Abstract
Objective Smoking induces changes in salivary pH and inflammatory biomarker levels associated with oral diseases. This study examined the effect of alkaline oral rinse to modify this effect of smoking on salivary study parameters.
Materials and Methods A double-blind randomized controlled trial (RCT) on systemically healthy smokers was conducted. A total of 78 smokers, aged 18 to 40 years, were enrolled as per exclusion/inclusion criteria. An alkaline mouthwash was provided to the intervention group and a placebo to control group. Salivary pH and inflammatory biomarker interleukin (IL)-1β levels were evaluated at baseline and at follow-up (14 ± 2 days).
Statistical Analysis Chi-squared test, independaent t -test, and paired t -test were used to observe the changes in parameters among and between groups before and after intervention using SPSS v16 with a significance level of p ≤0.050.
Results Sixty eight salivary samples were analyzed. All study parameters of the study sample were statistically insignificant between both intervention and control groups at baseline. pH level was 6.56 ± 0.53 at baseline and 6.62 ± 0.45 at follow-up in the intervention group; respective values for control group were 6.70 ± 0.36 and 6.83 ± 0.44 and the changes were not significant ( p ≥0.071). IL-1β level was 9.39 ± 10.23 pg/µL at baseline and 5.40 ± 6.62 pg/µL at a follow-up in the intervention group and the change was significant ( p = 0.001); respective values for the control group were 10.63 ± 11.50, and 9.33 ± 11.73 and the difference was nonsignificant ( p = 0.076).
Conclusion This randomized trial indicated that sodium bicarbonate mouth rinse is effective in decreasing IL-1β levels and increasing salivary pH favorable for prevention of oral diseases.
Keywords: saliva, smokers, alkaline mouth wash, pH levels, Interleukin-1β
Introduction
Oral diseases originate at the interface between host tissue and oral ecosystem resulting from environmental (salivary) and microbial dynamics. It may increase pathogenicity and later on initiate and exaggerate oral diseases. 1 Oral mucosa and teeth are washed and lubricated by human saliva, and several functions mediated by its dynamic composition. 2 It contains many proteins and peptides which help in defense and maintenance. 3 Buffering capacity of saliva prevents changes in pH levels (acid–base balance) of the oral environment and acids, thus produced, may neutralize salivary buffering nature. 4
The effects of tobacco consumption on some salivary features of flow rate, buffering capacity, pH, and consistency are still debatable. 5 The stimulation of saliva may affect the salivary pH and the concentrations of some constituents. 6 A study found that salivary pH increased significantly after sodium bicarbonate (SB) oral rinse. 7 Interleukin (IL)-1β is an essential biomarker of inflammatory status. IL-1β also regulates antigen-presenting cells, enhance antigen mediation of T-cells, and play a role in adaptive immunity. 8
Inflammatory biomarkers are influenced by supraregimen (oral) exposure and current trends emphasize the importance of supragingival biofilm control regimen and thus create the need to improve investigations in this field. 9 Prior studies have concentrated on a selected number of inflammatory markers such as fibrinogen, C-reactive protein (CRP), and IL- 6. 10 11 Several systemic alterations in the number of immune cells caused by cigarette smoking were noted. 12 Consistent with other observations of extensive alterations in immunity, Ouchida et al 13 reported that current smoking was associated with differences in systemic levels of multiple immune/inflammatory markers. Currently, human saliva was used as a liquid biopsy for the diagnosis of oral cancer biomarkers, periodontal diseases, dental caries, brain injury biomarkers, diabetics biomarkers, and lungs cancer. 14 15 16
The purpose of this noninvasive intervention trial was to examine the effect of alkaline (bicarbonate) mouthwash on saliva because salivary pH and inflammatory biomarker (IL-1β) levels are significant for dental caries and periodontal disease. 4 17
Materials and Methods
Study Design and Study Sample
The study was conducted as a double-blind randomized controlled trial (RCT). After screening and obtaining written informed consent, eligible patients were subjected to a baseline examination for oral health parameters. Whole mouth saliva was collected to analyze pH levels and IL-1β. Patients were randomly divided into the intervention group and control group at a ratio of 1:1. Both groups received a second examination and saliva collection at 2 weeks (14 ± 2 days) following completion of intervention to the first group and placebo to the second one. The study was conducted at a private institution in Lahore, and salivary specimens were transferred to the Department of Immunology, University of Health Science Lahore, Pakistan, for analysis. The investigators for saliva collection and laboratory procedures were blind from the study groups.
The target population selected for this project were smokers of age between 18 to 40 years. The sample size of this study was calculated using a statistical power calculator ( https://clincalc.com/stats/samplesize.aspx ). Seventy-eight patients were recruited and randomized into intervention and control groups with 20% drop out rate. Male smokers of the institution were screened as per inclusion/exclusion criteria. Smokers who fulfilled the inclusion criteria after general/medical/oral measures, were enlisted for the study. Validation and reproducibility of investigators, study tools, and procedure were established by conducting a pilot study.
Study Parameters and Intervention
General parameters of age, marital status, education, income, body mass index (BMI), smoking habits, dietary habits, and systemic conditions were noted at baseline. Oral health parameters of oral hygiene practices, missing teeth, carious teeth, calculus, gingivitis, periodontitis, and soft tissue lesions were examined at baseline only. Salivary specimens for pH level and IL-1β were collected both at baseline and follow-up examination. After the first examination, patients of the intervention group were provided SB mouthwash prepared for this study. Control group patients were provided with placebo mouthwash. All patients were explained about the use of mouthwash. Instructions for use of mouth wash were pasted on both bottles (test and control). Instructions included (1) use the mouth wash twice daily, after breakfast and after dinner; (2) if any untoward reaction develops, stop use and report to examiners; (3) keep out of reach of children; and (4) store at normal temperatures below 30°C. During follow-up, patients showing indications of any oral or systemic condition as per inclusion/exclusion criteria were dropped from the study.
Saliva Collection, Transportation, and Processing
Whole saliva (unstimulated) was collected using the Pure-SAL and RNA Pro-SAL from Oasis Diagnostics Corporation (Vancouver, Washington, United States) before breakfast, at 8 to 9 a.m., as patients were asked to refrain from drinking, eating, or any oral hygiene procedure 2 hours before sampling. 7 Before the collection of saliva, patients were invited to rinse with water for 15 seconds to remove any food debris, microorganisms, and desquamated epithelium cells. After this, patients were asked to sit straight in a chair and wait for 1 minute. Manufacturer instructions were strictly observed for the use of saliva device for the passive drooling method. Each sample was collected in two tubes: tube 1 was used for pH analysis and tube 2 for IL-1β analysis. Saliva samples were transported in the icebox, and within 2 hours after sampling, each sample was centrifuged at 60,000-100,000 Rpm for 20 minutes. The supernatant was separated into the new tube for storage at 80°C.
Laboratory Procedure
A trained technician who was blind to the source of sample performed the laboratory procedures. Total amounts of IL-1β were assessed for each subject on day 1, and at 2 weeks (14 ± 2 days) using the enzyme-linked immunosorbent assay (ELISA). The sandwich ELISA technique was used with IL-1β EIA Kit (Laboratory Science, United States). Plates were prepared as instructed by the manufacturer. Seventy-one ( n = 71) samples were tested in duplicate. Assay sensitivity was 4.69 pg/mL. The analysis was performed in the Department of Immunology, University of Health Sciences Lahore.
Data Processing and Analysis
Data for study parameters for each patient were noted on “data collection forms” including (1) screening form; (2) consent form; (3) form for demographic history, medical history, and oral parameters; and (4) salivary parameters. Data were analyzed using SPSS software (version 16.0, SPSS; Chicago, Illinois, United States) and presented as a proportion (%) for categorical variables and mean ± standard deviation (SD) for continuous variables. The Shapiro–Wilks test was used to determine the normality of the data distribution. All variables showed a normal distribution within the groups. Comparison of salivary parameters in intervention and control groups at 1st and 2nd stages of examinations were performed using the student’s t -test and paired t- test. The difference in number/proportions of patients with outcome measures (changes in pH levels and IL-1β levels) between intervention and control groups were analyzed using a c 2 test at all examination stages. For every analysis, the level of significance was considered as 5% ( p ≤ 0.050). Ethical and technical approval of the trial was obtained from The University of Faisalabad (vide letter no.: MPOM-FA16–001/2018).
Results
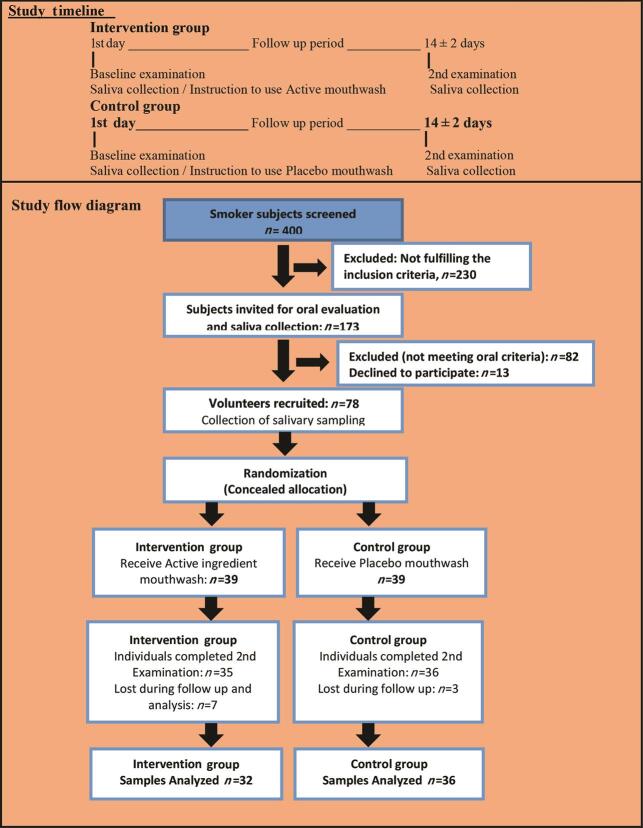
A total of 3,000 individuals were screened at a private institute of Lahore. Of them 400 smokers were invited for a general health examination. Among them, 230 were excluded because they did not fulfill the inclusion criteria. Then 173 eligible individuals were called for oral examination and saliva collection. After the oral examination, 82 individuals were excluded because they did not fulfill oral criteria and 13 individuals did not agree for participation. Seventy-eight healthy volunteer smokers were recruited for the trial. Sixty-eight individuals completed the study with 32 individuals in intervention and 36 individuals in the control group. Four individuals from the intervention group and three from the control group were dropped during follow-up. Three salivary samples of the intervention group were discarded from the analysis. Complete data of 32 individuals of the intervention group and 36 of the control group were finally analyzed ( Fig. 1 ).
Fig. 1.
Study flow diagram
General, Systemic, and Oral Health Study Parameters
A comparison of intervention and control groups at baseline, with respect to demographic parameters of age, BMI, marital status, education, and income revealed no significant differences ( p ≥0.182). Daily diet frequency, consumption of sweets, soft drinks, and fruits also showed no significant difference between groups (p≥0.092) At baseline, smoking status was analyzed for the number of cigarettes per day and smoking years. For both categories, statistical difference was nonsignificant ( p ≥0.077) between groups ( Table 1 ). Oral health parameters of calculus, gingivitis, periodontitis, oral lesions, dental caries, and missing teeth were observed with an insignificant difference ( p ≥0.81) between intervention and control groups. There was also no difference between groups concerning oral hygiene practices ( p ≥0.114; Table 2 ).
Table 1. General characteristics of study participants (mean ± SD/n [%]) at baseline.
| Parameters | Intervention group n = 39 |
Control group n = 39 |
Total n = 78 |
p -Value |
|---|---|---|---|---|
| Abbreviation: SD, standard deviation; PKR, Pakistani rupees. a Student’s t -test. b Chi-squared test. | ||||
| Age (y) | ||||
| Mean ± SD | 28.90 ± 6.96 | 30.97 ± 6.64 | 29.94 ± 6.84 | 0.182 a |
| 18–27 | 17 (21.8%) | 12 (15.4%) | 29 (37.2%) | |
| 28–37 | 16 (20.5%) | 19 (24.4%) | 35 (44.9%) | 0.495 b |
| ≥38 | 6 (7.7%) | 8 (10.3%) | 14 (17.9%) | |
| Body mass index | ||||
| Mean ± SD | 19.45 ± 4.47 | 20.76 ± 4.92 | 20.10 ± 4.71 | 0.221 a |
| Normal | 36 (46.8%) | 32 (41.6%) | 68 (88.3%) | |
| Overweight and above | 3 (2.6%) | 7 (9.1%) | 10 (11.7%) | 0.274 b |
| Marital status | ||||
| Married | 16 (57.1%) | 12 (43.0%) | 28 (36%) | 0.345 b |
| Education | ||||
| No education | 12 (15.4%) | 21 (26.9%) | 33 (42.3%) | |
| Up to Intermediate | 19 (24.4%) | 14 (18%) | 33 (42.3%) | 0.204 b |
| Bachelor and above | 8 (10.1%) | 4 (5.1%) | 12 (15.4%) | |
| Income (PKR) | ||||
| ≤15,000 | 14 (17.9%) | 12 (15.4%) | 26 (33.3%) | 0.562 b |
| >15,000 | 25 (32.1%) | 27 (34.6%) | 52 (66.8%) | |
| Exercise | ||||
| Yes | 6 (7.7%) | 3 (3.8%) | 9 (11.5%) | 0.288 b |
| Meals/day | ||||
| ≤2 meals | 6 (7.7%) | 4 (5.1%) | 10 (12.8%) | 0.374 b |
| ≥3 meals | 33 (42.3%) | 35 (44.9%) | 68 (87.2%) | |
| Use sweets daily | ||||
| Yes | 10 (12.8%) | 17 (21.8%) | 27 (34.6%) | 0.096 b |
| Use soft drinks daily | ||||
| Yes | 5 (6.4%) | 8 (10.3%) | 13 (16.7%) | 0.545 b |
| Use fruits daily | ||||
| Yes | 17 (21.8%) | 9 (11.5%) | 26 (33.3%) | 0.092 b |
Table 2. Oral health practices and status (mean ± SD/n [%]) of study participants at baseline.
| Parameters | Intervention group n = 39 |
Control group n = 39 |
Total n = 78 |
p -Value |
|---|---|---|---|---|
| Abbreviations: NA, not available; SD, standard deviation. a Student’s t -test. b Chi-squared test. | ||||
| Calculus | ||||
| Mild | 17 (21.8%) | 16 (20.5%) | 33 (42.3%) | |
| Moderate | 5 (12.8%) | 13 (16.7%) | 18 (23.1%) | 0.141 b |
| Severe | 6 (7.7%) | 4 (5.1%) | 10 (12.8%) | |
| No | 11 (14.1%) | 6 (7.7%) | 17 (21.8%) | |
| Gingivitis | ||||
| Mild | 9 (11.5%) | 15 (19.2%) | 24 (30.8%) | |
| Moderate | 5 (6.4%) | 5 (6.4%) | 10 (12.8%) | 0.275 b |
| Severe | 2 (2.6%) | 0 (0%) | 2 (2.7%) | |
| No | 23 (29.5%) | 19 (24.4%) | 42 (53.8%) | |
| Periodontitis | ||||
| Mild | 14 (17.9%) | 15 (19.2%) | 29 (37.2%) | |
| Moderate | 10 (12.8%) | 12 (15.4%) | 22 (28.2%) | 0.749 b |
| Severe | 4 (5.1%) | 5 (6.4%) | 9 (11.5%) | |
| No | 11 (14.1%) | 7 (9.0%) | 18 (23.1%) | |
| Oral lesions | ||||
| No | 39(50.0%) | 39 (50.0%) | 78 (100%) | NA |
| Missing teeth | ||||
| Mean ± SD | 0.36 ± 0.81 | 0.77 ± 1.20 | 0.56 ± 1.03 | 0.081 a |
| Yes | 8 (10.3%) | 16 (20.5%) | 24 (30.8%) | 0.085 b |
| No | 31 (39.7%) | 23 (29.5%) | 54 (69.2%) | |
| Dental caries | ||||
| Mean ± SD | 1.23 ± 1.56 | 0.97 ± 1.56 | 1.10 ± 1.55 | 0.471 a |
| Yes | 22 (28.2%) | 15 (19.2%) | 37(47.4%) | 0.173 b |
| No | 17 (21.8%) | 24 (30.8%) | 41 (52.6%) | |
| How clean teeth | ||||
| Toothbrush | 31 (39.7%) | 24 (30.8%) | 55 (70.5%) | 0.199 b |
| Miswak and others (manjan) | 8 (10.3%) | 15 (19.2%) | 23 (29.5%) | |
| Brushing time | ||||
| Once/day | 30 (38.5%) | 36 (46.2%) | 66 (84.6%) | 0.114 b |
| Twice/day | 9 (11.5%) | 3 (3.8%) | 12 (15.4%) | |
| Use toothpaste | ||||
| Yes | 27 (34.6%) | 20 (25.6%) | 47 (60.3%) | 0.165 b |
| Cigarettes/day | ||||
| Mean ± SD | 13.51 ± 5.61 | 15.90 ± 6.13 | 14.71 ± 5.96 | 0.077 a |
| ≤10 | 25 (32.1%) | 16 (20.5%) | 41 (52.6%) | |
| 11–20 | 12 (15.4%) | 20 (25.6%) | 32 (41.0%) | 0.124 b |
| ≥21 | 2 (2.6%) | 3 (3.8%) | 5 (6.4%) | |
| Smoking years | ||||
| Mean ± SD | 8.79 ± 5.56 | 9.54 ± 4.78 | 9.17 ± 5.16 | 0.529 a |
| ≤5 | 19 (24.4%) | 10 (12.8%) | 29 (37.2%) | 0.091 b |
| 6–10 | 10 (12.8%) | 17 (21.8%) | 27 (34.6%) | |
| ˃10 | 10 (12.8%) | 12 (15.4%) | 22 (28.2%) | |
Salivary Parameters at Baseline and Follow-up
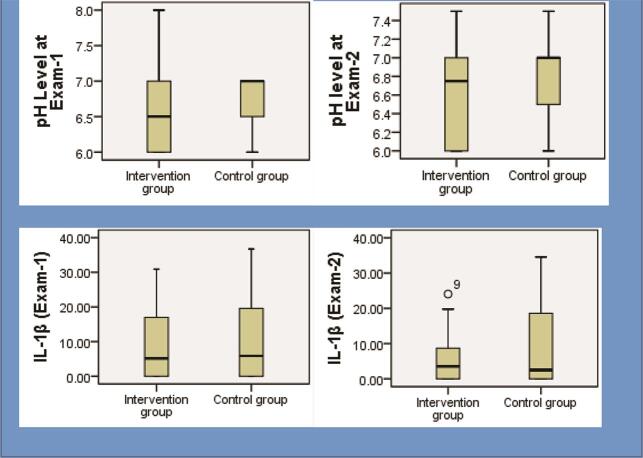
The status of salivary parameters of pH and IL-1β at baseline examination and follow-up examination is explained in Table 3 and Fig. 2 . In the intergroup analysis, there was an insignificant difference in pH (exam 1: p = 0.190; exam 2: p = 0.062) and IL-1β levels (exam 1: p = 0.641; exam 2: p = 0.099) between the two groups before and after the intervention. The IL-1β level was 9.39 ± 10.23 pg/µL at baseline, and 5.40 ± 6.62 pg/µL at follow-up examination in intervention group and the change was significant ( p = 0.001); respective values for control group were 10.63 ± 11.50 and 9.33 ± 11.73 pg/µL and change was nonsignificant ( p = 0.076). The intragroup analysis showed that changes in pH levels were not significant ( p ≥0.071).
Table 3. Salivary parameters (mean ± SD) of study participants before and after intervention.
| Salivary parameters | Intervention group n = 32 |
Control group n = 36 |
p -Value a |
|---|---|---|---|
| Abbreviations: IL, interleukin; SD, standard deviation. a Student’s t -test. | |||
| pH1 (exam-1) | 6.56 ± 0.53 | 6.70 ± 0.36 | t = 9.37, p = 0.190 |
| pH2 (exam-2) | 6.62 ± 0.45 | 6.83 ± 0.44 | t = 0.88, p = 0.062 |
| IL-1β (exam-1) | 9.39 ± 10.23 | 10.63 ± 11.50 | t = 0.91, p = 0.641 |
| IL-1β (exam-2) | 5.40 ± 6.62 | 9.33 ± 11.73 | t = 15.56, p = 0.099 |
Fig. 2.
Changes in salivary parameters before and after intervention. IL-1β and PH levels.
Discussion
Tobacco smoking is one of the leading causes of preventable deaths worldwide. Smoking induces inflammation and consequent immune modulation. Smokers also show a depression in tumor necrosis factor (TNF)-α and IL-8 levels, and IL-1 receptor antagonist and IL-1β levels are also altered. 18 Cigarette smoking affects the oral cavity first, so smoking has many adverse effects on the oral cavity. Studies report that oral cancer is high in smokers, oral precancerous lesions are associated with tobacco use, and there is a negative impact of smoking on periodontal health. Other conditions such as a reduction in smell and taste, hairy tongue, candidiasis, and leukoplakia are also associated with smoking. 19 20
To reduce the harms of continued smoking on general health and oral health, different strategies have been developing to cope with high smoking consumption worldwide. 21 Dentists need to play a vital role in preventing the damaging effects of smoking in the mouth. 22 Analysis of salivary flow rate is reported in scientific literature. 23 Cigarette smoking affects reactive free radicals and volatile aldehydes in saliva 24 and causes a transient decline in the availability of saliva, 25 while another study reports that salivary flow increases during tobacco use. 26 Decreased flow of saliva and buffering capacity (pH levels) is related to a higher risk for dental caries. 27 Salivary buffering by carbonates significantly affects Stephan curve, and salivary activities have a potential impact on a plaque that is the primary cause of oral diseases. 28 Ata-Ali et al showed that levels of IL-6, TNF-α, and IL-1β were statically higher in smokers than nonsmokers and another study reports that IL-1β helps to exaggerate periodontal tissue damage. 29
Tobacco smoking increases the incidence of dental caries, 30 the finding is inconsistent with this study, where smokers had fewer mean caries. Smoking is associated with an increased risk of tooth loss, 31 and tooth loss is reported to occur more frequently among current smokers (40.6%) than former (23.1%) and nonsmokers (27.9%). 32 Tooth loss status of these studies is coherent with that of this study. Current cigarette smokers showed the highest incidence of moderate or severe periodontitis (25.7%), compared with former cigarette smokers (20.2%) and nonsmokers (13.1%) 33 . This finding is slightly coherent with this study where the majority of smokers has mild or moderate periodontitis. Smokers had more severe gingivitis than those who did not smoke 34 that is comparable with this study that showed 58% of patients with gingivitis.
To our best knowledge, the present study might be the first one to observe the effect of SB on IL-1β concentration in smokers. Results of this study showed that levels of IL-1β significantly decreased (9.39 ± 10.23–5.40 ± 6.62 pg/µL) after intervention in the intervention group; however, in control group, the change (10.63 ± 11.50–9.33 ± 11.73 pg/µL) was not significant. This showed that placebo mouthwash was not effective as compared with the mouthwash with the active ingredient. Other studies also revealed a positive association between cigarette smoking and levels of IL-1β in vitro and animal models. 11 33 Although previous literature regarding the effect of cigarette smoking on local and systemic immune responses was mostly inconsistent, with reports of both increased, as well as decreased levels of cytokines. 12 35 Nevertheless, results may reflect an immunosuppressive effect of cigarette smoking on an important cytokine of inflammation, and that is consistent with an overall immune suppressive effect of nicotine. 36
Conclusion
This study demonstrated the importance of addition of SB in mouth wash for the prevention of oral diseases by increasing the salivary pH and decreasing the IL-1β. This study opens a new horizon for conduction of additional studies with large samples to gain more evidence in support of noninvasive oral local interventions.
Acknowledgments
We are grateful to The University of Faisalabad for the permission and help in conducting this study conduction and Sciences the University of Health, Lahore, for analyzing samples in the laboratory. We also acknowledge Pakistan Human Saliva Research Group (PakHSRG), for technical support in sampling procedure and manuscript preparation.
Funding Statement
Funding None.
Footnotes
Authors’ ContributionsConflict of Interest S.A.H. conceptualized this study. The methodology was shaped by A.W., N.A., and S.A.H. M.F.I. and Z.K. validated the facts of this study, and S.A.H., S.A., F.M., and S.A.H.B. provided a formal analysis of the present study. S.A.H. did the investigation. Data curation was done by A.W. and N.A., and the original draft was prepared by S.A.H. and S.A. The review and editing of this article was done by S.A., Z.K., M.F.I., and S.A.H. B. Finally, A.W. and N.A. supervised the entire research work and N.A., A.W., and M.F.I. conducted the project administration.
None declared.
References
- 1.Marsh P D. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8(02):263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 2.Tenovuo J. Salivary parameters of relevance for assessing caries activity in individuals and populations. Community Dent Oral Epidemiol. 1997;25(01):82–86. doi: 10.1111/j.1600-0528.1997.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 3.Khurshid Z, Naseem M, Sheikh Z, Najeeb S, Shahab S, Zafar M S. Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm J. 2016;24(05):515–524. doi: 10.1016/j.jsps.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cogulu D, Sabah E, Kutukculer N, Ozkinay F. Evaluation of the relationship between caries indices and salivary secretory IgA, salivary pH, buffering capacity and flow rate in children with Down’s syndrome. Arch Oral Biol. 2006;51(01):23–28. doi: 10.1016/j.archoralbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Grover N, Sharma J, Sengupta S, Singh S, Singh N, Kaur H. Long-term effect of tobacco on unstimulated salivary pH. J Oral Maxillofac Pathol. 2016;20(01):16–19. doi: 10.4103/0973-029X.180907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foglio-Bonda A, Pattarino F, Foglio-Bonda P L. Kinematic viscosity of unstimulated whole saliva in healthy young adults. Eur Rev Med Pharmacol Sci. 2014;18(20):2988–2994. [PubMed] [Google Scholar]
- 7.Chandel S, Khan M A, Singh N, Agrawal A, Khare V. The effect of sodium bicarbonate oral rinse on salivary pH and oral microflora: a prospective cohort study. Natl J Maxillofac Surg. 2017;8(02):106–109. doi: 10.4103/njms.NJMS_36_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker P M, Hennekens C H, Buring J E, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 9.Gomes S C, Abascal C C, Haas A N, Angst P DM, Oppermann R V, Marcantonio R A. Influence of supragingival biofilm control and smoking habit on Interleukin-1βconcentration. Braz Oral Res. 2015;29(01):S1806–8.32420150001003E16. doi: 10.1590/1807-3107BOR-2015.vol29.0115. [DOI] [PubMed] [Google Scholar]
- 10.Helmersson J, Larsson A, Vessby B, Basu S. Active smoking and a history of smoking are associated with enhanced prostaglandin F(2alpha), interleukin-6 and F2-isoprostane formation in elderly men. Atherosclerosis. 2005;181(01):201–207. doi: 10.1016/j.atherosclerosis.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Liang Q, Frost-Pineda K et al. Relationship between biomarkers of cigarette smoke exposure and biomarkers of inflammation, oxidative stress, and platelet activation in adult cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2011;20(08):1760–1769. doi: 10.1158/1055-9965.EPI-10-0987. [DOI] [PubMed] [Google Scholar]
- 12.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(03):J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Ouchida R, Mori H, Hase K et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci U S A. 2012;109(40):E2699–E2706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khurshid Z, Zohaib S, Najeeb S, Zafar M S, Slowey P D, Almas K. Human saliva collection devices for proteomics: an update. Int J Mol Sci. 2016;17(06):846–854. doi: 10.3390/ijms17060846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahibzada H A, Khurshid Z, Khan R S et al. Salivary IL-8, IL-6 and TNF-αas potential diagnostic biomarkers for oral cancer. Diagnostics (Basel) 2017;7(02):21–26. doi: 10.3390/diagnostics7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurshid Z. Salivary point-of-care technology. Eur J Dent. 2018;12(01):1–2. doi: 10.4103/ejd.ejd_376_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangbulla V, Nirola A, Gupta M, Batra P, Gupta M. Salivary IgA, interleukin-1β and MMP-8 as salivary biomarkers in chronic periodontitis patients. Chin J Dent Res. 2017;20(01):43–51. doi: 10.3290/j.cjdr.a37741. [DOI] [PubMed] [Google Scholar]
- 18.Rivera-Hidalgo F. Smoking and periodontal disease. Periodontol 2000. 2003;32:50–58. doi: 10.1046/j.0906-6713.2003.03205.x. [DOI] [PubMed] [Google Scholar]
- 19.Vellappally S, Fiala Z, Smejkalová J, Jacob V, Somanathan R. Smoking related systemic and oral diseases. Acta Med (Hradec Kralove) 2007;50(03):161–166. [PubMed] [Google Scholar]
- 20.Michaud D S, Fu Z, Shi J, Chung M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39(01):49–58. doi: 10.1093/epirev/mxx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindson-Hawley N, Hartmann-Boyce J, Fanshawe T R, Begh R, Farley A, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev. 2016;10:CD005231. doi: 10.1002/14651858.CD005231.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reibel J. Tobacco and oral diseases. Update on the evidence, with recommendations. Med Princ Pract. 2003;12 01:22–32. doi: 10.1159/000069845. [DOI] [PubMed] [Google Scholar]
- 23.Rehan F, Khan R S, Memon M S, Naqvi S. Analysis of resting mouth salivary flow rate and salivary pH of tobacco chewers and smokers. J Pak Dent Assoc. 2016;25(04):159–163. [Google Scholar]
- 24.Nagler R, Savulescu D, Gavish M. Cigarette smoke-induced reduction in binding of the salivary translocator protein is not mediated by free radicals. Biochimie. 2016;121:1–4. doi: 10.1016/j.biochi.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Aguilar-Zinser V, Irigoyen M E, Rivera G, Maupomé G, Sánchez-Pérez L, Velázquez C. Cigarette smoking and dental caries among professional truck drivers in Mexico. Caries Res. 2008;42(04):255–262. doi: 10.1159/000135670. [DOI] [PubMed] [Google Scholar]
- 26.Bayraktar G, Kazancioglu R, Bozfakioglu S, Ecder T, Yildiz A, Ark E. Stimulated salivary flow rate in chronic hemodialysis patients. Nephron. 2002;91(02):210–214. doi: 10.1159/000058394. [DOI] [PubMed] [Google Scholar]
- 27.Martins Mussi M C, Moffa E, Castro T et al. Salivary parameters and oral health in the Moebius syndrome. Spec Care Dentist. 2016;36(05):265–270. doi: 10.1111/scd.12175. [DOI] [PubMed] [Google Scholar]
- 28.Hay D I. Salivary factors in caries models. Adv Dent Res. 1995;9(03):239–243. doi: 10.1177/08959374950090030801. [DOI] [PubMed] [Google Scholar]
- 29.Ata-Ali J, Flichy-Fernández A J, Alegre-Domingo T, Ata-Ali F, Peñarrocha-Diago M. Impact of heavy smoking on the clinical, microbiological and immunological parameters of patients with dental implants: a prospective cross-sectional study. J Investig Clin Dent. 2016;7(04):401–409. doi: 10.1111/jicd.12176. [DOI] [PubMed] [Google Scholar]
- 30.Hou L T, Liu C M, Liu B Y, Lin S J, Liao C S, Rossomando E F. Interleukin-1beta, clinical parameters and matched cellular-histopathologic changes of biopsied gingival tissue from periodontitis patients. J Periodontal Res. 2003;38(03):247–254. doi: 10.1034/j.1600-0765.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki N, Nakanishi K, Yoneda M, Hirofuji T, Hanioka T. Relationship between salivary stress biomarker levels and cigarette smoking in healthy young adults: an exploratory analysis. Tob Induc Dis. 2016;14:20. doi: 10.1186/s12971-016-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krall E A, Dawson-Hughes B, Garvey A J, Garcia R I. Smoking, smoking cessation, and tooth loss. J Dent Res. 1997;76(10):1653–1659. doi: 10.1177/00220345970760100601. [DOI] [PubMed] [Google Scholar]
- 33.Ojima M, Hanioka T, Tanaka K, Aoyama H. Cigarette smoking and tooth loss experience among young adults: a national record linkage study. BMC Public Health. 2007;7:313. doi: 10.1186/1471-2458-7-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albandar J M, Rams T E. Global epidemiology of periodontal diseases: an overview. Periodontol 2000. 2002;29:7–10. doi: 10.1034/j.1600-0757.2002.290101.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim T H, Kim S J, Lee S M. Stimulation of the 7 nicotinic acetylcholine receptor protects against sepsis by inhibiting toll-like receptor via phosphoinositide 3-kinase activation. J Infect Dis. 2014;209(10):1668–1677. doi: 10.1093/infdis/jit669. [DOI] [PubMed] [Google Scholar]
- 36.Cui W Y, Li M D. Nicotinic modulation of innate immune pathways via α7 nicotinic acetylcholine receptor. J Neuroimmune Pharmacol. 2010;5(04):479–488. doi: 10.1007/s11481-010-9210-2. [DOI] [PubMed] [Google Scholar]