Abstract
Hospitalization represents a unique opportunity to re-engage out-of-care individuals, improve HIV outcomes and reduce health disparities. Electronic health records of HIV-positive individuals hospitalized at an urban, public hospital between September 2013- December 2015 were reviewed. In October 2014, a multidisciplinary HIV consult team (HIV specialist, case manager, and transitional care nurse (TCN)) was implemented. Engagement in care, retention in care and virologic suppression before and after hospitalization were compared between the pre- and post-intervention periods and by treatment received. Of 1056 inpatient admissions (pre-intervention=571, post-intervention=485), the majority were among males (69%) and racial/ethnic minorities (55% Black, 23% Hispanic). Each step of the HIV care cascade increased after hospitalization for both time periods (p<0.01 for each comparison). Those who received the HIV consult (N=131) or consult +TCN (N=128) had greater increases in engagement in care (23.7% and 30.5% v. 11.1%, p=0.04 and <0.01 respectively) and virologic suppression (28.3% and 29.7% v.7.1%, p <0.01 for both) than the no intervention (N=225) subgroup. Hospitalized patients with HIV have low rates of engagement in care, retention in care and virologic suppression, though all three outcomes improved after hospitalization. A multidisciplinary transitions team improved care engagement and virologic suppression in those who received the intervention.
Keywords: Hospitalization, HIV care cascade, transition of care, retention
Introduction
Despite major decreases in mortality and morbidity since the introduction of antiretroviral therapy (ART) (Metsch et al., 2009; Zhao, Encinosa, & Hellinger, 2006), fewer than half of people living with HIV (PLWH) in the United States are engaged in ongoing medical care (Hall et al., 2012; Mugavero, 2016; Mugavero et al., 2012). Low rates of engagement and retention in HIV care result in decreased access to ART, uncontrolled HIV infection and poor health outcomes (Mugavero, 2016; Thompson et al., 2012). Certain subgroups are at higher risk for non-engagement in care, including PLWH who are black or Hispanic (CDC, 2014, 2017; Dasgupta, Oster, Li, & Hall, 2016; Horberg et al., 2015), in younger age groups (CDC, 2017; Horberg, et al., 2015) and who are living in the Southern US (Philbin et al., 2018). Various individual and structural barriers to engagement and retention in care have been identified, including housing instability (Holtzman, Brady, & Yehia, 2015), stigma (Holtzman, et al., 2015; Taylor et al., 2018), substance use disorders (Holtzman, et al., 2015), mental illness (Bengtson et al., 2018; Holtzman, et al., 2015; Yehia et al., 2015), limited social support (Taylor, et al., 2018) and transportation. New interventions in diverse settings are needed to overcome these barriers to reduce disparities in HIV outcomes.
Hospitalization represents a critical period to re-engage a vulnerable subset of PLWH (Buchacz et al., 2008; Lazar, Kersanske, Xia, Daskalakis, & Braunstein, 2017; Metsch et al., 2016) who are more likely to experience gaps in the HIV care cascade (Bell et al., 2010; E. M. Gardner and Haukoos, 2015; Kerr, Stephens, Gibson, & Duffus, 2012; Metsch, et al., 2016), but who demonstrate a high willingness to participate in re-engagement in care (Davila et al., 2017). Most interventions aiming to improve engagement in care and virologic suppression have been conducted in the outpatient setting through combinations of patient education (Cabral et al., 2018; Giordano et al., 2016), addressing specific barriers to care (Cabral, et al., 2018; Cunningham et al., 2018; L. I. Gardner et al., 2005; Giordano, et al., 2016; Irvine et al., 2015), telephone follow-up (Gentry, van-Velthoven, Tudor Car, & Car, 2013) and patient navigation (Craw et al., 2008). Several re-engagement interventions have focused on hospitalized PLWH, though with modest success (Giordano, et al., 2016; Metsch, et al., 2016). A multidisciplinary intervention, which addresses both medical and social factors associated with HIV outcomes as well as care coordination, has the potential to build on evidence-based interventions from the outpatient setting and extend this to inpatients.
In this study, we aim to (a) assess changes in the HIV care cascade (engagement in care, retention in care, virologic suppression) before and after hospitalization and (b) measure the impact of a multidisciplinary HIV inpatient intervention (medical consultation +/− transitional care nurse (TCN)) on care engagement and clinical outcomes.
Methods
We conducted a retrospective review of electronic health records (EHR) of PLWH hospitalized in the Parkland Health and Hospital System (PHHS) one year before and after the implementation of a multidisciplinary HIV inpatient team. Parkland is an 870 bed urban, public hospital and the primary safety net health system in Dallas, TX. This study was approved by the UT Southwestern Medical Center Institutional Review Board.
The multidisciplinary HIV transitions team, supported in part through the Center for Medicare and Medicaid Services (CMS) 1115 waiver program, was implemented in October 2014 with the goal of reducing 30-day hospital readmissions for HIV-positive inpatients. Members of the team address different aspects of patient care: medical (HIV specialists and advanced practice providers), social (TCNs) and care coordination (HIV case managers). The medical HIV team provides diagnostic and therapeutic recommendations and post-discharge follow-up and is activated when an HIV consult is placed by the primary treatment team. The TCN approaches patients within this consult group who are deemed high-risk for readmission (new HIV diagnosis, prior admission, psychosocial needs) to review barriers to care, complete patient education using a teach-back method and develop an individualized transitional care plan. HIV case managers provide care coordination (arrange funding, follow-up visits, referrals) for all hospitalized PLWH. Interdisciplinary team rounds are conducted each weekday. Prior to October 2014, medical consults for HIV inpatients were performed by the general infectious diseases team without formal coordination with HIV case management, and the TCN and advanced practice provider positions did not exist.
Data were obtained from the EHR (Epic systems, Verona, WI) and included all individuals with a diagnosis of HIV (as per ICD-9, ICD-10 code, positive HIV test result, or an HIV viral load ≥ 20 copies/mL); aged 18 or older; who had an inpatient admission to PHHS between 9/1/2013 and 12/30/2015 and who had had 1 or more outpatient HIV clinic visits within the hospital system prior to admission. Outpatient data (clinic visits and HIV viral loads) were obtained for one year preceding and following the inpatient admissions (i.e. 9/1/2012 through 12/30/2016).
Variables collected included: demographics (age, gender, race, ethnicity, marital status, primary language), socio-economic and behavioral variables (insurance status, homelessness, substance use, mental illness), clinical variables (CD4 count, HIV viral load) and hospitalization characteristics (primary inpatient diagnoses, length of stay, discharge status). Mental health was categorized into the following categories: depression/suicidality/mania; anxiety, schizophrenia/psychosis, other/multiple diagnoses based on ICD-9/10 classification. Substance use was determined by drug screen result.
Similar to previously published methods (Berry, Fleishman, Moore, Gebo, & Network, 2012; Berry et al., 2013), we determined the primary admitting diagnosis using the first listed ICD-9/ICD-10 code assigned at discharge, unless it was a code for HIV (042, B20, V08, Z21), in which case the second code was used. Clinical Classification Software (CCS) was used to assign primary ICD-9 and ICD-10 codes into one of 18 clinically meaningful categories (Elixhauser A, 2015) and modified as per previous studies (Berry, et al., 2012; Berry, et al., 2013). We reassigned end-organ infections to the non-AIDS-related infection category and defined a separate category for AIDS-defining illnesses (CDC, 1992).
Between March 2015 to June 2017 TCNs collected a checklist of barriers to HIV care including: incarceration, funding, mental health, substance use, social support, stigma, health literacy, homelessness, transportation, pill burden, and compatibility with provider from.
Engagement in care was defined as ≥1 HIV clinic visit within the six months prior to hospitalization or following discharge; retention in care was defined as two HIV clinic visits > 90 days apart within twelve months prior to hospitalization or following discharge; HIV virologic suppression was defined as <200 copies/mL (most recent value up to 90 days prior to admission and within 6 months after discharge).
The cohort was separated into two “treatment” groups for evaluation: those hospitalized before the team was implemented on October 1, 2014 (team=0), and those hospitalized after this date (team=1). Comparisons between groups were performed based on the Student’s t-test for continuous variables and Chi-square test for categorical variables. Nonparametric methods such as the Wilcoxon rank-sum test were employed where appropriate. The proportion of patients who were engaged in care, retained in care and had virologic suppression were determined for each group before and after hospitalization. For each binary outcome, we constructed GEE models (Zeger, Liang, & Albert, 1988) where the log link function was used. Covariates included time, intervention, and their interaction, and subject random effects were included to account for correlation among observations from the same subjects. Such models allow us to make inference about (a) the difference in each of outcome before and after hospitalization and (b) the difference in differences in each outcome between treatment groups (e.g. the difference in engagement in care before and after hospitalization compared between the pre- and post-intervention groups) based on the interaction terms.
To assess the impact of the HIV team, a “dose response, treatment received” approach focused on those who were hospitalized after the implementation of multidisciplinary team (team=1 only) and divided them into three groups: 1) not evaluated by the team (no consult), 2) evaluated by medical team but not TCN (consult only), and 3) evaluated by medical team and TCN (consult +TCN). Individuals who were approached by the TCN but who did not receive TCN services (e.g. patient refused), N=10, were included in the consult only group. Similar GEE logistic models were employed to assess the difference in outcomes (engagement, retention and suppression) before and after hospitalization between each of the three groups (no consult, consult only, and consult +TCN).
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
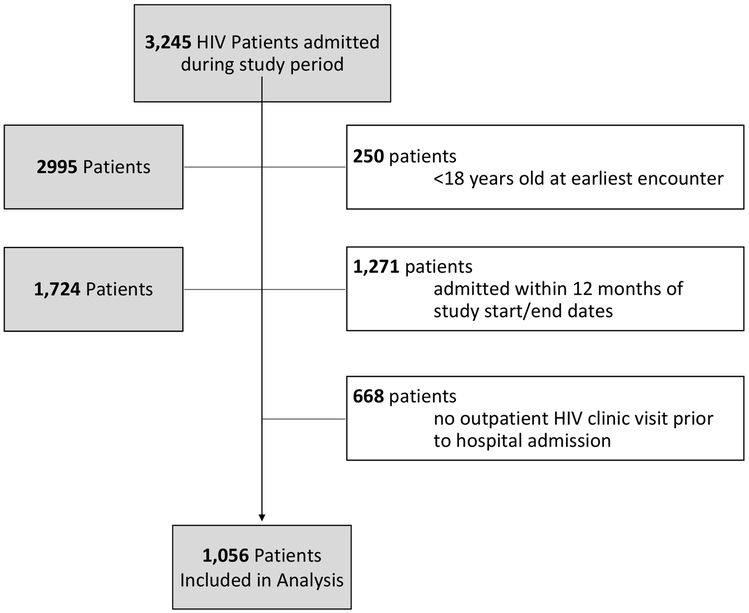
Overall 3245 individuals with HIV had an inpatient admission during the entire study period (9/1/2012–12/30/2016). Of these, we excluded: 250 were <18 years old, 1271 hospitalized before 9/1/2013 or after 12/30/2015, and 668 did not have any HIV clinic visit data prior to hospitalization, leaving 1056 individuals included in the final analysis (Figure 1).
Figure 1.
Flow diagram of final study cohort
The cohort was majority male (69%), non-Hispanic black (55%) and single (77%). Health insurance included Medicaid (33%), Medicare (27%), charity care (37%) and private insurance (3%). Nearly 40% had AIDS by CD4 criteria (18% CD4<50; 20% CD4 50– 200), and only 45% had an HIV viral load < 200 copies/mL. The most common causes for admission were: non AIDS-defining Infections (20%), followed by Respiratory (9%), Digestive (9%) and Circulatory (7%) systems. AIDS defining illnesses made up 5% of primary admitting diagnoses overall. The median length of hospital stay was longer in the post-intervention period (5 v 4 days, p<0.01), but otherwise baseline characteristics were similar between the two time periods (Table 1).
Table 1.
Baseline characteristics of HIV-positive patients at time of index admission
| Variable | Total HIV Admissions N=1056 |
Admitted before Transitions Team n=571 |
Admitted after Transitions Team n=485 |
P value |
|---|---|---|---|---|
| N (%) | n (%) | n (%) | ||
| Age study start, median (range) | 45 (18, 80) | 46 (18, 80) | 45 (19, 76) | 0.65 |
| Sex | ||||
| Male | 729 (69) | 388 (68) | 341 (70) | 0.41 |
| Female | 327 (31) | 183 (32) | 144 (30) | |
| Race/Ethnicity | ||||
| Non-Hispanic Black | 582 (55) | 308 (54) | 274 (56) | 0.55 |
| Non-Hispanic White | 211 (20) | 118 (21) | 93 (19) | |
| Hispanic | 240 (23) | 135 (24) | 105 (22) | |
| Other | 23 (2) | 10 (2) | 13 (3) | |
| Language | ||||
| English | 914 (87) | 488 (85) | 426 (88) | 0.43 |
| Spanish | 122 (12) | 70 (12) | 52 (11) | |
| Other | 20 (2) | 13 (2) | 7 (1) | |
| Marital status | ||||
| Single | 812 (77) | 442 (77) | 370 (76) | 0.77 |
| Married | 122 (11) | 57 (10) | 55 (11) | |
| Divorced/widowed/other | 132 (13) | 72 (13) | 60 (12) | |
| Insurance | ||||
| Medicaid | 346 (33) | 202 (35) | 144 (30) | 0.16 |
| Medicare | 284 (27) | 146 (26) | 138 (28) | |
| Charity/Self-pay | 392 (37) | 202 (35) | 190 (39) | |
| Private | 34 (3) | 21 (4) | 13 (3) | |
| Any Psychiatric Diagnoses | ||||
| Yes | 252 (24) | 126 (22) | 126 (26) | 0.14 |
| No | 804 (76) | 445 (78) | 359 (74) | |
| Psychiatric Diagnosis Class | ||||
| Depression/ Suicidality/ Mania | 92 (9) | 51 (9) | 41 (8) | 0.30 |
| Anxiety | 42 (4) | 17 (3) | 25 (5) | |
| Schizophrenia / Psychosis | 25 (2) | 12 (2) | 13 (3) | |
| Other/ Multiple Diagnoses | 93 (9) | 46 (8) | 47 (10) | |
| Drug Screen Result | ||||
| Positive | 119 (11) | 60 (11) | 59 (12) | 0.40 |
| Negative/ No Test | 937 (89) | 511 (89) | 426 (88) | |
| Positive Result Drug Class | ||||
| Amphetamines | 16 (2) | 7 (1) | 9 (1) | 0.61 |
| Benzodiazepines | 5 (0) | 3 (1) | 2 (0) | |
| Cocaine | 34 (3) | 16 (3) | 18 (4) | |
| Opiates | 38 (4) | 23 (4) | 15 (3) | |
| Multiple Drugs | 26 (2) | 11 (2) | 15 (3) | |
| Any History of Homelessness | ||||
| Yes | 89 (8) | 43 (8) | 46 (9) | 0.25 |
| No | 967 (92) | 528 (92) | 439 (91) | |
| CD4 | ||||
| <50 | 191 (18) | 103 (18) | 88 (18) | 0.75 |
| 51-200 | 214 (20) | 112 (20) | 102 (21) | |
| 201-499 | 315 (30) | 180 (32) | 135 (28) | |
| >500 | 221 (21) | 117 (20) | 104 (21) | |
| Unknown/ Missing | 115 (11) | 59 (10) | 56 (12) | |
| Viral load (preadmission) | ||||
| <20 | 341 (32) | 171 (30) | 170 (35) | 0.06 |
| 21-200 | 133 (13) | 83 (15) | 50 (10) | |
| 201-1000 | 48 (5) | 31 (5) | 17 (4) | |
| 1001-10,000 | 51 (5) | 28 (5) | 23 (5) | |
| >10,001 | 200 (19) | 115 (20) | 85 (18) | |
| Unknown/ Missing | 283 (27) | 145 (25) | 140 (29) | |
| Length of stay, days, median (range) | 4 (0, 75) | 4 (0, 75) | 5 (0, 66) | .01 |
| Primary admitting diagnosis | ||||
| AIDS-defining illness | 54 (5) | 34 (6) | 20 (4) | .01 |
| Non-ADI infections | 207 (20) | 99 (17) | 108 (22) | |
| Neoplasms | 40 (4) | 24 (4) | 16 (3) | |
| Endocrine/ nutrition/ metabolic/ immunity | 36 (3) | 24 (4) | 12 (2) | |
| Blood blood-forming organs | 15 (1) | 8 (1) | 7 (1) | |
| Mental Illness | 18 (2) | 11 (2) | 7 (1) | |
| Nervous system/ sense organs | 57 (5) | 35 (6) | 22 (5) | |
| Circulatory system | 71 (7) | 38 (7) | 33 (7) | |
| Respiratory system | 100 (9) | 43 (8) | 57 (12) | |
| Digestive system | 90 (9) | 47 (8) | 43 (9) | |
| Genitourinary system | 31 (3) | 15 (3) | 16 (3) | |
| Pregnancy/ childbirth/ puerperium | 49 (5) | 28 (5) | 21 (4) | |
| Skin subcutaneous tissue | 19 (2) | 11 (2) | 8 (2) | |
| Musculoskeletal system/ connective tissue | 54 (5) | 15 (3) | 39 (8) | |
| Injury poisoning | 33 (3) | 24 (4) | 9 (2) | |
| Misc. Health Status | 148 (14) | 92 (16) | 56 (12) | |
| Residual Codes | 34 (3) | 23 (4) | 11 (2) |
Self-reported barriers to care as collected by the TCN are presented in Table 2. The vast majority (85%), reported at least one barrier to care, and 39% had 3 or more barriers. Mental health (44%) and substance use (42%) were the most common, followed by funding issues (26%), lack of social support (26%) and incarceration (18%).
Table 2.
Barriers to care as recorded by Transitional Care Nurse
| Proportion with barrier N=882 |
|
|---|---|
| Any barrier to care | 85% |
| 1-2 barriers | 46% |
| >=3 barriers | 39% |
| Mental Health | 44% |
| Substance Use | 42% |
| Funding | 26% |
| Social support | 26% |
| Incarceration | 18% |
| Transportation | 16% |
| Homelessness | 14% |
| Pill Burden | 13% |
| Stigma | 11% |
| Child Care | 6% |
| Job Schedule | 3% |
| Health Literacy | 5% |
| Provider compatibility | 2% |
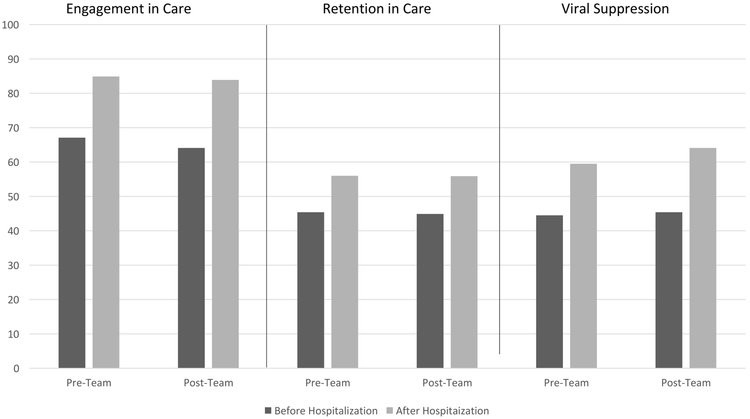
Each step of the HIV care cascade increased significantly after hospitalization for both time periods (p<0.01 for each comparison). At the population level (examining all-comers regardless of treatment received), the gains in the care cascade were not significantly different after the multidisciplinary intervention team compared to before its implementation. Post-discharge engagement in care increased 17.9% (95%CI 13.1–22.6; from 67.1% (383/571) to 85.0% (485/571)) in the pre-intervention period and 19.8% (14.8–24.8; from 64.1% (311/485) to 83.9% (407/485)) in the post-intervention period, p=0.79; retention in care increased 10.7% (5.4–16.0; from 45.3% (259/571) to 56% (320/571)) v. 10.9% (5.3–16.6; from 45% (218/485) to 55.9% (271/485)), p=0.95, and viral suppression increased 15.1% (10.7–19.5; from 44.4% (254/571) to 59.5%(340/571)) v. 18.8% (13.9–23.5; from 45.3% (220/485) to 64.1% (311/485)), p=0.25 (Figure 2).
Figure 2.
HIV Care Cascade before and after Hospitalization, Stratified by Pre- and Post-Transitions Team Time Period
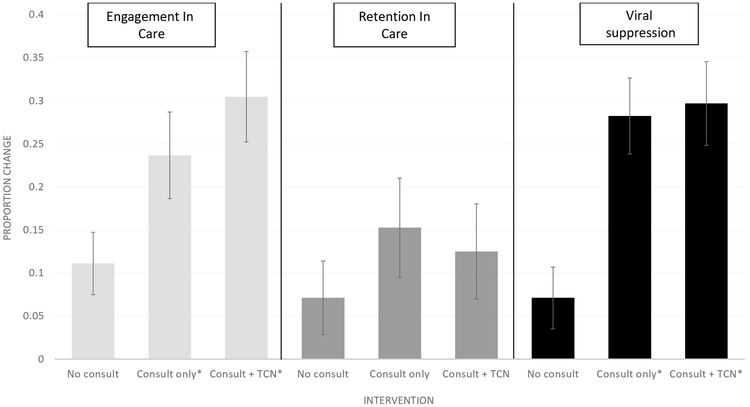
For analyses of the post-intervention group only (N=485), differences in each step of the care cascade were compared before and after hospitalization between three groups: 1) no consult N=225, 2) consult alone N=131, and 3) consult + TCN N=128. For engagement in care, patients who received these interventions had a significantly larger increase in post-discharge engagement when compared to those who did not receive any intervention components (consult (23.7%, 95% CI 14.2–33.1 from 61% (80/131) to 84.7% (111/131)) v. no consult (11.1%, 4.0–18.2, from 72.9% (164/225) to 84% (189/225)), p=0.04; TCN+ consult (30.5%, 20.2–40.8, from 52.3% (67/128) to 82.8% (106/128)) v. no consult (11.1%), p <0.01). There was no significant difference between those who received the consult alone (23.7%) vs. consult +TCN (30.5%), p=0.34. Increases in retention in care were not significantly different between the three groups (consult (15.3%,4.4–26.2 from 35.9% (47/131) to 51% (67/131)) v. no consult (7.1%, 1.3–15.5 from 55.6% (125/225) to 62.7% (141/225), p=0.24); consult +TCN (12.5%, 1.7–23.3, from 35.9% (46/128) to 48.4% (62/128) v. no consult (7.1%), p=0.44; and consult (15.3%) v. consult +TCN (12.5%), p=0.72. Increases in rates of virological suppression were significantly greater in consult group (28.3%, 20.0–36.5, from 30.5% (40/131) to 58.8% (77/131)) v. no consult (7.1%, 0.1–14.1, from 64.4% (145/225) to 71.5% (161/225), p<0.01) and in consult +TCN (29.7%, 20.2–39.2, from 26.6% (34/128) to 56.3% (72/128)) v. no consult (7.1%), p<0.01. There was no significant difference in increases in post-discharge virologic suppression between the two intervention groups, consult v. consult +TCN (28.3% v. 29.7%, p=0.82) (Figure 3).
Figure 3. Differences in Engagement in Care, Retention in Care, and Virologic Suppression Before and After Hospitalization During Intervention Period by Treatment Group.
Asterix indicates significant difference when compared to no consult group.
Discussion
We describe changes in the HIV care cascade for a large population of hospitalized PLWH who are predominantly male, of minority race/ethnicity, and either uninsured or receiving Medicaid. Despite prior linkage to HIV care, at the time of hospitalization this group had suboptimal retention in care (45%), low virologic suppression rates (45%) and a high proportion had AIDS (38%). As anticipated, we found that inpatient hospitalization was a key opportunity to improve re-engagement in HIV care, retention in care and virologic suppression. These improvements in the HIV care cascade after hospitalization were consistent over multiple years, from 2013–2015. We also found that implementation of a multi-disciplinary inpatient HIV team had a significantly larger impact on care engagement and virologic suppression among patients evaluated by the HIV medical team with or without a TCN visit (N=260 combined) compared to those who did not receive these services (N=225).
When comparing the entire post-intervention hospitalized group to historical controls, the improvements in the care cascade were not significantly different between the two time periods, though nearly half of the HIV-positive inpatients in the post-intervention time frame did not receive any additional services due to the HIV team not being consulted and/or limited TCN availability. In addition, when analyses were limited to those in the intervention time period, retention in care, using the Institute of Medicine definition of 2 visits >90 days apart in a 12 month period (Rebeiro et al., 2014), did not change significantly in the intervention groups compared to the no consult group. These results may be explained by limited sustainability of the intervention many months after hospital discharge, but may also be related to challenges in accurately measuring retention in care (Mugavero, Amico, Horn, & Thompson, 2013; Mugavero, et al., 2012), for which there is no gold standard.
Nearly all patients who meet with the TCN reported at least one barrier to HIV care continuity, especially mental health and substance use, followed by funding, social support, incarceration, transportation and homelessness. Over a third, 39%, reported ≥3 barriers to care, underscoring the social complexity of this inpatient cohort. Traditionally, mental healthcare in the inpatient setting focuses on acute symptom management (e.g. psychosis, suicidality), but does not address subacute problems, such as chronic depression, which may be a key contributor to hospitalization through nonadherence to medications, missed clinic visits, and substance use (Mitchell et al., 2010; Quinlivan et al., 2017; Zuniga, Yoo-Jeong, Dai, Guo, & Waldrop-Valverde, 2016). Similarly, acute medical issues related to substance use (e.g. overdose, withdrawal), may demand immediate medical management, whereas addiction treatment, such as medical assisted therapy or counseling, was until recently deferred to the post-discharge outpatient setting. New models of care which integrate mental health care and addiction services into the inpatient setting and provide a transition of care to continue this treatment after discharge are being implemented with positive results (Marks et al., 2018; Trowbridge et al., 2017; Wakeman, Metlay, Chang, Herman, & Rigotti, 2017), including in patients with HIV.
In our cohort, the primary medical reasons for admission were similar to other studies of PLWH, particularly those which include uninsured or safety net populations (Lazar, et al., 2017). We found that non-AIDS defining infections were most common, followed by respiratory, cardiovascular and gastro-intestinal causes. Our findings align with national trends documenting steady hospitalization rates among PLWH overall, with a decline in AIDS defining illnesses and an increase in other co-morbid conditions (Berry, et al., 2012; Gebo, Fleishman, & Moore, 2005).
Several other studies have examined the impact of interventions initiated in the inpatient setting on the HIV care cascade. A randomized intervention of peer mentoring sessions did not impact a combined outcome of virologic suppression and retention in care at 6 months (28% in each group) (Giordano, et al., 2016). In a multicenter study of hospitalized PLWH and substance use disorder randomized to patient navigation with or without financial incentives compared to treatment as usual, virologic suppression at 12 months (primary outcome), was not significantly different between groups. However, virologic suppression at 6 months (end of intervention) was 50% in those receiving navigation and incentives versus 38% in those receiving treatment as usual (p=0.03) (Metsch, et al., 2016). Both of these studies focused on high-risk subgroups (uncontrolled HIV, substance use disorders) and underscore the challenges with addressing multiple needs (peer support, education, care coordination) and sustainability of resource-intense interventions.
Our study has several limitations. First, it was conducted at a single site, which may limit generalizability. However, our safety-net population is representative of the HIV epidemic in other urban areas especially in the South, a region which leads the US in new HIV diagnoses but has worse clinical outcomes. Second, our intervention was not randomized, rather, it was delivered based on need (primary team requesting consult, readmission risk), and therefore our analyses are focused on a retrospective pre-post comparison using historical controls. However, our before and after populations were similar in terms of baseline characteristics. Lastly, we restricted our analyses to patients who at least one prior HIV clinic visit, thereby excluding new/recent diagnoses, patients who receive care from multiple institutions, and patients who had been lost to care.
At the time of hospital admission, many patients in our study had suboptimal engagement in outpatient HIV care and low rates of virologic suppression. The HIV care cascade improved after hospitalization in the overall study population, though these gains were not significantly greater in the post-intervention period compared to prior to implementation of the multidisciplinary transitions team. However, patients receiving one or more intervention component (HIV specialist consultation +/− TCN) had significantly greater improvements in engagement in care and virologic suppression than those who did not receive any intervention. The most common self-reported barriers to care were mental health and substance use. Hospitalization remains a key time and venue to re-engage out-of-care patients and improve clinical outcomes among PLWH. Future efforts to examine integration of routine mental health care and addiction medicine into inpatient care and coordination of post-hospitalization social services are needed.
Acknowledgments:
NIH K23 AI 112477, NIH R34 DA 045592, AHRQ R24 HS 022418
Footnotes
Conflicts: AN receives research funding from the Gilead FOCUS program.
Contributor Information
AE Nijhawan, Department of Internal Medicine, Division of Infectious Diseases, Department of Clinical Sciences, Division of Outcomes and Health Services Research, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, Texas 75390-9169; Parkland Health and Hospital Systems, Dallas, Texas.
M Bhattatiry, University of Texas Southwestern Medical Center, Dallas, Texas.
M Chansard, Department of Clinical Sciences, University of Texas Southwestern Medical Center, Dallas, Texas.
S Zhang, Department of Clinical Sciences, University of Texas Southwestern Medical Center, Dallas, Texas.
EA Halm, Department of Internal Medicine, Division of General Internal Medicine, Department of Clinical Sciences, Division of Outcomes and Health Services Research, University of Texas Southwestern Medical Center, Dallas, Texas.
References:
- Bell C, Metsch LR, Vogenthaler N, Cardenas G, Rodriguez A, Locascio V, . . . del Rio C (2010). Never in care: characteristics of HIV-infected crack cocaine users in 2 US cities who have never been to outpatient HIV care. J Acquir Immune Defic Syndr, 54(4), pp. 376–380. doi: 10.1097/QAI.0b013e3181d01d31 Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2888612/pdf/nihms181147.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson AM, Pence BW, Mimiaga MJ, Gaynes BN, Moore R, Christopoulos K, . . . Mugavero M (2018). Depressive Symptoms and Engagement in HIV Care following ART Initiation. Clin Infect Disdoi: 10.1093/cid/ciy496 Retrieved from https://academic.oup.com/cid/advance-article-abstract/doi/10.1093/cid/ciy496/5036558?redirectedFrom=fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SA, Fleishman JA, Moore RD, Gebo KA, & Network HIVR (2012). Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr, 59(4), pp. 368–375. doi: 10.1097/QAI.0b013e318246b862 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22240460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SA, Fleishman JA, Yehia BR, Korthuis PT, Agwu AL, Moore RD, . . . Network, H. I. V. R. (2013). Thirty-day hospital readmission rate among adults living with HIV. AIDS, 27(13), pp. 2059–2068. doi: 10.1097/QAD.0b013e3283623d5f Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23612008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchacz K, Baker RK, Moorman AC, Richardson JT, Wood KC, Holmberg SD, & Brooks JT (2008). Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. Aids, 22(11), pp. 1345–1354. doi: 10.1097/QAD.0b013e328304b38b Retrieved from https://insights.ovid.com/pubmed?pmid=18580614 [DOI] [PubMed] [Google Scholar]
- Cabral HJ, Davis-Plourde K, Sarango M, Fox J, Palmisano J, & Rajabiun S (2018). Peer Support and the HIV Continuum of Care: Results from a Multi-Site Randomized Clinical Trial in Three Urban Clinics in the United States. AIDS Behavdoi: 10.1007/s10461-017-1999-8 [DOI] [PubMed] [Google Scholar]
- CDC. (1992). 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep, 41(RR-17), pp. 1–19. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1361652 [PubMed] [Google Scholar]
- CDC. (2014). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2012. HIV surveillance supplemental report 2014. . 19. Retrieved. [Google Scholar]
- CDC. (2017). Social determinants of health and selected HIV care outcomes among adults with diagnosed HIV infection in 37 states and the District of Columbia, 2015. 22. Retrieved. [Google Scholar]
- Craw JA, Gardner LI, Marks G, Rapp RC, Bosshart J, Duffus WA, . . . Schmitt K (2008). Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr, 47(5), pp. 597–606. doi: 10.1097/QAI.0b013e3181684c51 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18285714 [DOI] [PubMed] [Google Scholar]
- Cunningham WE, Weiss RE, Nakazono T, Malek MA, Shoptaw SJ, Ettner SL, & Harawa NT (2018). Effectiveness of a Peer Navigation Intervention to Sustain Viral Suppression Among HIV-Positive Men and Transgender Women Released From Jail: The LINK LA Randomized Clinical Trial. JAMA Intern Med, 178(4), pp. 542–553. doi: 10.1001/jamainternmed.2018.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Oster AM, Li J, & Hall HI (2016). Disparities in Consistent Retention in HIV Care−-11 States and the District of Columbia, 2011–2013. MMWR Morb Mortal Wkly Rep, 65(4), pp. 77–82. doi: 10.15585/mmwr.mm6504a2 [DOI] [PubMed] [Google Scholar]
- Davila JA, Hartman C, Cully J, Stanley M, Amico KR, Soriano E, . . . Giordano TP (2017). Feasibility of identifying out of care HIV-positive patients in a hospital setting and enrolling them in a retention intervention. HIV Clin Trials, 18(2), pp. 75–82. doi: 10.1080/15284336.2017.1287536 Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5611820/pdf/nihms905042.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elixhauser A, S. C, Palmer L (2015). Clinical Classifications Software (CCS). U.S. Agency for Healthcare Research and Quality. Retrieved. [Google Scholar]
- Gardner EM, & Haukoos JS (2015). At the Crossroads of the HIV Care Continuum: Emergency Departments and the HIV Epidemic. Ann Emerg Med, 66(1), pp. 79–81. doi: 10.1016/j.annemergmed.2015.04.032 Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4478202/pdf/nihms696547.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LI, Metsch LR, Anderson-Mahoney P, Loughlin AM, del Rio C, Strathdee S, . . . Holmberg SD (2005). Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. Aids, 19(4), pp. 423–431. [DOI] [PubMed] [Google Scholar]
- Gebo KA, Fleishman JA, & Moore RD (2005). Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr, 40(5), pp. 609–616. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16284539 [DOI] [PubMed] [Google Scholar]
- Gentry S, van-Velthoven MH, Tudor Car L, & Car J (2013). Telephone delivered interventions for reducing morbidity and mortality in people with HIV infection. Cochrane Database Syst Rev(5), p Cd009189. doi: 10.1002/14651858.CD009189.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Cully J, Amico KR, Davila JA, Kallen MA, Hartman C, . . . Stanley M (2016). A Randomized Trial to Test a Peer Mentor Intervention to Improve Outcomes in Persons Hospitalized With HIV Infection. Clin Infect Dis, 63(5), pp. 678–686. doi: 10.1093/cid/ciw322 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27217266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall HI, Gray KM, Tang T, Li J, Shouse L, & Mermin J (2012). Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr, 60(1), pp. 77–82. doi: 10.1097/QAI.0b013e318249fe90 [DOI] [PubMed] [Google Scholar]
- Holtzman CW, Brady KA, & Yehia BR (2015). Retention in care and medication adherence: current challenges to antiretroviral therapy success. Drugs, 75(5), pp. 445–454. doi: 10.1007/s40265-015-0373-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horberg MA, Hurley LB, Klein DB, Towner WJ, Kadlecik P, Antoniskis D, . . . Silverberg MJ (2015). The HIV Care Cascade Measured Over Time and by Age, Sex, and Race in a Large National Integrated Care System. AIDS Patient Care STDS, 29(11), pp. 582–590. doi: 10.1089/apc.2015.0139 [DOI] [PubMed] [Google Scholar]
- Irvine MK, Chamberlin SA, Robbins RS, Myers JE, Braunstein SL, Mitts BJ, . . . Nash D (2015). Improvements in HIV care engagement and viral load suppression following enrollment in a comprehensive HIV care coordination program. Clin Infect Dis, 60(2), pp. 298–310. doi: 10.1093/cid/ciu783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JC, Stephens TG, Gibson JJ, & Duffus WA (2012). Risk factors associated with inpatient hospital utilization in HIV-positive individuals and relationship to HIV care engagement. J Acquir Immune Defic Syndr, 60(2), pp. 173–182. doi: 10.1097/QAI.0b013e31824bd55d Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22293549 [DOI] [PubMed] [Google Scholar]
- Lazar R, Kersanske L, Xia Q, Daskalakis D, & Braunstein SL (2017). Hospitalization Rates Among People With HIV/AIDS in New York City, 2013. Clin Infect Dis, 65(3), pp. 469–476. doi: 10.1093/cid/cix343 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28444155 [DOI] [PubMed] [Google Scholar]
- Marks LR, Munigala S, Warren DK, Liang SY, Schwarz ES, & Durkin MJ (2018). Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Disdoi: 10.1093/cid/ciy924 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30357363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsch LR, Bell C, Pereyra M, Cardenas G, Sullivan T, Rodriguez A, . . . del Rio C (2009). Hospitalized HIV-infected patients in the era of highly active antiretroviral therapy. Am J Public Health, 99(6), pp. 1045–1049. doi: 10.2105/ajph.2008.139931 Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2679801/pdf/1045.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsch LR, Feaster DJ, Gooden L, Matheson T, Stitzer M, Das M, . . . del Rio C (2016). Effect of Patient Navigation With or Without Financial Incentives on Viral Suppression Among Hospitalized Patients With HIV Infection and Substance Use: A Randomized Clinical Trial. JAMA, 316(2), pp. 156–170. doi: 10.1001/jama.2016.8914 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27404184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SE, Paasche-Orlow MK, Forsythe SR, Chetty VK, O’Donnell JK, Greenwald JL, . . . Jack BW (2010). Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med, 5(7), pp. 378–384. doi: 10.1002/jhm.673 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20577971 [DOI] [PubMed] [Google Scholar]
- Mugavero MJ (2016). Elements of the HIV Care Continuum: Improving Engagement and Retention in Care. Top Antivir Med, 24(3), pp. 115–119. [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Amico KR, Horn T, & Thompson MA (2013). The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis, 57(8), pp. 1164–1171. doi: 10.1093/cid/cit420 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23797289 [DOI] [PubMed] [Google Scholar]
- Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni ML, Gardner LI, . . . Retention in Care Study, G. (2012). Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr, 61(5), pp. 574–580. doi: 10.1097/QAI.0b013e318273762f Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23011397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbin MM, Feaster DJ, Gooden L, Duan R, Das M, Jacobs P, . . . Metsch LR (2018). The North-South divide: substance use risk, care engagement, and viral suppression among hospitalized HIV-infected patients in 11 U.S. cities. Clin Infect Disdoi: 10.1093/cid/ciy506 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29920584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlivan EB, Gaynes BN, Lee JS, Heine AD, Shirey K, Edwards M, . . . Pence BW (2017). Suicidal Ideation is Associated with Limited Engagement in HIV Care. AIDS Behav, 21(6), pp. 1699–1708. doi: 10.1007/s10461-016-1469-8 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27380390 [DOI] [PubMed] [Google Scholar]
- Rebeiro PF, Horberg MA, Gange SJ, Gebo KA, Yehia BR, Brooks JT, . . . Althoff KN (2014). Strong agreement of nationally recommended retention measures from the Institute of Medicine and Department of Health and Human Services. PLoS One, 9(11), p e111772. doi: 10.1371/journal.pone.0111772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BS, Fornos L, Tarbutton J, Munoz J, Saber JA, Bullock D, . . . Nijhawan AE (2018). Improving HIV Care Engagement in the South from the Patient and Provider Perspective: The Role of Stigma, Social Support, and Shared Decision-Making. AIDS Patient Care STDS, 32(9), pp. 368–378. doi: 10.1089/apc.2018.0039 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30179530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, . . . Nachega JB (2012). Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med, 156(11), pp. 817–833, W-284, W-285, W-286, W-287, W-288, W-289, W-290, W-291, W-292, W-293, W-294. doi: 10.7326/0003-4819-156-11-201206050-00419 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22393036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge P, Weinstein ZM, Kerensky T, Roy P, Regan D, Samet JH, & Walley AY (2017). Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat, 79, pp. 1–5. doi: 10.1016/j.jsat.2017.05.007 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28673521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman SE, Metlay JP, Chang Y, Herman GE, & Rigotti NA (2017). Inpatient Addiction Consultation for Hospitalized Patients Increases Post-Discharge Abstinence and Reduces Addiction Severity. J Gen Intern Med, 32(8), pp. 909–916. doi: 10.1007/s11606-017-4077-z Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28526932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia BR, Stephens-Shield AJ, Momplaisir F, Taylor L, Gross R, Dube B, . . . Brady KA (2015). Health Outcomes of HIV-Infected People with Mental Illness. AIDS Behav, 19(8), pp. 1491–1500. doi: 10.1007/s10461-015-1080-4 Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4527875/pdf/nihms-686504.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Encinosa W, & Hellinger F (2006). HIV Hospitalizations in 1998 and 2005: Statistical Brief #41 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]
- Zuniga JA, Yoo-Jeong M, Dai T, Guo Y, & Waldrop-Valverde D (2016). The Role of Depression in Retention in Care for Persons Living with HIV. AIDS Patient Care STDS, 30(1), pp. 34–38. doi: 10.1089/apc.2015.0214 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26544915 [DOI] [PMC free article] [PubMed] [Google Scholar]