Abstract
Regeneration is a post-embryonic developmental process that ensures complete morphological and functional restoration of lost body parts. The repair phase is a key step for the effectiveness of the subsequent regenerative process: in vertebrates, efficient re-epithelialisation, rapid inflammatory/immune response and post-injury tissue remodelling are fundamental aspects for the success of this phase, their impairment leading to an inhibition or total prevention of regeneration. Among deuterostomes, echinoderms display a unique combination of striking regenerative abilities and diversity of useful experimental models, although still largely unexplored.
Therefore, the brittle star Amphiura filiformis and the starfish Echinaster sepositus were here used to comparatively investigate the main repair phase events after injury as well as the presence and expression of immune system and extracellular matrix (i.e. collagen) molecules using both microscopy and molecular tools.
Our results showed that emergency reaction and re-epithelialisation are similar in both echinoderm models, being faster and more effective than in mammals. Moreover, in comparison to the latter, both echinoderms showed delayed and less abundant collagen deposition at the wound site (absence of fibrosis). The gene expression patterns of molecules related to the immune response, such as Ese-fib-like (starfishes) and Afi-ficolin (brittle stars), were described for the first time during echinoderm regeneration providing promising starting points to investigate the immune system’s role in these regeneration models.
Overall, the similarities in repair events and timing within the echinoderms and the differences with what has been reported in mammals suggests that effective repair processes in echinoderms play an important role for the subsequent ability to regenerate. Targeted molecular and functional analyses will shed light on the evolution of these abilities in the deuterostomian lineage.
Keywords: starfishes, brittle stars, emergency reaction, wound healing, collagen, immune/inflammatory response
1. Introduction
All animals face and heal wounds regardless of their phylogenetic position and the life stage of individuals, though the final result of the restoration process can be remarkably different. The first post-traumatic events and the specific regulation and cross talk of the numerous cytotypes and molecules involved are fundamental to address the final outcome: tissue repair versus tissue regeneration and functional recovery (White et al., 2009). In vertebrates, the main steps of wound repair are re-epithelialisation, inflammatory/immune response, formation of the granulation tissue, and extracellular matrix (ECM) deposition and remodelling (Xue and Jackson, 2015). The impairment of these events, such as the absence/reduction of re-epithelialisation, the misregulation of the inflammatory/immune response and the occurrence of fibrosis, can be correlated with limited regenerative ability. Wound healing via a complete and functional epithelial layer is a critical step to ensure effective repair (Pastar et al., 2014): for example, in mammals impaired epidermal restoration leads to chronic non-healing wounds, causing severe medical problems such as ulcers and absence of tissue regeneration (Sivamani et al., 2007).
Functional repair is achieved also thanks to a highly tuned inflammatory and immune response. The immune system is fundamental during haemostasis and throughout the whole inflammation phase (Park and Barbul, 2004; MacLeod and Mansbridge, 2015). In mammals, several molecules, such as fibrinogen, lectins, ficolins, cytokines (i.e. TNF-α and TGF-β) and interleukins (i.e. IL-1, Il-2, IL-6, IL-8), are key players during the inflammation process and their misregulation as well as local and systemic factors, may affect proper wound healing (Guo and DiPietro, 2006) and subsequent tissue restoration.
The constant and finely regulated remodelling of the ECM components (mainly collagen) is a further key event needed for effective wound healing (Xue and Jackson, 2015). Exaggerated inflammatory response during the first phase of repair can lead to fibro-proliferative disorders (Tredget et al., 1997; Singer and Clark, 1999) which in turn result in excessive deposition of collagen and other ECM molecules (fibrosis) (Ben Amar and Bianca, 2016) and occasionally also in pathological hypertrophic scar or keloid formation. Over-deposition of collagen and its reduced remodelling are known to impair proper healing and regeneration of the damaged tissues (Bock and Mrowietz, 2002; Rahban and Garner, 2003; Diegelmann and Evans, 2004).
It is noteworthy that vertebrates are able to heal minor injuries but most of them possess restricted ability to completely restore lost body parts (Sánchez Alvarado, 2000). Some fishes (Akimenko et al., 2003), amphibian urodeles (Brockes and Kumar, 2002) and reptiles (Bateman and Fleming, 2009) can repair and regenerate after severe or debilitating wounds but the most striking regenerative abilities are still and by far found among the invertebrate clades. Cnidarians (Bosch, 2007), planarians (Saló et al., 2009), annelids (Bely, 2006), and echinoderms (Candia Carnevali, 2006) are the most representative examples. Echinoderms (Arnone et al., 2015) in particular show the maximum extent of regenerative potential among deuterostomes: indeed, they can regenerate body appendages such as arms (Candia Carnevali, 2006), internal organs (Mozzi et al., 2006; Mashanov and García-Arrarás, 2011), and even whole animals from an isolated body fragment (Ducati et al., 2004). Moreover, representatives of all the five extant classes display regenerative capabilities (Hyman, 1955) with clear examples also found in fossils (Oji, 2001), suggesting that these are ancient and widespread features of the phylum. Therefore, echinoderms are promising models to study this phenomenon and, thus, they provide us with a valid comparative perspective with non-regenerating models, humans included.
Arm regeneration is one of the most extensively studied processes in echinoderms (for a review see Candia Carnevali and Bonasoro, 2001; Biressi et al., 2010; Ben Khadra et al., 2017). Regardless of the species, different critical events take place during the first hours/days post amputation, including wound closure, re-epithelialisation and a rapid inflammatory response. As for mammals (Stroncek and Reichert, 2008), tissue remodelling at the wound site is also observed. During sea cucumber gut regeneration tissue remodelling is one of the last phenomena occurring in the repair phase and this was suggested to be directly related to their high efficiency of regeneration (Quiñones et al., 2002; Cabrera-Serrano and García-Arrarás, 2004). Furthermore, immune-related molecules have been described in sea urchins and sea cucumbers (Pancer et al., 1999; Rast et al., 2006; Ramírez-Gómez et al., 2008, 2009, 2010; Ramírez-Gómez and García-Arrarás, 2010; Smith et al., 2010) and their presence/role needs to be comparatively investigated in the repair processes of other echinoderms. This should lead to a deeper understanding of the process and to shed light on evolutionary divergences/similarities within the phylum and with non-regenerating models.
Among the different echinoderm models, starfishes (Asteroidea) and brittle stars (Ophiuroidea) are becoming valid experimental models to study arm regenerative process (Ben Khadra et al., 2017; Biressi et al., 2010; Czarkwiani et al., 2013, 2016). Nevertheless, in both classes, the cellular/tissue and molecular aspects of the repair phase have never been simultaneously and comparatively investigated and with a multidisciplinary approach. Therefore, this research aims to describe and compare the phenomena occurring during the repair phase after traumatic arm amputation using both the brittle star Amphiura filiformis (Ophiuroidea) and the starfish Echinaster sepositus (Asteroidea). Classical histological and ultrastructural methods are employed for the description of the main repair events from a cell/tissue perspective, whereas molecular techniques are used to investigate the involvement of inflammatory/immune responses and the ECM (mainly collagen). Overall, a detailed knowledge on how echinoderms heal severe wounds, and actually regenerate, will possibly shed light on similarities and/or differences with other animals able to regenerate whole lost body parts and, also, with those unable to do it, humans included.
2. Materials and Methods
2.1. Animal collection, maintenance and regeneration tests
Adult (disc diameter ~ 0.5 cm) specimens of Amphiura filiformis were collected at the Sven Lovén Centre for Marine Sciences in Kristineberg (Sweden). Adult (diameter ~ 12 cm) specimens of Echinaster sepositus were collected by SCUBA divers at depth of 5-8 m in the Marine Protected Areas of Portofino (Ligurian Sea, Italy) and of Bergeggi Island (Ligurian Sea, Italy). All experimental animals were left to acclimatise for about one-two weeks and maintained in aerated aquaria of artificial sea water (ASW) (Instant Ocean®) at 14°C and 34‰ salinity (brittle stars) or 18°C and 37‰ salinity (starfishes). Chemical-physical ASW parameters were constantly checked. Animals were fed twice a week with Microvore Microdiet (Brightwell Aquatics; brittle stars) or small pieces of cuttlefish (starfishes). Traumatic arm amputation was performed using a scalpel: for brittle stars a maximum of two arms per animal were amputated at 1 cm from the disc, whereas for starfishes the distal third of one arm was removed. Brittle stars were previously anaesthetised in 3.5% MgCl2 (6H2O) solution (pH 8.3) in a 1:1 mix of filtered ASW and milliQ water. Animals were then left to regenerate in the aquaria for pre-determined periods, namely 24 and 72 hours (h) and 1 week (w) post-amputation (p.a.) for E. sepositus and 8, 16, 24, 48, 72 hours (h) and 5 days (d) p.a. (corresponding to stage 2 of Czarkwiani et al., 2016) for A. filiformis. Brittle star samples at 8d (stage 4) and 2-3 weeks (w) p.a. (>50% DI; Dupont and Thorndyke, 2006; from now on called >50%) were collected and processed as well in order to confirm/complete some in situ hybridisation results (see below and Supplementary Materials). Regenerating arms were collected including part of the stump and differently processed according to the subsequent analyses.
2.2. Microscopy analyses
2.2.1. Light (LM) and transmission electron microscopy (TEM)
For Epon resin embedding regenerating samples were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate (pH about 7.4) with 1.2% (brittle stars) or 1.4% (starfishes) NaCl and washed overnight at 4°C in 0.1 M cacodylate buffer. They were then processed as described by Ben Khadra and co-workers (2015a) with only slight modifications in decalcification step that was performed after osmium tetroxide post-fixation at 4°C for at least 2-3 days using a 1:1 solution (v/v) of 2% L-ascorbic acid and 0.3 M NaCl in distilled water. Semi-thin sections (1 μm) were obtained using a Reichert-Jung Ultracut E with glass knives, stained with crystal violet and basic fuchsin and then observed under a Jenaval light microscope provided with a DeltaPix Invenio 3S 3M CMOS camera and DeltaPix Viewer LE Software or a Zeiss AxioImager M1 microscope equipped with a Zeiss AxioCamHRc camera.
For transmission electron microscopy (TEM) the same samples used for semi-thin sections were used to obtain ultra-thin sections (0.07-0.1 μm) which were collected on copper grids, stained with 1% uranyl acetate followed by lead citrate and finally carbon coated with an EMITECH K400X Carbon Coater. Grids were observed and photographed using a Jeol 100SX, a Zeiss EFTEM Leo912ab or a PHILIPS CM 10 transmission electron microscope.
2.3. Gene expression analyses
Gene expression analysis is of paramount importance to understand the process of wound healing and regeneration; however, little or no protocols have been so far adapted to detect genes expressed during starfish regeneration. To optimise and validate the protocols of ISH on paraffin sections for starfishes, two genes were identified and cloned (see below): an actin gene (Ese-actin) and the transcription factor ets1/2 (Ese-ets1/2). The same genes were selected as positive controls also for WMISH on brittle star samples: Afi-actin was identified and cloned for the first time, whereas Afi-ets1/2 was already available (Czarkwiani et al., 2013). For all the positive controls specific fragments were isolated by PCR and cloned in bacteria vector to transcribe antisense RNA probes, as detailed below and in the Supplementary Materials.
2.3.1. Candidate gene identification
Gene identification in both species was performed looking for markers of the regenerative process with a specific focus on those involved in the collagen deposition regulation and the immune/inflammatory response during the repair phase. Since it was not always possible to clone the candidate genes in both species, we will show the data of different markers (see below).
2.3.1.1. Candidate gene identification in E. sepositus
The identified gene of interest was the collagen biosynthesis enzyme prolyl-4-hydroxylase (p4h). Due to the absence of any transcriptome for this species, degenerate primers (see Table S2) were manually designed on protein multialignment built on sequences retrieved from EchinoBase for Strongylocentrotus purpuratus and Patiria miniata genomes, and EchinoDB (http://echinodb.uncc.edu/) and National Center for Biotechnology Information (NCBI) databases. After cloning a specific fragment by PCR using these primers, Ese-p4h sequence was checked performing a Basic Local Alignment Search Tool (BLAST) against the NCBI non-redundant database (https://blast.ncbi.nlm.nih.gov/Blast.cgi), identifying as best BLAST hit the alpha-1 subunit of the Atlantic herring (Clupea harengus, XP_012689665.1; Table S1). Furthermore, the conserved domain architecture retrieval tool (cDART, NCBI) showed the 2OG-Fe(II) oxygenase superfamily domain is encoded on the Ese-p4h isolated fragment. This domain is characteristic of P4H therefore confirming it was the desired collagen biosynthesis enzyme.
Degenerate primers from Zhang and Cohn (2006) for vertebrate collagen were tested as well (see Table S2). Ese-fibrinogen-like (Ese-fib-like) is a gene belonging to the fibrinogen-related (FReD) domain superfamily. Using the cDART tool (NCBI) the presence of a FReD domain was confirmed. This is usually present in fibrinogen, a glycoprotein that helps in the formation of blood clotting in vertebrates forming bridges between platelets and being the precursor of fibrin.
As previously mentioned, actin 1 and ets1/2 were selected as positive controls: specific primers were designed based on the nucleotide sequence of actin 1 (NCBI accession number: KC858258.1, GI: 525327359; see Supplementary Materials), whereas degenerate primers already available in the laboratory were used to clone ets1/2 (see Table S2). For actin 1, since the expected product length was shorter than 300 bp, 3’RACE was performed using a mixed cDNA samples from regenerate stages with the FirstChoice® RLM-RACE Kit (Ambion) according to manufacturer’s instructions (see Supplementary Materials and Table S3). We cloned a longer fragment that was used to obtain a longer RNA antisense probe for in situ hybridisation (see below). Table S1 summarises the best BLAST hits of the identified genes in EchinoBase (SPU best BLAST) and in NCBI (NCBI best BLAST).
2.3.1.2. Candidate gene identification in A. filiformis
Genes of interest were identified from EchinoBase (http://www.echinobase.org), starting with a targeted gene search in Strongylocentrotus purpuratus database (http://www.echinobase.org/Echinobase/) using Gene Name or Gene Synonym as searching words. BLAST-X analyses were performed over the Afi transcriptome (Dylus et al., 2017) in order to obtain the corresponding gene sequences in A. filiformis. The genes of interest were Afi-p4h and Afi-ficolin, whereas actin (Afi-actin) was used as positive control (see Supplementary Materials).
The Afi-p4h (AfiCDS.id43946.tr460) similarly identified as best BLAST hit in the sea urchin genome (EchinoBase; http://www.echinobase.org/Echinobase/) the prolyl-4-hydroxylase alpha-1 subunit precursor (SPU_027669), whereas in the NCBI non-redundant database the Atlantic herring prolyl-4-hydroxylase subunit alpha-1 (Clupea harengus, XP_012689665.1). The cDART tool confirmed the presence of a prolyl-4-hydroxylase alpha subunit domain. Therefore, this transcript was considered as prolyl-4-hydroxylase (p4h).
The Afi-ficolin gene (AfiCDS.id39565.tr647) was isolated from an A. filiformis cDNA pool. The clone sequence was analysed using BLAST-X against the sea urchin genome (S. purpuratus; EchinoBase) and NCBI non-redundant database and confirmed to belong to the FReD superfamily and to be a closely related gene to the sea urchin Sp-Fic1 (SPU_000045). Table S1 summarises the best BLAST hits of the identified genes in EchinoBase (SPU best BLAST) and in NCBI (NCBI best BLAST) with corresponding scores and E-values.
2.3.2. Primer design
Different design strategies were followed depending on the gene of interest and sequence availability. For specific primers in both species PRIMER3 Software version 0.4.0 (http://primer3.ut.ee/) was used, optimising the following parameters: max 3’ stability was set at 8.0 and max polyX at 3. For brittle stars their specificity was checked performing a BLAST to the A. filiformis developmental transcriptome (Dylus et al., 2017). Degenerate primers were manually designed as described above. Tables S2 and S3 summarises all E. sepositus and A. filiformis primers.
2.3.3. RNA extraction, cDNA synthesis, gene cloning and antisense probe transcription
For A. filiformis, RNA was extracted, genes were cloned and antisense probes were prepared as described by Czarkwiani and co-workers (2013). RNA of E. sepositus was extracted at the different regenerating stages (24 hours, 72 hours and one week p.a.) from 5 specimens per stage with the RiboPure Kit (Ambion) following manufacturer’s instructions. cDNA synthesis was performed using the RETROscript kit (Ambion) following manufacturer’s instructions and using 1 μg of total RNA. A pool of cDNA was prepared and used to perform subsequent PCRs. The amplification reaction protocol using Invitrogen reagents (Taq DNA Polymerase (Invitrogen) or Q5 High-Fidelity DNA Polymerase (New England BioLabs)) was optimised for each gene of interest (see Supplementary Materials). Moreover, when necessary 3’RACE was performed (see Supplementary Materials). All PCR products were subsequently ligated into pGEM®-T Easy Vector System I (Promega) and transformed in Subcloning Efficiency Invitrogen DH5α (Life Technologies) or Top 10 Competent Cells E. coli (Fisher Scientific) according to manufacturer’s instructions. The presence of the correct fragment was checked by sequencing (Source BioScience). RNA antisense digoxigenin (DIG) labelled probes were transcribed in vitro using the Sp6/T7 Transcription Kit (Roche) and the DIG RNA labelling Mix (Roche) following manufacturer’s guidelines.
2.3.4. Whole mount in situ hybridisation (WMISH) on A. filiformis
Brittle star in situ hybridisations were performed in whole mount and then samples were embedded in paraffin wax and sectioned for detailed analysis. A. filiformis regenerating samples were fixed in 4% PFA in 1X PBS with 0.1% Tween-20 (PBST) overnight at 4°C and stored in 100% methanol at -20°C until use.
Chromogenic WMISH was performed with antisense probes as previously described along with positive and negative controls (Czarkwiani et al., 2013) with the following modifications: hybridisation temperature was raised to 50-55°C depending on the probe length and all washes were conducted in 1X MABT (0.1 M maleic acid pH 7.5, 0.15 M NaCl, 0.1% Tween-20). Samples were stored in 50% glycerol at 4°C and subsequently observed under a Zeiss AxioImager M1 microscope equipped with a Zeiss AxioCam HRc camera.
After imaging, WMISH samples were embedded in paraffin wax and sectioned in order to better understand the tissue-specific expression patterns. Briefly, samples stored in 50% glycerol were washed in 1x PBS or 1x MABT at room temperature (RT) and decalcified for 1-2 days in 0.5 M EDTA in 1x PBS (pH 8) or in 1:1 solution (v/v) of 2% L-ascorbic acid and 0.3 M NaCl in distilled water at 4°C. After washes in 1x PBS or 1x MABT, they were post-fixed in 4% PFA in 1x PBS or 2% glutaraldehyde in 1x MABT at RT, washed twice in 1x PBS or 1x MABT, de-hydrated in an increasing scale of ethanol, cleared in xylene and embedded in paraffin wax following classical procedures. Samples were then sectioned (10 μm thickness) and sections were de-waxed in xylene, mounted with Eukitt® and observed under a Jenaval light microscope provided with a DeltaPix Invenio 3S 3M Pixel CMOS camera and DeltaPix ViewerLE Software.
2.3.5. In situ hybridisation (ISH) on E. sepositus sections
Because of the limited number of starfish regenerating arm samples, their large size (around 1 cm) and the bright orange pigmentation typical of this species, an ISH on paraffin wax sections was optimised. Samples were fixed in 4% PFA in 0.1 M MOPS (pH 7) and 0.5 M NaCl for at least one week at 4°C or in 4% PFA in PBST, decalcified in Morse’s solution (10% sodium citrate and 20% formic acid in DEPC-treated water) overnight at 4°C and embedded in paraffin wax as described by Ben Khadra and co-workers (2015a). Samples were sectioned at 10 μm thickness using a Leica RM2155 microtome. Since no ISH technique is reported in the literature for E. sepositus paraffin sections, two different protocols were tested and optimised, giving us comparable results. In parallel, negative controls were run performing the hybridisation without probes in order to check potential anti-DIG antibody cross-reactivity. ISH protocols are detailed in the Supplementary Materials. After ISH, sections were imaged under a Zeiss AxioImager M1 microscope equipped with a Zeiss AxioCamHRc camera.
3. Results
A brief description of the gross morphology of starfish and brittle star arms is re-called in the Supplementary Materials to facilitate the understanding of the subsequent results (Fig. S1). Since the epidermis plays a key role during the repair phase (see below) and no data is currently available for Amphiura filiformis, a new ultrastructural description of the non-regenerating epidermis is here briefly provided. For the description of the non-regenerating epidermis of Echinaster sepositus see Ben Khadra and co-workers (2015a).
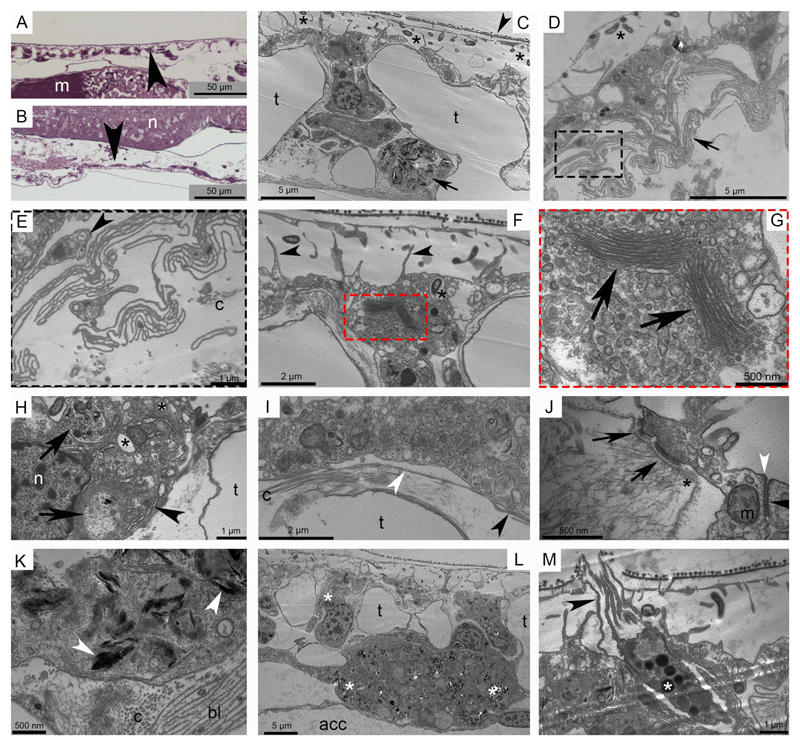
In A. filiformis, the aboral and oral epidermis lines the trabeculae of the skeletal shields (Fig. 1A, B, C). This epithelium is composed of an external cuticle, the epidermal cells and the underlying basal lamina (Fig. 1C, D, F). The epidermal cells and the subcuticular space house numerous bacteria (Fig. 1C, D, F). A sub-epithelial nerve plexus is occasionally detectable underneath the basal lamina (Fig. 1E). The cuboidal epidermal cells present different organelles and inclusions (Fig. 1F, G, H) and are connected to each other by apical junctional complexes and to the underlying basal lamina (Fig. 1I) and the dermal layer by hemidesmosomes (Fig. 1J). Secretory cells (granulated cells) are observable (Fig. 1M) all scattered within the epidermis. Presumptive pigment cells (or chromatophores) containing spindle-like electron-dense structures are visible in the dermal layer (Fig. 1C, K, L). These structures, whose specific nature is still unknown, are sometimes present also in the epidermal cells (Fig. 1L).
Fig. 1. Ultrastructure of the brittle star stump (non-regenerating) epidermis.
Light microscopy (LM) and transmission electron microscopy (TEM). A) Semi-thin sagittal section of the aboral epidermis (arrowhead). B) Semi-thin sagittal section of the oral epidermis (arrowhead). C) The aboral epidermis shows the cuboid epidermal cells nested in the skeletal trabeculae and covered by a well-defined cuticle (arrowhead). The subcuticular space hosts numerous bacteria (asterisks) and beneath the epidermis a presumptive pigment cell is visible (arrow). D) In the oral epidermis bacteria are visible in the subcuticular space (asterisk) and the pleats and folds of the basal lamina (arrow) are present immediately beneath the epidermal cells. E) Detail of Fig. D showing the pleats and folds of the basal lamina and the presence of scattered nervous processes (arrowhead). F) The epidermal cells show microvilli branching in the subcuticular space (arrowheads) and a bacterium inside the cell and surrounded by a membrane (asterisk). G) Detail of Fig. F showing the abundant apical Golgi apparatus (arrows). H) Inclusions of different types (arrows), electron-lucent vesicles (asterisks) and abundant RER (arrowhead) are visible in the epidermal cells. I) The basal lamina shows both thin (white arrowhead) and thick (black arrowhead) structure. Thin collagen fibrils are present immediately underneath. J) In the apical portion of the epidermis the apical zonulae (white arrowhead) and subjacent septate junction (black arrowhead) are visible between two adjacent epidermal cells. Hemidesmosomes (arrows) are connecting the epidermal cells with the underlying basal lamina (asterisk) to maintain epidermis integrity. K) In the presumptive pigment cells the spindle-like electron-dense structures (arrowheads) are present both surrounded or not by a thin membrane. L) The aboral epidermis shows a big presumptive pigment cell underneath the epidermis. Spindle-shaped electron-dense structures (asterisks) are spread in the cytoplasm and are present in lower amount also in some epidermal cells. M) A presumptive secretory cell is scattered among epidermal cells showing long microvilli in the subcuticular space apically breaking the cuticle (arrowhead) and compact electron-dense material packed in roundish membrane-bound vesicles (asterisk) in the cytoplasm. Junction complexes connect this cells to the adjacent epidermal cells. Abbreviations and symbols: acc - aboral coelomic cavity; bl - basal lamina; c - collagen fibril; m in A - muscle; m in J - mitochondrion; n in B - radial nerve cord; n in H - nucleus; t - trabecula; asterisk in C, D, F - bacteria; asterisk in J - basal lamina; asterisk in H - electron-lucent vesicle; asterisk in L - spindle-shaped electron-dense structure; asterisk in M - electron-dense granule.
3.1. Microscopic anatomy of the repair phase
Ben Khadra and co-workers (2015a) provided a general overview of the main events of E. sepositus repair phase after traumatic arm amputation. Some key concepts are re-called in Fig. S2 (Supplementary Materials) in order to make more immediate the comparison with the repair events of A. filiformis reported below.
3.1.1. Wound closure
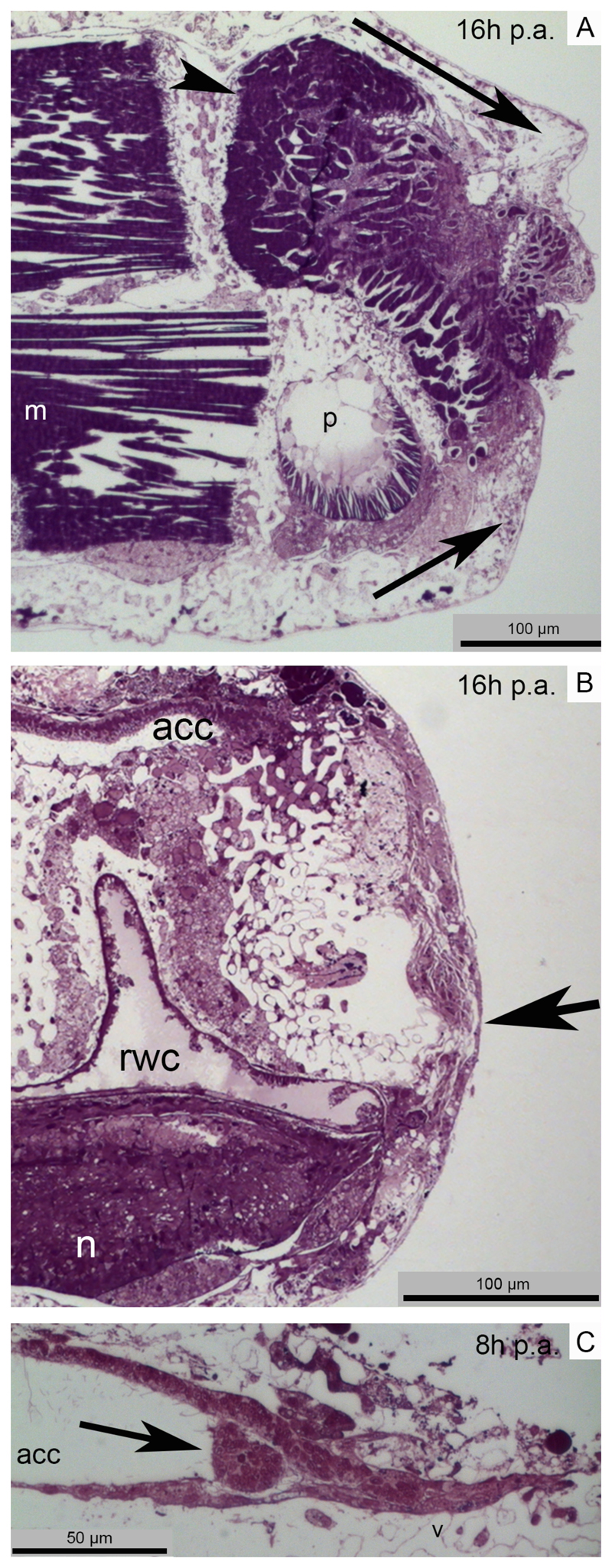
As for starfishes, within few hours p.a. brittle stars respond to injury by limiting coelomic fluid loss and microorganism entrance. However, contrary to the former (Fig. S2A), brittle stars do not form a haemostatic ring but seal the coelomic cavities and vessels (i.e. the aboral coelomic cavity and the radial water canal) by bending the first aboral and oral shields proximal to the amputation plane (Fig. 2A). Clotting phenomena of circulating cells (mainly coelomocytes) are immediately visible in the coelomic cavity close to the wound site (Fig. 2C) together with the first signs of histolysis and remodelling of injured tissues (mainly muscle bundles) (Fig. 2A).
Fig. 2. Main events of A. filiformis repair phase.
Light microscopy (LM). A) Semi-thin parasagittal section showing the downward and upward movements of the aboral shield and of the oral shield respectively (arrows) to help wound closure. The intervertebral muscles involved in the amputation already show rearrangement phenomena (arrowhead). B) Semi-thin sagittal section where the new epithelium covers the whole wound surface (arrow) and the main body cavities (aboral coelomic cavity and radial water canal) are already sealed. C) Semi-thin sagittal section showing that cells (possibly coelomocytes) are clotting in the aboral coelomic cavity lumen in order to seal it and avoid loss of fluid (arrow). Abbreviations and symbols: acc - aboral coelomic cavity; m - muscle; n - radial nerve cord; p - podium; rwc - radial water canal.
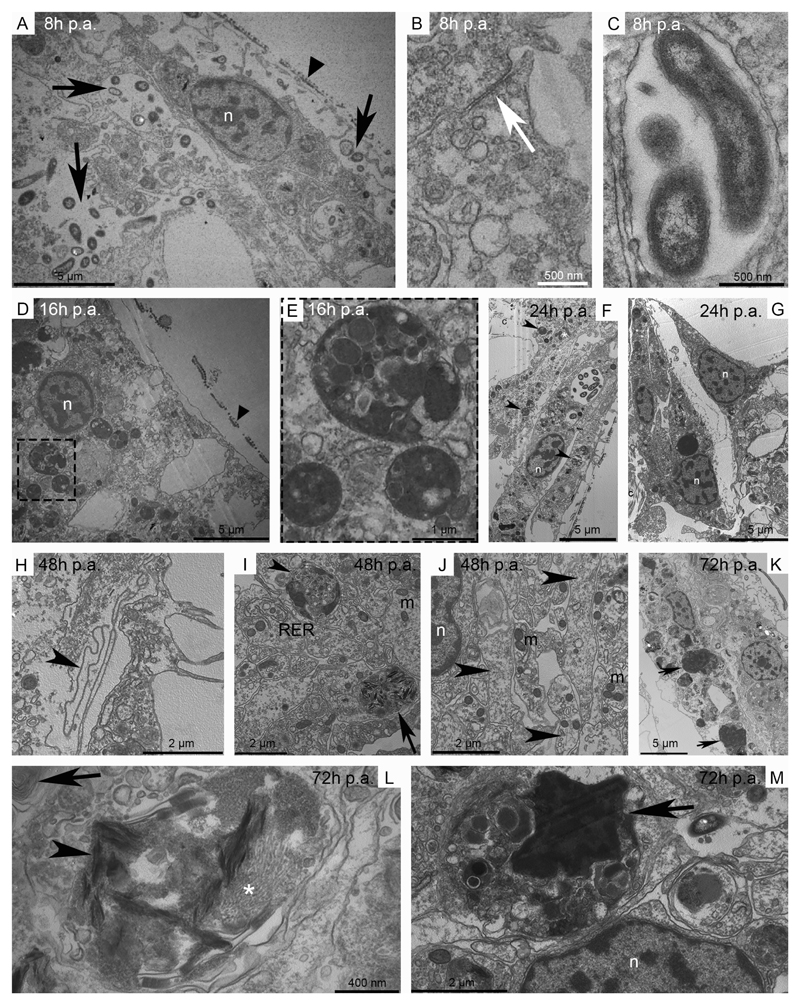
Simultaneously to the first emergency responses, and in agreement with absence of cell proliferation in the first 48 hours p.a. (Czarkwiani et al., 2016), in brittle stars healing of the injury begins with migration of stump epidermal cells. An almost complete wound epidermis, provided with microvilli and cuticle, is visible within 8 hours p.a. (Fig. 2B, 3A). It is composed by a monolayer of slightly elongated epidermal cells characterised by big oval/roundish and patched nucleus and the presence of junctional complexes in their apical portion (Fig. 3B, D). Analysis of serial sections of samples at different regenerative stages suggests that, similarly to starfishes (Fig. S2B), the new epidermis migrates centripetally over the wound. The basal membrane becomes visible only at the middle/late repair phase (after 48-72 hours p.a.), initially as a collection of fragmented pleats and folds rather than a continuous and well-defined layer (Fig. 3H). Increasing number of bacteria are present in the subcuticular layer as well as deep in the wound area at all stages: they are widely spread in the intercellular spaces as well as inside vesicles of the epidermal cells and underlying phagocytes (Fig. 3A, C, F, K). While re-epithelialisation occurs, a layer of different cytotypes (i.e. phagocytes and presumptive pigment cells) forms beneath the new epidermis starting at 8 hours p.a. and being visible till 72 hours p.a. (Fig. 2B, 3). During this period, cells of this layer (and of the epidermis) present several cytoplasmic inclusions, such as heterogeneous phagosomes, spindle-shaped electron-dense structures, myelin figures and several types of both electron-lucent and electron-dense inclusions/vesicles (Fig. 3D-G, I-M); these inclusions, together with numerous mitochondria and well-developed rough endoplasmic reticulum (RER), suggest an intense phagocytic and tissue remodelling activity. Junctional complexes do apparently not connect cells which create a thick and compact layer (but not a syncytium) resembling, in position and function, the phagocyte syncytium and the granulation tissue-like observable in starfishes (Fig. S2E; Ben Khadra et al., 2017). Besides the removal of cell debris, this cell layer provides support for the migration of the overlying epidermal cells and acts as cell barrier between the stump tissues and the wound area (Fig. 3G). Numerous nervous processes become visible, scattered among this layer, during the middle/late repair phase (48 hours p.a.; Fig. 3J).
Fig. 3. Main events of the A. filiformis repair phase.
Transmission electron microscopy (TEM). A) The new epithelium presents cells with an oval/roundish nucleus and well-defined nucleolus. The cuticle is already observable (arrowhead) and numerous bacteria (arrows) are present both underneath the epithelium and in the subcuticular space. B) Detail of an apical junction complex (arrow) between adjacent cells of the new epithelium. C) Detail of bacteria enveloped by a thin membrane. D) New epithelial cells show a well-defined cuticle (arrowhead) and patchy nuclei; several phagosomes are detectable. E) Detail of D on phagosomes. F) The new epidermis presents elongated epidermal cells and a well-defined cuticle. Numerous phagosomes (arrowheads) and mitochondria (asterisk) are visible in both epidermal cells and in the underneath thick layer of cells. G) Different cytotypes are present beneath the new epidermis and create a layer dividing the rearranging/regenerating area from the stump extracellular matrix mainly composed of collagen fibrils. H) The new basal lamina (arrowhead) is visible as pleats and folds beneath the epidermal cells. I) Different cytotypes are observable underneath the new epidermis: cells do not form a syncytium and present abundant RER, phagosomes (arrowhead), spindle-shaped electron-dense structures (arrow) and numerous mitochondria. J) Numerous nervous processes (arrowheads) with mitochondria are visible scattered among the different cytotypes. K) In the regenerating area new epidermal cells present a flat-cubic shape and the rearranging contractile apparatus of several myocytes (arrows) is phagocytised by cells underneath the new epidermis. L) In the rearranging/regenerating area spindle-shaped electron-dense structures (arrowhead) are visible together with myofilaments (asterisk). Myelin figures are present as well (arrow). M) The rearranging contractile apparatus of a myocyte (arrow) inside the phagosome of a cell underneath the new epidermis. Abbreviations and symbols: c - collagen; m - mitochondrion; n - nucleus; RER - rough endoplasmic reticulum; asterisk in F - mitochondria; asterisk in L - myosin filaments.
3.1.2. Collagen appearance
Only after re-epithelialisation and the main remodelling/phagocytosis events are finished, the new extracellular matrix (ECM) is deposited.
In starfishes a sparse micro-fibrillar collagenous material is observed from 72 hours p.a. in the oedematous (granulation tissue-like) area (Fig. S2F; Ben Khadra et al., 2017), whereas small bundles of collagen fibrils appear only at the end of the repair phase (one week p.a.; Fig. S2G; Ben Khadra et al., 2017).
In brittle stars a comparable oedematous area is never detected. A thin collagenous layer becomes visible below the epidermis starting at 2-3 days p.a. (middle/late repair phase) (Czarkwiani et al., 2016). TEM analyses indicate the absence of organised collagen fibrils till the middle/late repair phase.
3.2. Gene expression in the repair phase
Molecular techniques on adult echinoderms are still not widely established with the expression patterns of the genes here presented being described for the first time. The methods here used are essentially new for starfishes and they provide a new perspective to the study of echinoderm regeneration. Positive and negative controls were performed in both species in order to validate in situ hybridisation results. The description of the selected controls and their expression patterns are detailed in the Supplementary Materials (Fig. S5, S6, S7, S8). Here, it is important to stress that the localised expression patterns of the positive controls showed the effectiveness of the techniques in both model systems. Therefore, the analyses of some genes relevant for the repair phase were performed, as detailed below.
3.2.1. Immune/inflammatory response-related genes
The precise regulation of the immune response after injury is a critical factor. Therefore, the expression patterns of two relevant genes, known to be involved in human wound healing (Zuliani-Alvarez and Midwood, 2015), were here investigated: a fibrinogen-like (Ese-fib-like) for starfishes and a ficolin (Afi-ficolin) for brittle stars (Fig. 4). Both proteins contain a fibrinogen-related domain.
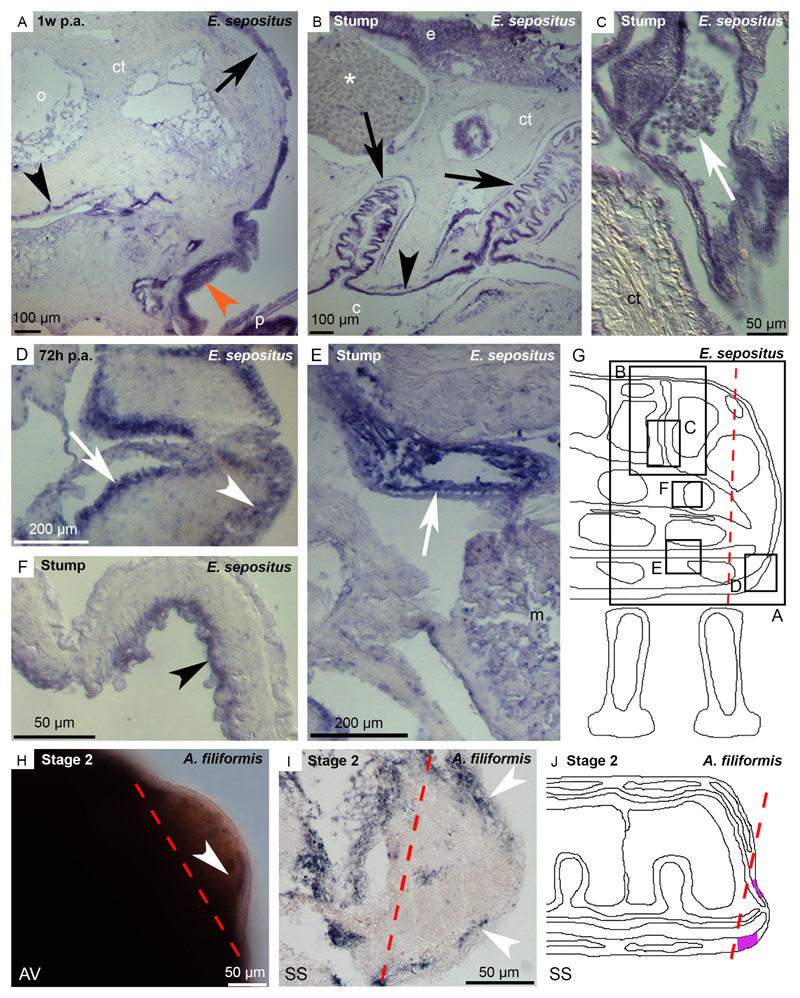
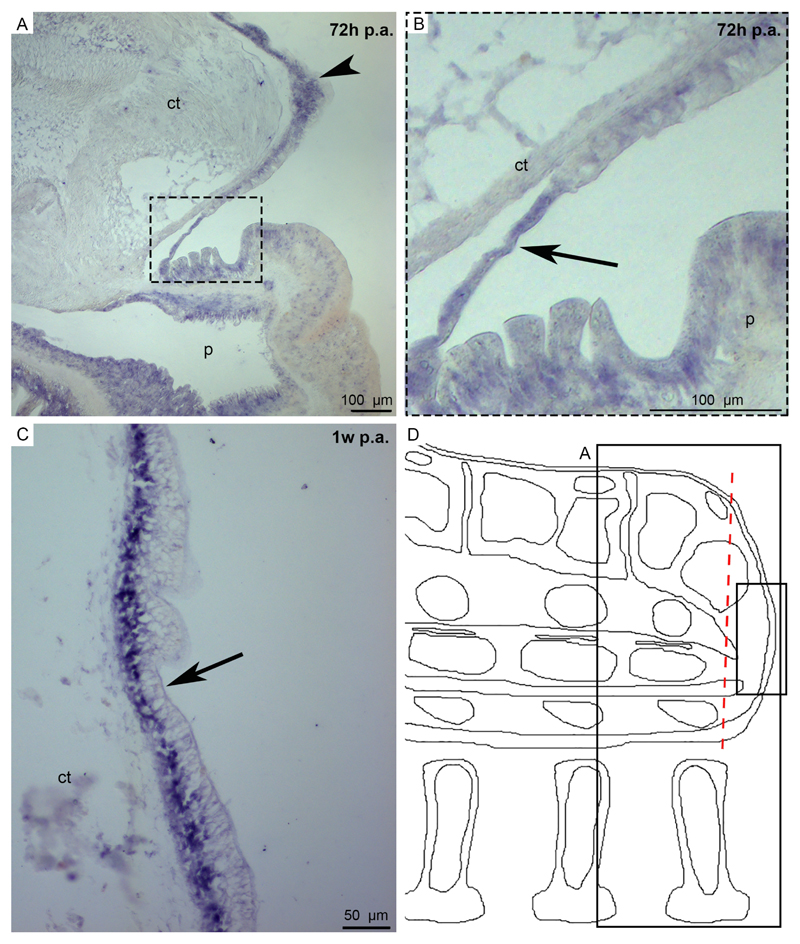
Fig. 4. Expression pattern of Ese-fib-like on E. sepositus regenerating arms (A-G) and of Afi-ficolin on A. filiformis regenerating arms (H-J).
A) Ese-fib-like is expressed in the new epidermis (orange arrowhead), in the circular coelomic muscles (black arrowhead) and in the epidermis of the stump (arrow). B) In the stump expression is detectable in the coelomic epithelium (arrowhead), in the coelomic lining of the papulae (arrows) but no signal is present in the mucous gland (asterisk). C) Cells in the papulae (possibly coelomocytes) are stained (arrow). D) The regenerating radial nerve cord is stained in both ectoneural (arrowhead) and hyponeural (arrow) systems. E) Ese-fib-like is expressed at the level of the radial water canal epithelium (arrow) of the stump. F) The inner lining of the stump ampullae (arrowhead) expresses this transcript. G) Sagittal section scheme where black boxes indicate corresponding images of this figure to facilitate the understanding of the expression pattern location. H) WMISH sample showing that Afi-ficolin is expressed in the dermal layer below the epidermis (arrowhead). I) Post in situ paraffin section showing the expression of Afi-ficolin in the dermal layer of the regenerative bud (arrowheads). J) Sagittal section scheme showing Afi-ficolin expression pattern in the regenerative bud. Signal is highlighted in violet. Red dotted lines: amputation plane. Abbreviations and symbols: AV - aboral view; c - coelom; ct - connective tissue; e - epidermis; m - muscle; o - ossicle; p - podium; SS - sagittal section; asterisk - mucous gland.
Fibrinogen is the precursor of fibrin, important for coagulation and granulation tissue formation after wound in vertebrates (Laurens et al., 2006: Drew et al., 2001). A fibrinogen-like gene, belonging to the FReD superfamily, was isolated in starfishes (see Table S1). The FReD domain was confirmed also using the cDART tool (NCBI). ISH of Ese-fib-like shows a distinct staining in the new epithelium covering the wound area at one week p.a. (Fig. 4A) and in the regenerating radial nerve cord in the ectoneural and hyponeural systems at 72 hours p.a. (Fig. 4D). In the stump area, Ese-fib-like expression is localised in the epidermis (Fig. 4A, B), in the coelomic epithelium lining the perivisceral cavity (Fig. 4B), the papulae (Fig. 4B, C), the radial water canal (Fig. 4E) and the ampullae (Fig. 4F). Free-circulating coelomocytes express also this gene (Fig. 4C) as well as the circular coelomic muscles (Fig. 4A).
Ficolins are considered part of the echinoderm immune gene repertoire (Hibino et al., 2006) as they encode for proteins that are involved in different aspects of innate immunity (Matsushita et al., 2001). A ficolin gene, belonging to the FReD superfamily, was isolated in A. filiformis (see Table S1). Afi-ficolin is expressed in the dermal lining of the epidermis in the regenerative bud at the end of the repair phase (stage 2; Fig. 4H-J). In the stump tissues this transcript is localised in the radial water canal epithelium (Fig. S3).
3.2.2. Collagen biosynthesis enzyme gene
Collagen is a key protein of the repair phase and its biosynthesis necessarily needs to be finely regulated. For this reason, the biosynthetic enzyme prolyl-4-hydroxylase (p4h; Myllyharju, 2003) was here investigated. The genes of the alpha-1-subunit of p4h were identified in both experimental models and their expression patterns analysed during the repair phase.
In starfish regenerating tissues Ese-p4h expression is detected in the new epidermis at both 72 hours and one week p.a. (Fig. 5). The signal in the stump tissues is further described in the Supplementary Materials (Fig. S4A-C) and suggests that other epithelial tissues, such as the coelomic lining and the radial nerve cord, may have a role in collagen biosynthesis. In brittle stars, besides the stump tissues (Fig. S4D), in the regenerative bud Afi-p4h is expressed in the coelomic lining but only after the repair phase is finished (Fig. S4E-I).
Fig. 5. Expression pattern of Ese-p4h on E. sepositus regenerating arms.
A) In a 72 hours p.a. sample Ese-p4h is expressed in the regenerating epidermis (dotted square) and in the epidermis of the stump (arrowhead). B) Detail of A on the signal in the regenerating epidermis (arrow). C) The new epidermis at one week p.a. shows a signal (arrow). D) Sagittal section scheme where black boxes indicate corresponding images of this figure to facilitate the understanding of the expression pattern location. Red dotted line: amputation plane. Abbreviations: ct - connective tissue; m - muscle; p - podium.
4. Discussion
In this article we present data on the first events of the regenerative processes in two classes of echinoderms, the Ophiuroidea and the Asteroidea. A comparative approach, with the introduction, for the first time, of molecular and histological analyses, is used, providing us with a new vantage point to understand the high regenerative potential of these systems. The information gathered on the different repair events is discussed below.
4.1. Wound closure
After arm amputation a series of emergency reactions are immediately activated to prevent the loss of body fluids and decrease the wound exposed surface. Differently from E. sepositus (Ben Khadra et al., 2015a; Ben Khadra et al., 2017) and from starfishes in general (Mladenov et al., 1989; Candia Carnevali et al., 1993; Moss et al., 1998), in brittle stars no evident circular constriction of the arm-tip is detectable. This is consistent with the different brittle star arm anatomy (i.e. conspicuous skeletal elements and the absence of a circular muscle layer surrounding the coelomic cavity). Here the apical contraction of the body wall is sufficient for sealing the narrow fluid-filled vessels/cavities (aboral coelomic cavity and radial water canal). In comparison, blood vessel constriction and wound contraction are fundamental events also in mammal wound healing (Pastar et al., 2014; Ibrahim et al., 2015) but while the former is an almost immediate reaction, the latter is delayed comparing to the events happening in both echinoderm models. In humans, skin wound shrinkage slowly starts almost immediately after injury but its main peak of activity occurs around 10 days after the damage (Shultz et al., 2005), different from echinoderms, where it is visible within 1-2 days p.a. (Fig. 2A, C). The delay observed in mammals might be due to the “time consuming” activation of fibroblasts resident in the injury’s neighbourhood which have to leave their quiescent state, migrate towards the wound and be transformed into myofibroblasts, the ultimate responsible of wound contraction (Martin, 1997).
Besides constriction, in both echinoderm species, loss of fluid prevention is also mediated by rapid clotting of circulating cells (coelomocytes), a phenomenon analogous to mammalian platelet clot formation (Peacock, 1984; Clark, 1988; Ibrahim et al., 2015). Noteworthy, in starfishes, coelomocytes displaying platelet-like ultrastructure and function are present (personal observations).
Delays or defects in re-epithelialisation can prevent functional wound healing and regeneration (Sivamani et al., 2007). In both A. filiformis and E. sepositus this step is very rapid though in the former it is accomplished earlier (8-16 hours p.a. versus 48-72 hours p.a.), most likely a consequence of the smaller arm size. In mammals, skin re-epithelialisation is accomplished later (around 4 days; Pastar et al., 2014). Noteworthy, in both echinoderm models the new epithelium is formed by elongation of stump epithelial cells present in the adjacent wound edges, without any initial contribution of local proliferation: the onset of cell cycle activity, indeed, occurs far after re-epithelialisation is accomplished (Mladenov et al., 1989; Czarkwiani et al., 2016). Similarly to starfishes (Ben Khadra et al., 2015a), in A. filiformis regenerating epidermal cells retain their junctional complexes. This common feature of echinoderms markedly distinguishes them from mammals where cell-cell junction disruption is a pre-requisite for migration of keratinocytes over the wound area (Pastar et al., 2014). In both echinoderms and mammals (Clark et al., 1982; Larjava et al., 1993) a well-defined basal lamina is not detectable until after the complete differentiation of epidermal cells, which facilitates their migratory movements.
The events occurring after re-epithelialisation slightly differ in the histological organisation between the two echinoderm models. Indeed, the wound area of starfish arm is characterised by the presence of a temporary (3-7 days p.a.) oedematous area (Ben Khadra et al., 2015a), not detectable in brittle stars. This area has the aspect of the mammalian granulation tissue and it is characterised by the presence of sparse inflammatory cytotypes (mainly coelomocytes/phagocytes) which can be considered the functional and ultrastructural analogous of monocytes/macrophages (Ryter, 1985; Martin, 1997; Pastar et al., 2014). In the outermost part, phagocytes form a continuous syncytial layer underlying the wound epithelium (Ben Khadra et al., 2015a). In brittle stars, a proper oedematous area is lacking, although the compact and persistent phagocyte layer underlying the wound epidermis can be considered, functionally and cytologically, comparable. However, in the latter model cells are separated and never form a syncytium. In both echinoderms the wound is therefore covered by an active and temporary “cellular scar” (i.e. a scar mainly composed by cells rather than fibrous matrix), which protects and isolates the delicate underling wound tissues from external insults and pathogens. As for the granulation tissue of mammals, this “tissue” progressively matures in the subsequent days: new cytotypes appear, including nerve elements and presumptive pigment cells, while the ECM is reorganised (see below).
4.2. Immune/inflammatory responses
It is well known that the immune system plays a crucial role during the inflammation phase occurring after injury (Park and Barbul, 2004; MacLeod and Mansbridge, 2015). Two inflammatory/immune response-related genes of echinoderms were here identified: Ese-fib-like (starfishes) and Afi-ficolin (brittle stars).
Ese-fib-like is a fibrinogen-related (FReD) domain-containing gene. This domain is typical of fibrinogen, the precursor of fibrin in vertebrates. During wound healing fibrin acts as network-forming molecule fundamental for blood coagulation (Laurens et al., 2006) and also for granulation tissue formation and cell migration (Drew et al., 2001). The presence of fibrinogen-like proteins in echinoderms has been described only by Xu and Doolittle (1990) in the sea cucumber Parastichopus parvimensis though no expression data is available. The signal detected in the new epidermis and in both the regenerating and the stump coelomic epithelium suggests that these tissues could be involved in the production of fibrinogen-like proteins during the repair phase. Interestingly, the coelomic epithelium is considered one of the “hematopoietic” tissues of echinoderms (Holm et al., 2008), responsible of coelomocytes production, the cells that are involved in clot formation after wound production (see above). However, no clear ultrastructural evidences of fibrin-like network around coelomocyte clot was found. Therefore, deeper investigations are now necessary to understand the functional role of this newly identified fibrinogen-like molecule.
In brittle stars Afi-ficolin encodes for a protein also containing a FReD domain. In both vertebrates and invertebrates ficolin is a lectin important in the innate immune response (Fujita, 2002; Iwanaga and Lee, 2005; Matsushita, 2009, Zuliani-Alvarez and Midwood, 2015). Its presence in the genome/proteome of other echinoderms has been previously reported (Hibino et al., 2006; Franco, 2011). The expression of this transcript at stage 2 in the proximal dermal layer suggests that cells of the new connective tissue may be involved in the immune response after injury. Whether these are new cells or cells recruited from the surrounding stump tissues needs to be further investigated, preferentially through cell tracking. The localised expression in the stump in the radial water canal epithelium suggests that proteins might be synthetized there and subsequently released in the coelomic fluid of the water vascular system, mobilised towards the regenerating area.
4.3. Extracellular matrix deposition and remodelling: a focus on collagen
In line with the general higher speed of regeneration, appearance of an organised fibrous extracellular matrix (ECM) occurs earlier in brittle stars than in starfishes. In both echinoderm models nonfibrillar collagen-like molecules are firstly deposited. However, it is at the end of the repair phase that collagen fibrils and fibril bundles become visible (Fig. S2; Ben Khadra et al., 2015a, b, 2017).
To better define collagen production/deposition, the gene expression of a key collagen biosynthetic enzyme (prolyl-4-hydroxylase; p4h) was investigated. Till now, few studies have focused on the expression of p4h in invertebrates (Veijola et al., 1994; and Andrew, 2002) and in particular in marine invertebrates (Pozzolini et al., 2015). In this context, this study represents a pioneering work. In brittle stars this gene is not apparently expressed in the first phase after injury (it becomes visible only at advanced stages in the coelomic epithelium), whereas in starfishes it is localised in the regenerating epidermis at the middle/late repair phase, supporting a role of this tissue in early collagen biosynthesis/deposition. For A. filiformis the apparent incongruences between the absence of Afi-p4h expression till the onset of the regenerative phase and the microscopic detection of collagen from the end of the repair phase need to be further investigated through quantitative PCR (also at earlier stages).
Noteworthy, in both echinoderms ECM deposition starts later than in mammals (Clore et al., 1979): indeed, in the latter new collagen appears at the very beginning of the repair phase (from about 10 hours after injury). A reticular and disorganised fibrillar network of collagen type III is firstly deposed and then replaced by thick, dense and parallel fibres of collagen type I that are constantly remodelled (Xue and Jackson, 2015). Differently, in echinoderms collagen deposition begins only at the end of the repair phase and it initially occurs as non-fibrillar loose ECM, possibly providing a more “dynamic and plastic” environment for tissue regeneration. Moreover, as already suggested for sea cucumbers (Quiñones et al., 2002) and contrary to mammals (Bock and Mrowietz, 2002; Rahban and Garner, 2003), in both brittle stars and starfishes no fibrotic scar is normally detected.
4.4. Conclusions
In this study the brittle star Amphiura filiformis and the starfish Echinaster sepositus were used as models to describe and compare the repair phase phenomena after arm amputation within echinoderms as well as with mammals’ healing events after wound. The main similarities and differences between them are summarised in Table 1 and Fig. 6.
Table 1. Comparison of the events occurring during the repair phase of echinoderms and mammals.
Symbol: * - data from Martin, 1997; Werner and Grose, 2003; Pastar et al., 2014.
| EVENT | STARFISHES | BRITTLE STARS | MAMMALS* |
|---|---|---|---|
| Constriction of the cavities/canals | Sealing of the coelomic cavities (haemostatic ring) | Sealing of the coelomic cavities (no haemostatic ring, bending of the shields) | Vasoconstriction of the blood vessels |
| Wound contraction | Aboral body wall moves towards the oral side (within 24 hours p.a.) | Aboral and oral body walls move towards the wound (within 24 hours p.a.) | Contraction of the wound edges (after 3-4 days post injury) |
| Cell clotting in the cavities/canals | Coelomocytes | Coelomocytes | Platelets |
| Phagocytosis | Phagocytes/coelomocytes | Phagocytes/coelomocytes/epidermis | Macrophages |
| Re-epithelialisation direction | Centripetal | Centripetal | Centripetal |
| Epidermal cell junction disruption | No | No | Yes |
| Oedematous area (granulation tissue-like) formation | Yes | No | Granulation tissue |
| Canal/vasa infiltration | No | No | Yes (angiogenesis) |
| Fibrosis | No | No | Yes |
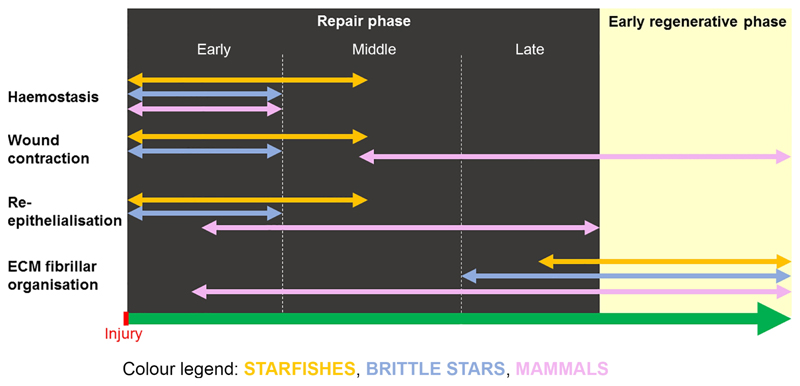
Fig. 6. Main similarities/differences in the events of the repair phase among starfishes (E. sepositus), brittle stars (A. filiformis) and mammals.
See colour legend embedded in the figure.
Taken together, our results show that:
both echinoderm models display similar haemostasis, wound contraction and re-epithelialisation phenomena and, in comparison to mammals, they are overall more efficient during the emergency reaction after injury in terms of timing and efficacy;
the regenerating epidermis of echinoderms is apparently a highly active and multi-functional tissue, involved in both inflammatory/immune response (phagocytosis), plus in collagen biosynthesis;
the extracellular matrix (ECM) fibrillar organisation after injury is comparable in the two echinoderm models and it is delayed and less conspicuous than in mammals. Moreover, over-deposition of collagen (fibrosis) is never detectable. Overall, the temporary loose configuration of the ECM is likely to be more “plastic” than the collagenous scar of mammals, therefore possibly facilitating the subsequent regenerative process, as suggested for sea cucumbers (Quiñones et al., 2002).
It is important to point out that re-epithelialisation, inflammatory/immune system-related genes and ECM fibrillar organisation/deposition during brittle star and starfish repair phases were here deeply described. Furthermore, interesting differences and similarities in repair events and timing within echinoderms and between echinoderms and mammals were highlighted. The comparison between animals able or unable to regenerate after injury suggests that regenerative abilities are mechanistically diverse, from the very first repair events. These differences, contrary to what is assumed, are not just differences in the subsequent re-growth capacities. In the future, perturbation tests aimed to impair/block re-epithelialisation, immune response or ECM deposition should be performed to test the hypothesis that specific repair events are strictly necessary to permit an efficient regenerative process. Moreover, our findings show that echinoderms, and starfishes especially, can be considered valid alternative models to study wound healing and regeneration in light of human health future applications (Gurtner et al., 2008).
Supplementary Material
Highlights.
Echinoderms are valid models to study repair phase and regeneration post amputation
Quick re-epithelialisation and wound contraction characterise echinoderm wound healing
Echinoderm epidermis has a multi-functional role during the repair phase
Delayed collagen deposition and no fibrosis differentiate echinoderms from mammals
Acknowledgements
The authors thank the Sven Lovén Centre for Marine Sciences in Kristineberg (Sweden), especially Sam Dupont and Bengt Lundve, for the collection of experimental brittle stars and the Marine Protected Areas of Portofino and Bergeggi Island (Ligurian Sea, Italy) for the permission to collect experimental starfishes. A special thanks to the SCUBA divers Marco Benati, Marco Di Lorenzo and Paola Fasano for starfish collection. We thank Prof. Max Telford, Fraser Simpson and Wendy Hart for help with gene cloning, Dr. Avi Lerner for providing ets1/2 degenerate primers, Laura Piovani and Mark Turmaine for help with transmission electron microscopy protocols.
Footnotes
Conflict of interest disclosure
The authors certify that there is no conflict of interest. All authors contributed to and approved the final manuscript.
Authors' contributions and funding
CF, PO and MS conceived the study. CF, AC, AZ, YBK, GC carried out the experiments. YBK and PM provided the sequence of Ese-actin. CF, AC, FB, MS, PO and MDCC analysed the data. CF, PO, PM and MS wrote the manuscript. This work was partly funded by the KVA fund SL2015-0048 from the Royal Swedish Academy of Science. CF was funded by an Erasmus Placement Fellowship. AC was funded by a Wellcome Trust PhD studentship. MS was funded by a Young Researcher Grant of the University of Milan.
Contributor Information
Cinzia Ferrario, Email: cinzia89.ferrario@alice.it.
Yousra Ben Khadra, Email: youssra_benkhadra@yahoo.fr.
Anna Czarkwiani, Email: a.czarkwiani@ucl.ac.uk.
Anne Zakrzewski, Email: a.zakrzewski@ucl.ac.uk.
Pedro Martinez, Email: pedro.martinez@ub.edu.
Graziano Colombo, Email: graziano.colombo@unimi.it.
Francesco Bonasoro, Email: francesco.bonasoro@unimi.it.
Maria Daniela Candia Carnevali, Email: daniela.candia@unimi.it.
Paola Oliveri, Email: p.oliveri@ucl.ac.uk.
References
- Abrams EW, Andrew DJ. Prolyl 4-hydroxylase alpha-related proteins in Drosophila melanogaster: tissue-specific embryonic expression of the 99F8-9 cluster. Mech Dev. 2012;112:165–171. doi: 10.1016/s0925-4773(01)00636-0. [DOI] [PubMed] [Google Scholar]
- Akimenko MA, Marì-Beffa M, Becerra J, Géraudie J. Old Questions, New Tools, and Some Answers to the Mystery of Fin Regeneration. Developmental Dynamics. 2003;226:190–201. doi: 10.1002/dvdy.10248. [DOI] [PubMed] [Google Scholar]
- Arnone MI, Byrne M, Martinez P. Echinodermata. In: Wanninger A, editor. Evolutionary Developmental Biology of Invertebrates Vol 6 (Deuterostomia) Springer-Verlag Wien; 2015. [DOI] [Google Scholar]
- Bateman PW, Fleming PA. To cut a long tail short: a review of lizard caudal autotomy studies carried out over the last 20 years. Journal of Zoology. 2009;277:1–14. [Google Scholar]
- Bely AE. Distribution of segment regeneration ability in the Annelida. Integr Comp Biol. 2006;46:508–518. doi: 10.1093/icb/icj051. [DOI] [PubMed] [Google Scholar]
- Ben Amar M, Bianca C. Towards a unified approach in the modeling of fibrosis: A review with research perspectives. Physics of Life Reviews. 2016;17:61–85. doi: 10.1016/j.plrev.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Ben Khadra Y, Ferrario C, Di Benedetto C, Said K, Bonasoro F, Candia Carnevali MD, Sugni M. Wound repair during arm regeneration in the red starfish Echinaster sepositus. Wound Repair Regen. 2015a;23:611–622. doi: 10.1111/wrr.12333. [DOI] [PubMed] [Google Scholar]
- Ben Khadra Y, Ferrario C, Di Benedetto C, Said K, Bonasoro F, Candia Carnevali MD, Sugni M. Re-growth, morphogenesis, and differentiation during starfish arm regeneration. Wound Repair Regen. 2015b;23:623–634. doi: 10.1111/wrr.12336. [DOI] [PubMed] [Google Scholar]
- Ben Khadra Y, Sugni M, Ferrario C, Bonasoro F, Varela Coelho A, Martinez P, Candia Carnevali MD. An integrated view of asteroid regeneration: tissues, cells and molecules. Cell and Tissue Research. 2017 doi: 10.1007/s00441-017-2589-9. [DOI] [PubMed] [Google Scholar]
- Biressi A, Ting Z, Dupont S, Dahlberg C, Di Benedetto C, Bonasoro F, Thorndyke M, Candia Carnevali MD. Wound-healing and arm regeneration in Ophioderma longicaudum and Amphiura filiformis (Ophiuroidea, Echinodermata): comparative morphogenesis and histogenesis. Zoomorphology. 2010;129:1–19. [Google Scholar]
- Bock O, Mrowietz U. Keloids. A fibroproliferative disorder of unknown etiology. Hautarzt. 2002;53:515. doi: 10.1007/s00105-001-0316-6. [DOI] [PubMed] [Google Scholar]
- Bosch TCG. Why polyps regenerate and we don't: Towards a cellular and molecular framework for Hydra regeneration. Developmental Biology. 2007;303:421–433. doi: 10.1016/j.ydbio.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Molecular Cell Biology. 2002;3:566–574. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- Cabrera-Serrano A, García-Arrarás JE. RGD-containing peptides inhibit regeneration in the sea cucumber Holothuria glaberrima. Dev Dyn. 2004;231:171–178. doi: 10.1002/dvdy.20112. [DOI] [PubMed] [Google Scholar]
- Candia Carnevali MD. Regeneration in echinoderms: repair, regrowth, cloning. ISJ. 2006;3:64–76. [Google Scholar]
- Candia Carnevali MD, Bonasoro F. A microscopic overview of crinoid regeneration. Microsc Res Techniq. 2001;55:403–426. doi: 10.1002/jemt.1187. [DOI] [PubMed] [Google Scholar]
- Candia Carnevali MD, Lucca E, Bonasoro F. Mechanisms of arm regeneration in the feather star Antedon mediterranea: healing of wound and early stages of development. The Journal of Experimental Zoology. 1993;267:299–317. [Google Scholar]
- Clark RA. Overview and general considerations of wound repair. In: Clark RAF, Henson PM, editors. The molecular and cellular biology of wound repair. Plenum; New York: 1988. pp. 3–23. [Google Scholar]
- Clark RAF, Lanigan JM, DellaPelle P, Manseau E, Dvorak HF, Colvin RB. Fibronectin and Fibrin Provide a Provisional Matrix for Epidermal Cell Migration During Wound Reepithelialization. The Journal of Investigative Dermatology. 1982;79:264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Clore JN, Cohen IK, Diegelmann RF. Quantitation of Collagen Types I and III during Wound Healing in Rat Skin. Experimental Biology and Medicine. 1979;161 doi: 10.3181/00379727-161-40548. [DOI] [PubMed] [Google Scholar]
- Czarkwiani A, Dylus DV, Oliveri P. Expression of skeletogenic genes during arm regeneration in the brittle star Amphiura filiformis. Gene Expression Patterns. 2013;13:464–472. doi: 10.1016/j.gep.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarkwiani A, Ferrario C, Dylus DV, Sugni M, Oliveri P. Skeletal regeneration in the brittle star Amphiura filiformis. Frontiers in Zoology. 2016;13:18. doi: 10.1186/s12983-016-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Frontiers in Bioscience. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97:3691–3698. doi: 10.1182/blood.v97.12.3691. [DOI] [PubMed] [Google Scholar]
- Ducati CC, Candia Carnevali MD, Barker MF. Regenerative potential and fissiparity in the starfish Coscinasterias muricata. Echinoderms: Munchen: Proceedings of the 11th International Echinoderm Conference; 6-10 October 2003; Munich, Germany. CRC Press; 2004. pp. 112–118. [Google Scholar]
- Dupont S, Thorndyke MC. Growth or differentiation? Adaptive regeneration in the brittle star Amphiura filiformis. The Journal of Experimental Biology. 2006;209:3873–3881. doi: 10.1242/jeb.02445. [DOI] [PubMed] [Google Scholar]
- Dylus DV, Blowes LM, Czarkwiani A, Elphick MR, Oliveri P. Developmental transcriptome of the brittlestar Amphiura filiformis reveals gene regulatory network rewiring in echinoderm larval skeleton evolution. BioRxiv. 2017 doi: 10.1101/166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Franco CDMF. Proteomics based approach to understand tissue regeneration. Starfish as a model organism. PhD thesis; 2011. [Google Scholar]
- Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nature Reviews. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Modrell MS, Northcutt RG, Catania KC, Luer C, Baker CVH. Electrosensory ampullary organs are derived from lateral line placodes in cartilaginous fishes. Development. 2012;139:3142–3146. doi: 10.1242/dev.084046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Europe PMC. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Hibino T, Loza-Coll M, Messier C, Majeske AJ, Cohen AH, Terwilliger DP, Buckley KM, Brockton V, Nair SV, Berney K, Fugmann SD, et al. The immune gene repertoire encoded in the purple sea urchin genome. Developmental Biology. 2006;300:349–365. doi: 10.1016/j.ydbio.2006.08.065. [DOI] [PubMed] [Google Scholar]
- Holm K, Dupont S, Sköld HN, Stenius A, Thorndyke MC, Hernroth B. Induced cell proliferation in putative haematopoietic tissues of the sea star, Asterias rubens (L.) J Exp Biol. 2008;211:2551–2558. doi: 10.1242/jeb.018507. [DOI] [PubMed] [Google Scholar]
- Hyman LH. The Invertebrates. Vol. 4. Echinodermata. Mc Graw Hill Book Company Inc.; New York, Toronto, London: 1955. [Google Scholar]
- Ibrahim MM, Chen L, Bond JE, Medina MA, Ren L, Kokosis G, Selim AM, Levinson H. Myofibroblasts contribute to but are not necessary for wound contraction. Laboratory Investigation. 2015;95:1429–1438. doi: 10.1038/labinvest.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga S, Lee BL. Recent Advances in the Innate Immunity of Invertebrate Animals. Journal of Biochemistry and Molecular Biology. 2005;38:128–150. doi: 10.5483/bmbrep.2005.38.2.128. [DOI] [PubMed] [Google Scholar]
- Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of Integrins and Basement Membrane Components by Wound Keratinocytes. J Clin Invest. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens N, Koolwijk P, De Maat PM. Fibrin structure and wound healing. Journal of Thrombosis and Haemostasis. 2006;4:932–939. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- MacLeod AS, Mansbridge JN. The Innate Immune System in Acute and Chronic Wounds. Advances in Wound Care Mary Ann Liebert, Inc. 2015;5:65–78. doi: 10.1089/wound.2014.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Mashanov VS, García-Arrarás JE. Gut Regeneration in Holothurians: A Snapshot of Recent Developments. The biological bulletin. 2011;221:93–109. doi: 10.1086/BBLv221n1p93. [DOI] [PubMed] [Google Scholar]
- Matsushita M. Ficolins: Complement-Activating Lectins Involved in Innate Immunity. J Innate Immun. 2009;2:24–32. doi: 10.1159/000228160. [DOI] [PubMed] [Google Scholar]
- Mladenov PV, Bisgrove B, Asotra S, Burke RD. Mechanisms of arm-tip regeneration in the sea star, Leptasterias hexactis. Roux’s Arch Dev Biol. 1989;198:19–28. doi: 10.1007/BF00376366. [DOI] [PubMed] [Google Scholar]
- Moss C, Hunter J, Thorndyke MC. Pattern of bromodeoxyuridine incorporation and neuropeptide immunoreactivity during arm regeneration in the starfish Asterias rubens. Phil Trans R Soc London B. 1998;353:421–436. [Google Scholar]
- Mozzi D, Dolmatov IY, Bonasoro F, Candia Carnevali MD. Visceral regeneration in the crinoid Antedon mediterranea: basic mechanisms, tissues and cells involved in gut regrowth. Cent Eur J Biol. 2006;1:609–635. [Google Scholar]
- Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biology. 2003;22:15–24. doi: 10.1016/s0945-053x(03)00006-4. [DOI] [PubMed] [Google Scholar]
- Oji T. Fossil records of echinoderm regeneration with special regard to crinoids. Micr Res Tech. 2001;55:397–402. doi: 10.1002/jemt.1186. [DOI] [PubMed] [Google Scholar]
- Pancer Z, Rast JP, Davidson EH. Origins of immunity: transcription factors and homologues of effector genes of the vertebrate immune system expressed in sea urchin coelomocytes. Immunogenetics. 1999;49:773–86. doi: 10.1007/s002510050551. [DOI] [PubMed] [Google Scholar]
- Park JE, Barbul A. Understanding the role of immune regulation in wound healing. The American Journal of Surgery. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle) 2014;3:445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock EE. Wound repair. Saunders; Philadelphia: 1984. Wound repair; pp. 38–55. [Google Scholar]
- Pozzolini M, Scarfì S, Mussino F, Ferrando S, Gallus L, Giovine M. Molecular Cloning, Characterization, and Expression Analysis of a Prolyl 4-Hydroxylase from the Marine Sponge Chondrosia reniformis. Mar Biotechnol. 2015;17:393–407. doi: 10.1007/s10126-015-9630-3. [DOI] [PubMed] [Google Scholar]
- Quiñones JL, Rosa R, Ruiz DL, García-Arrarás JE. Extracellular matrix remodelling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Dev Biol. 2002;250:181–197. doi: 10.1006/dbio.2002.0778. [DOI] [PubMed] [Google Scholar]
- Rahban SR, Garner WL. Fibroproliferative scars. Clin Plast Surg. 2003;30:77. doi: 10.1016/s0094-1298(02)00069-x. [DOI] [PubMed] [Google Scholar]
- Ramírez-Gómez F, Ortiz-Pineda PA, Rojas Cartagena C, Suarez-Castillo EC, García-Arrarás JE. Immune-related genes associated with intestinal tissue in the sea cucumber Holothuria glaberrima. Immunogenetics. 2008;60:57–71. doi: 10.1007/s00251-007-0258-y. [DOI] [PubMed] [Google Scholar]
- Ramírez-Gómez F, Ortiz-Pineda PA, Rivera Cardona G, García-Arrarás JE. LPS-induced genes in intestinal tissue of the sea cucumber Holothuria glaberrima. PLoS ONE. 2009;4:e6178. doi: 10.1371/journal.pone.0006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Gómez F, Aponte-Rivera F, Mendez Castaner L, García-Arrarás JE. Changes in holothurian coelomocyte populations following immune stimulation with different molecular patterns. Fish Shellfish Immunol. 2010;29:175–185. doi: 10.1016/j.fsi.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Gómez F, García-Arrarás JE. Echinoderm immunity. ISJ. 2010;7:211–220. [Google Scholar]
- Rast JP, Smith LC, Loza-Coll M, Hibino T, Litman GW. Genomic Insights into the Immune System of the Sea Urchin. Science. 2006;314:952–956. doi: 10.1126/science.1134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Relationship between ultrastructure and specific functions of macrophages. Comparative Immunology, Microbiology and Infectious Diseases. 1985;8:119–133. doi: 10.1016/0147-9571(85)90039-6. [DOI] [PubMed] [Google Scholar]
- Saló E, Abril JF, Adell T, Cebrià F, Eckelt K, Fernàndez-Taboada E, Handberg-Thorsager M, Iglesias M, Molina MD, Rodrìguez-Esteban G. Planarian regeneration: achievements and future directions after 20 years of research. Int J Dev Biol. 2009;53:1317–1327. doi: 10.1387/ijdb.072414es. [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A. Regeneration in the metazoans: why does it happen? BioEssays. 2000;22:578–590. doi: 10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Shultz GS, Ladwig G, Wysocki A. Extracellular matrix: review of its roles in acute and chronic wounds. World Wide Wounds. 2005 [Google Scholar]
- Singer AJ, Clark RAF. Cutaneous wound healing. The New England Journal of Medicine. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Sivamani RK, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849–2868. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- Smith LC, Ghosh C, Buckley KM, Clow LA, Dheilly NM, Haug T, Henson JH, Li C, Lun CM, Majeske AJ, Matranga V, et al. Echinoderm immunity. Söderhäll Kenneth., editor. Invertebrate Immunity. © 2010 Landes Bioscience and Springer Science+Business Media. [Google Scholar]
- Stroncek JD, Reichert WM. Overview of Wound Healing in Different Tissue Types. In: Reichert WM, editor. Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. Chapter 1. [PubMed] [Google Scholar]
- Tredget EF, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids and contractures: the cellular and molecular basis for therapy. Surg Clin North Am. 1997;77:701–730. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- Veijola J, Koivunen P, Annunen P, Pihlajaniemi T, Kivirikko KI. Cloning, baculovirus expression, and characterization of the alpha subunit of prolyl 4-hydroxylase from the nematode Caenorhabditis elegans. This alpha subunit forms an active alpha beta dimer with the human protein disulfide isomerase/beta subunit. J Biol Chem. 1994;269:26746–26753. [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- White LM, Roy S, Gordillo GM, Kalliainen LK, Melvin WS, Ellison EC, Sen CK. Wound healing and regeneration. Physiology and Maintenance. 2009;1 [Google Scholar]
- Xu X, Doolittle RF. Presence of a vertebrate fibrinogen-like sequence in an echinoderm. Proc Natl Acad Sci USA. 1990;87:2097–2101. doi: 10.1073/pnas.87.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Jackson CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Advances in Wound Care. 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Cohn MJ. Hagfish and lancelet fibrillar collagens reveal that type II collagen-based cartilage evolved in stem vertebrates. PNAS. 2006;103:16829–16833. doi: 10.1073/pnas.0605630103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani-Alvarez L, Midwood KS. Fibrinogen-Related Proteins in Tissue Repair: How a Unique Domain with a Common Structure Controls Diverse Aspects of Wound Healing. Advances in Wound Care. 2015;4:273–285. doi: 10.1089/wound.2014.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.