Human IL-4 macrophages promote epithelial wound recovery and suppress colitis, supporting their use as a cell therapy for IBD.
Abstract
Murine alternatively activated macrophages can exert anti-inflammatory effects. We sought to determine if IL-4–treated human macrophages [i.e., hM(IL4)] would promote epithelial wound repair and can serve as a cell transfer treatment for inflammatory bowel disease (IBD). Blood monocytes from healthy volunteers and patients with active and inactive IBD were converted to hM(IL4)s. IL-4 treatment of blood-derived macrophages from healthy volunteers and patients with inactive IBD resulted in a characteristic CD206+CCL18+CD14low/− phenotype (RNA-seq revealed IL-4 affected expression of 996 genes). Conditioned media from freshly generated or cryopreserved hM(IL4)s promoted epithelial wound healing in part by TGF, and reduced cytokine-driven loss of epithelial barrier function in vitro. Systemic delivery of hM(IL4) to dinitrobenzene sulphonic acid (DNBS)–treated Rag1−/− mice significantly reduced disease. These findings from in vitro and in vivo analyses provide proof-of-concept support for the development of autologous M(IL4) transfer as a cellular immunotherapy for IBD.
INTRODUCTION
Elegant studies in mice have revealed the processes of tissue seeding and replenishment with macrophages, how the microenvironment affects macrophage function, and the spectrum of macrophage phenotype, ranging from the interferon-γ (IFN-γ)/lipopolysaccharide (LPS) classically activated through a variety of alternatively activated macrophage (AAM) subtypes; the most studied being interleukin-4 (IL-4)–treated macrophages [M(IL4)] (1–3). While capable of producing pro-inflammatory mediators, the AAM, or regulatory macrophage (Mreg), is considered anti-inflammatory, with important roles in homeostasis and tissue restitution/recovery after injury (4, 5).
Adoptive transfer of M(IL4)s reduces the severity of colitis in murine animal models (6, 7), an observation recapitulated with other Mreg subtypes (8). Arginase and IL-10 and recruitment to the gut are implicated in the anti-colitic effect observed after systemic delivery of murine AAMs (7, 9–11). Therapeutic benefits of AAMs have also been shown in, for example, models of inflammatory renal disease and diabetes (12, 13). Furthermore, AAMs are capable of promoting induction or development of regulatory T cells (Tregs) (14), and one consequence of mesenchymal stem cell transfer (MSCT) is the induction of Mregs (15). A detailed awareness of the mouse macrophage has accrued, where, for example, stimuli such as IL-6 and dead neutrophils enhance an M(IL4) phenotype (16, 17); however, precise understanding of human AAMs has lagged significantly behind knowledge of their murine counterparts.
The accumulation of macrophages in the mucosa of patients with inflammatory bowel disease (IBD) led to the concept that monocytes recruited to the gut are more pathogenic than resident macrophages that perform their phagocytic duties in a nonphlogistic manner (18). Consistent with this, patients with IBD can have elevated numbers of circulating pro-inflammatory CD14+CD16+ monocytes, and apheresis of these cells has some efficacy in Crohn’s disease (19). In addition, examination of biopsies from inflamed regions of the colon of patients with Crohn’s disease revealed reduced numbers of CD68+CD206+ cells, presumptive Mregs (6).
Thus, the goals of the current study were as follows: (i) using RNA sequence analysis, to assess the impact of IL-4 on human blood-derived macrophages and determine the ability of blood-derived macrophages from healthy donors and patients with IBD to convert to M(IL4s); (ii) to assess the human (h) M(IL4)’s wound healing ability in an in vitro epithelial injury model; and (iii) to determine the hM(IL4)’s capacity to alleviate colitis in T cell– and B cell–deficient Rag1−/− mice treated with dinitrobenzene sulfonic acid (DNBS).
RESULTS
RNA sequencing reveals significant transcriptional regulation by IL-4
RNA sequence analysis revealed statistically significant changes in expression of 996 genes in IL-4–treated macrophages, corroborating the changes in 90 immune-related genes reported by Martinez et al. (20) (Fig. 1A): 510 genes were up-regulated and 486 down-regulated (Fig. 1A). Consistent with various reports, markers indicative of an alternatively activated M(IL4) were altered (Fig. 1B), and a panel of genes associated with immune signaling and tissue repair was up-regulated (Fig. 1B). Increased expression of CD206 and CCL18 and down-regulation of CD14 in M(IL4)s were confirmed at the mRNA and protein levels by quantitative polymerase chain reaction (qPCR) and enzyme-linked immunosorbent assay (ELISA) or flow cytometry, respectively (Fig. 1, C and D). Specificity was confirmed using IFN-γ, which did not evoke this response from the macrophages (fig. S1, A and B). Gene pathway analytics showed up-regulation of signaling networks related to IL-4 and IL-10 signaling, fatty acid metabolism, and degranulation (Fig. 1E). Comparison of an immune-panel gene array on murine M(IL4) with the human RNA sequence data revealed significant changes in 18 common genes, and of these, 12 showed a similar increase or decrease (Fig. 1F). hM(IL4)s were hyporesponsive to LPS as defined by tumor necrosis factor–α (TNFα), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and MCP-1 (monocyte chemoattractant protein-1) output (fig. S2).
Fig. 1. IL-4 evokes substantial gene regulation in human macrophages.
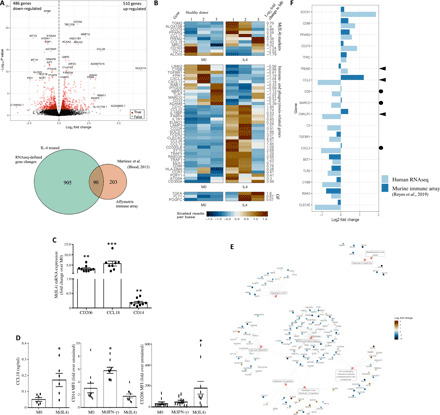
Blood monocytes from three healthy donors were converted to macrophages (2 × 105) with macrophage colony-stimulating factor (M-CSF) and then left untreated [M(0)] or treated with IL-4 [M(IL4)] or IFN-γ [M(IFN-γ)] for 48 hours (both 10 ng/ml). (A) RNA sequence (RNAseq) analysis revealed IL-4–evoked significant changes in 996 genes as shown in the volcano plot and confirmed some of the previously reported gene changes (Venn diagram). (B) Heat maps showing IL-4 regulation of selected genes related to M(IL4) polarization and immune function (GF, growth factors). (C) IL-4–evoked increased expression of CD206 and CCL18, and decreased CD14 mRNA was confirmed by qPCR (D) and at the protein level by ELISA or flow cytometry (MFI, mean fluorescence intensity; each dot represents macrophages from an individual donor; mean ± SEM; *, **, and ***P < 0.05, 0.01, and 0.001 compared to M0, respectively). (E) Reactome network analysis shows clusters of gene changes increased in M(IL4). (F) Comparison of hM(IL4) RNA sequence data with murine mRNA immune array shows good alignment [►, gene change is opposite direction; ●, no response in mouse M(IL4)].
hM(IL-4) promotes in vitro epithelial wound repair
Monolayers of Caco2 epithelia treated with hM(IL4) conditioned medium (CM) showed enhanced wound recovery compared to culture medium only, similar to that evoked by transforming growth factor–β (TGFβ) (Fig. 2, A and B) (data not shown). hM(IFN-γ)-CM did not enhance epithelial wound recovery (fig. S1B). Analysis of cryopreserved hM(IL4) revealed maintenance of the CD206+CCL18+CD14low/− phenotype, although expression was often less than in freshly differentiated hM(IL4) from the same individual (fig. S3A). CM from cryopreserved hM(IL4)s promoted epithelial wound repair (fig. S3B).
Fig. 2. Human blood–derived M(IL4)s promote epithelial wound repair in vitro.
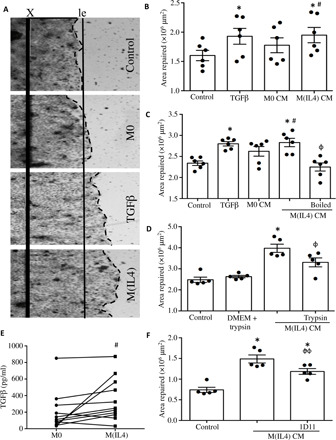
(A) Representative images of epithelia showing the original margin of wounds (X), leading edge of wounds (dashed line), and leading edge (le) of the control monolayer. (B) Treatment with TGFβ (10 ng/ml) or CM from IL-4–treated macrophages [M(IL4) CM] increased epithelial repair. The M(IL4) CM was also (C) boiled or (D) treated with trypsin, which blocked repair. (E) The hM(IL4)s spontaneously produced more TGFβ than nonactivated macrophages (M0) from the same donor. (F) Addition of TGFβ-neutralizing antibodies (1D11) to the M(IL4) CM significantly impaired epithelial repair [mean ± SEM; n = 6 monolayers from three experiments; *, #, and ɸP < 0.05 compared to control (culture medium only), ɸɸ, P < 0.01 compared to M(IL4) CM; M0 CM, and M(IL4) CM, respectively].
Boiling or trypsin treatment of the hM(IL4)-CM reduced its ability to enhance epithelial wound recovery (Fig. 2, C and D). TGFβ was increased in hM(IL4)-CM compared to M(0)-CM from the same individual (Fig. 2E), and immunoneutralization of TGFβ significantly reduced the hM(IL4)-CM capacity to promote epithelial wound recovery (Fig. 2F). Boiling the M(IL4)-CM appeared to be more effective than the immunoneutralization of TGFβ in reducing wound repair, and while this may represent variation between experiments, it may suggest that heat-sensitive factors other than TGFβ contribute to M(IL4)-CM–evoked wound repair. Redundancy in wound repair in the gut would be advantageous, given the importance of gut barrier function to health.
The addition of IL-6 enhanced the M(IL4) phenotype, with the hM(IL4 + IL6) displaying increased CD206 mRNA and CCL18 production (fig. S4, A and B). hM(IL4 + IL6)-CM produced the greatest increase in epithelial monolayer repair after wounding (fig. S4C). Comparison of hM(IL4)-CM from the same donor revealed a significant increase in epithelial recovery following treatment with hM(IL4 + IL6)-CM (n = 4, P < 0.05).
IFN-γ decreases epithelial barrier function when applied to the basolateral side of filter-grown epithelial monolayers, as gauged by decreased transepithelial resistance (TER) and increased transcytosis of fluorescein isothiocyanate (FITC)–dextran, indicators of paracellular permeability (21). This cytokine-evoked reduction in epithelial barrier function was significantly reduced by hM(IL4)-CM (Fig. 3, A and B).
Fig. 3. CM from hM(IL4)s reduced IFN-γ–evoked increases in epithelial permeability.
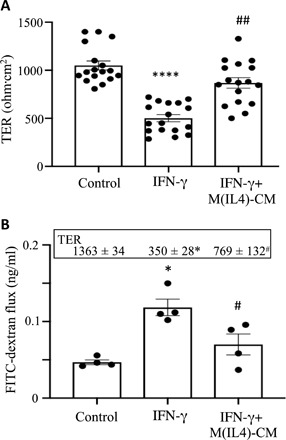
Confluent, filter-grown T84 epithelial cell monolayers were treated with IFN-γ (10 ng/ml) ± 50% CM from hM(IL4)s (1 × 106 cultured for 24 hours), and TER was measured 24 hours later (A) (n = 17 monolayers from six experiments; ****P < 0.001 compared to control; ##P < 0.01 compared to IFN-γ). (B) Following 24 hours of exposure to IFN-γ ± M(IL4) CM, 70-kDa FITC-dextran was added to the lumen aspect of the monolayers, and samples from the basolateral compartment of the culture well were collected 4 hours later and assessed (n = 4 monolayers, one experiment; inset shows TER of the monolayers under the corresponding conditions; *P < 0.001 compared to control; #P < 0.01 compared to IFN-γ).
M(IL4) from individuals with IBD promotes epithelial wound healing
Macrophages differentiated from blood monocytes from patients with inactive Crohn’s disease or ulcerative colitis were converted to an M(IL4) that was indistinguishable from healthy donor M(IL4) based on CD206, CCL18, and CD14 mRNA expressions (Fig. 4, A to C). There was significant variability in the responses of macrophages from patients with active IBD, with results suggesting IL-4 responders and nonresponders (Fig. 4, A to C). Review of patient characteristics did not reveal any feature(s) that predicted responsiveness to IL-4. Flow cytometry of the blood-derived macrophages from healthy volunteers and patients with Crohn’s disease or ulcerative colitis revealed no differences in IL-4Rα expression (data not shown). Exposure to pro-inflammatory cytokines in the blood could render cells hyporesponsive to IL-4; macrophages from healthy volunteers were exposed to a mixture of IFN-γ, IL-1β, and TNFα for 48 hours. When challenged with IL-4, these cells still showed increases in CD206 and CCL18 mRNA (fig. S5).
Fig. 4. Macrophages from patients with inactive disease convert to M(IL4)s that can enhance epithelial wound repair.
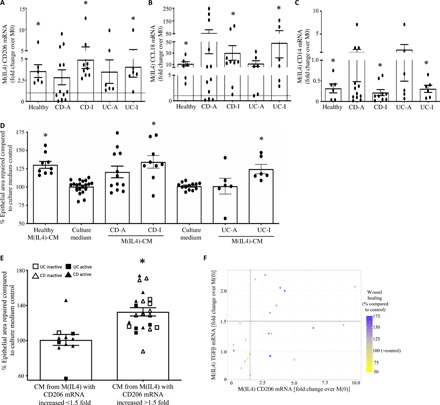
Blood-derived macrophages from healthy volunteers and patients with Crohn’s disease (CD) or ulcerative colitic (UC) with active (CD-A) or inactive (CD-I) disease was exposed to IL-4 (10 ng/ml, 48 hours) and (A) CD206, (B) CCL18, and (C) CD14 mRNA assessed by qPCR and normalized to nontreated macrophages (M0) from the same individual. (D) Confluent monolayers of the Caco2 epithelial cell line were mechanically wounded and exposed to culture media or a 50% CM from the various macrophage groups, and the area recovered was measured 24 hours later. (E) Setting a 1.5-fold increase in CD206 mRNA in response to IL-4 predicted an M(IL4) CM that would significantly increase epithelial wound repair [mean ± SEM; *P < 0.05 compared to M0 (A to C) or culture medium only (D) by analysis of variance (ANOVA) followed by Tukey’s test; each dot represents macrophages from a different donor]. (F) Correlation of patient mRNA expression of TGFβ and CD206 with their wound healing capacity (% wound healing compared to control).
Paralleling the mRNA expression analysis, M(IL4)-CM from patients with inactive Crohn’s disease or ulcerative colitis significantly improved repair in wounded Caco2 epithelial monolayers (Fig. 4D). While the size of repaired epithelial monolayer was on average small, this was consistent with data from healthy donor M(IL4)-CM. Addressing the variability within patient-derived M(IL4) to promote wound repair in the in vitro assay (as with mRNA responses, there appeared to be responders and nonresponders in blood monocytes from patients with active disease), CD206 and TGFβ mRNAs were postulated as predictive of enhanced epithelial wound healing capacity. Using CD206 expression as a canonical hM(IL4) marker, cells with ≥50% increase in CD206 mRNA had the ability to significantly enhance epithelial wound healing (n = 22), whereas macrophages below this threshold had a negligible capacity to promote healing (n = 10) (Fig. 4E). Adding in consideration of M(IL4) TGFβ mRNA returned the finding that CM from M(IL4)s with >1.5-fold increases in CD206 and TGFβ mRNA had the greatest ability to promote wound healing (Fig. 4F).
hM(IL4) reduces the severity of colitis in Rag1−/− mice
Intrarectal administration of DNBS to Rag1−/− mice produces an acute inflammatory response as defined by macroscopic disease score, shortening of the colon, and histopathology (Fig. 5 and fig. S6). Disease was significantly less severe in mice given either freshly differentiated (Fig. 5, A to C) or cryopreserved hM(IL4)s (Fig. 5, D to F) by intraperitoneal or intravenous administration [cryopreservation of hM(IL4) resulted in 74 ± 7% cell survival (n = 3)]. Adoptive transfer of hM(0) did not affect the outcome of DNBS-induced colitis (Fig. 5, A to C). Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining confirmed major cell death in DNBS-treated mice that was less apparent in the hM(IL4)-treated mice (Fig. 6A). Mice receiving hM(IL4)s had increased tissue levels of IL-10 (Fig. 6B) mRNA that resulted in a shift in the murine TNFα:IL-10 mRNA ratio (Fig. 6C) in favor of anti-inflammatory conditions. In accordance, hM(IL4) + DNBS–treated mice had reduced colonic protein levels of TNFα (Fig. 6D). In contrast, murine TGFβ protein levels were increased in the colon hM(IL4)-treated mice (Fig. 7A). Murine epithelia are responsive to human TGFβ as demonstrated by increased SMAD-phosphorylation, which was also increased when M(IL4) CM was added to a murine epithelial cell line (Fig. 7B). In this acute model of colitis, the benefit of hM(IL4) was not accompanied by any obvious increase in markers of fibrosis in the colon (table S1), liver, lung, heart, or kidney (fig. S7A).
Fig. 5. hM(IL4)s reduce DNBS-induced colitis in mice.
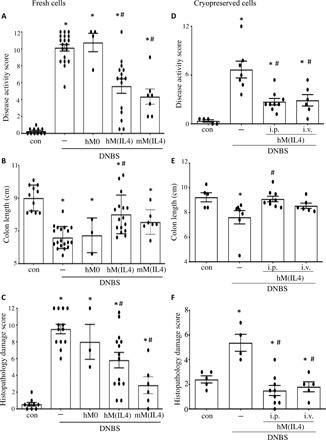
Male C57BL/6 Rag1−/− mice were treated with freshly generated hM(IL4)s or murine (m) M(IL4)s or unstimulated macrophages (M0) [106 cells intraperitoneally (i.p.)] 48 hours before intrarectal delivery of 5 mg of DNBS. Seventy-two hours later, mice were necropsied and (A) a macroscopic disease score calculated, (B) colon length recorded, and (C) histopathology assessed on H&E sections. In separate studies, hM(IL4)’s cryopreserved for 1 month were thawed and delivered by i.p. or intravenous (i.v.) injection 48 hours before DNBS and subsequently, (D) disease activity score, (E) colon length, and (F) histopathology were assessed [mean ± SEM; each dot represents a mouse; macrophages from 10 healthy donors in five experiments (A to C) and 5 to 6 donors in two experiments for cryopreserved hM(IL4) (D to F); * and #P < 0.05 compared to control (con) and DNBS, respectively, by ANOVA followed by Tukey’s test (colon length) or the Kruskal-Wallis test (disease and histopathology scores)].
Fig. 6. hM(IL4)s reduce apoptosis and promote anti-inflammatory conditions in mice with DNBS-induced colitis.
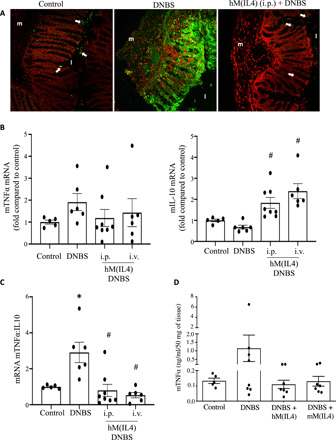
Male C57/bl6 Rag1−/− were treated with cryopreserved hM(IL4)s (106 cells i.p. or i.v.) and 48 hours later received 5 mg of DNBS intrarectally. Seventy-two hours later, mice were necropsied and (A) cryosections of mid-colon assessed by TUNEL staining for apoptotic cells [red, 4′,6-diamidino-2-phenylindole; green, TUNEL+; original objective, 20×)], (B) murine TNFα and IL-10 mRNA assessed by qPCR, (C) the ratio of TNFα:IL-10, and protein levels of (D) murine TNFα assessed by ELISA [mean ± SEM; each dot represents a mouse; macrophages from four to six healthy donors in two experiments; * and #P < 0.05 compared to control (con) and DNBS, respectively, by ANOVA followed by Tukey’s test; m, muscle; l, lumen; arrow, TUNEL+ cell].
Fig. 7. Administration of hM(IL4) increases colonic TGFβ.
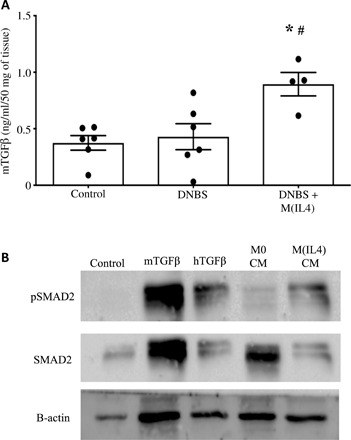
Fresh or cryopreserved hM(IL4) (106 cells i.p.) were administered to male C57BL/6 Rag1−/− mice and 48 hours later received intrarectal delivery of 5 mg of DNBS. Three days later, mice were necropsied and (A) colonic protein levels of TGFβ assessed by ELISA (mean ± SEM; each dot represents a mouse; macrophages from four healthy donors in two experiments; * and #P < 0.05 compared to control and DNBS, respectively, by ANOVA followed by Tukey’s test). (B) CM (50%) from unstimulated M(0)s and M(IL4)s, and mouse and human TGFβ (10 ng/ml) were applied to serum-starved (1 hour) confluent mouse IEC.4 cells, total protein was isolated at 30 min, and SMAD2 phosphorylation was detected by immunoblotting. β-actin was used as a control.
Rag1−/− mice injected (intraperitoneally) with hM(IL4)s 4 weeks before DNBS were protected from the pro-colitic stimulus as assessed by disease activity scores, colon length, and degree of histopathology (Fig. 8, A to D) [a similar phenomenon occurred with wild-type BALB/c mice treated with murine M(IL4)s (Fig. 8E)]. Analysis of lung, liver, and colon of the mice given hM(IL4)s showed no increase in markers of fibrosis compared to controls (fig. S7B) as gauged by assessment of Mason trichrome–stained sections.
Fig. 8. Mice receiving hM(IL4) 1 month before DNBS have reduced disease severity.
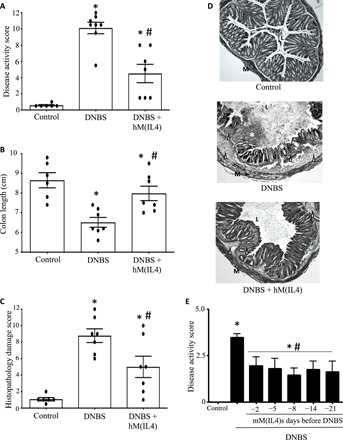
Male C57BL/6 Rag1−/− mice were administered cryopreserved hM(IL4)s (106 cells i.p.) and 30 days later received intrarectal delivery of 5 mg of DNBS. Seventy-two hours later, mice were necropsied and (A) a macroscopic disease score was calculated, (B) colon length was recorded, and (C) histopathology assessed on H&E sections was assessed [data are mean ± SEM; each dot represents a mouse; macrophages from three healthy donors in two experiments; * and #P < 0.05 compared to control (con) and DNBS, respectively, by ANOVA followed by Tukey’s test (colon length) or the Kruskal-Wallis test (disease and histopathology scores)]. (D) presents representative histological images from the treatment groups (M, outer layers of muscle; L, gut lumen; *, ulceration; triangle, inflammatory infiltrate; original objective, 20×). (E) shows that male BALB/c mice treated with murine M(IL4)s 2 to 21 days before DNBS are protected from the pro-colitic stimulus [mean ± SEM; * and #P < 0.05 compared to control (con) and DNBS, respectively, by ANOVA followed by Kruskal-Wallis test].
Soluble mediators from hM(IL4) increase fibroblast growth without obvious fibrosis
Fibrosis is not a major feature of acute models of colitis, but could be initiated by M(IL4)s. Treatment of a human fibroblast cell line with hM(IL4)-CM did not result in enhanced proliferation but did increase total protein levels in the cells (table S1). qPCR revealed no increase in α–smooth muscle actin (α-SMA) or P4-hydroxylase mRNA in the treated fibroblasts (table S1). Assessment of murine colon 5 days after receiving hM(IL4)s revealed no increase in α-SMA, P4-hydroxylase, and oe collagen type III α1 mRNA (table S1).
DISCUSSION
Despite advances in IBD treatment, the best therapies can induce and maintain remission in approximately one-third of patients leaving an unmet need for safe and effective therapies. The search for a single therapy that is transformative for all patients remains elusive, and given the heterogeneous nature of IBD, an individualized approach to the patient is desirable. A substantial body of evidence reveals that many subtypes of murine AAMs are anti-inflammatory and promote healing (6, 17, 22). However, while an hM(IL4) phenotype has been defined, little is known of its function, and the markers that distinguish it from a pro-inflammatory macrophage differ from those that characterize its murine counterpart (20). We present data in support of the hypothesis that autologous M(IL4) transfer can treat IBD.
Multiple markers have been used to define hM(IL4)s, and these were confirmed and others identified by RNA sequence analysis. Setting CD206high/CCL18+/CD14−/low expression as indicating an hM(IL4), CM from these cells significantly improved epithelial wound healing in a reductionist in vitro model. In addition, CM from cyropreserved hM(IL4)s was almost as effective in promoting wound healing as freshly differentiated hM(IL4)s. This adds to the practicality of hM(IL4) therapy, such that patients’ M(IL4)s can be stored for use upon disease relapse (11). While the increased recovery of the damaged epithelium was small in magnitude, this was a consistent finding. Marginal, but significant, increases in wound recovery when translated to the surface area of the gut could benefit to the patient. The hM(IL4)-evoked wound recovery was mediated, at least partially by TGFβ, adding to the postulate that manipulating TGFβ signaling could be therapeutic in IBD (23). Aligning with this, the reduction in barrier function caused by exposure to IFN-γ was significantly reduced in T84 cell monolayers cotreated with hM(IL4)-CM: findings compatible with TGFβ antagonism of IFN-γ–evoked increase in epithelial permeability (24). As a caveat, TGFβ therapy has the specter of fibrosis, and Mregs can be fibrotic in murine models. However, not all studies report a profibrotic effect of murine M(IL4)s, even with repeated administrations, (9), while others suggest an antifibrotic effect of mouse Mregs (10).
Distinct differences exist between circulating and tissue-resident immune cells from healthy individuals and people with IBD (19). A prerequisite for autologous cell transfer is that a patient’s blood-derived macrophages convert to M(IL4)s. Differentiation of M(IL4)s from monocytes of patients with inactive IBD was readily achieved, and the CM promoted epithelial wound healing to a degree not appreciably different from M(IL4)s from healthy individuals. In contrast, and not unexpectedly, ~50% of patients with active IBD had an impaired ability to produce M(IL4)s with wound healing capability, an inability that correlated with reduced expression of CD206 mRNA and, in some instances, TGFβ mRNA. Together, the latter suggests that M(IL4) CD206 and TGFβ expression could be a biomarker to predict responsiveness to hM(IL4)-transfer therapy.
Many mechanisms could account for monocytes from patients with active IBD not converting to M(IL4)s (e.g., reduced signal transducers and activators of transcription 6 signaling or increased expression of suppressor of cytokine-signaling proteins). Can this inability to produce M(IL4)s be overcome? From a translational perspective, the simplest solution would be to obtain monocytes from patients in remission for conversion to M(IL4) and cryopreservation. Alternatively, the potential to drive an M(IL4)-type cell via synergism with additional stimuli could be pursued. For instance, IL-6 increases the murine M(IL4) phenotype (16), and hM(IL4 + IL6) from healthy individuals displayed the greatest increase in CD206 mRNA expression, and hM(IL4 + IL6)-CM led to enhancement of wound recovery compared to hM(IL4)-CM from the same individual.
The use of human cells while instructive and yielding proof-of-principle data must be viewed within the limitations of in vitro analyses. Using mice lacking T and B cells, adoptive transfer of freshly differentiated or cryopreserved hM(IL4)s protected Rag1−/− mice from DNBS-induced colitis. Aligning with the in vitro analyses, colon from protected mice had increased levels of TGFβ, which could be of mouse or human origin, and participated in the suppression of disease, noting that human TGFβ can activate murine cells (Fig. 7B). In demonstrating in vivo efficacy, this finding also reveals that the anti-colitic effect of hM(IL4) transfer is not dependent on adaptive immunity in the recipient (11). Extrapolating to IBD, this suggests that hM(IL4) transfer could be effective in patients with deficits in their T cells (25). Furthermore, the anti-colitic effect of hM(IL4)s occurred when transfer was performed 4 weeks before treatment with DNBS. A similar sustained response was reported for a murine Mreg in a mouse model of diabetes (13). Mechanistically, this implies either prolonged establishment of the cells (perhaps due to a lack of adaptive immunity in Rag1−/− mice) or that the M(IL4) initiates a long-lived program in the recipient, rendering them less susceptible to DNBS; these possibilities should be assessed. In addition, we cannot exclude the possibility that death of M(IL4)s in vivo has an anti-colitic effect and should be addressed in subsequent mechanistic studies. The longevity of the hM(IL4) effect in the Rag1−/−-DNBS model, if translatable, presents the intriguing possibility that autologous transfer in patients could enhance recovery from a flare in disease and extend the period of remission. Experiments designed to determine the location of the transferred cells could be used to enhance hM(IL4) therapy by, for example, using cells in which mucosal (or gut specific) addressins are increased.
When proposing a new therapy, the issue of safety is omnipresent. Assessment of direct interaction between hM(IL4)s and human fibroblasts, albeit in an acute in vitro setting, revealed a small increase in fibroblast total protein, consistent with increased growth, but with negligible changes in markers of fibrosis. Also, Rag1−/− mice displayed no overt fibrosis following hM(IL4) treatment. Nevertheless, fibrosis remains an unknown risk with hM(IL4) and requires further study. Susceptibility to bacterial infection is a concern (26), as with any immunosuppressive therapy (e.g., anti-TNFα therapy), and while mouse M(IL4)s retain a phagocytic capacity that can be boosted by butyrate (27), we should be cognizant of this possibility with hM(IL4) transfers for IBD. Also, immunosuppressive macrophages can promote cancer (28), and studies to assess hM(IL4) transfer as a potential oncogenic stimulus need to be conducted.
Cellular immunotherapy for IBD is not unprecedented, such as MSCT for fistulizing disease (29). Intriguingly, murine and human MSCTs increase AAMs in mice and people, (15) which can, in turn, promote development of Tregs: (30) reciprocally, and Tregs can promote AAM development (31). Thus, AAMs may be at the center of a regulatory pathway with MSCs and Tregs that protects tissues from inflammatory damage. Many of the issues surrounding MSCT for IBD (e.g., sex, disease location, and concomitant therapy) (32) also apply to hM(IL4) transfer to treat IBD and need to be addressed in rigorous studies.
Having shown that IL-4 evokes major transcriptome changes in human blood monocyte–derived macrophages and that hM(IL4)s promote epithelial wound repair in an in vitro assay, reduce cytokine-induced epithelial barrier defects, and are beneficial in a murine model of acute colitis, we have demonstrated the cells’ pro-healing/anti-inflammatory ability and present this as proof-of-concept support for M(IL4) immunotherapy for IBD.
MATERIALS AND METHODS
Study design
Our overall objective was to determine whether hM(IL4)s promote epithelial wound healing and suppress colitis, as proof-of-principle data to support future analysis of autologous cell transfer therapy with M(IL4)s for treating IBDs. Blood-derived macrophages were isolated from healthy donors and from patients with active and inactive Crohn’s disease and ulcerative colitis, and conversion to an M(IL4) phenotype was assessed via qPCR and flow cytometry; in addition, RNA-sequencing analysis was applied to M(IL4)s from three healthy donors for a broader appreciation of M(IL4) phenotype. An in vitro assay was used to determine the wound healing capacity of M(IL4)s by applying CM from these cells to mechanically wounded Caco2 epithelial monolayers for 48 hours, followed by measurement of total area repaired. To assess the protective effect of M(IL4)s in vivo, Rag1−/− mice were administered fresh or cryopreserved hM(IL4)s (intraperitoneally or intravenously) 2 days or 1 month before intrarectal instillation of the pro-colitic agent, DNBS, and colitis was assessed 3 days later by macroscopic disease activity score, histopathology scores, TUNEL staining, and qPCR and ELISA for IL-10, TGFβ, and TNFα. Scoring of disease was performed in a blinded fashion. The processing of samples and statistical analysis was executed simultaneously for all experimental groups. For all experiments, a minimum of three experiments and a minimum n value of four were chosen. The amount of replicates and statistics are referred to in each figure legend.
Animal studies
Animal experiments adhered to the Canadian Council on Animal Welfare as administered by the University of Calgary Animal Care Committee under protocol AC17-0115. Male 10- to 14-week-old C57BL/6 Rag1−/− mice (breeding colony, University of Calgary) were injected intraperitoneally or intravenously with 106 hM(IL4) or hM(0) in 250 μl of phosphate-buffered saline 48 hours before intrarectal delivery of 2,4-DNBS (5 mg in 100 μl of 50% ethanol; MP Biomedicals) to induce colitis (11). In another set of experiments, mice received hM(IL4) 1 month before DNBS treatment. Murine M(IL4) served as a positive control (11).
On day 3 after DNBS, necropsies were performed, and disease was assessed by a macroscopic disease activity score. A segment of mid-colon was excised, fixed, and processed for H&E staining, and a histopathology score was calculated. Collagen deposition was examined in Masson’s trichrome–stained sections of formalin-fixed colon, liver, heart, lung, and kidney (6, 9). In some experiments, colonic and liver levels of collagen were measured using the Sircol Soluble Collagen Assay (Biocolor assays). Additional portions of colon were excised and extracted for total protein to determine TNFα and TGFβ levels by ELISA or placed in TRIzol (Invitrogen) and TNFα and IL-10 mRNA assessed by qPCR.
Human studies
Blood from healthy volunteers [male = 15, female = 9; age range, 20 to 66, mean = 31 ± 12 (SD)] was obtained with approval by the University of Calgary Conjoint Health Research Ethics Board (REB15-1270_REN3). Patients [table S2; male = 17, female = 16; age range, 20 to 87, mean = 47 ± 17 (SD)] were consented, and blood was obtained through the University of Calgary Intestinal Inflammation Tissue bank (REB15-0586_REN3). Peripheral blood mononuclear cells were collected, and following a 4-hour 37°C incubation on petri dishes, nonadherent cells were removed, and the adherent cells were incubated in culture medium (RPMI-1640 medium), 2% penicillin/streptomycin, Hepes (20 mM; all from Gibco), and 10% pooled normal human heat-inactivated and sterile-filtered AB serum (Innovative Research, Novi, MI) with recombinant hM-CSF (10 ng/ml) for 7 days. Medium was changed on day 5, with the addition of hM-CSF. Size, granularity, and CD68 expression by flow cytometry revealed that the cultures were 97.5 ± 0.1% macrophages (n = 3). Macrophages (2.5 × 105) were differentiated for 48 hours with recombinant IL-4 (±IL-6) or IFN-γ (all 10 ng/ml) and then assessed. Differentiated macrophages (2.5 × 105) received 1 ml of fresh culture medium, and 24 hours later, the CM was collected for use and cytokine determinations. Differentiated macrophages are not a rapidly dividing cell type; however, we assessed cell numbers 72 hours and 1 week after cytokine treatment and found no significant change in cell numbers [i.e., proliferation; M(0) = 2.6 ± 0.7 compared to M(IL4) = 2.7 ± 0.07 × 105] and % cell survival [i.e., viability; M(0) = 88 ± 16 compared to M(IL4) = 95 ± 26], respectively (n = 4; mean ± SD). Similarly, testing of viability via trypan blue exclusion revealed no differences between the two groups at the end of the in vitro culture when either CM was collected or cells harvested for delivery to mice.
For cryopreservation, M(IL4)s were resuspended (5 × 105/ml) in 10% dimethyl sulfoxide in human serum and stored at −80°C overnight in Mr. Frosty containers (Thermo Fisher Scientific, Wilmington, DE) with isopropanol and then transferred to liquid nitrogen.
Real-time qPCR
Macrophage RNA was isolated using the Aurum Total RNA Mini Kit (Bio-Rad Laboratories, Hercules, CA), quantified using the Nanodrop 1000 Spectrophotometer (Thermo Fisher Scientific), and 0.5 μg of RNA converted to complementary DNA (cDNA) using an iScript kit (Bio-Rad Laboratories Canada, Mississauga, ON, Canada). Real-time qPCR of human and murine macrophages was performed (9) using primers listed in table S3. Data are presented as the target gene: 18S ribosomal RNA gene ratio for M(0) that was normalized to a value for 1 for comparison with M(IL4) from the same individual. A correlation graph of the expression of human TGFβ mRNA, CD206 mRNA, and % epithelial wound healing from M(IL4)-CM was made in R (version 3.5.3) using the ggplot2 (version 3.2.1).
RNA sequencing
RNA from M(0) and M(IL4)s from three healthy donors was quality assessed using the Agilent Tapestation 2200, with RNA integrity values 8 to 10 indicating samples free of DNA contamination (21). RNA sequencing was performed at the Centre for Health Genomics and Informatics, University of Calgary, where samples were made into libraries using the Illumina TruSeq Stranded mRNA LT library preparation kit and were sequenced on a 75-cycle high-output NextSeq 500 run. Briefly, transcripts were quantified with Kallisto 0.43.1 (33) using Homo sapiens GRCh38 (Ensembl release 90) cDNA, with sequence bias correction turned on and 50 bootstraps. All downstream analyses were done in R 3.5.1 (34). Sleuth 0.30.0 was used for differential expression, with treatment as the main effect and donor included as a covariate (35). The M(IL4)s were compared to nonstimulated M(0) using the Wald test, and differentially expressed genes were selected on the basis of a q value cutoff of 0.05. Enriched gene sets for both KEGG (36) and Reactome (37) pathways were identified with overrepresentation tests (P < 0.05) using clusterProfiler 3.10.0 (38) and ReactomePA 1.26.0 (39), respectively. Data were deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE132732 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE132732).
In vitro wound repair assays
Human colon–derived Caco2 or T84 epithelial cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) [supplemented with 10% fetal bovine serum (FBS), 2% penicillin/streptomycin, and 0.00144% sodium bicarbonate]. Cells (5 × 105) were seeded in six-well plates for 48 hours and transferred to DMEM (0.5% FBS) for 24 hours, and monolayers were wounded with a razor blade. Macrophage CM was diluted 1:1 with DMEM culture medium (0.5% human serum) and applied to wounded epithelia for 48 hours. Wounds were observed using the Nikon Eclipse Ti-U inverted microscope at ×4 magnification (Melville, NY) (40). Analysis of repair was measured in three fields of view from the initial wound edge to the leading edge for total area repaired in micrometer2. When CM from patient cells was used, wound repair was normalized for % baseline increase of total area repaired compared to medium control. TGFβ (10 ng/ml) served as a positive control. In some experiments, CM was (i) boiled (20 min) and (ii) subjected to trypsin (2 U/μl; 37°C; 2 hours) and then trypsin inhibitors (2 U/μl for 2 ml of CM; 37°C; 30 min), or (iii) TGFβ-neutralizing antibodies were added (100 ng/ml; 2 hours at 37°C; 1d11 clone Cedarlane Labs).
Epithelial permeability
T84 epithelial cells were seeded onto 3-μm porous filter supports and cultured until confluent (24) as determined by a TER of ≥1000 ohm.cm2. Monolayers were then treated basolaterally with IFN-γ (10 ng/ml) ± 50% M(IL4)-CM, and TER was measured 24 hours later via voltmeter and chopstick electrodes. Then, 70-kDa FITC-dextran (200 μg/ml; Sigma Chemical Co.) was added to the apical side of the monolayer, samples (500 μl) of basolateral medium were collected 4 hours later, fluorescence was measured, and amount of FITC-dextran was read off a standard curve.
Cytokines
Cell culture medium was collected from nonactivated M(0) or M(IL4) or LPS (10 ng/ml for 24 hours)–activated macrophages and was subjected to the Luminex human cytokine array pro-inflammatory focused 13-plex (HDF13) panel (EveTechnologies, Calgary, Canada). ELISA DuoSet kits (R&D Systems Inc.) were used to measure CCL18, IL-10, TGFβ, and TNFα in macrophage supernatants according to the manufacturer’s protocols. All samples were assessed in duplicate.
Protein detection on immunoblotting
Confluent mouse IEC4.1 intestinal epithelial cells were serum starved for 1 hour and then treated with CM from hM(0) and hM(IL4)s and mouse or human recombinant TGFβ (10 ng/ml, 30 min; eBioscience). Whole extracts were analyzed by immunoblotting protocol with the following antibodies: rabbit polyclonal phospho-SMAD2 (1:1000, Cell Signaling Technology, #3108), rabbit polyclonal SMAD2 (1:2500, Cell Signaling Technology, #3102), and rabbit polyclonal β-actin (1, 1000, Abcam, #8227).
M(IL4)-fibroblast interaction
The human colonic fibroblast cell line CCD-18Co (American Type Culture Collection, CRL-1459) was seeded (5 × 105) in six-well plates for 48 hours and then treated with M(0)-CM or M(IL4)-CM (50%), and mRNA, total protein, and proliferation were assessed.
Statistics
Parametric data were analyzed using one-way analysis of variance (ANOVA) with Tukey’s posttest or by Student’s t test for two group comparisons. When data were normalized, analysis was one-way ANOVA with Kruskal-Wallis posttest or Wilcoxon signed-rank test. Statistical comparisons were performed using GraphPad Prism 5.0 (GraphPad Prism Software, La Jolla, CA), and a level of statistically significant difference was accepted at P < 0.05.
Supplementary Material
Acknowledgments
T.S.J. was supported, in part, by a stipend from the NSERC CREATE Host-Parasite Interactions (HPI) program at the University of Calgary. G.L. held an AI-HS PhD studentship. A.S. was supported by the Beverly Philips Snyder Institute University of Calgary, NSERC CREATE HPI, and the Canadian Association of Gastroenterology studentships. D.M.M. holds a Canada Research Chair (Tier 1) in Intestinal Immunophysiology in Health and Disease. We acknowledge support from the Intestinal Tissue Bank and G. Bindra at the University of Calgary for access to patient material and the flow cytometry suite (University of Calgary). Funding: Funding for this study was provided by a Crohn’s Colitis Canada Grant-in-Aid to D.M.M. Author contributions: T.S.J.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and statistical analysis; G.L.: acquisition of data and analysis and interpretation of data; A.W.: acquisition of data; B.E.C.: acquisition of data; M.L.W.: analysis and interpretation of data, statistical analysis, and technical support; S.R.: acquisition of data; A.S.: acquisition of data; N.M.: acquisition of data; P.L.B.: study concept and design and critical revision of manuscript for important intellectual content; R.P.: study concept and design and critical revision of manuscript for important intellectual content; D.M.M.: study concept and design, interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtained funding, and study supervision. Competing interests: All authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/23/eaba4376/DC1
REFERENCES AND NOTES
- 1.Jenkins S. J., Ruckerl D., Cook P. C., Jones L. H., Finkelman F. D., van Rooijen N., MacDonald A. S., Allen J. E., Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J., Kubes P., A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165, 668–678 (2016). [DOI] [PubMed] [Google Scholar]
- 3.De Schepper S., Verheijden S., Aguilera-Lizarraga J., Viola M. F., Boesmans W., Stakenborg N., Voytyuk I., Schmidt I., Boeckx B., de Casterlé I. D., Baekelandt V., Gonzalez Dominguez E., Mack M., Depoortere I., De Strooper B., Sprangers B., Himmelreich U., Soenen S., Guilliams M., Vanden Berghe P., Jones E., Lambrechts D., Boeckxstaens G., Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175, 400–415.e13 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Hamidzadeh K., Christensen S. M., Dalby E., Chandrasekaran P., Mosser D. M., Macrophages and the recovery from acute and chronic inflammation. Annu. Rev. Physiol. 79, 567–592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minutti C. M., Jackson-Jones L. H., García-Fojeda B., Knipper J. A., Sutherland T. E., Logan N., Ringqvist E., Guillamat-Prats R., Ferenbach D. A., Artigas A., Stamme C., Chroneos Z. C., Zaiss D. M., Casals C., Allen J. E., Local amplifiers of IL-4Rα–mediated macrophage activation promote repair in lung and liver. Science 356, 1076–1080 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter M. M., Wang A., Parhar K. S., Johnston M. J. G., van Rooijen N., Beck P. L., McKay D. M., In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 138, 1395–1405 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Weisser S. B., Kozicky L. K., Brugger H. K., Ngoh E. N., Cheung B., Jen R., Menzies S. C., Samarakoon A., Murray P. J., Lim C. J., Johnson P., Boucher J. L., van Rooijen N., Sly L. M., Arginase activity in alternatively activated macrophages protects PI3Kp110δ deficient mice from dextran sodium sulfate induced intestinal inflammation. Eur. J. Immunol. 44, 3353–3367 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Shouval D. S., Biswas A., Goettel J. A., McCann K., Conaway E., Redhu N. S., Mascanfroni I. D., al Adham Z., Lavoie S., Ibourk M., Nguyen D. D., Samsom J. N., Escher J. C., Somech R., Weiss B., Beier R., Conklin L. S., Ebens C. L., Santos F. G. M. S., Ferreira A. R., Sherlock M., Bhan A. K., Müller W., Mora J. R., Quintana F. J., Klein C., Muise A. M., Horwitz B. H., Snapper S. B., Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40, 706–719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung G., Wang A., Fernando M., Phan V. C., McKay D. M., Bone marrow-derived alternatively activated macrophages reduce colitis without promoting fibrosis: Participation of IL-10. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G781–G792 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Pesce J. T., Ramalingam T. R., Mentink-Kane M. M., Wilson M. S., el Kasmi K. C., Smith A. M., Thompson R. W., Cheever A. W., Murray P. J., Wynn T. A., Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLOS Pathog. 5, e1000371 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung G., Petri B., Reyes J. L., Wang A., Iannuzzi J., McKay D. M., Cryopreserved Interleukin-4-treated macrophages attenuate murine colitis in an integrin β7-dependent manner. Mol. Med. 21, 924–936 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Wang Y. P., Zheng G., Lee V. W. S., Ouyang L., Chang D. H. H., Mahajan D., Coombs J., Wang Y. M., Alexander S. I., Harris D. C. H., Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 72, 290–299 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Parsa R., Andresen P., Gillett A., Mia S., Zhang X.-M., Mayans S., Holmberg D., Harris R. A., Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes 61, 2881–2892 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Q., Tsuboi N., Shi Y., Ito S., Sugiyama Y., Furuhashi K., Endo N., Kim H., Katsuno T., Akiyama S., Matsuo S., Isobe K. I., Maruyama S., Transfusion of CD206+ M2 macrophages ameliorates antibody-mediated glomerulonephritis in mice. Am. J. Pathol. 186, 3176–3188 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Melief S. M., Schrama E., Brugman M. H., Tiemessen M. M., Hoogduijn M. J., Fibbe W. E., Roelofs H., Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 31, 1980–1991 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Fernando M. R., Reyes J. L., Iannuzzi J., Leung G., McKay D. M., The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLOS ONE 9, e94188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosurgi L., Cao Y. G., Cabeza-Cabrerizo M., Tucci A., Hughes L. D., Kong Y., Weinstein J. S., Licona-Limon P., Schmid E. T., Pelorosso F., Gagliani N., Craft J. E., Flavell R. A., Ghosh S., Rothlin C. V., Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smythies L. E., Sellers M., Clements R. H., Mosteller-Barnum M., Meng G., Benjamin W. H., Orenstein J. M., Smith P. D., Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115, 66–75 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanai H., Iida T., Takeuchi K., Watanabe F., Yamada M., Kikuyama M., Maruyama Y., Iwaoka Y., Hirayama K., Nagata S., Takai K., Adsorptive depletion of elevated proinflammatory CD14+CD16+DR++ monocytes in patients with inflammatory bowel disease. Am. J. Gastroenterol. 103, 1210–1216 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Martinez F. O., Helming L., Milde R., Varin A., Melgert B. N., Draijer C., Thomas B., Fabbri M., Crawshaw A., Ho L. P., Ten Hacken N. H., Cobos Jiménez V., Kootstra N. A., Hamann J., Greaves D. R., Locati M., Mantovani A., Gordon S., Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: Similarities and differences. Blood 121, e57–e69 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Connelly M., Boland D., Graf E., Tissot C., Padmanabanm A., Performance of the agilent RNA ScreenTape and high sensitivity RNA ScreenTape assay for the agilient 2200 TapeStation system. TApeStation, 1–4 (2012). [Google Scholar]
- 22.Vos A. C., Wildenberg M. E., Arijs I., Duijvestein M., Verhaar A. P., de Hertogh G., Vermeire S., Rutgeerts P., van den Brink G. R., Hommes D. W., Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm. Bowel Dis. 18, 401–408 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Feagan B. G., Sands B. E., Rossiter G., Li X., Usiskin K., Zhan X., Colombel J. F., Effects of mongersen (GED-0301) on endoscopic and clinical outcomes in patients with active Crohn’s disease. Gastroenterology 154, 61–64.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 24.McKay D. M., Singh P. K., Superantigen activation of immune cells evokes epithelial (T84) transport and barrier abnormalities via IFN-γ and TNFα: Inhibition of increased permeability, but not diminished secretory responses by TGF-β2. J. Immunol. 159, 2382–2390 (1997). [PubMed] [Google Scholar]
- 25.Goldberg R., Scotta C., Cooper D., Nissim-Eliraz E., Nir E., Tasker S., Irving P. M., Sanderson J., Lavender P., Ibrahim F., Corcoran J., Prevost T., Shpigel N. Y., Marelli-Berg F., Lombardi G., Lord G. M., Correction of defective T-regulatory cells from patients with Crohn’s disease by ex vivo ligation of retinoic acid receptor-α. Gastroenterology 156, 1775–1787 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Weng M., Huntley D., Huang I. F., Foye-Jackson O., Wang L., Sarkissian A., Zhou Q., Walker W. A., Cherayil B. J., Shi H. N., Alternatively activated macrophages in intestinal helminth infection: Effects on concurrent bacterial colitis. J. Immunol. 179, 4721–4731 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernando M. R., Saxena A., Reyes J. L., McKay D. M., Butyrate enhances antibacterial effects while suppressing other features of alternative activation in IL-4-induced macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G822–G831 (2016). [DOI] [PubMed] [Google Scholar]
- 28.DeNardo D. G., Ruffell B., Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369–382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panés J., García-Olmo D., Van Assche G., Colombel J. F., Reinisch W., Baumgart D. C., Dignass A., Nachury M., Ferrante M., Kazemi-Shirazi L., Grimaud J. C., de la Portilla F., Goldin E., Richard M. P., Leselbaum A., Danese S.; ADMIRE CD Study Group Collaborators , Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 388, 1281–1290 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Schmidt A., Zhang X. M., Joshi R. N., Iqbal S., Wahlund C., Gabrielsson S., Harris R. A., Tegnér J., Human macrophages induce CD4+Foxp3+ regulatory T cells via binding and re-release of TGF-β. Immunol. Cell Biol. 94, 747–762 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Tiemessen M. M., Jagger A. L., Evans H. G., van Herwijnen M. J. C., John S., Taams L. S., CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. U.S.A. 104, 19446–19451 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lightner A. L., Cell-based therapy for Crohn’s disease: Time to consider optimization. Nat. Rev. Gastroenterol. Hepatol. 16, 137–138 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Bray N. L., Pimentel H., Melsted P., Pachter L., Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Team RDC, R: A language and environment for statistical computing, R Foundation for Statistical Computing 1; 2011.
- 35.Pimentel H., Bray N. L., Puente S., Melsted P., Pachter L., Differential analysis of RNA-seq incorporating quantification uncertainty. Nat. Methods 14, 687–690 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K., KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45, D353–D361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabregat A., Sidiropoulos K., Garapati P., Gillespie M., Hausmann K., Haw R., Jassal B., Jupe S., Korninger F., McKay S., Matthews L., May B., Milacic M., Rothfels K., Shamovsky V., Webber M., Weiser J., Williams M., Wu G., Stein L., Hermjakob H., D'Eustachio P., The reactome pathway knowledgebase. Nucleic Acids Res. 44, D481–D487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu G., Wang L. G., Han Y., He Q. Y., clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu G., He Q. Y., ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 12, 477–479 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Hudson G. M., Flannigan K. L., Erickson S. L., Vicentini F. A., Zamponi A., Hirota C. L., Alston L., Altier C., Ghosh S., Rioux K. P., Mani S., Chang T. K., Hirota S. A., Constitutive androstane receptor regulates the intestinal mucosal response to injury. Br. J. Pharmacol. 174, 1857–1871 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/23/eaba4376/DC1


