | 59 (38, 115) |
| Health centre/dispensary | 44 (28, 68) |
| Mission/foundation | 290 (182, 398) |
| Staffing | |
| Number of staff at HIV clinic | 15 (10, 21) |
| County | 19 (16, 23) |
| Subcounty | 15 (11, 20) |
| Health centre/dispensary | 12 (9, 19) |
| Mission/foundation | 40 (24, 55) |
| Has specific staff for AYA clinic | 7 (7) |
| Has staff who see only children or AYA | 1 (1) |
| Staff type for HIV clinic | |
| Clinical officers | 100 (98) |
| Counsellors | 87 (85) |
| Nurses/nurse counsellors | 82 (80) |
| Peer educator | 91 (89) |
| Laboratory technician | 75 (74) |
| Nutritionists | 63 (62) |
| Social workers | 39 (38) |
| Community health workers | 66 (65) |
| Psychologists/psychiatrists | 10 (10) |
| Medical officers | 8 (8) |
| Clinic model of care | |
| AYA day* | 93 (91) |
| Weekend AYA day | 53 (57) |
| In separate AYA clinic | 19 (20) |
| Integrated clinic | 9 (9) |
| Definition of adolescent | |
| Lower limit, 10 years of age | 79 (77) |
| Upper limit, 19 years of age | 73 (72) |
| AYA records | |
| Has method to identify records | 75 (74) |
Specific day of the week the clinics select that is dedicated to adolescents and young adults.
Patient volume differed by clinic type, with HIV clinics in county and mission/foundation hospitals serving the largest number of AYA (260 [IQR: 120, 365] and 290 [IQR: 182, 398], respectively), whereas subcounty hospitals and health centres/dispensaries served over 4 times fewer AYA (59 [IQR: 38, 115] and 44 [IQR: 28, 68], respectively) (Table 1). Overall, AYA comprised 12% of the total clinic population. The proportion of AYA was slightly lower, but not significantly different, in larger clinics (county/ mission/ foundation hospitals) than smaller clinics (13% vs. 12%, P < 0.001). Clinics in county hospitals had significantly lower AYA-to-staff ratios than clinics in health centres, dispensaries or subcounty hospitals (4:1 vs. 11:1, P < 0.001), as did clinics in mission/foundation hospitals (7:1 vs. 11:1, P < 0.001).
AYA services
It was rare to have staff who specialised solely on AYA-specific care. Only 7 clinics had specific staff who were designated to work in the AYA clinic; however, these same staff also provided care for adults in six of the seven clinics. Clinic staff distribution was weighted more towards general medical care and general counselling, with few psychosocial specialists and few high-cadre medical specialists. Almost all clinics (98%) had a clinical officer (equivalent to physician assistants in the USA). The majority had counsellors (85%), nurses or nurse counsellors (80%), peer educators (89%) and laboratory technicians (74%). Almost two-thirds had community health workers (65%) or nutritionists (62%), and more than a third had social workers (38%). Only 10% of clinics had psychologists or psychiatrists, and only 8% had medical doctors (Table 1).
While AYA-specific staffing was uncommon, designating specific times and spaces to AYA-specific care was relatively common. Almost all clinics (91%) had a separate day or days dedicated to provision of AYA services. In 57% of those clinics, the AYA day was on a weekend (Saturday/ Sunday). Only 20% had a separate space in the clinic where AYA services were provided. AYA services were provided all day in the majority of clinics (74%), and did not differ by whether clinics had AYA-specific days (72% in those with AYA-specific days and 89% in those without, P = 0.27). Clinics in smaller hospitals tended to be less likely to have weekend clinic days (36% vs. 55%, P = 0.19) and separate spaces (20% vs. 7%, P = 0.235) than larger clinics, but these differences were not significant.
During AYA-specific days, it was common for AYA to have access to supportive services aimed primarily at treatment adherence. A majority of clinics with an AYA-specific day also held support groups or teen clubs (97%) and treatment literacy meetings (93%) on the dedicated AYA clinic day, and 15% preferentially posted staff with AYA training to the clinic on AYA days. Most facilities (n = 92) had other activities/support including snacks/lunch (13%), games (10%), psychosocial support (16%) or career education (2%). Seventeen per cent of clinics with selected AYA day(s) had extra staff to support additional services, the majority being peer educators or adolescent champions (71%). Other staff who specially attended the AYA-specific days included programme officers, counsellors, psychologists, religious leaders or children officers.
Scheduling AYA visits was generally tailored towards supporting AYA to receive AYA-specific services and to meet their clinical needs. Clinical officers who largely provided medical care were also responsible for scheduling AYA visits in 83% of clinics. Medication adherence (91%) and poor clinical condition (49%) were commonly considered when deciding clinic visit frequency. AYA faring poorly were given shorter intervals between visits. Visit scheduling also took into account the availability of the AYA (81%), availability of the caregiver (23%) or whether the clinic was overbooked (12%). Almost all (94%) clinics with an AYA clinic day informed AYA during their current visit that they were scheduled to attend clinic on the AYA day.
In recognition of the unique AYA needs and risk of poor outcomes, the majority of clinics defined adolescents largely by WHO guidelines (age 10 [78%] to age 19 [72%]), 69% of clinics had started using the Kenyan MoH adolescent package of services including the adolescent checklist (62%), and had developed systems to uniquely identify AYA records (74%).
Disclosure and transition services
Most clinics (89%) had age-based policies for guiding when healthcare providers should begin disclosure discussions with caregivers and children. However, there was a gap between policy and practice regarding the age of disclosure initiation discussions. Almost half (44%) of the 91 clinics that specified age to begin disclosure discussions reported that discussions with caregivers began in practice at a median of 2 years (IQR: 2, 4) later than the clinic policy-recommended ages. Similarly, gaps existed with age to begin disclosure discussions with children, with disclosure occurring at a median of 2 years later in practice versus what was stated in clinic disclosure policy (IQR: 2, 4) reported in 45 clinics (Figure 2). The median typical age at full disclosure was reported by HCW to occur at 12 years (IQR: 10, 15) (Figure 3).
Figure 2.
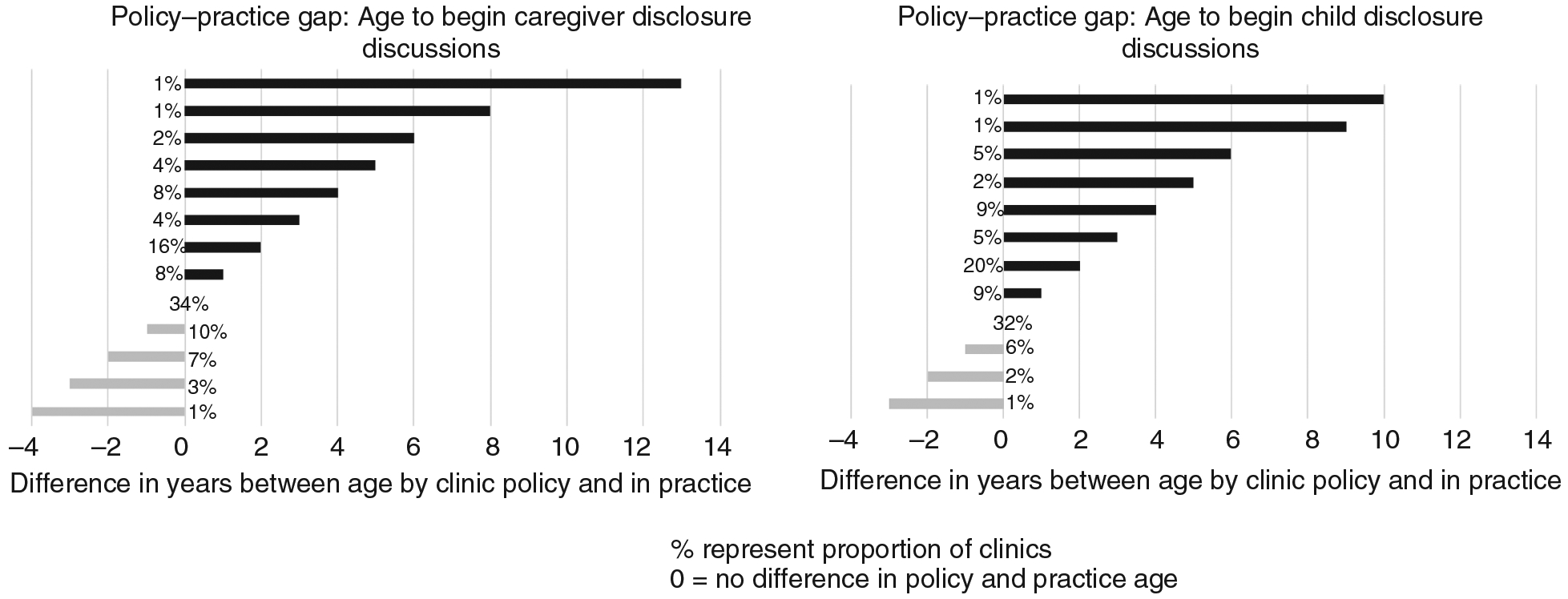
Practice–policy gap in age to begin caregiver and child disclosure discussions.
Figure 3.
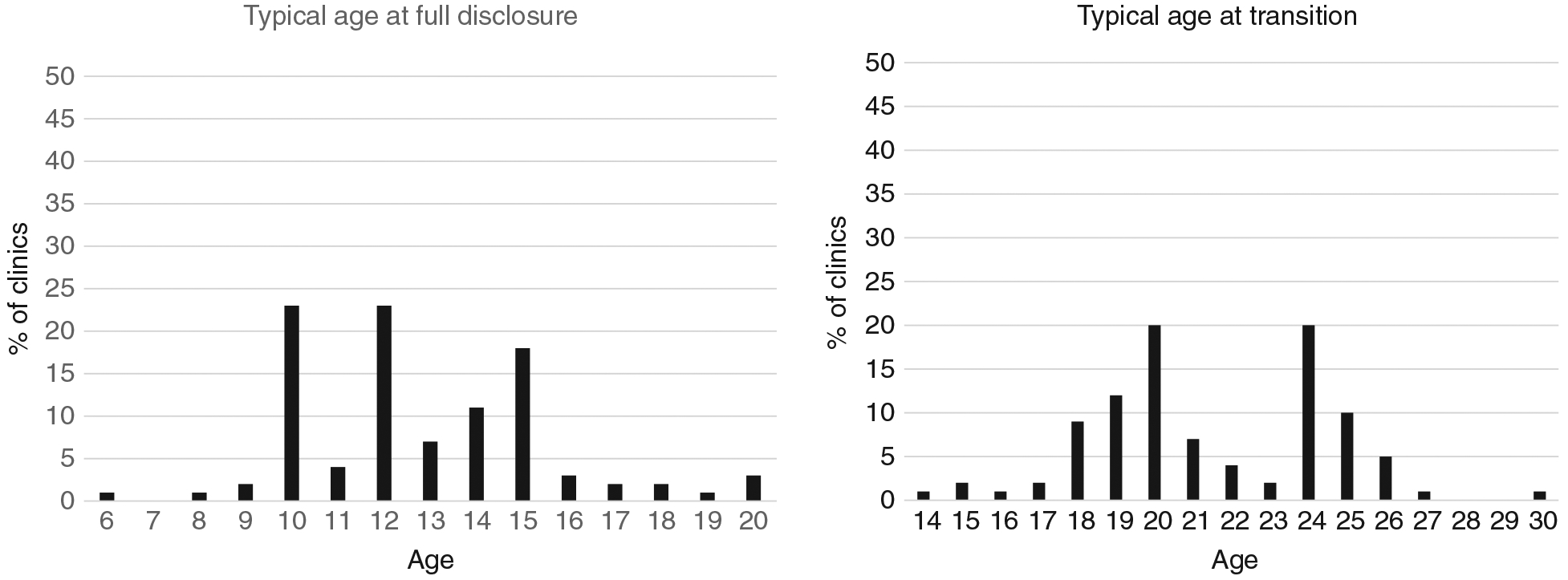
Typical age at full disclosure and transition (healthcare worker reported).
Age was an important component of defining when transition began and was completed. Almost all clinics had policies that specified age at which transition discussions should begin (93%) and end (94%), despite heterogeneous transition definitions. By policy, median age at the start of discussions was 18 years (IQR: 15–19) and completion of transition was at 20 years (IQR: 19–24). Transition occurred at a median age of 21 (IQR: 19–24), with ages 20 and 24 most frequently reported by facilities (Figure 3). In the majority (78%) of clinics, transition was defined by an AYA, demonstrating independence in their own care. Other definitions included the following: reaching a certain age (58%), independently collecting antiretroviral medication (49%), completing an adult clinic visit (27%), completing a formal transition process (25%), becoming pregnant (5%) and switching to adult ART regimens (1%).
Where policies were available, national guidelines were most commonly used, with disclosure guidelines more commonly used than transition guidelines (97% and 69%, respectively) (Table 2). The MoH adolescent checklist was commonly used to track disclosure (67%) and transition (41%). While clinics using the checklist (n = 67) reported to achieve full disclosure slightly earlier (median 12 years [IQR: 10–15]) compared to those not using (n = 34; median 12.5 years [IQR: 12–15]), the difference was not significant (t-test, P = 0.15). A few clinics used additional tools such as disclosure and transition readiness assessment checklists (42% and 19%, respectively) (Table 2). Many staff cadres were involved in initiating disclosure and transition discussions. Disclosure discussions were typically initiated by clinical officers (77%), counsellors (63%), nurses (52%), caregivers (58%) and peer educators (50%), while transition discussions were typically initiated by clinical officers (88%), nurses (60%), counsellors (58%) and peer educators (51%). Only 52% of clinic documented the transfer to adult services, and 66% tracked AYA post-transfer to adult clinics.
Table 2.
Disclosure and transition services
| Disclosure and transition services N = 102 | n (%)/Median (IQR) |
|---|---|
| Disclosure | |
| Has disclosure guidelines | 99 (97) |
| National guidelines | 98 (99) |
| Has disclosure tools | 87 (85) |
| MoH adolescent checklist | 68 (78) |
| Tracks individual disclosure start | 93 (91) |
| Tracks individual disclosure status | 89 (87) |
| Transition | |
| Clinic definition of transition | |
| Shows independence in their own care | 80 (78) |
| Specific age | 59 (58) |
| Picks medication on their own | 50 (49) |
| Has attended adult clinic | 28 (27) |
| Completion of a formal transition ceremony | 25 (25) |
| Becomes pregnant | 5(5) |
| Switch from paediatric to adult ART | 1 (1) |
| regimen | |
| Has transition guidelines | 70 (69) |
| National guidelines | 67 (97) |
| Has transition tools | 50 (49) |
| MoH adolescent checklist | 42 (84) |
| Readiness assessment tools | 19 (38) |
| Tracking tool | 7(14) |
| Transition booklet | 3(6) |
| Other transition tools | 6(12) |
| Tracks transition completion | 61 (60) |
| Documents transfer to adult services | 53 (52) |
| Tracks AYA post-transfer | 67 (66) |
Discussion
In this national survey of 102 clinics in Kenya, we found that HIV clinics had adopted models of care to address unique AYA needs. Despite largely utilising the same clinic staff and space for all HIV-positive clients, specific AYA-focused activities, including AYA clinic days, flexible scheduling and weekend clinic days, were common. While national guidelines offered guidance for disclosure and transition, there were delays in offering disclosure services and transition guidelines were less commonly used. Clinics had methods of defining transition, relying mostly on age, though the definitions were heterogeneous. Individualised transition tracking, readiness assessment, planning and monitoring outcomes were uncommon, and use of disclosure and transition tools beyond those in the national guidelines was rare.
Clinic organisation and staffing was as expected[29]; in Kenya, enrolled nurses provide care in dispensaries, 1–3 clinical officers and one medical officer provide care in health centres, while higher level facilities have more medical officers and specialists. We found that clinics in county hospitals had lower staff–AYA ratios, potentially influencing AYA contact time. Future studies to understand differences in outcomes by staff contact time or facility type may be warranted.
Our findings on the models of AYA care reflect those of other settings in SSA, where AYA clinic days are common, with the same staff caring for all age groups[7,19]. In a survey of Nigerian HIV clinics in 2016, only 28% of clinics had an AYA clinic day, which is substantially lower than in Kenya and could be explained by growing interest and focus for AYA-friendly services in Kenya[26]. While AYA clinic days help to focus AYA services, there is evidence of poor utilisation of particularly non-clinical services with only a third of Ugandan AYA regularly utilising the clinics and services offered[30]. This could be a result of conflicting school or work schedules. Indeed, a majority of surveyed clinics reported AYA availability as the most important consideration during scheduling. Understanding barriers to attending AYA clinic days and outcomes of AYA who do not utilise them may be important in developing approaches that meet the needs of all AYA. We found that clinical officers who primarily provided medical care also provided disclosure and transition services in almost all clinics. The median number of clinical officers was 1 per clinic; therefore, task shifting for disclosure and transition services may be beneficial. We noted very few mental health experts – only 10% of clinics had access to a psychologist or psychiatrist despite well-described mental health challenges among AYA[31].
Late age at disclosure is common in SSA[22]. We found that the majority of clinics typically reached full disclosure by age 10–15, which is later than the national target of full disclosure by age 9–12[32]. Inclusion of disclosure guidelines in national HIV guidelines and expanding the use of the APOC may improve age at disclosure[1,32]. While many clinics had disclosure policies, we found a lag in actual practice, with full disclosure typically occurring much later than recommended by clinic policies and national guidelines[32]. Caregiver support for disclosure or task shifting in addition to child-friendly tools to support disclosure may promote planned, early disclosure. Standard toolkits proven to improve outcomes[24] may provide a more comprehensive approach to disclosure.
As in many SSA settings, transition definitions were heterogeneous[19]. There was little time between the typical age at initiation of transition discussion and age at transition, and this may contribute to AYA discomfort in adult services[33]. The goal of transition is to support AYA to gain life skills and knowledge to independently manage their care[34], which could be viewed as a process. Implementing transition services at an earlier age and within existing support services outside clinic services, such as during support groups or treatment education meetings, strategies already implemented in majority of clinics, may be a feasible approach. Task-shifting transition roles may be useful if clinic volumes are high or depending on staffing configurations.
While the Kenya MoH APOC[35] checklist provides a cue to remind healthcare workers to initiate discussions on transition, only one question, ‘Has transition preparation discussion began (yes/no)’, is dedicated to transition and one to disclosure, ‘Disclosure done (partial/full)’. These questions do not provide tracking of components within the process of transition and disclosure. Clinics did not differentiate perinatally and non-perinatally infected AYA (PHIV), who may require different approaches to care. The WHO and chronic non-infectious literature define transition as ‘gaining knowledge and skills to independently manage care’[9,34], which would be difficult to assess with this tool. However, the APOC tool is an excellent start, as it provides a foundation that can be expanded to capture additional transition process milestones. The APOC tool is already widely used in AYA clinics, and its use has been associated with improved viral suppression [36]. Data used in this analysis were self-reported and may be prone to recall and social desirability bias.
Our study is timely with data collected up to January 2019. However, AYA clinic practices are dynamic and are likely to continue to evolve in the coming years. We sampled clinics based on clinic volume and use of electronic medical records. These clinics may more likely be better-resourced and may not generalise to the whole country. However, our results may provide important current evidence of AYA models of care, and disclosure and transition practices in clinics in programme settings in Kenya.
In conclusion, HIV care programmes in SSA have implemented a variety of innovative approaches to address unique AYA needs. However, there is limited evidence regarding the impact of these diverse models. It will be important to understand ideal models of AYA care that result in improved outcomes and the impact of these models on post-transition outcomes. Although there is a lack of standard transition definitions and inadequate information on tracking tools, making it challenging to evaluate transition, there is promising progress in implementing tools to capture disclosure and transition practices. Research to close the policy–practice gap for disclosure, and develop consistent transition definitions, tracking and monitoring systems is urgently needed.
Acknowledgements
We wish to thank the ATTACH study team, study clinics and staff who made this study possible. We thank the National HIV and STI Control Program (NASCOP), Kenya, and County governments for their support. This publication was made possible with support from the National Institutes of Health (NIH), The National Institute of Child Health and Development (NICHD) 1R01HD089850-01 and F32HD088204 to ADW, 5K24HD054314-09 to GJS and the Fogarty International Center (FIC) D43TW009783 to INN. Additional support was provided by the UW Global Center for Integrated Health of Women, Adolescents and Children (Global WACh), the University of Washington CFAR (P30 AI027757). Partial support for this research came from a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, P2C HD042828, to the Center for Studies in Demography & Ecology at the University of Washington. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
References
- 1.Enane LA, Vreeman RC, Foster C. Retention and adherence: global challenges for the long-term care of adolescents and young adults living with HIV. Curr Opin HIV AIDS 2018: 13: 212–219. [DOI] [PubMed] [Google Scholar]
- 2.Kim S-H, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS (London, England) 2014: 28: 1945–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanoni BC, Archary M, Buchan S, Katz IT, Haberer JE. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: the Cresting Wave. BMJ Glob Health 2016: 1: e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slogrove AL, Mahy M, Armstrong A, Davies MA. Living and dying to be counted: What we know about the epidemiology of the global adolescent HIV epidemic. J Int AIDS Soc 2017: 20(Suppl 3): 21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS (London, England) 2014: 28(4): 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koech E, Teasdale CA, Wang C et al. Characteristics and outcomes of HIV-infected youth and young adolescents enrolled in HIV care in Kenya. AIDS 2014: 28: 2729–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahourou DL, Gautier-Lafaye C, Teasdale CA et al. Transition from paediatric to adult care of adolescents living with HIV in sub-Saharan Africa: challenges, youth-friendly models, and outcomes. J Int AIDS Soc 2017: 20(Suppl 3): 21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maturo D, Powell A, Major-Wilson H, Sanchez K, De Santis JP, Friedman LB. Transitioning adolescents and young adults with HIV infection to adult care: pilot testing the “movin’ out” transitioning protocol. J Pediatr Nurs 2015: 30: e29–35. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Roberts P, Dhaliwal S, Della P. Transitioning adolescent and young adults with chronic disease and/or disabilities from paediatric to adult care services – an integrative review. J Clin Nurs 2016: 25: 3113–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okumura MJ, Ong T, Dawson D et al. Improving transition from paediatric to adult cystic fibrosis care: programme implementation and evaluation. BMJ Qual Saf 2014: 23 (Suppl 1): i64–i72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyons SK, Libman IM, Sperling MA. Diabetes in the Adolescent: Transitional Issues. J Clin Endocrinol Metab 2013: 98: 4639–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treadwell M, Telfair J, Gibson RW, Johnson S, Osunkwo I. Transition from pediatric to adult care in sickle cell disease: establishing evidence-based practice and directions for research. Am J Hematol 2011: 86: 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabriel P, McManus M, Rogers K, White P. Outcome evidence for structured pediatric to adult health care transition interventions: a systematic review. J Pediatr 2017: 188: 263–269. [DOI] [PubMed] [Google Scholar]
- 14.Pyatak EA, Sequeira PA, Vigen CLP et al. Clinical and psychosocial outcomes of a structured transition program among young adults with type 1 diabetes. J Adolesc Health 2017: 60: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Transition GOT. (Available from: http://www.gottransition.org/). [23 April 2018]. [Google Scholar]
- 16.AIDSTAR-One Adolescents Living with HIV Toolkit. (Available at https://aidsfree.usaid.gov/collections/aidstar-one-adolescents-living-hiv-toolkit). [27 Sep 2018]. [Google Scholar]
- 17.Transition Got. (Available from: http://www.gottransition.org/) [Google Scholar]
- 18.Wagner AD, Shah SK, Njuguna IN et al. Financial incentives to motivate pediatric HIV testing-assessing the potential for coercion, inducement, and voluntariness. J Acquir Immune Defic Syndr 2018: 78: e15–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badejo OA, Menson WNA, Sam-Agudu NA et al. Pediatric to adult healthcare transitioning for adolescents living with HIV in Nigeria: A national survey. PLoS ONE 2018: 13: e0198802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Making health services adolescent friendly Developing national quality standards for adolescent friendly health services. WHO; 2012. (Available from: https://apps.who.int/iris/bitstream/handle/10665/75217/9789241503594_eng.pdf;jsessionxml:id=B4FA7138F1E97A315BFEDE5FE9E47BB2?sequence=1). [11 May 2019]. [Google Scholar]
- 21.Arrive E, Dicko F, Amghar H et al. HIV status disclosure and retention in care in HIV-infected adolescents on antiretroviral therapy (ART) in West Africa. PLoS ONE 2012: 7: e33690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vreeman RC, Gramelspacher AM, Gisore PO, Scanlon ML, Nyandiko WM. Disclosure of HIV status to children in resource-limited settings: a systematic review. J Int AIDS Soc 2013: 16: 18466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vreeman RC, Scanlon ML, Mwangi A et al. A cross-sectional study of disclosure of HIV status to children and adolescents in western Kenya. PLoS ONE 2014: 9: e86616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beima-Sofie KM, Brandt L, Hamunime N et al. Pediatric HIV disclosure intervention improves knowledge and clinical outcomes in HIV-infected children in Namibia. J Acquir Immune Defic Syndr 2017: 75: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenya HIV Estimates: Report 2018. National AIDS Control Council. (Available from: https://nacc.or.ke/wp-content/uploads/2018/11/HIV-estimates-report-Kenya-20182.pdf) [14 May 2019]. [Google Scholar]
- 26.National AIDS and STI Control Program (NASCOP). Adolescent Package of Care in Kenya: A health care provider guide to adolescent care. Nairobi, Kenya: 2014. (Available from: https://faces.ucsf.edu/sites/faces.ucsf.edu/files/AdolescentPackage.pdf). [7 April 2019]. [Google Scholar]
- 27.Mburu M, Ong’wen P, Guzé Met al.Evaluating The Implementation And Impact Of The Adolescent Package Of Care At Health Facilities In Former Nyanza Province, Kenya. International AIDS Society (IAS); 2017. Paris, France. [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009: 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Human resources for health (HRH) assessment report for northern Kenya: Overview of Health Workforce Distribution across 10 Counties. May 2013. [Google Scholar]
- 30.Nyabigambo A, Muliira JK, Atuyambe L, Babikako HM, Kambugu A, Ndoleriire C. Determinants of utilization of a no-cost HIV transition clinic: a cross-sectional study of young adults living with HIV/AIDS. Adolesc Health Med Ther 2014: 5: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaitho D, Kumar M, Wamalwa D, Wambua GN, Nduati R. Understanding mental health difficulties and associated psychosocial outcomes in adolescents in the HIV clinic at Kenyatta National Hospital, Kenya. Ann Gen Psychiatry 2018: 17: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ministry of Health, National AIDS & STI Control Program. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2018 Edition. Nairobi, Kenya: NASCOP, August 2018. Print. [Google Scholar]
- 33.Grewal G, Obimbo E, Wamalwa D, Inwani IW. Prevalence, barriers and facilitators of age appropriate transition from paediatric to adult care among HIV infected adolescents at Kenyatta National hospital. Paper presented at: 9th International Workshop on HIV Pediatrics2017; 21–22 July 2017, Paris, France. [Google Scholar]
- 34.The World Health Organization. Adolescent HIV Testing Counselling and Care. Implementation guidance for health providers and planners (Available from: http://apps.who.int/adolescent/hiv-testing-treatment/page/Transition. [23 April 2018] [Google Scholar]
- 35.Jellinek MS, Murphy JM, Burns BJ. Brief psychosocial screening in outpatient pediatric practice. J Pediatr 1986: 109: 371–378. [DOI] [PubMed] [Google Scholar]
- 36.Mburu M, Ongwen P, Guze M et al. Evaluating the implementation and impact of the adolescent package of care at health facilities in former Nyanza province, Kenya. Paper presented at: International AIDS Society (IAS) 2017 2017; Paris, France. [Google Scholar]


