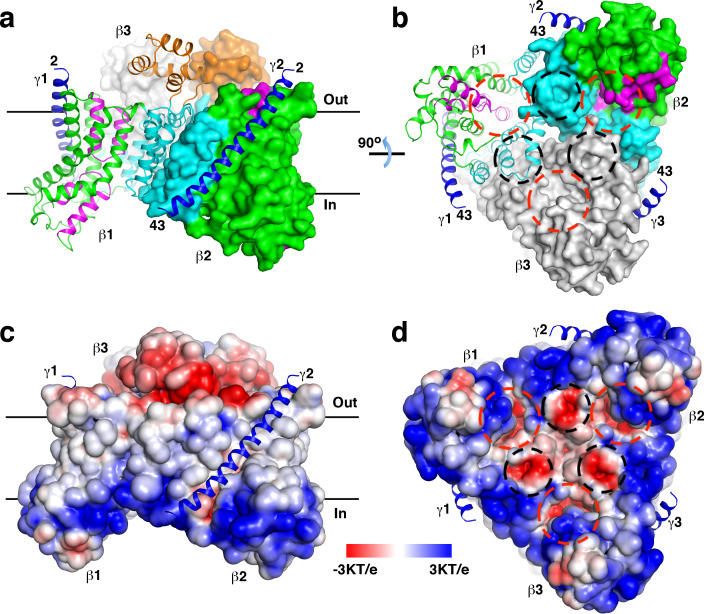
Figure 1. Overall structure of the StOAD βγ sub-complex.
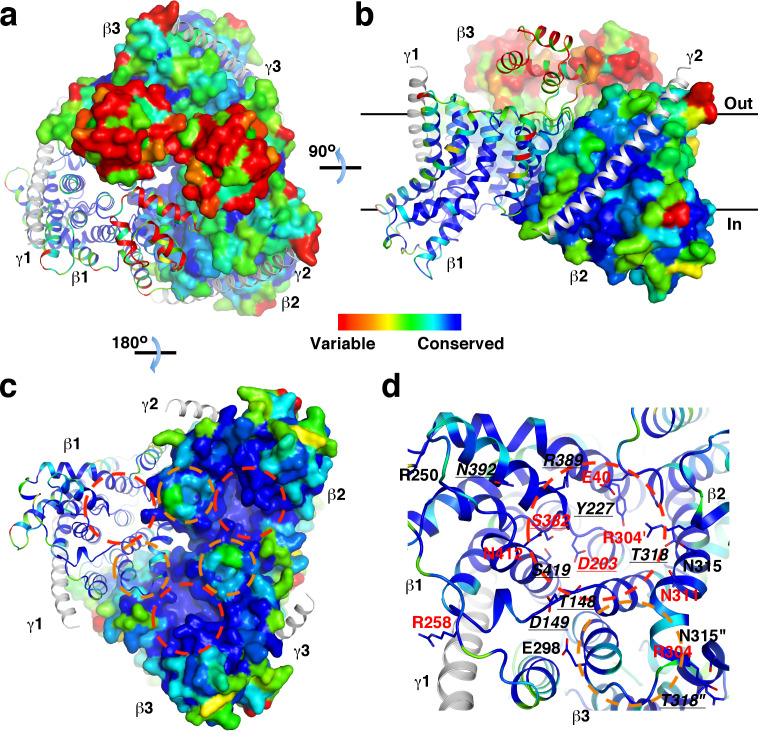
(a)-(b) Structure of the StOAD β3γ3 hetero-hexamer. The γ subunits are colored in blue and shown in cartoon representation. The first and second β subunits (β1 and β2) are colored with the scaffold domain in cyan, core domain in green, domain E in orange and the helical hairpins in magenta. This coloring scheme is used throughout the manuscript unless otherwise indicated. The third β subunit (β3) is colored in gray. Cartoon representation of β1 and the molecular surfaces of β2 and β3 are shown. The first and last residues in the γ subunit visible in our structure are indicated (residues 2 and 43, respectively). Structural figures were prepared with PyMOL (www.pymol.org). (c)-(d) Electrostatic potential at the solvent assessable surface of the StOAD β subunit trimer. The views of panels (c) and (d) are identical to the views of panels (a) and (b), respectively. The γ subunits are presented in cartoon representation and colored in blue. The red and black circles in panels (b) and (d) indicate the first and second negatively charged regions in the β subunit cytoplasmic face, respectively. The black lines in panels (a) and (c) indicate the boundaries of the membrane bilayer.
Figure 1—figure supplement 1. Sequence alignment of the OAD β (a) and γ (b) subunits and their equivalents in other decarboxylase sodium pumps.
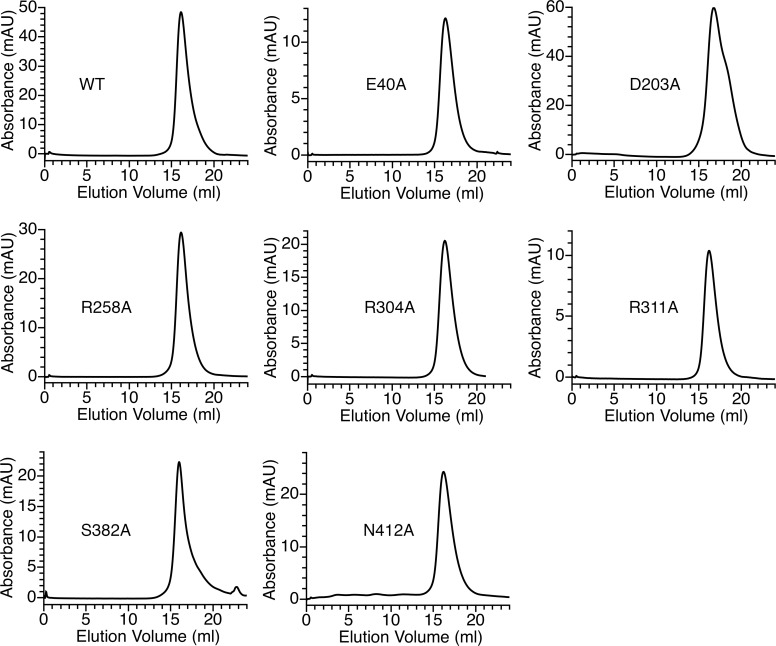
Figure 1—figure supplement 2. Gel filtration characterization of the wild type and substituted StOAD βγ sub-complex.
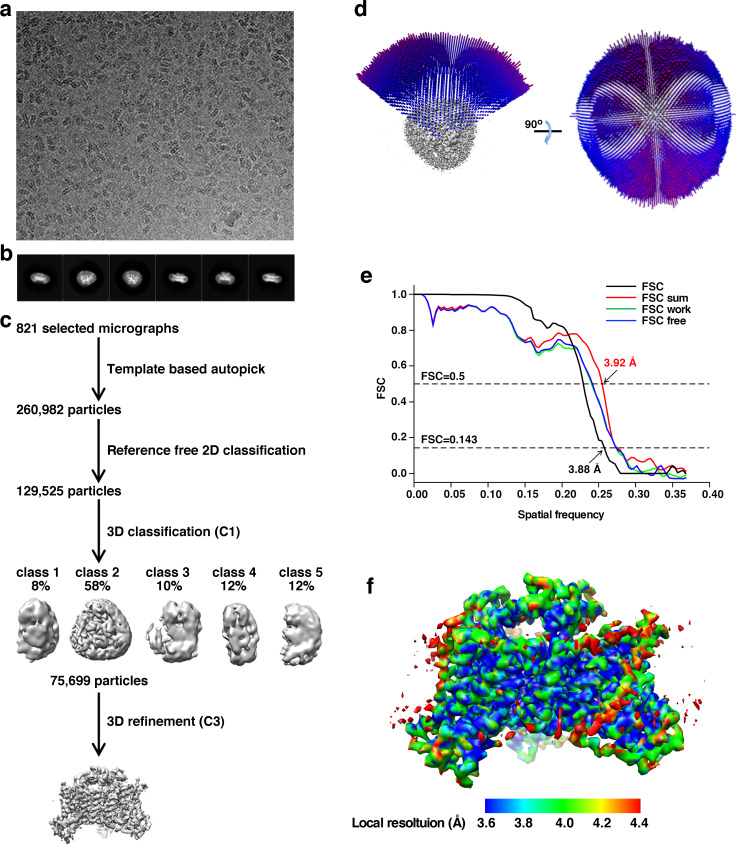
Figure 1—figure supplement 3. Structure determination of the StOAD βγ sub-complex by cryo-EM.
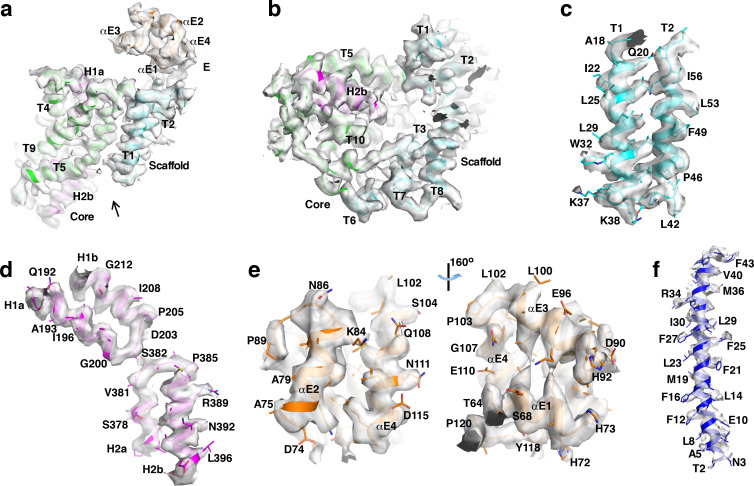
Figure 1—figure supplement 4. Cryo-EM densities.
Figure 1—figure supplement 5. Conservation of individual residues in the β subunit.
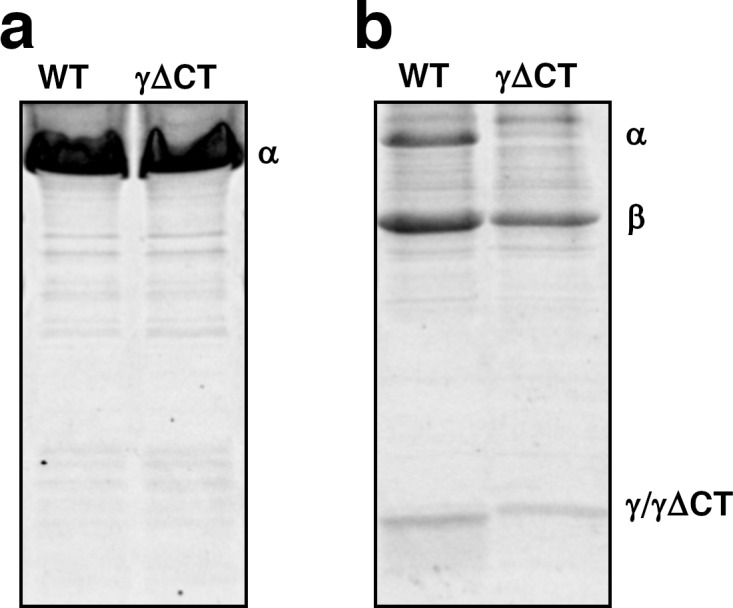
Figure 1—figure supplement 6. The γ subunit C-terminal tail plays a critical role in the interaction between the α subunit and the βγ sub-complex.