Abstract
Background and aims
The prevalence of psychiatric disease in eosinophilic esophagitis (EoE) patients is not fully characterized. We aimed to determine the prevalence of psychiatric disease and centrally acting medication use in a cohort of EoE children and adults and evaluated whether psychiatric disease impacts EoE clinical presentation.
Methods
We conducted a retrospective study of newly diagnosed EoE cases at the University of North Carolina from 2002–2018. Psychiatric comorbidities and relevant treatments were extracted from medical records. The demographic and clinical features of EoE patients with and without psychiatric diagnoses, as well as those with and without psychiatric medication use, were compared.
Results
Of 883 patients (mean age 26.6, 68% male, 79% white), 241 (28%) had a psychiatric comorbidity. The most common diagnosis was anxiety (23%) followed by depression (17%); 28% of patients were treated pharmacologically. There were 45 patients (5%) treated pharmacologically without a psychiatric diagnosis for chronic pain syndromes, insomnia, and/or epilepsy. EoE cases with a psychiatric diagnosis were more likely to be female, white, ≥ 18 years old, and to have longer symptom duration prior to diagnosis.
Conclusions
Psychiatric comorbidities were common in EoE, seen in a third of adults and more than 1 in 7 children, and with similar proportions receiving a prescription medication. These illnesses affected EoE presentation, as psychiatric comorbidities were more likely in older, female, and white patients with a longer duration of symptoms preceding diagnosis.
Introduction
Eosinophilic esophagitis (EoE) is a chronic immune and antigen-mediated disease characterized clinically by symptoms of esophageal dysfunction and histologically by eosinophilic-predominant esophageal inflammation. The condition is diagnosed when there are at least 15 eosinophils per high-power field (eos/hpf) on esophageal biopsies following the exclusion of alternative etiologies of esophageal eosinophilia (1–4). Over the preceding two decades, EoE emerged as a major contributor to esophageal morbidity and now represents a leading cause of esophagitis, strictures, and food impaction (5,6).
Symptoms of EoE impact patients’ health-related quality of life (HRQOL) (7–10). While several studies address this association, especially in pediatric and adolescent populations, the impact of EoE on patients’ mental health, which is distinct from though related to HRQOL, remains poorly understood (11–13). As a consequence, the prevalence and spectrum of psychosocial problems, psychiatric comorbidities, and centrally acting medication use have not been extensively described in EoE patients. Knowledge related to psychosocial issues has also been highlighted as an area of need by patients (14). Moreover, no study has assessed whether the presence of a co-morbid psychiatric disorder associates with the clinical, endoscopic, or histologic features of EoE.
It has been our clinical impression that there is a substantial overlap between EoE and concomitant psychiatric/psychological conditions. Therefore, this study aimed to assess the prevalence and spectrum of psychiatric co-morbidities and centrally acting medication use in a large cohort of EoE children, adolescents, and adults. It also aimed to assess whether the presence of a co-morbid psychiatric disorder was associated with clinical, endoscopic, and/or histologic features of EoE at the time of EoE diagnosis.
Methods
We conducted a retrospective cohort study utilizing the University of North Carolina (UNC) EoE Clinicopathologic Database from 2002–2018. The UNC IRB approved this study. This database contains data extracted from the electronic medical records of incident cases of EoE seen at UNC diagnosed per consensus guidelines (1,2), as previously described (15–19).
The electronic medical record of every patient contained within the UNC EoE Clinicopathologic Database was searched to identify each EoE patient with a psychiatric comorbidity. Psychiatric comorbidities captured for this study included depression, anxiety, bipolar depression, post-traumatic stress disorder (PTSD), and schizophrenia. Psychiatric comorbidities were considered present if diagnosed by a clinician in the medical record. For each patient captured within the database, we extracted receipt of a centrally acting medication (e.g. selective serotonin reuptake inhibitor [SSRI], serotonin norepinephrine reuptake inhibitor [SNRI], tricyclic antidepressant, neuroleptic, benzodiazepine, buspirone, and other [e.g. trazodone, pregabalin, gabapentin, mirtazapine) as well as a history of counseling for a psychiatric diagnosis. We also confirmed that certain medications were not used for other indications (e.g. a benzodiazepine given during a procedure was not counted; gabapentin prescribed for a seizure disorder or diabetic neuropathy was not counted; etc.) among patients diagnosed with a psychiatric disorder.
Using a standardized data collection form, we also collected cohort demographics, symptoms, endoscopic findings, and histologic findings from the electronic medical records and the UNC Clinicopathologic database for all EoE patients at the time of EoE diagnosis. Of note, the EoE Endoscopic Reference Score was available for only 1/3 of our database (since our database includes many cases from prior to when EREFS was established). As such, we utilized endoscopic descriptor terms.
Descriptive statistics were used to characterize the patient cohort. Bivariate statistics with Pearson’s chi-square test were performed to compare categorical variables. Similarly, bivariate statistics with student’s t-test were performed to compare continuous variables. Analysis of variance was used to compare continuous variables for non-dichotomous categories. We then used multivariate regression models to assess the relationship between the presence of a psychiatric diagnosis and the clinical features of EoE. To identify potential confounders, we assessed whether any of the covariates were unequally distributed between patients with and without a psychiatric comorbidity by using means for continuous variables and percent’s for categorical variables. For each model created, we also performed bivariate analyses to assess for unadjusted relationships between each of the independent variables and the predictor variable of interest (e.g. gender, race, age at EoE diagnosis, atopic disease diagnosis, and baseline eosinophil count). We then used logistic regression to estimate the dependent variable by outcome odds ratio for that model, adjusted for confounding. Change-in-effect was used to remove any variables from the model(s) that did not meaningfully change the adjusted odds ratio estimate(s), and thus were not founders of the relationship(s). Any factors that changed the fully adjusted odds ratio(s) by > 10% when dropped were considered confounders and retained in the final model.
All statistical analyses were performed using Stata 14.2 (Stata Corp, College Station, TX). Additional non-parametric testing did not substantively change the conclusions of this study. Non-statistical analyses comparing our data with nationally representative figures (20–25) were also included in this study in order to assess the relative frequency of psychiatric comorbidities in a general population with those in an EoE population.
Results
Baseline cohort characteristics at time of EoE diagnosis
We analyzed 883 patients with an initial diagnosis of EoE with active disease, and 241 (28%) had a psychiatric comorbidity. In the overall EoE cohort, the mean age at EoE diagnosis was 26.6 years, and most patients were male (68%) and white (79%). There were 506 (57%) adults and 377 (43%) children or adolescents.
Spectrum of psychiatric comorbidities and burden of medication use
Of the 241 EoE patients diagnosed with a psychiatric comorbidity, the most common diagnoses were anxiety (23%) and depression (17%). Bipolar depression (3%), PTSD (1%), and schizophrenia (0.3%) were infrequently reported. Overall, adults were more likely to have received a psychiatric diagnosis than children (33% vs. 15%; p < 0.001). Individual psychiatric diagnoses were also significantly more prevalent in adults than children, with anxiety (25% vs. 13%, p<0.001) and depression (24% vs. 6%, p<0.001) being most common.
For this cohort, 28% of patients were treated medically, and we had documentation of 6% receiving counseling for a psychiatric comorbidity. Centrally acting medication use was similarly more common in adults compared to children, with SSRIs (23% vs. 9%, p < 0.001) and benzodiazepines (16% vs. 5%, p <0.001) being frequently prescribed. Overall, adults with a psychiatric diagnosis were more likely than children to have received any medicinal treatment (36% vs. 17%; p < 0.001) but not counseling (6% vs. 5%; p = 0.42). There were also 45 (5%) patients treated with a psychiatric medication without diagnosis of a psychiatric disorder (Table 1). Indications for treatment included history of seizures (16%), insomnia (18%), and history of chronic pain syndrome (60%), and the most commonly prescribed medication among this subset was gabapentin (36%).
Table 1.
Baseline demographics, symptoms, endoscopy, and eosinophil counts (n = 883).
| Psychiatric diagnosis (n = 241) | No diagnosis or psychiatric medications (n = 583) | No diagnosis, on psychiatric medications (n = 45) | P value | |
|---|---|---|---|---|
| Demographics (N, %) | ||||
| Age (mean y ± SD)1 | 33.9 ± 18.3 | 23.5 ± 17.7 | 28.6 ± 18.6 | 0.06 |
| Symptom length (mean y ± SD)2 | 8.94 ± 8.9 | 6.74 ± 7.75 | 7.61 ± 7.63 | 0.01 |
| Male | 127 (53) | 433 (74) | 31 (69) | < 0. 001 |
| White | 211 (88) | 444 (77) | 30 (67) | < 0.001 |
| Atopic diagnosis | 107 (45) | 234 (42) | 18 (40) | 0.31 |
| Symptoms (N, %) | ||||
| Dysphagia | 187 (79) | 397 (69) | 33 (73) | 0.02 |
| Food impaction | 66 (28) | 179 (32) | 12 (27) | 0.23 |
| Heartburn | 109 (46) | 192 (34) | 10 (22) | 0.002 |
| Chest pain | 35 (15) | 47 (8) | 6 (13) | 0.03 |
| Abdominal pain | 56 (24) | 109 (19) | 9 (20) | 0.26 |
| Endoscopy findings (N, %) | ||||
| Normal | 21 (9) | 67 (12) | 12 (27) | 0.003 |
| Exudates | 90 (37) | 233 (40) | 13 (29) | 0.27 |
| Rings | 121 (50) | 258 (45) | 25 (56) | 0.16 |
| Edema | 80 (33) | 218 (38) | 14 (31) | 0.36 |
| Furrows | 149 (62) | 365 (63) | 23 (51) | 0.27 |
| Narrow | 39 (17) | 92 (16) | 5 (11) | 0.68 |
| Stricture | 57 (24) | 130 (22) | 11 (24) | 0.91 |
| Dilation | 66 (27) | 129 (22) | 11 (24) | 0.31 |
| Peak eosinophil count3 | ||||
| 63.8 ± 46.2 | 68.6 ± 44.8 | 59.6 ± 43.1 | 0.004 | |
Age at diagnosis;
Symptom length: Length of symptomatic period preceding diagnosis;
Max peak eosinophil count: eosinophils per high-power field ± SD standard deviation
Within this cohort, 96 (40%) patients were diagnosed with a psychiatric illness following the diagnosis of EoE. Moreover, patients diagnosed with a psychiatric illness prior to EoE diagnosis had a longer duration of symptoms preceding EoE diagnosis compared to those with a psychiatric diagnosis after EoE diagnosis (11.0 vs 7.2 years; p = 0.01).
In regards to patients ultimately treated with a corticosteroid prescription, we found no association between corticosteroid use and diagnosis of a psychiatric condition (p = 0.98).
Comparison of EoE patients with and without a psychiatric comorbidity
Compared to those without a psychiatric diagnosis, EoE patients with a psychiatric diagnosis were more likely to be female (47% vs. 26%; p < 0.001), white (88% vs. 76%; p < 0.001), and to be ≥ 18 years old at the time of EoE diagnosis (75% vs. 51%; p <0.001). These patients were also found to have a longer symptom duration preceding EoE diagnosis (8.9 years vs. 6.8 years; p = 0.002). Prior to adjustment for covariates, EoE patients with a psychiatric diagnosis more commonly reported symptoms of dysphagia (79% vs. 69%; p = 0.005), heartburn (46% vs. 33%; p = 0.001), and chest pain (15% vs. 9%; p = 0.02). Endoscopic findings did not differ between the two groups. While peak eosinophil counts differed statistically, this difference was not clinically significant.
Comparisons were also made between patients with a psychiatric diagnosis, patients without receipt of a psychiatric diagnosis or psychiatric medications, and those without a psychiatric diagnosis or receipt of psychiatric medications. Similar findings were found for these three groups as for dichotomously comparing those with and without a psychiatric diagnosis alone (Table 1).
After controlling for covariates, adult age, longer symptom length, female sex, and white race were found to be independently associated with a psychiatric diagnosis (Table 2). As compared with the bivariate analysis, predicted baseline eosinophil counts remained comparable (61.0 vs. 68.6 eos/hpf; p=0.02) for patients with and without psychiatric comorbidities, though no longer statistically different.
Table 2.
Adjusted odds ratios for associations between psychiatric diagnosis and demographics, symptoms, endoscopic, and histologic features
| Adjusted OR (95% CI) | |
|---|---|
| Demographics (N, %) | |
| Adult1 | 2.78 (1.95 – 3.96) |
| Symptom length2 | 1.03 (1.01 – 1.05) |
| Male | 0.45 (0.32 – 0.63) |
| White | 1.86 (1.17 – 2.94) |
| Atopic diagnosis | 0.96 (0.78 – 1.19) |
| Symptoms (N, %) | |
| Dysphagia | 1.00 (0.66 – 1.52) |
| Food impaction | 0.65 (0.46 – 0.93) |
| Heartburn | 1.05 (0.86 – 1.29) |
| Chest pain | 0.86 (0.65 – 1.15) |
| Abdominal pain | 0.92 (0.72 – 1.17) |
| Endoscopy findings (N, %) | |
| Normal | 0.86 (0.48 – 1.54) |
| Exudates | 1.01 (0.72 – 1.41) |
| Rings | 0.66 (0.45 – 0.95) |
| Edema | 0.88 (0.63 – 1.24) |
| Furrows | 0.91 (0.65 – 1.29) |
| Narrow | 0.82 (0.53 – 1.28) |
| Stricture | 0.78 (0.53 – 1.17) |
| Dilation | 0.85 (0.57 – 1.26) |
| Max peak eosinophil count3 | |
| 63.6 vs. 68.1; p = 0.22 | |
Adult: comparison of proportion of patients ≥ 18 years old at diagnosis and symptom length adjusted
Symptom length: increased odds for psychiatric diagnosis per each year of age added;
Max peak eosinophil count: comparison of predicted max eosinophil count
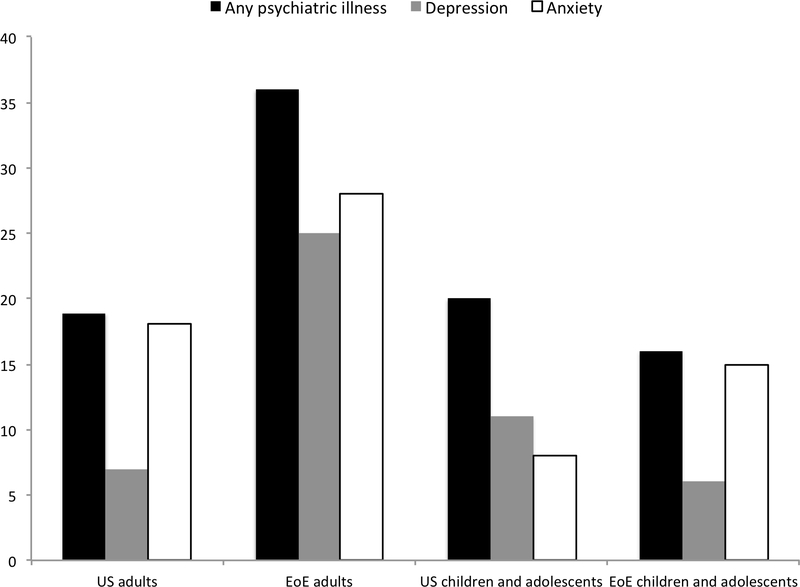
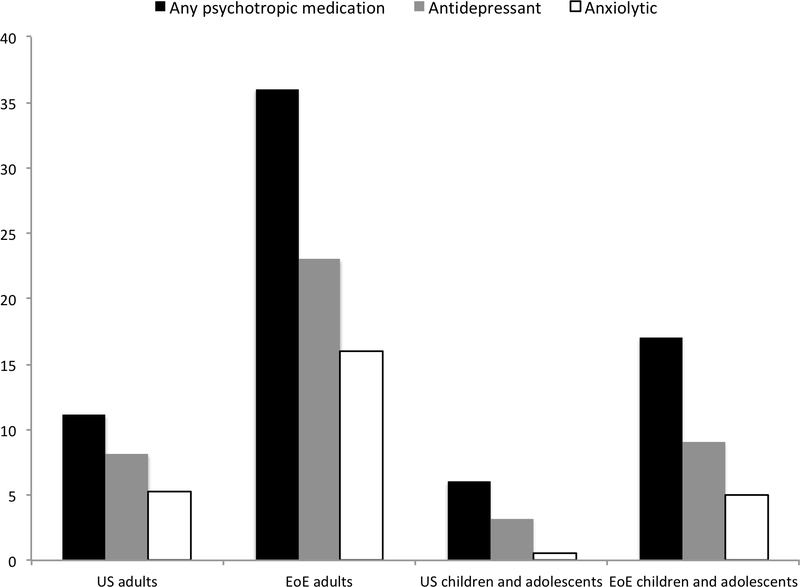
The prevalence of a psychiatric condition(s) and the prescription of psychotropic medications were also compared with nationally representative data. In our cohort, 36% of adult EoE patients had received a diagnosis of any psychiatric condition, which compares to 18.9% of the general US adult population (20). Similarly, depression (25% EoE patients vs. 6.9% US population) (20) and anxiety (18% vs. 5.2%) (20), specifically, were more common in our cohort. This trend continued when comparing the use of centrally acting medications. The use of any centrally acting medication was found in 36% of our EoE adult patients versus 11% of the US adult population (21). Individual categories of centrally acting medication use were also more common. For example, 16% of EoE patients versus 5.2% of the US population have received benzodiazepines for psychiatric illness (22). The prevalence of psychotropic medication use, but not psychiatric diagnoses, was also higher in children and adolescents with EoE compared to US children and adolescents overall. National figures report that approximately 20% of children and adolescents have been diagnosed with any psychiatric diagnosis, which compares to 16% of our cohort (20). However, any centrally acting medication use (17% vs. 6%), antidepressant use (9% SSRI vs. 3.2% any anti-depressant), and benzodiazepine use (5% vs. 0.5%) was considerably more common in our EoE adolescents and children compared to national figures (23–25).
Discussion
Though EoE has been shown to impact the HRQOL of affected patients (7–10), limited data explore the association with psychiatric disease (11–13). This holds particularly true for adults diagnosed with EoE. In this study, we aimed to determine the prevalence and spectrum of psychiatric diseases and centrally acting medication use in a large cohort of EoE patients. We also aimed to explore the association between the presence of a psychiatric comorbidity and presenting features of EoE. We found that psychiatric comorbidities were common in adults and children with EoE. There were one-third of adults and more than 1 in 7 children who had received a diagnosis of a psychiatric condition. Moreover, similar proportions of adults and children with EoE in our cohort had received a prescription medication for a psychiatric illness. We also found that EoE patients with psychiatric comorbidities are more likely to be older, female, white, and to have a longer duration of symptoms preceding EoE diagnosis. However, endoscopic findings did not differ by the presence of a psychiatric comorbidity and histologic findings did not meaningfully differ. Of note, patients described in this study had prior non-response to a trial of proton pump inhibition, given their addition to the database before the 2017 and 2018 guidelines removing this diagnostic requirement, and as such, generalizability to patients with a histologic response to proton pump inhibition should be made cautiously (3,4). It’s also interesting to note that patients diagnosed with a psychiatric illness prior to EoE diagnosis had a longer duration of symptoms preceding EoE diagnosis. This raises the question of the impact of long-standing and undiagnosed symptoms on patients’ wellbeing (26).
Symptoms of EoE impact patients’ health-related quality of life (HRQOL) (7–10). The most comprehensive assessment of this association was described in a 2018 systematic review of 22 studies showing that the condition disrupts and restricts the daily life of patients (7). Moreover, EoE likely negatively impacts the wellbeing of patients’ caregivers and patients’ families. The authors also found that EoE symptom severity likely correlates with EoE’s impact on HRQOL, and that this impact appears to decrease with successful treatment of EoE itself.
Less robust data exist for psychosocial dysfunction experienced by EoE patients, which largely has pertained to and been described in children and adolescents (11–13). In one retrospective study of 64 children with EoE (12), patients were evaluated to identify psychosocial problems experienced from infancy through adolescence. Here, 2 in 3 children were found to have difficulties in at least one of the areas assessed (e.g. sleep, social, school, anxiety, and depression). For instance, 41% and 28% of included patients had experienced issues with anxiety and depression, respectively. An additional study of a multi-center registry assessed the clinical features of a cohort of EoE patients largely consisting of children and adolescents (median age: 10.7 years; interquartile range: 6.9 – 16.9 years) (11). Similar to values presented in our study, they found that approximately 15% of the patients within their registry had reported depression and/or anxiety, and that this prevalence increased with patient age.
For the majority of psychiatric conditions and medication classes assessed within this study, their diagnosis and use were greater in our cohort of EoE patients compared with nationally representative data. This finding was particularly pronounced for adult EoE patients (Figure 1). In our cohort of adult EoE patients, the diagnosis of any psychiatric condition as well as the use of any centrally acting medication were considerably more common than the prevalence reported for the US population. Though the proportion of children with a psychiatric diagnosis mirrored national numbers, we also saw that the prevalence of psychotropic medication use was higher in our cohort of children and adolescents.
Figure 1.
A.National versus cohort prevalence of psychiatric diagnoses. Nationally representative data obtained from the National Alliance on Mental Illness and published medical literature.20–25
B. National versus cohort prevalence of psychotropic medication use. Nationally representative data obtained from the National Alliance on Mental Illness and published medical literature.20–25
There are limitations to this study. Given that this study was retrospective, there was the potential for measurement bias. However, we attempted to minimize this by requiring a confirmed diagnosis made by a health care provider, and confirmed that medication was relevant and directed at the psychiatric comorbidity rather than a different indication. Because of this, reported values may be an underestimate of psychiatric comorbidities. We also did not collect data using validated symptom measures and standardized instruments were not used to diagnose conditions or record patient symptoms, as data were not collected in a prospective manner and some data collection preceded the validation of symptom measures. Symptom findings should be interpreted with caution. Despite these issues, there are multiple strengths to this paper. This study, according to our understanding of the current literature base, now represents the largest and only cohort study directly assessing the association between psychosocial dysfunction and clinical features of EoE. It is also the only study to assess the prevalence and spectrum of psychiatric disorders in a cohort of adults with EoE. Data extraction was rigorous, the cohort consisted of only patients meeting a consensus diagnosis of EoE, and all patients were assessed for a psychiatric condition throughout their inclusion in the UNC EoE Clinicopathologic Database. This helped capture the full contingent of patients in our cohort with co-morbid psychiatric illness.
In conclusion, we found that psychiatric comorbidities were common in a large cohort of EoE patients. There were a third of adults and more than 1 in 7 children receiving a psychiatric diagnosis, and a similar proportion of patients receiving a prescription medication for a psychiatric disease. After adjusting for potential confounders, we also found that EoE patients with psychiatric comorbidities are more likely to be older, female, white, report heartburn, and to have a longer duration of symptoms. This study suggests that psychiatric disease should be screened for in all patients with EoE, and it provides the initial evidence that psychiatric illness may influence the presentation of EoE. Future studies should further explore whether psychiatric disease activity drives EoE clinical features or whether psychiatric disease activity is a consequence of living with EoE itself.
Study highlights.
1. WHAT IS CURRENT KNOWLEDGE
The prevalence of psychiatric disease in eosinophilic esophagitis patients is not fully characterized.
Symptoms of eosinophilic esophagitis impact patients’ health-related quality of life.
2. WHAT IS NEW HERE
Psychiatric comorbidities and psychotropic medication use were common in eosinophilic esophagitis patients, seen in a third of adults and more than 1 in 7 children.
Psychiatric illness influenced eosinophilic esophagitis presentation.
This study suggests that psychiatric disease should be screened for in all patients with eosinophilic esophagitis.
Acknowledgments
Financial support
This research was supported by NIH Awards T35 DK007386 (MT) and R01 DK101856 (ESD).
Potential competing interests: Dr. Dellon is a consultant for Adare, Aimmune, Alivio, Allakos, AstraZeneca, Banner, Biorasi, Calypso, Enumeral, EsoCap, Gossamer Bio, GSK, Receptos/Celegene, Regeneron, Robarts, Salix, and Shire, receives research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire, and has received an educational grant from Allakos, Banner, and Holoclara. None of the other authors report and potential conflicts of interest with this study.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679–692. [DOI] [PubMed] [Google Scholar]
- 3.Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5(3):335–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018;155(4):1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellon ES, Kim HP, Sperry SLW, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79(4):577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol. 2015;110(5):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukkada V, Falk GW, Eichinger CS, et al. Health-Related Quality of Life and Costs Associated With Eosinophilic Esophagitis: A Systematic Review. Clin Gastroenterol Hepatol. 2018;16(4):495–503. [DOI] [PubMed] [Google Scholar]
- 8.Debrosse CW, Franciosi JP, King EC, et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol. 2011;128(1):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franciosi J, Hommel K, DeBrosse C, et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern E, Taft T, Zalewski A, Gonsalves N, Hirano I. Prospective assessment of disease-specific quality of life in adults with eosinophilic esophagitis. Dis Esophagus. 2018;131(4). [DOI] [PubMed] [Google Scholar]
- 11.Chehade M, Jones SM, Pesek RD, et al. Phenotypic Characterization of Eosinophilic Esophagitis in a Large Multicenter Patient Population from the Consortium for Food Allergy Research. J Allergy Clin Immunol Pract. 2018;6(5):1534–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RF, Menard-Katcher C, Atkins D, et al. Psychosocial dysfunction in children and adolescents with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2013;57(4):500–5. [DOI] [PubMed] [Google Scholar]
- 13.Hommel KA, Franciosi JP, Gray WN, et al. Behavioral functioning and treatment adherence in pediatric eosinophilic gastrointestinal disorders. Pediatr Allergy Immunol. 201223(5):494–9. [DOI] [PubMed] [Google Scholar]
- 14.Hiremath G, Kodroff E, Strobel MJ, et al. Individuals affected by eosinophilic gastrointestinal disorders have complex unmet needs and frequently experience unique barriers to care. Clin Res Hepatol Gastroenterol. 2018; 42(5):483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed C, Fan C, Koutlas N, et al. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46(9):836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Runge TM, Eluri S, Cotton CC, et al. Outcomes of esophageal dilation in eosinophilic esophagitis: Safety, efficacy, and persistence of the fibrostenotic phenotype. Am J Gastroenterol. 2016;111(2):206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eluri S, Runge TM, Hansen J, et al. Diminishing Effectiveness of Long-Term Maintenance Topical Steroid Therapy in PPI Non-Responsive Eosinophilic Esophagitis. Clin Transl Gastroenterol. 2017;8(6):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eluri S, Selitsky S, Perjar I, et al. Clinical and Molecular Factors Associated With Histologic Response to Topical Steroid Treatment in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2019;17(6):1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed CC, Fan C, Koutlas N, et al. Compounded Oral Viscous Budesonide is Effective and Provides a Durable Response in Eosinophilic Esophagitis. HSOA J Gastroenterol Hepatol Res. 2018;7(1):2509–2515. [PMC free article] [PubMed] [Google Scholar]
- 20.Retrieved June 6 2019 Mental health by the numbers. from Https//www.nami.org/Learn-More/Mental-Health-By-the-Numbers.
- 21.Paulose-Ram R, Safran MA, Jonas BS, et al. Trends in psychotropic medication use among U.S. adults. Pharmacoepidemiol Drug Saf. 2007;16(5):560–70. [DOI] [PubMed] [Google Scholar]
- 22.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–42. [DOI] [PubMed] [Google Scholar]
- 23.Jonas BS, Gu Q, Albertorio-Diaz JR. Psychotropic medication use among adolescents: United States, 2005–2010. NCHS Data Brief. 2013; 135:1–8. [PubMed] [Google Scholar]
- 24.Olfson M, He JP, Merikangas KR. Psychotropic medication treatment of adolescents: Results from the national comorbidity survey-adolescent supplement. J Am Acad Child Adolesc Psychiatry. 2013;52(4):378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Leon S, Lopez-Gomez MI, Warner B, Ruiter-Lopez L. Psychotropic medication in children and adolescents in the United States in the year 2004 vs 2014. DARU, J Pharm Sci. 2018;26(1):5–10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed CC, Koutlas NT, Robey BS, et al. Prolonged Time to Diagnosis of Eosinophilic Esophagitis Despite Increasing Knowledge of the Disease. Clin Gastroenterol Hepatol. 2018; 16(10):1667–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]