Abstract
Objective
To evaluate the association between long-term exposure to ambient air pollution and cognitive decline in older adults residing in an urban area.
Methods
Data for this study were obtained from 2 prospective cohorts of residents in the northern Manhattan area of New York City: the Washington Heights–Inwood Community Aging Project (WHICAP) and the Northern Manhattan Study (NOMAS). Participants of both cohorts received in-depth neuropsychological testing at enrollment and during follow-up. In each cohort, we used inverse probability weighted linear mixed models to evaluate the cross-sectional and longitudinal associations between markers of average residential ambient air pollution (nitrogen dioxide [NO2], fine particulate matter [PM2.5], and respirable particulate matter [PM10]) levels in the year prior to enrollment and measures of global and domain-specific cognition, adjusting for sociodemographic factors, temporal trends, and censoring.
Results
Among 5,330 participants in WHICAP, an increase in NO2 was associated with a 0.22 SD lower global cognitive score at enrollment (95% confidence interval [CI], −0.30, −0.14) and 0.06 SD (95% CI, −0.08, −0.04) more rapid decline in cognitive scores between visits. Results were similar for PM2.5 and PM10 and across functional cognitive domains. We found no evidence of an association between pollution and cognitive function in NOMAS.
Conclusion
WHICAP participants living in areas with higher levels of ambient air pollutants have lower cognitive scores at enrollment and more rapid rates of cognitive decline over time. In NOMAS, a smaller cohort with fewer repeat measurements, we found no statistically significant associations. These results add to the evidence regarding the adverse effect of air pollution on cognitive aging and brain health.
Age-related cognitive decline is a growing public health concern as increases in life expectancy and the aging of the population are expected to substantially increase the prevalence of cognitive impairment and dementia.1,2 An estimated 47 million individuals live with dementia, with the global prevalence expected to double every 20 years.3 Poor cognitive function is a key cause of disability among older adults and can have profound social, economic, and health implications.4 Global health care expenditures for cognitive impairment reached $818 billion in 2015 and are expected to reach a staggering $2 trillion by 2030.3 Risk of accelerated cognitive decline increases with age, cerebrovascular disease, and the presence of traditional cardiovascular risk factors, but these factors do not fully account for risk of cognitive decline in the population. Identification of novel risk factors, particularly modifiable risk factors, is of great importance.
Air pollution, a ubiquitous environmental exposure, is a widespread public health hazard, particularly in urban areas. Despite noteworthy improvements in overall levels of ambient air pollution in high-income countries over the last decade, more than 124 million US residents live in areas that do not meet the US Environmental Protection Agency (EPA) National Ambient Air Quality Standards.5 Long-term exposure to ambient air pollution has been highlighted as a risk factor for cognitive decline in addition to its association with other cardiovascular and neurologic outcomes.6–11 The purpose of this study was to examine the association between long-term exposure to ambient residential air pollution and cognitive decline among older adults in 2 multiethnic, urban cohorts within the northern Manhattan neighborhood of New York City. We hypothesized that individuals exposed to higher levels of air pollution would have lower levels of cognitive function at baseline and steeper trajectories of cognitive decline.
Methods
Data collection
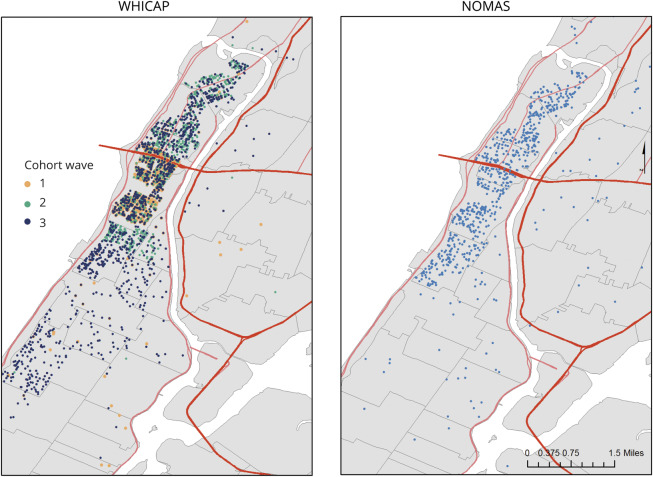
Data for this study were obtained from 2 prospective, community-based cohorts located in northern Manhattan, New York City: the Washington Heights–Inwood Community Aging Project (WHICAP) and the Northern Manhattan Study (NOMAS) (figure 1).
Figure 1. Residential location of cohort participants throughout northern Manhattan.
NOMAS = Northern Manhattan Study; WHICAP = Washington Heights–Inwood Community Aging Project.
WHICAP is a prospective, population-based study of aging and dementia. Established in 3 recruitment waves, the first wave of participants was recruited in 1992 from a random sample of Medicare-eligible adults (age ≥65) residing in the neighborhoods of Washington–Hamilton Heights and Inwood in northern Manhattan. The second and third waves were recruited from the same communities in 1999 and 2010, with a goal to recruit a cohort of ethnically and educationally diverse elderly without dementia. These cohorts were not randomly identified, but chosen based on the following criteria: (1) the final sample would be equally divided among Hispanic, non-Hispanic black, and non-Hispanic white participants; (2) the cohort would represent equal proportions of 65–74 and ≥75-year-old participants; and (3) individuals would be excluded if they had substantial cognitive problems, had been diagnosed with dementia, or did not speak English or Spanish. To date, at least one neuropsychological battery has been collected on 6,261 older adults. Participants are evaluated longitudinally every 18–24 months, with a comprehensive neuropsychological battery, medical and neurologic examination, and survey about health-related behaviors, medication, comorbidities, and cardiovascular risk factors. While some participants in this cohort had up to 13 neuropsychological examinations, given the recruitment in waves over time, fewer than 10% of participants had data available from more than 6 examinations. To ensure model stability, we limited our analyses to data from examinations 1–6. Detailed sampling strategies, recruitment outcomes, and examination methodology have been discussed previously.12
NOMAS is a prospective, population-based cohort study of 3,298 participants designed to measure cardiovascular risk factors and outcomes in a multiethnic urban population. Initial eligibility for the cohort included age ≥40 years, permanent residency in one of the 5 zip codes representing northern Manhattan, and no history of clinical stroke. Detailed methods of recruitment, baseline evaluation, and follow-up have been described previously.13 Initial study recruitment occurred from 1993 to 2001 and participants continue to be followed up annually by telephone. During annual follow-up between 2003 and 2008, participants were invited to join a subcohort to receive neuropsychological testing and MRI scans if they met the following eligibility criteria at time of enrollment: free of clinical stroke, free of clinically identified dementia, aged ≥50 years, and no contraindications to MRI. A total of 1,091 participants were enrolled from 2003 to 2008. In addition, a sample of household members of NOMAS participants (n = 199) were enrolled using the same eligibility criteria from 2006 to 2008 in order to increase sample size, creating a final subcohort sample without dementia of 1,290. Those participating in this subcohort underwent a standardized medical examination to ascertain risk factor status, MRI, and detailed neuropsychological examination at time of enrollment. NOMAS participants received one follow-up neuropsychological testing after 5 years.
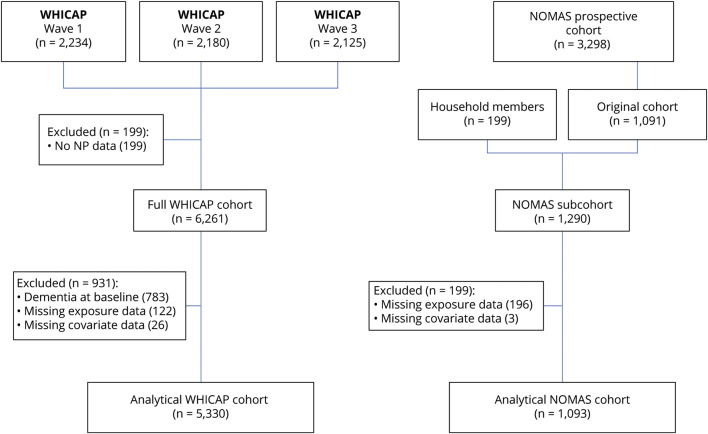
The final analytical samples comprised individuals from each cohort who (1) were free of dementia at baseline, (2) had at least one neuropsychological examination at any point during the study period, (3) had primary address in New York City, allowing for measurement of exposure, and (4) had no missing data for any of the confounding variables. These exclusion criteria resulted in a total sample size of 5,330 individuals in the WHICAP cohort and 1,093 in NOMAS (figure 2).
Figure 2. Creation of Washington Heights–Inwood Community Aging Project (WHICAP) and Northern Manhattan Study (NOMAS) analytical cohorts.
NP = neuropsychological.
Standard protocol approvals, registrations, and patient consents
All activities pertaining to NOMAS and WHICAP were approved by the institutional review board at Columbia University Medical Center. Written informed consent was provided by each participant at enrollment.
Assessment of residential ambient air pollution
Participants’ residential addresses at enrollment (taken as the time of the first neuropsychological evaluation in each cohort) were geocoded using Geosupport Batch Address Translator Desktop Edition (NYC Department of City Planning, New York, NY). We restricted analyses to those with primary addresses in New York City.
Estimates of residential air pollution exposure in the calendar year prior to enrollment at each geocoded address were ascertained using regionalized universal kriging models for nitrogen dioxide (NO2; ppb), fine particulate matter less than 2.5 µm in diameter (PM2.5; μg/m3), and respirable particulate matter (PM10; μg/m3), as previously described.14,15 Measurements of NO2, PM2.5, and PM10 were obtained from the US EPA Air Quality System and annual average values were used in a universal kriging regression framework to predict concentrations at addresses without monitoring. Partial least square methods were used to include geographic covariates (roadway density, population density, urban land, agricultural land, forests, bodies of water), land use, and roadway proximity to improve predictions. The NO2 model incorporated satellite data to improve predictions.15 We ran sensitivity analyses in a subset of WHICAP participants enrolled after 2000 using air pollution models developed for the Multi-Ethnic Study of Atherosclerosis and Air Pollution Study (MESA Air).16 MESA Air pollution models utilized monitoring data from the US EPA Air Quality System, monitors placed by MESA Air in the region, monitors maintained by the New York City Community Air Survey at sites throughout the New York City area, and at MESA Air participants' homes, which may lead to better spatial resolution in the small geographic area of northern Manhattan. All exposures were obtained and analyzed as continuous variables and included in models per interquartile range (IQR).
We calculated distance from participant residence to the nearest major roadway as a secondary marker of long-term exposure to traffic pollution. ArcGIS (version 10.3.1; ESRI, Inc., Redlands, CA) was used to calculate the Euclidean distance from geocoded residence to nearest major roadway, defined as US Census Features Class A1 (primary highway with limited access) or A2 roadway (nationally and regionally important highways that do not have limited access), which include most federal and interstate highways and some larger state and county highways. We modeled residential distance to roadway as a log-transformed continuous variable (per IQR) based on prior studies.17,18
Outcome ascertainment
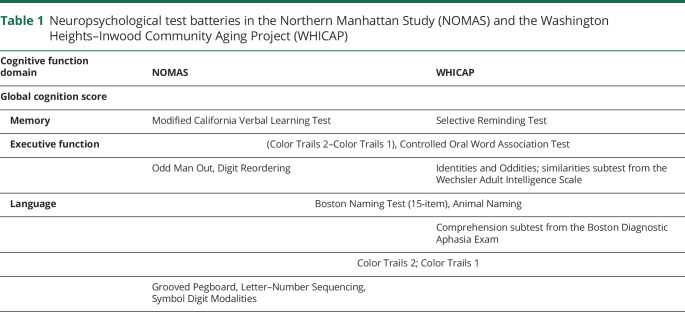
Neuropsychological batteries used in WHICAP and NOMAS were very similar; both were designed to capture key cognitive domains in both English- and Spanish-speaking older adults and developed to permit the calculation of domain-specific z scores. A complete list of tests included in each cohort is shown in table 1.
Table 1.
Neuropsychological test batteries in the Northern Manhattan Study (NOMAS) and the Washington Heights–Inwood Community Aging Project (WHICAP)
Cognitive function was calculated the same way in both cohorts. Individual neuropsychological tests were first standardized into z scores using cohort-specific means and SDs at baseline. We explored performance in global cognitive scores constructed using the mean of the z scores of all available neuropsychological tests. Subsequent analyses looked at performance in 3 individual functional domains, previously identified through factor analyses performed previously in each of the individual cohorts.12,19,20 Domain-specific scores were expressed as the mean of the individual test z scores loading into that domain. To enhance interpretability, the global cognitive score and domain-specific scores were standard-normal transformed using means and SDs at baseline such that results reflect a change in outcome relative to the SD of each outcome.
Sociodemographic risk factors
In each cohort, age was self-reported at time of neuropsychological assessment. Race–ethnicity was collected through self-identification using the format of the 2000 US Census. All individuals were first asked to report their racial group and then, in a second question, were asked whether they were of Hispanic origin. For the purpose of analysis, individuals were characterized into white non-Hispanic, black non-Hispanic, Hispanic, and other. Education was collected through self-report as total years of education completed. A summary z score for socioeconomic status (SES) was derived at the census tract level as a neighborhood measure of wealth, education, and occupation.21 In WHICAP analyses, a cohort indicator for wave was included in the analysis to account for cohort and secular trends.
Statistical analysis
The WHICAP and NOMAS cohorts were analyzed separately. Distributions of sociodemographic characteristics and cardiovascular risk factors were calculated as mean (±SD) for continuous variables and proportions (n [%]) for categorical variables. Estimates of pollutant exposure are presented as mean (IQR).
Stabilized inverse probability of censoring weights (IPCW) accounted for possible selection bias due to nonrandom loss to follow-up over time.22,23 To estimate the IPCW, we fit pooled logistic models at each time point predicting the probability that an individual remained in the study up until that time point. At each time point, we regressed a binary indicator for censorship on baseline pollutant levels as well as a series of available time-invariant sociodemographic covariates (sex, race–ethnicity, education, and neighborhood socioeconomic status) and a set of time-varying covariates that included age at the time of assessment and cognitive function at previous visit. Stabilized weights were calculated as the marginal probability of each individual’s censorship status (yes or no) divided by the probability of each individual’s censorship status conditional on the set of time-invariant and time-varying covariates. Using this method, individuals with a larger probability of being lost to follow-up would be weighted more heavily in the final analysis. The use of IPCW results in a pseudopopulation in which the censorship is marginally independent of treatment. IPCW were created separately within each cohort, and for each exposure and outcome combination to create a series of pseudopopulations for analysis.
We used linear mixed models for repeated measures weighted by the IPCW to study the relation of residential air pollution to both baseline cognitive function and cognitive decline.24 Separate analyses were performed for each pollutant in relation to both global cognitive scores and individual cognitive functional domain scores. All models adjusted for pollutant, visit, visit by pollutant interaction, and the series of sociodemographic characteristics as described above (age, sex, race–ethnicity, education, and neighborhood SES). The models, analyzed using PROC MIXED procedures, fitted participants as a random effect and used a compound symmetry covariance matrix. We analyzed pollutant exposures as both continuous measures and by quartile.
Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Data availability
The data that support these findings are available with approved data request. Data procurement processes, study protocol, and statistical analysis coding will be shared upon request from the corresponding author of this study.
Results
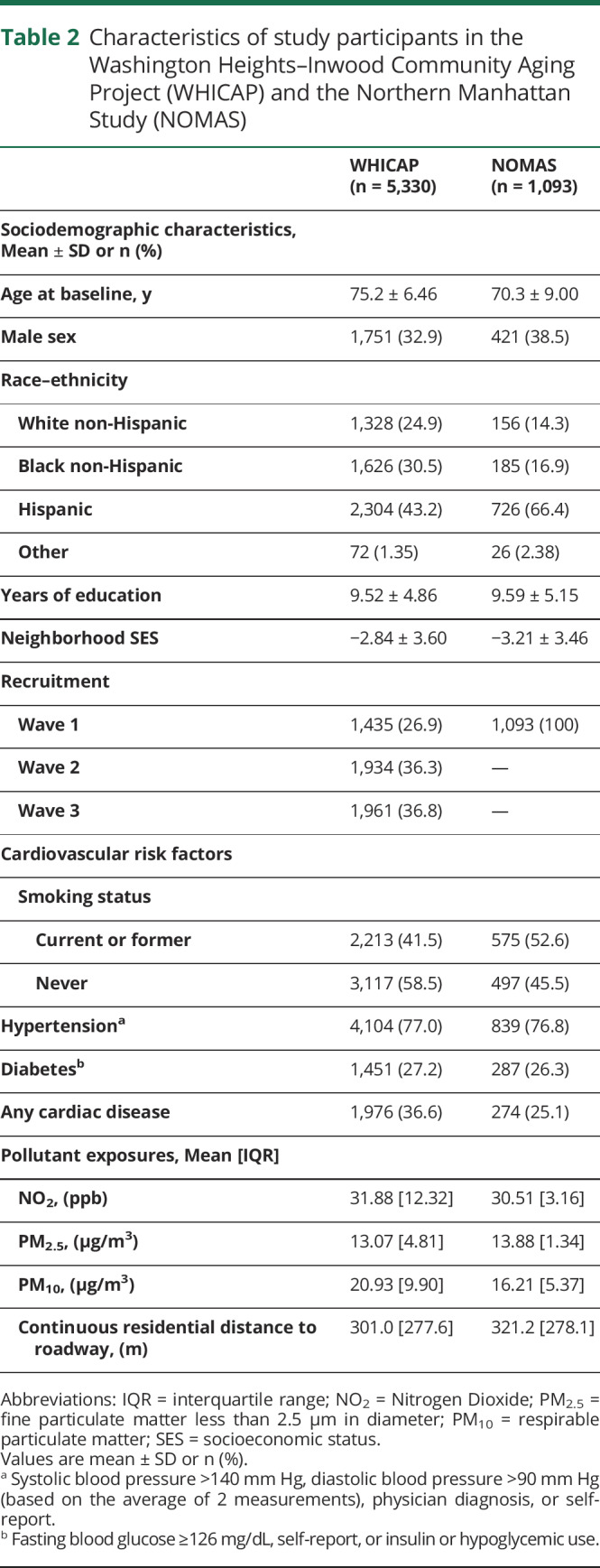
WHICAP participants were predominately women (66%), Hispanic, with a median age at enrollment of 75.2 (±6.46) years and high prevalence of traditional cardiovascular risk factors (table 2). Mean (IQR) annual estimates of ambient air pollution were 31.9 (12.3) ppb NO2, 13.1 (4.8) μg/m3 PM2.5, and 20.9 (9.9) μg/m3 PM10. Characteristics of the NOMAS population were similar, except that NOMAS participants were slightly younger, with a median age of 70 years (±9.0), had lower prevalence of cardiovascular disease, and were more likely to be of Hispanic ethnicity (table 2). Mean levels of residential air pollutants were similar in the 2 cohorts, but with less variability in pollution levels among NOMAS vs WHICAP participants. Demographic characteristics of the cohorts did not differ substantially across quantiles of pollutants (table e-1, dryad-migration.cdlib.org/stash/share/clJtz9D38gDCJx74LRTqtTjdLD6SQJxVYp1IvpKC7No).
Table 2.
Characteristics of study participants in the Washington Heights–Inwood Community Aging Project (WHICAP) and the Northern Manhattan Study (NOMAS)
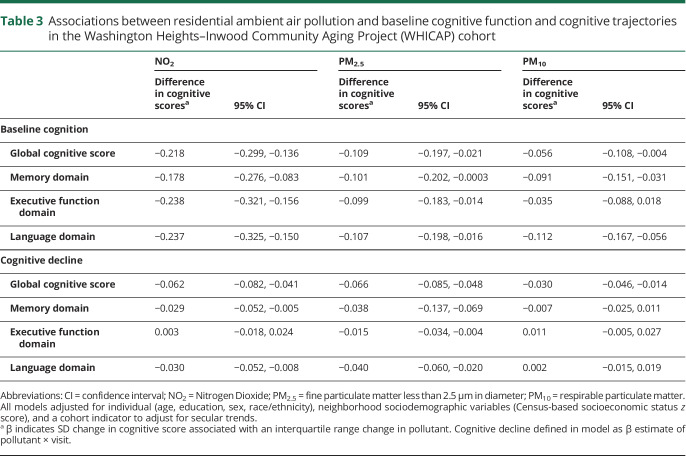
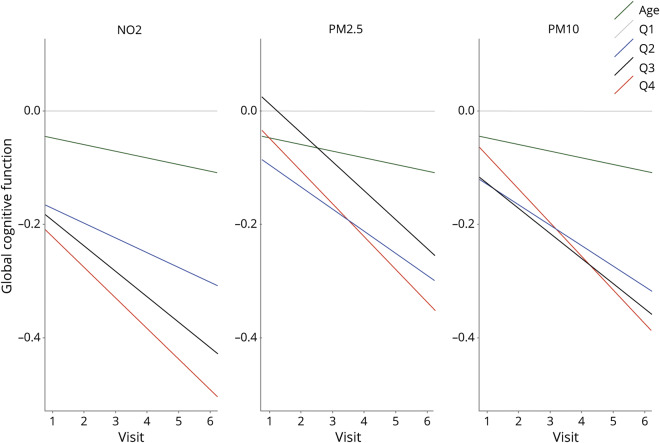
In WHICAP, higher levels of residential ambient air pollution were associated with both global cognitive function at baseline and more rapid rates of decline of global cognitive function between visits. In fully adjusted models, a 1 IQR increase in residential NO2 was predictive of a 0.22 SD (95% confidence interval [CI], −0.30, −0.14) lower global cognitive score at baseline and more rapid decline (−0.06 SD; 95% CI −0.08, −0.04) in global cognitive function between biennial visits (table 3 and figure 3). Estimates for residential particulate matter were similar. A 1 IQR increase in PM2.5 and PM10 was predictive of lower baseline global cognitive scores and more rapid rates of decline in global cognitive scores between visits (table 3). Results were similar across individual cognitive domains (table 3) and when analyzed by quartiles (table e-2, dryad-migration.cdlib.org/stash/share/clJtz9D38gDCJx74LRTqtTjdLD6SQJxVYp1IvpKC7No). Residential distance from roadway was not associated with either baseline cognitive scores or change in cognition over time (table e-3, dryad-migration.cdlib.org/stash/share/clJtz9D38gDCJx74LRTqtTjdLD6SQJxVYp1IvpKC7No). Sensitivity analyses using MESA Air models for exposure estimates showed slight attenuation of the association between NO2, and PM2.5 and global cognitive function, but not substantially (table e-4, dryad-migration.cdlib.org/stash/share/clJtz9D38gDCJx74LRTqtTjdLD6SQJxVYp1IvpKC7No).
Table 3.
Associations between residential ambient air pollution and baseline cognitive function and cognitive trajectories in the Washington Heights–Inwood Community Aging Project (WHICAP) cohort
Figure 3. Associations between ambient air pollutants, baseline global cognition, and cognitive decline with effects of age as comparison.
NO2 = Nitrogen Dioxide; PM2.5 = fine particulate matter less than 2.5 µm in diameter; PM10 = respirable particulate matter.
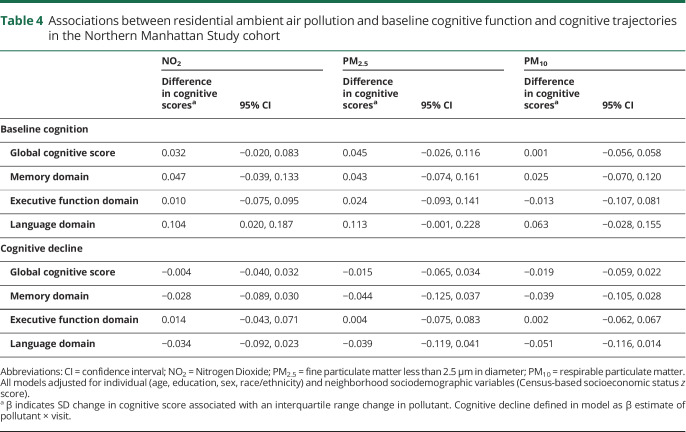
In contrast to the results seen in the WHICAP cohort, in NOMAS, we found no significant associations between residential ambient air pollution and either cognitive function at baseline or cognitive decline over time (table 4).
Table 4.
Associations between residential ambient air pollution and baseline cognitive function and cognitive trajectories in the Northern Manhattan Study cohort
Discussion
We examined the associations between residential levels of multiple air pollutants and cognitive function and decline in 2 racially and ethnically diverse urban cohorts in northern Manhattan, with differing results. In WHICAP, we saw a cross-sectional association between ambient air pollution and cognitive functioning as well as evidence supporting the hypothesis that higher levels of air pollutants lead to more rapid cognitive decline over time. However, we did not find evidence of these associations in the NOMAS cohort.
While the existing research generally supports the hypothesis that there is an association between air pollution and cognition, previous studies have also reported mixed evidence. Several large cohort studies have found statistically significant cross-sectional associations between ambient air pollution and cognitive function,8,25,26 but there is less evidence regarding a longitudinal association between air pollution and within-person rates of cognitive decline over time. Higher levels of long-term exposure to both PM2.5 and PM2.5–10 were associated with faster rates of cognitive decline in the Nurses' Health Study.27 Similarly, PM10 was analyzed longitudinally over several time points among men and women enrolled in the Cardiovascular Health Study, where a 10 μg/m3 increase in PM10 was associated with −2.6 lower Mini-Mental State Examination score and −1.1 point lower Digit Symbol Substitution Test score.28 In contrast, several studies done in large, population-based cohorts did not find significant associations between ambient air pollution and cognitive function and decline, including recent studies done in the large UK biobank database,6 the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort,29 and a study of episodic memory in the longitudinal Betula study in northern Sweden.30
While we found significant associations between residential air pollution and cognitive function and decline in the WHICAP cohort, these results were not consistent in the NOMAS cohort. This may have been due to several key characteristics of the NOMAS cohort, beginning with selection into the subcohort of individuals receiving neuropsychological testing. The selection of individuals with no dementia, stroke, or cardiac events from a preexisting cohort is likely inducing substantial selection bias into our results, particularly the cross-sectional estimates. In addition, the NOMAS cohort only has 2 time points from which to analyze cognitive change over time, in contrast to 6 follow-up neuropsychological examinations in WHICAP. A final key difference between the 2 cohorts is the distribution of exposure. In an urban study area with limited geographic extent such as northern Manhattan, there may have been limited spatial variability in pollutant levels, particularly in the NOMAS cohort, which had narrower IQRs across all pollutants, despite mean levels being similar. However, the urban study area is also a strength of both WHICAP and NOMAS, since this is one of the few studies to focus primarily on intraurban variation in measures of ambient air pollution, eliminating many potential unmeasurable confounders that may have existed in prior studies. In addition, although we adjusted for neighborhood and individual-level measures of SES in our analysis, we were unable to adjust for individual income levels.
Our study found relatively consistent associations among several measures of air pollution and cognitive function in the WHICAP study. In several other studies, this relationship was less consistent. A study of 1,496 adults living in the greater Los Angeles area found no association between air pollutants and a global cognitive score, although PM2.5 was associated with lower verbal learning scores, and NO2 was inversely associated with logical memory.31 Similarly, analysis of the Heinz Nixdorf Recall Study found significant associations between PM2.5 and diagnosis of mild cognitive impairment, though the study found no significant associations between PM10, PM10–2.5, NOx, and NO2 and cognitive function.26 The 2 cohorts including in this analysis, WHICAP and NOMAS, are located in a relatively small geographic area. It is possible that the similarity across pollutant estimates is due to the fact that these pollutants do not exist in isolation and these analyses are likely measuring the effect a mixture of these pollutants is having on cognitive function. Further studies should attempt to differentiate the individual effects of each pollutant. In all analyses, residential distance from roadway had nonsignificant measures of effect. This may have been due to limited variability of the exposure measure or to the fact that in this cohort, it is a nonspecific measure of air pollution.
While the magnitude of the observed associations in this study may appear small, if these associations represent a causal effect, the implications for global public health would be pronounced. Specifically, the association between NO2 and the accelerated rate of cognition decline was comparable to approximately 1 year of aging. With the global prevalence of dementia expected to reach almost 90 million individuals within the next 20 years,3 even a small reduction in ambient air pollution could have a substantial effect on cognitive health.
As concern regarding the deleterious health effects of ambient air pollution is growing, several biological mechanisms behind the adverse effects on the brain and cerebral vasculature have been proposed. A series of experimental animal studies indicate that ambient particles may have their effect on the CNS either through a systemic response via the circulatory system or intranasally by direct translocation to the brain through the olfactory bulb.32–34 Once in the nervous system, pollutant particles activate a series of systemic inflammatory pathways leading to vascular inflammation,35–37 impaired microvascular reactivity,38 and changes in cerebral hemodynamics.39 Further evidence of these mechanisms comes from a series of studies done in Mexico City, where strong histologic evidence of cerebral microvascular damage, systemic inflammatory markers, and brain pathology has been observed in autopsied brains of dogs and children residing in high vs low pollutant areas.40,41
The current study adds to the growing scientific evidence supporting the importance of air pollution in brain health of older adults, although this analysis had several important limitations.
The use of 2 long-term population-based cohorts may have introduced several potential limitations. It is likely that selection of participants in and out of the study could be biasing the results. Selection bias may have occurred with the original cohort enrollment, with individuals with lower cognitive function never making it into the study. In addition, there is the potential for informative censoring occurring throughout the study, with participants unable to come back for follow-up due to reduction in cognitive function. We attempted to address bias due to informative censoring by weighting the original cohorts using inverse probability of censoring weights, created at each follow-up using a series of sociodemographic factors, exposure levels, and cognitive function scores. It is possible, however, that we were unable to capture all potential sources of censoring with the data available. In addition, inverse probability of censoring weights would not account for bias due to differential enrollment or survival to enrollment. However, any residual bias would likely bias our estimates towards the null.
A key limitation of this study is that many of the physiologic processes preceding cognitive decline have been found to begin much earlier in life, and risk factors at midlife have been shown to be more important for the process of accelerated cognitive decline.3,42 Assessing midlife risk factors is not possible in these 2 cohorts, but the results from the proposed study can be used to inform future studies that look at a life course approach of environmental effects on cognitive aging. We chose not to adjust for traditional cardiovascular risk factors as confounders in our models. Ambient air pollution has been shown to be associated with higher risk of risk factors such as hypertension,43 stroke,10,44 cardiovascular disease,11 and chronic obstructive pulmonary disease,45 thus they are more likely to be mediators of this association between air pollution and cognition. The association between air pollution and cognition is further complicated by the high prevalence of multimorbidity in aging populations.46
Individuals with chronic obstructive pulmonary disease and other respiratory disorders have higher rates of comorbid conditions including cardiovascular diseases, diabetes, stroke, and asthma, which are known to be exacerbated by high levels of air pollution.47 This offers an interesting opportunity for future studies to assess these relationships.
The estimates of residential air pollution did not include data on time spent in locations outside the home or measure lifetime or occupational exposure. Levels of traffic-related air pollutants have decreased by almost 70% in the United States since the implementation of the Clean Air Act in 1970,5 therefore individuals may have been exposed to much higher levels of pollution throughout their lives as compared as to what was measured for the purpose of this study. In addition, many of the participants grew up in other countries and may have had different exposures to pollutants at younger ages. We included a cohort and year indicator variable in all final models to adjust for secular trends in decreasing levels of air pollution, but there may be some residual bias. The majority of participants were retired at the time of the study and there are limited data on lifetime workplace pollution exposures. Occupational exposures seem unlikely to be associated with residential outdoor levels of air pollutants, and thus unlikely to confound the analyses.
Practice effects, or improvements in neuropsychological test performance due to repeated examinations, may also have been a source of error in this analysis.48 However, because these effects are not likely to be influenced by pollutant measures, any bias caused by these effects would be towards the null.
The use of 2 large, prospective cohorts, WHICAP and NOMAS, for this study has provided a unique opportunity to evaluate multidimensional data in a population of over 6,000 residents of northern Manhattan and there are several key strengths to this study. Earlier studies have shown that prevalence of cognitive decline and dementia vary by sex and race–ethnic group; the prevalence of cognitive decline and dementia is higher in women and non-Hispanic whites, while older African Americans are twice as likely and Hispanics are 1.5 times as likely as older non-Hispanic whites to develop incident dementia.49,50 It is important to have a racially and ethnically diverse population of older adults that is not limited by sex to be able to ascertain differences in higher risk groups and also be able to generalize results to an aging urban population. Another benefit of these 2 large cohorts was the ability to analyze trajectories of cognitive decline over time using well-validated neuropsychological tests, a key limitation of many of the current studies of air pollution and cognition.
This study found that participants in the WHICAP study living in areas of northern Manhattan with higher levels of ambient air pollutants have lower cognitive scores at baseline and more rapid rate of decline over time. In contrast, we saw no significant associations in the NOMAS cohort. These results add to the evidence base surrounding the role of air pollution on accelerated cognitive aging and brain health.
Glossary
- CI
confidence interval
- EPA
Environmental Protection Agency
- IPCW
inverse probability of censoring weights
- IQR
interquartile range
- MESA Air
Multi-Ethnic Study of Atherosclerosis and Air Pollution Study
- NOMAS
Northern Manhattan Study
- PM2.5
fine particulate matter less than 2.5 µm in diameter
- PM10
respirable particulate matter
- SES
socioeconomic status
- WHICAP
Washington Heights–Inwood Community Aging Project
Appendix. Authors
Footnotes
Study funding
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the NIH under award number T32HL134625, the National Institute of Environmental Health Sciences under award numbers R01-ES020871 and P30-ES007033, the National Institute of Aging under award R01-AG016206, and Environmental Protection Agency grants RD-831697 and RD-83830001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsoring institutions.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75. [DOI] [PubMed] [Google Scholar]
- 3.Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015: the Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer's Disease International; 2015. [Google Scholar]
- 4.Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer's disease. Arch Med Res 2012;43:600–608. [DOI] [PubMed] [Google Scholar]
- 5.US Environmental Protection Agency. Our Nation's Air Status and Trends through 2010. Research Triangle Park, NC: US Environmental Protection Agency; 2012. [Google Scholar]
- 6.Cullen B, Newby D, Lee D, et al. Cross-sectional and longitudinal analyses of outdoor air pollution exposure and cognitive function in UK Biobank. Nat Sci Rep 2018;8:12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salinas-Rodríguez A, Fernández-Niño JA, Manrique-Espinoza B, et al. Exposure to ambient PM2.5 concentrations and cognitive function among older Mexican adults. Environ Int Pergamon 2018;117:1–9. [DOI] [PubMed] [Google Scholar]
- 8.Tallon LA, Manjourides J, Pun VC, Salhi C, Suh H. Cognitive impacts of ambient air pollution in the national social health and aging Project (NSHAP) cohort. Environ Int 2017;104:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Power MC, Adar SD, Yanosky JD, Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology 2016;56:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulick ER, Wellenius GA, Boehme AK, Sacco RL, Elkind MS. Residential proximity to major roadways and risk of incident ischemic stroke in the Northern Manhattan Study. Stroke 2018;49:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 12.Manly JJ, Bell-Mcginty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol 2005;62:1739–1746. [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors: The Northern Manhattan Stroke Study. Stroke 2001;32:1725–1731. [DOI] [PubMed] [Google Scholar]
- 14.Sampson PD, Richards M, Szpiro AA, et al. A regionalized national universal kriging model using partial least squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos Environ 2013;75:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young MT, Bechle MJ, Sampson PD, et al. Satellite-based NO2 and model validation in a national prediction model based on universal kriging and land-use regression. Environ Sci Technol 2016;50:3686–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller JP, Olives C, Kim SY, et al. A unified spatiotemporal modeling approach for prediction of multiple air pollutants in the Multi-Ethnic Study of Atherosclerosis and Air Pollution. Environ Health Perspect 2015;1:41–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilker EH, Mostofsky E, Lue SH, et al. Residential proximity to high-traffic roadways and poststroke mortality. J Stroke Cerebrovasc Dis 2013;22:e366–e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulick ER, Wellenius GA, Kauffman JD, et al. Long-term exposure to ambient air pollution and subclinical cerebrovascular disease in NOMAS (the Northern Manhattan Study). Stroke 2017;48:1966–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong C, Nabizadeh N, Caunca M, et al. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology 2015;85:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MSV, Wright CB. Construct validity of cognitive reserve in a multiethnic cohort: the Northern Manhattan Study. J Int Neuropsychol Soc 2009;15:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 22.Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ. Selection bias due to loss to follow up in cohort studies. Epidemiology 2016;27:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernán MA, McAdams M, McGrath N, Lanoy E, Costagliola D. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res 2009;18:27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laird NM, Ware JH. Random-effects models for longitudinal data author(s). Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- 25.Ailshire JA, Crimmins EM. Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol 2014;180:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzivian L, Dlugaj M, Winkler A, et al. Long-term air pollution and traffic noise exposures and mild cognitive impairment in older adults: a cross-sectional analysis of the Heinz Nixdorf Recall study. Environ Health Perspect 2016;124:1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 2012;172:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semmens EO. Effects of traffic-related air pollution on cognitive function, dementia risk and brain MRI findings in the Cardiovascular Health Study. Available at: hdl.handle.net/1773/21903. Accessed October 2016.
- 29.Loop MS, Kent ST, Al-Hamdan MZ, et al. Fine particulate matter and incident cognitive impairment in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. PLoS One 2013;8:e75001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oudin A, Forsberg B, Lind N, et al. Is long-term exposure to air pollution associated with episodic memory? A longitudinal study from northern Sweden. Nat Sci Rep 2017;7:12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gatto NM, Henderson VW, Hodis HN, et al. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 2014;40:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol Taylor Francis 2004;16:437–445. [DOI] [PubMed] [Google Scholar]
- 33.Oberdörster G, Utell MJ. Ultrafine particles in the urban air: to the respiratory tract—and beyond? Environ Health Perspect 2002;110:A440–A441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters A, Veronesi B, Calderón-Garcidueñas L, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol Biomed Cent 2006;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell A, Oldham M, Becaria A, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 2005;26:133–140. [DOI] [PubMed] [Google Scholar]
- 36.Levesque S, Taetzsch T, Lull ME, et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect 2011;119:1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 2008;36:289–310. [DOI] [PubMed] [Google Scholar]
- 38.Adar SD, Klein R, Klein BEK, et al. Air pollution and the microvasculature: a cross-sectional assessment of in vivo retinal images in the population-based Multi-Ethnic Study of Atherosclerosis (MESA). Plos Med Public Libr Sci 2010;7:e1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wellenius GA, Boyle LD, Wilker EH, et al. Ambient fine particulate matter alters cerebral hemodynamics in the elderly. Stroke 2013;44:1532–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calderón-Garcidueñas L, Reynoso-Robles R, Vargas-Martínez J, et al. Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer's disease. Environ Res 2016;146:404–417. [DOI] [PubMed] [Google Scholar]
- 41.Calderón-Garcidueñas L, Mora-Tiscareño A, Styner M, et al. White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. J Alzheimer's Dis 2012;31:183–191. [DOI] [PubMed] [Google Scholar]
- 42.Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimer's Dis 2014;42:1295–1310. [DOI] [PubMed] [Google Scholar]
- 43.Kingsley SL, Eliot MN, Whitsel EA, et al. Residential proximity to major roadways and incident hypertension in post-menopausal women. Environ Res 2015;142:522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ljungman PL, Mittleman MA. Ambient air pollution and stroke. Stroke 2014;45:3734–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schou L, Østergaard B, Rasmussen LS, Rydahl-Hansen S, Phanareth K. Cognitive dysfunction in patients with chronic obstructive pulmonary disease: a systematic review. Respir Med 2012;106:1071–1081. [DOI] [PubMed] [Google Scholar]
- 46.Vassilaki M, Aakre JA, Cha RH, et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc 2015;63:1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raherison C, Girodet P. Epidemiology of COPD. Eur Respir Rev Eur Respir Soc 2009;18:213–221. [DOI] [PubMed] [Google Scholar]
- 48.Vivot A, Power MC, Glymour MM, et al. Jump, hop, or skip: modeling practice effects in studies of determinants of cognitive change in older adults. Am J Epidemiol 2016;183:302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manly JJ, Mayeux R. Ethnic differences in dementia and Alzheimer's disease. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support these findings are available with approved data request. Data procurement processes, study protocol, and statistical analysis coding will be shared upon request from the corresponding author of this study.