Abstract
Various lifestyle factors including physical activity and obesity, stress, sleep and smoking may modify risk of developing inflammatory bowel diseases (IBD). In patients with established IBD, these lifestyle factors may significantly impact natural history and clinical outcomes. Recreational exercise decreases risk of flare and fatigue in patients with IBD. In contrast, obesity increases the risk of relapse, and is associated higher anxiety, depression, fatigue and pain and higher healthcare utilization. Obesity also modifies pharmacokinetics of biologic agents unfavorably and is associated with higher risk of treatment failure. Sleep disturbance is highly prevalent in patients with IBD, independent of disease activity, and increases the risk of relapse and chronic fatigue. Similarly, stress, particularly perceived stress rather than major life events, may trigger symptomatic flare in patients with IBD, though it’s impact on inflammation is unclear. Cigarette smoking is associated with unfavorable outcomes including risk of corticosteroid-dependence, surgery and disease progression in patients with Crohn’s disease; in contrast, smoking does not significantly impact outcomes in patients with ulcerative colitis, though some studies suggest that it may be associated with lower risk of flare. The effect of alcohol and cannabis use in patients with IBD is inconsistent, with some studies suggesting cannabis may decrease chronic pain in patients with IBD, without a significant effect of biological remission. While these lifestyle factors are potentially modifiable, only a few interventional studies have been conducted. Trials of structured exercise and psychological therapy including mindfulness-based therapies such as meditation and yoga, and gut-directed hypnotherapy have not consistently demonstrated benefit in clinical and/or endoscopic disease activity in IBD, though may improve overall quality of life.
Keywords: Prognosis, stress, lifestyle, adiposity, Crohn’s disease
The global incidence and prevalence of inflammatory bowel diseases (IBD) is rising, with steep increase in incidence in newly industrialized countries.1 Various lifestyle factors besides diet including physical activity (and obesity) and smoking have been implicated in the recent epidemiological trends in the risk of developing IBD, and have been covered elsewhere.2, 3 This review focuses on how important lifestyle factors – physical activity (and obesity), smoking, alcohol consumption and cannabis use, sleep, and stress – affect the natural history and outcomes in patients with established IBD, and how modifying lifestyle may improve IBD outcomes. The impact of diet and dietary modification in patients with established IBD has been covered elsewhere.
PHYSICAL ACTIVITY AND OBESITY IN PATIENTS WITH IBD
Physical activity and exercise may be associated with decreased risk of developing IBD, particularly Crohn’s disease (CD). However, the development of IBD, particularly presence of active symptoms, impairs patients’ ability to exercise and participation in sports activities.4, 5 In survey studies, approximately 50% patients report that IBD moderately or significantly impacted their fitness.4 One of the consequences of lack of exercise is obesity. Approximately 15–40% adult patients with IBD are obese (body mass index [BMI] ≥30 kg/m2) and an additional 20–40% are overweight, with a comparable distribution of obesity in CD and ulcerative colitis (UC).6
Impact of Physical Activity and Obesity on Natural History of IBD
Physiologically, the benefits of exercise in boosting immune response and reducing pro-inflammatory cytokines is well known.7 Voluntary exercise in animal models of chemical-induced colitis have also shown to mitigate symptoms and reduce inflammatory burden.8 However, there have been limited studies on the impact of physical activity and exercise on the natural history of IBD. Most cross-sectional studies have focused on how IBD disease activity may negatively impact ability to exercise, and are confounded by disease severity. In a prospective cohort study of 1857 patients with IBD, Jones and colleagues observed that higher level of exercise was independently associated with 24–32% lower risk of symptomatic relapse over 6 months among patients in remission.9
Obesity, in contrast, has been associated with a lower prevalence of clinical remission and higher anxiety, depression, fatigue, pain and inferior social function scores on PROMIS measures, as compared to non-obese patients with IBD.10 Longitudinal studies suggest that obesity may negatively impact clinical course and healthcare utilization (Table 1). In a large internet-based cohort study of 7296 patients with IBD (4748 patients with CD, 19.5% obese; 2548 patients with UC, 20.3% obese), Jain and colleagues observed that obesity was independently associated with increased risk of persistent disease activity or relapse in patients with CD (class II or III obesity vs. normal BMI: OR, 1.86; 95% CI, 1.30–2.68) and UC (OR, 2.97; 95% CI, 1.75–5.17), with a dose-response relationship.10 In a propensity score-matched, nationally representative cohort study of 42,285 patients with IBD (12.4% obese), Nguyen and colleagues observed that obese patients with IBD had a higher annual burden and costs of hospitalization, as compared to non-obese patients.11 Besides overall obesity, visceral adiposity has been more consistently associated with adverse outcomes in patients with IBD. High visceral adipose tissue volume was associated with increased risk of penetrating or stricturing complications (OR, 1.7; 95% CI, 1.1–2.9), hospitalization (OR, 1.9; 95% CI, 1.2–3.4) and shorter time interval to surgery (HR, 1.4; 95% CI, 1.0–2.0), after adjusting for age and BMI in a pediatric cohort of patients with CD.12 High visceral fat area has also been associated with increased risk of recurrence of CD after surgical resection.13
Table 1.
Key longitudinal studies on the impact of obesity on disease course and complications in patients with inflammatory bowel diseases
| Author, Year | Location, Time Period | Patient characteristics | Prevalence of obese and overweight | Key Findings |
|---|---|---|---|---|
| Flores,89 2015 | 2 centers, retrospective, cohort study; Dallas, TX, USA; 2000–12; median follow-up, 5.2y | CD: 297; 31% female • Disease duration, 15y; • Ileocolonic disease, 47%; • Penetrating disease, 20%; • Biologics, 45% UC: 284; 24% female • Extensive UC, 59%; • Disease duration, 12y; • Biologics, 16% |
CD: 30.3% obese; 33.3% overweight (based on average BMI of each patient during the study period) UC: 35.2%; 71.5% (based on average BMI of each patient during the study period) |
Crohn’s Disease 1. No significant differences between phenotype of CD (age at onset, location, behavior) in obese vs. overweight vs. normal/underweight patients 2. Lower rates of a composite endpoint of CD-related surgery/hospitalization and/or initiation of anti-TNF therapy in obese (73%) and overweight patients (77%), as compared to normal/underweight patients (94%) Ulcerative Colitis 1. No significant in differences in UC disease extent (age at onset, location, behavior) in obese vs. overweight vs. normal/underweight patients 2. Lower rates of a composite endpoint of UC-related surgery/hospitalization and/or initiation of anti-TNF therapy in obese (40%) and overweight patients (49%), as compared to normal/underweight patients (67%) |
| Seminerio,90 2015 | Single center, retrospective cohort study; Pittsburgh, PA, USA; 2009–11; median follow-up, NR | 1494 patients with IBD (860 CD, 634 UC); 51% female • Disease duration, ~16y |
31.5% obese (including 4.4% with severe obesity – BMI≥40kg/m2); 40% overweight (based on average BMI of each patient during the study period) | 1. No significant difference in the risk of IBD-related surgery, hospitalization, emergency department use in obese vs. overweight vs. normal BMI adults 2. Obesity, in particular BMI≥35kg/m2 was associated with poorer IBD-related quality of life, as compared to normal BMI, and higher frequency of elevation in C-reactive protein 3. Actual dose of prescribed medication for weight-based therapies was lower than anticipated based on body weight (for example, mean infliximab doses in patients with class III obesity was 3.96 kg/m2 and that of azathioprine was 1.1mg/kg) 4. Increasing BMI was associated with higher rates of diabetes, hypertension and hyperlipidemia |
| Pringle,91 2015 | Single-center, cross-sectional study; Boston, MA, USA; 2004- | 846 patients with CD; 55% females • Disease duration, 12y; • Penetrating disease, 29%; • Biologic use, 56% |
16% obese; 30% overweight (at CD diagnosis) | 1. Compared to adults with BMI<25kg/m2, obese patients had lower prevalence of penetrating disease (OR, 0.56; 95% CI, 0.31–0.99), but comparable rates of perianal and stricturing disease, and prior surgery 2. Genetic predisposition did not appear to modify the effect of obesity on CD-related complications |
| Hass,92 2006 | Single-center, retrospective cohort study; Philadelphia, PA, USA; 1997–2002 | 138 patients with CD; 59% females • Disease duration, 13y • Perianal CD, 10% • Biologic use, 45% |
32% overweight or obese (at CD diagnosis) | 1. No significant difference in the number of CD-related surgeries or escalation of medical therapy in patients with BMI≥25kg/m2 vs. BMI<25kg/m2. 2. No significant difference in the time to first surgery between overweight vs. normal weight adults, although time to first surgery was shorted in overweight vs. underweight adults (2y vs. 21y, p=0.04) |
Impact of Physical Activity and Obesity on Treatment Response and Surgery in IBD
There have been limited studies on how usual physical activity may modify pharmacologic treatment response in patients with IBD. Biologically, exercise has been shown to reduce tumor necrosis factor-α (TNFα), which may conceivably augment response to TNFα antagonists though this has not been studied.14 Physical fitness is associated with superior outcomes after major surgery, including abdominal surgery. Several randomized trials and meta-analyses have also demonstrated the benefit of physical exercise prehabilitation, including aerobic exercises and/or resistance training in reducing post-operative morbidity and pulmonary complications in patients undergoing major abdominal surgery.15, 16
In contrast, population pharmacokinetic studies of all biologic agents used in IBD have identified high body weight as a risk factor associated with increased drug clearance, resulting in shorter half-lives and low trough drug concentrations.6 This effect might be related to impaired absorption of subcutaneously administered agents, rapid proteolysis and to a ‘TNF-sink’ phenomenon with higher inflammatory burden due to adipose tissue in obese patients. Kurnool and colleagues observed that each 1kg/m2 increase in BMI was associated with 4% increase in the risk of treatment failure, 8% increase in the risk of surgery or hospitalization and 6% lower odds of achieving endoscopic remission in a cohort 160 biologic-treated patients with UC, with comparable effects seen with fixed-dose therapies and weight-based agents.17 In a prospective cohort of adalimumab-treated patients with CD, Bultman and colleagues observed that over one-third of patients were dose-escalated to weekly adalimumab within a median 5 months of initiating therapy, and BMI was the only independent predictor of dose escalation.18 However, in a post-hoc analyses of clinical trials of infliximab in IBD, demonstrated no association between obesity and short-term clinical remission and mucosal healing in infliximab- or placebo-treated patients.19, 20 In a systematic review of 54 cohorts including 19,372 tumor necrosis factor-α (TNFα) antagonist-treated patients with immune-mediated inflammatory diseases (23% obese), Singh and colleagues observed that patients with obesity had 60% higher odds of failing therapy (OR,1.60; 95% CI,1.39–1.83), with a dose-response relationship; each 1kg/m2 increase in BMI was associated with 6.5% higher odds of treatment failure.19 Whether a similar negative effect of obesity on response to targeted small molecule inhibitors, such as tofacitinib, is unclear.
Intra-abdominal surgeries in patients with obesity are both technically challenging and are associated with higher rates of post-operative complications than surgeries in patients with a normal BMI. In a systematic review, Makino and colleagues observed longer operative times, an increased likelihood of conversion to open procedures, more comorbidities, a higher risk of postoperative complications (in particular wound infection) and a longer length of hospital stay in obese patients undergoing colorectal resection compared to individuals who were not obese.21 Two aspects of surgery that might be particularly challenging in patients with IBD merit special mention. First, obesity makes creating a stoma challenging due to stomal retraction, higher rates of complications such as parastomal hernia, mucocutaneous separation and stoma prolapse. Second, the mesentery of patients with obesity tends to be foreshortened by the mesenteric fat, making it more challenging to create a J-pouch in patients with UC. Obesity increases risk of short-term postoperative complications in patients undergoing ileal pouch–anal anastomosis, although long-term outcomes might be comparable to those in patients without obesity in experienced centers.22
Can Structured Exercise Interventions and Treating Obesity Help IBD?
Several small studies have evaluated the impact of diverse structured exercise interventions, including cardiovascular training, strength training, yoga, and mind-body therapies, on outcomes in patients with IBD, albeit or poor methodological quality and short duration of follow-up.14 These studies have generally demonstrated improvement in overall fitness, increase bone mineral density and reduction in stress and anxiety. However, they have variably demonstrated modest benefits in IBD-related clinical disease activity or biochemical markers of inflammation. Experts have recommended maintaining an active lifestyle with moderate intensity endurance and resistance exercise in an enjoyable activity at least 30 minutes per day, three times per week in patients with mild to moderately active IBD.14
Though there are no interventional studies of intentional weight loss in IBD, trials of diet and/or lifestyle-induced weight loss in other autoimmune diseases suggest improvement in disease outcomes with weight loss. In trials in obese patients with psoriasis, those randomized to weight loss intervention were 2.9 times more likely to achieve remission, compared to those without intervention (OR, 2.92; 95% CI, 1.39–6.13).23 In a randomized trial comparing low calorie or free-managed diet in 126 patients with psoriatic arthritis starting anti-TNF therapy, Di Munno et al observed that regardless of type of intervention, patients who achieved ≥5% weight loss were 4.2 times more likely to achieve clinical remission, as compared to those who experienced <5% weight loss.24 Similar benefits with weight loss in obese patients with IBD are conceivable. Case series have shown that bariatric procedures in selected morbidly obese patients with IBD result in improvement in disease activity after weight loss.25 Diet and lifestyle modifications to achieve weight loss may be challenging in IBD patients due to active gastrointestinal symptoms and a high prevalence of comorbid irritable bowel syndrome. Endoscopic bariatric interventions are currently contraindicated in patients with IBD; pharmacological weight loss interventions are attractive. A phase 2 clinical trial an FDA-approved weight loss medication, phentermine-topiramate in obese biologic-treated patients with UC is ongoing.
SLEEP AND SLEEP DISTURBANCE IN PATIENTS WITH IBD
Sleep disturbance is prevalent in patients with IBD. Between 47% and 82% of patients with IBD report disrupted sleep, night-time awakenings and nonrestorative sleep as compared with one-third of the general population.26, 27 In a large study of 3173 patients with IBD, 60% reported sleep disturbance at baseline.28 Strongest risk factors for sleep disturbance were depressive symptoms and active disease, with female sex, smoking and diagnosis of CD (vs. UC) also being associated with sleep disturbance. The relationship between sleep, sleep disturbance and IBD is bi-directional. Active disease is invariably associated with poor sleep quality. In a prospective study, all patients with symptomatically active IBD had poor sleep quality, as compared to 73% patient with quiescent disease.27 This includes more awakenings, longer sleep latency and reduced stage 3 sleep using electroencephalographic recordings, compared to patients with quiescent disease and healthy controls. Even among patients with quiescent disease, patients with IBD have inferior sleep quality as compared to healthy controls, similar to patients with irritable bowel syndrome, with prolonged sleep latency, greater sleep fragmentation, shorter sleep duration and greater use of sleep aids. Rates of sleep disturbance are higher in patients with objective evidence of inflammation, independent of symptoms.29
Circadian Misalignment in Patients with IBD
Circadian homeostasis maintains the sleep-wake cycle and plays a key role in preserving healthy immune function.30 Disruption of this process, or circadian misalignment, can promote inflammatory diseases such as IBD. Contributors to circadian misalignment including social jet lag (discrepancy between innate circadian rhythms and actual sleep time due to social obligations), sleep debt (difference between average sleep duration during the work week and work-free days) and chronotype (a person’s biological inclination to sleep during a certain time of day). Cross-sectional studies have demonstrated that, as compared to healthy controls, patients with IBD have higher social jet lag and sleep debt.31 Moreover, this circadian misalignment has been associated with more aggressive CD with stricturing and fistulizing behavior, and CD-related surgery.
Impact of Sleep Disturbance on Natural History and Outcomes in IBD
Prospective cohort studies have demonstrated the negative impact of sleep disturbance on risk of relapse and impaired quality of life in patients with IBD. In a cohort study, Ananthakrishnan and colleagues observed that sleep disturbance was independently associated with 1.6 to 2-fold higher risk of disease flare over 6m in 651 patients with CD in clinical remission at baseline.28 In contrast, they did not observe any significant association between sleep disturbance and subsequent risk of relapse in patients with asymptomatic UC. Poor sleep quality is also a significant contributor to fatigue observed in patients with IBD. In a prospective, population-based Manitoba IBD cohort study, fatigue was a common symptom reported in 72% patients with active disease and 30% patients with inactive disease.26 In these patients, poor sleep quality was independently associated with fatigue, in both patients with active and inactive IBD. Similarly, Borren and colleagues observed that sleep disruption in patients with inactive IBD is associated with persistent fatigue.32 A positive feedback loop might exist whereby active disease leads to poor sleep that in turn worsens inflammation, with both factors leading to fatigue.
Can Improving Sleep Hygiene Improve Outcomes in Patients with IBD?
Treating active IBD with pharmacotherapy has been associated with improved sleep hygiene in patients with IBD. However, it remains unclear how improving sleep hygiene impacts outcomes in patients with IBD. No clinical trials have evaluated whether structured interventions to improve sleep quality decrease the risk of clinical relapse and improve objective measures of inflammation in patients with IBD. However, it is conceivable the improving sleep hygiene would improve fatigue and quality of life in patients with IBD. Experts recommend systematic evaluation of sleep hygiene in patients with IBD with fatigue.33, 34 Patients with poor sleep quality should be screened for comorbid conditions like obstructive sleep apnea, restless legs syndrome and insomnia and educated on sleep hygiene. Patients with persistent sleep disturbance should be referred to a sleep specialist for potential pharmacotherapy and behavioral therapy.
STRESS IN PATIENTS WITH IBD
In contrast to psychiatric illnesses like depression and anxiety, stress is broadly defined as threat to a steady state of homeostasis in an individual’s life, and implicates both a stressor (i.e., an environmental demand) and an individual’s physiological and emotional response to the stressor.35 Stress is pervasive in modern lifestyle with approximately 60% of Americans reporting stress. Exaggerated response to a stressor may be functionally disruptive and evolve into a psychiatric illness; conversely, in a patient with anxiety, stress perception may be disproportionate to environmental demand. While the negative impact of major psychological comorbidities on disease activity and healthcare utilization in patients with IBD is increasingly being recognized, the impact of daily stressors and perceived stress on disease course and outcomes in patients with IBD is less clear.36 Pathophysiologically, stress has been demonstrated to negatively impact gastrointestinal function and increase gut permeability through its impact on the immune, endocrine and nervous system.
Impact of Stress on the Natural History and Outcomes in Patients with IBD
Patients with chronic health conditions, like IBD, have higher prevalence of stress than the general population, in common domains of finances, work and family. Stress, particularly ‘perceived stress’, can impact IBD disease activity. Early retrospective case-control studies have variably suggested that major stressful life events may lead to a higher risk of developing IBD, though were limited by recall bias.37, 38 More importantly, measuring stress based only on occurrence of life events captures burden of conventional stressors and not an individual’s response to it, often referred to as ‘perceived stress’. Prospective cohort studies have suggested that high perceived stress in the preceding 3–6 months may be associated with an increased risk of symptomatic flares.39–42 In a survey study of 600 patients in the population-based University of Manitoba IBD Research Registry, high perceived stress in the preceding 3 months, as measured by the Cohen’s Perceived Stress Scale, and not occurrence of a major life event or negative mood, was associated with 2.4-fold higher odds of developing a flare, based on clinical disease activity indices.39 However, perceived stress was not associated with increased fecal calprotectin, as a biomarker of inflammation, suggesting that the impact of stress may be mediated through it’s impact of gastrointestinal function and not necessarily an abnormal immune response.43 In a comprehensive prospective cohort study evaluating clinical, biological and psychosocial parameters predictive of clinical relapse in 101 patients with quiescent CD, Bitton and colleagues observed that besides conventional factors of high-risk phenotype (elevated C-reactive protein, presence of fistulizing disease), patients with high perceived stress and ineffective avoidance coping had a shorter time to relapse.44 Besides the impact of an acutely stressful event, chronically high perceived stress is independently associated with a higher risk of relapse. In a prospective cohort study of 62 patients with UC followed over 5 years, Levenstein and colleagues observed that high long-term (>2y) perceived stress tripled the risk of UC flare.45
The relationship between stress and IBD symptoms is bidirectional. Approximately 30% patients with symptomatic IBD report higher rates of perceived stress due to their IBD; in contrast, patients with IBD who are asymptomatic are significantly less likely to report stress due to their IBD.46
Can Decreasing Stress Improve Outcomes in Patients with IBD?
Stress management is challenging, with the pervasive nature and diverse settings and sources of stress. RCTs of psychological therapy including cognitive behavioral therapy, mindfulness-based therapies such as meditation and yoga, and gut-directed hypnotherapy have not been shown to be effective in decreasing perceived stress in patients with IBD.47 While these therapies may improve quality of life and depression over a short-term, they have not been shown to significantly improve disease activity or decrease risk of clinical relapse.
SMOKING IN PATIENTS WITH IBD
Cigarette smoking has been associated with an increased risk of developing CD, particularly in Western countries, although with a lower risk of developing UC across all regions.2 Continued smoking, smoking cessation and nicotine replacement may variably modify outcomes in patients with established IBD.
Impact of Smoking on Outcomes in IBD
Crohn’s Disease
Smoking has consistently been associated with a more severe phenotype, complicated behavior and adverse outcomes in CD.48–50 In a recent Spanish registry-based cohort of 3224 patients with CD, current smokers were more likely to have ileum-dominant CD, perianal disease and stricturing phenotype. Smoking has also been associated with progression from inflammatory to penetrating or stricturing behavior.51 In a meta-analysis of 33 high quality cohort studies with ~11,000 patients with CD, To and colleagues observed that, compared with non-smokers, smokers had 56–85% increase CD flares, a nearly twofold increase in clinical recurrence after surgery, a 54–68% increase in the need for first surgery and a twofold increase in rates of second surgery.52 Smoking has been associated with higher corticosteroid use, corticosteroid-requiring disease flares and corticosteroid-dependency.53 Smokers also achieve higher 6-thioguanine nucleotide levels as compared to non-smokers, though it has not been consistently associated with higher effectiveness of thiopurines.54, 55 In the TOPPIC trial comparing mercaptopurine vs. placebo for preventing recurrence of CD after surgery, mercaptopurine was effective in decreasing risk of clinical recurrence only in patients who were smokers; no benefit was observed in non-smokers.56 However, smoking has not been shown to modify response to biologic therapy.57, 58
Ulcerative Colitis
Unlike CD, smoking does not negatively impact clinical outcomes in patients with established UC and may potentially be protective against adverse outcomes. In a nationally representative study of 6754 patients with UC (13% smokers at diagnosis), Blackwell and colleagues observed no significant difference in risk of severe UC flare requiring corticosteroids, corticosteroid-dependency and need for thiopurines between smokers, ex-smokers and non-smokers.59 In a meta-analysis of 16 high quality cohort studies, there was no significant difference in risk of UC flare (smokers vs. non-smokers: 4 studies; OR, 1.26; 95% CI, 0.65–2.44), colectomy (5 studies; OR, 0.89; 95% CI, 0.62–1.26), proximal disease extension (4 studies; OR, 0.57; 95% CI, 0.20–1.66) and development of pouchitis in patients with ileal pouch anal anastomosis (3 studies; OR, 0.57; 95% CI, 0.21–1.53).60 In contrast, a 2015 meta-analysis of 20 observational studies in patients with UC, including case-control studies, smokers had a lower risk of colectomy, as compared to non-smokers (OR, 0.55; 95% CI, 0.33–0.91).61
Impact of Smoking Cessation on Outcomes in IBD
Crohn’s Disease
Smoking cessation appears to mitigate risk and adverse outcomes associated with smoking in patients with CD. In a prospective cohort study of 899 patients with CD (474 non-smokers, 59 quitters) followed over 29 months, Cosnes and colleagues observed that risk of disease flare, corticosteroid exposure, and treatment modification was comparable between quitters and never smokers, and was significantly lower as compared to continued smokers.62 In another population-based inception cohort study of 749 patients with CD who were smokers at diagnosis, 44% quit within 2 years of CD diagnosis.53 As compared to persistent smokers, those who quit smoking were less likely to be corticosteroid-dependent on follow-up, though rates of intestinal resection were not different. Meta-analyses have also suggested no significant differences in former vs. never smokers in risks of disease flare, initial and repeat surgery in patients with CD.52
Despite evidence on the detrimental effects of smoking, and beneficial effects of smoking cessation in patients with CD, smoking prevalence remains high, with many patients being unaware of the detrimental effects of smoking on CD course and outcomes.63 While passive education on smoking cessation may not be helpful, structured smoking cessation programs involving individual counseling, nicotine replacement, and/or pharmacotherapy have been effective in helping patients with CD quit, and may improve disease outcomes.64
Ulcerative Colitis
Anecdotally, patients have associated quitting smoking with triggering a flare of UC, though it has been challenging to establish a true temporal relationship in robust clinical studies. In a case-control study of 32 patients with UC who quit smoking after diagnosis, matched with nonsmoker and continuing smoker controls, Beaugerie and colleagues observed that patients who quit smoking were more likely to have active disease, be hospitalized, and require treatment with steroids and immunomodulators.65 More recent systematic studies have failed to demonstrate the negative impact of quitting smoking in patients with UC. In a UK population-based cohort, there was no significant difference in rates of flares requiring corticosteroids, corticosteroid-dependence, UC-related hospitalization or colectomy between patients who quit smoking within 2 years of UC diagnosis vs. persistent smokers, though crude rates of corticosteroid use and thiopurine use was higher in those who quit smoking.59 In a meta-analysis, To and colleagues found no significant difference in risk of colectomy between ex-smokers, current smokers, and never smokers.60
Impact of Nicotine Replacement in UC
With the protective association between smoking and risk of UC, nicotine replacement therapy has been studied as adjunctive therapy in patients with UC. Early clinical trials of high-dose transdermal nicotine (>15mg/d) in non-smoking patients with mild to moderately active UC despite mesalamine therapy suggested benefit in inducing clinical remission, but not in maintaining remission, over placebo;66, 67 however, it was not as effective as low dose prednisone.68 Nicotine enemas, 6mg/d were not effective in inducing remission in patients with distal UC.69 Treatment-limiting side effects are common with nicotine, which leads to withdrawal of therapy, and hence, is not recommended for routine management.70 It’s role in modern management of UC, with availability of multiple immunosuppressive therapies has not been evaluated.
ALCOHOL AND CANNABIS USE IN PATIENTS WITH IBD
Alcohol Use in IBD
Alcohol has not been linked definitively as either a risk factor for development of IBD71, 72 or to any specific adverse outcomes.73, 74 Two separate meta-analyses, one each for CD and UC, found no association between alcohol consumption and risk of developing CD (6 studies; OR, 0.85; 95% CI, 0.68–1.08) or UC (9 studies; OR, 0.95; 95% CI, 0.65–1.39).72, 75 However, 30–60% patients with IBD often voluntarily avoid alcohol.76, 77 Survey studies have variably suggested either subjective worsening of gastrointestinal symptoms or no change in clinical disease activity or biomarkers of inflammation with alcohol consumption.76, 78 Magee and colleagues observed that dietary intake of wine and beer, but not spirits, in patients with UC across disease spectrum, is significantly associated with worse endoscopic disease activity.79 In a prospective cohort study of 183 patients with UC in remission, Jowett and colleagues observed that higher intake of alcoholic beverages, particularly sulfite-containing beverages, was associated with a 2.4-fold higher risk of clinical relapse.80 However, these findings have been inconsistent. In an experimental study in 23 patients with IBD in remission, moderate intake of alcohol (1–3 glasses for red wine per day for 1 week) was not associated with clinical relapse, and in fact, was associated with a significant decline in fecal calprotectin.81 Overall, it seems that moderate alcohol consumption may be safe in patients with IBD.
Cannabis Use in IBD
Approximately 10–20% patients with IBD are active cannabis users, though this is probably underestimated with increasing legalization across North America.82 In survey studies, chronic abdominal pain and prior abdominal surgery were associated with 3.5 to 5-fold higher odds of marijuana use, with self-reported improvement in pain, cramping and diarrhea, and increased appetite, with marijuana.83, 84
Small interventional studies have suggested that cannabis use may improve IBD-related symptoms in patients with CD. In the first RCT of cannabis in 21 patients with active CD, Naftali and colleagues demonstrated that, as compared to placebo, smoking 2 cannabis cigarettes per day for 8 weeks was associated with significantly higher rates of clinical response (decline in Crohn’s disease activity index [CDAI] by 100 points) (90% vs. 40%, p=0.03) and numerically higher rates of clinical remission (CDAI <150; 40% vs. 10%, p=0.11); however, cannabis use was not associated with any significant change in biochemical markers of inflammation.85 A subsequent RCT of low-dose oral cannabidiol (CBD) RCT of 19 patients with active CD from the same group of investigators, failed to demonstrate any positive effect in clinical and biochemical parameters.86 In a placebo-controlled trial in patients with active UC on mesalamine, Irving and colleagues assessed the efficacy, safety, and tolerability of once-daily oral CBD for 10 weeks.87 They observed no significant difference in rates of clinical remission (CBD vs. placebo: 28% vs. 26%), although there were considerable protocol deviations, with 90% patients receiving CBD extract reporting treatment-related adverse events. In contrast, Naftali and colleagues observed that smoking 2 cannabis cigarettes per day for 8 weeks was associated with clinical and endoscopic response, but no biochemical response, in an RCT of 28 patients with moderate to severely active UC. While there is considerable enthusiasm for the potential benefit of cannabis, particularly in the patient community, long-term effect of cannabis in these patients with IBD is unknown. In the general population, long-term use of cannabis is associated increased risk of addiction to other substances, diminished life achievement, increase in motor vehicle accidents, symptoms of chronic bronchitis, abnormal brain development in a younger population, psychiatric disturbances, depression, and anxiety, and risk of cannabis hyperemesis syndrome in frequent cannabis users.88 At this point, there is very limited evidence to inform the routine use of cannabis in patients with IBD.
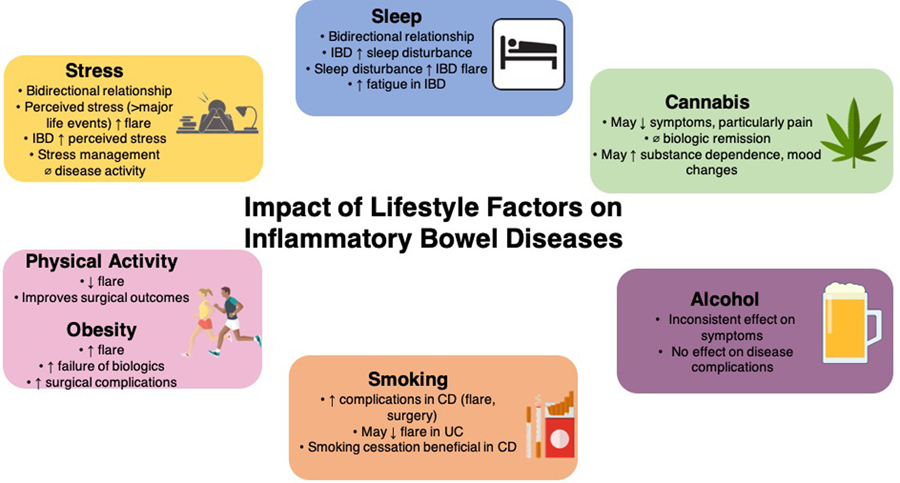
In summary, several modifiable lifestyle factors play a vital role in the course and outcomes of patients with IBD. Figure 1 summarizes key findings on impact of these lifestyle factors in patients with established IBD. Well-designed prospective cohort studies to better understand their impact on the natural history and randomized interventional studies of lifestyle factor modification are warranted to augment the effectiveness of current pharmacological therapies. In the meantime, a conscious and deliberate assessment of these lifestyle factors is warranted for every patient with IBD, and serves as a key aspect in engaging patients in adjunctive self-management of their disease.
Figure 1.
Effect of lifestyle factors on the course of inflammatory bowel diseases
Acknowledgments
Disclosures: Dr. Singh is supported by NIH/NIDDK (K23DK117058), ACG Junior Faculty Development Award, the Crohn’s and Colitis Foundation Career Development Award (#404614), Litwin IBD Pioneers Grant (#623346) and AGA-Pfizer Young Investigator Pilot Research Award in Inflammatory Bowel Disease.
SS – Research grants from AbbVie; Consulting fees from AbbVie, Takeda, Pfizer
Footnotes
Conflicts of Interest:
JJR – None to declare
AH – None to declare
REFERENCES
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017;152:313–321 e2. [DOI] [PubMed] [Google Scholar]
- 3.Piovani D, Danese S, Peyrin-Biroulet L, et al. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019;157:647–659 e4. [DOI] [PubMed] [Google Scholar]
- 4.Gatt K, Schembri J, Katsanos KH, et al. Inflammatory Bowel Disease [IBD] and Physical Activity: A Study on the Impact of Diagnosis on the Level of Exercise Amongst Patients With IBD. J Crohns Colitis 2019;13:686–692. [DOI] [PubMed] [Google Scholar]
- 5.Greenley RN, Naftaly JP, Walker RJ, et al. Sports Participation in Youth With Inflammatory Bowel Diseases: The Role of Disease Activity and Subjective Physical Health Symptoms. Inflamm Bowel Dis 2018;24:247–253. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Dulai PS, Zarrinpar A, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nature Reviews Gastroenterology & Hepatology 2016;30:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci 2019;8:201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook MD, Martin SA, Williams C, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun 2013;33:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones PD, Kappelman MD, Martin CF, et al. Exercise decreases risk of future active disease in patients with inflammatory bowel disease in remission. Inflamm Bowel Dis 2015;21:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Nguyen NH, Proudfoot JA, et al. Impact of Obesity on Disease Activity and Patient-Reported Outcomes Measurement Information System (PROMIS) in Inflammatory Bowel Diseases. Am J Gastroenterol 2019;114:630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen NH, Ohno-Machado L, Sandborn WJ, et al. Obesity Is Independently Associated With Higher Annual Burden and Costs of Hospitalization in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2019;17:709–718 e7. [DOI] [PubMed] [Google Scholar]
- 12.Uko V, Vortia E, Achkar J-P, et al. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohn’s disease. Inflammatory Bowel Diseases 2014;20:2286–91. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Zhu W, Gong J, et al. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Colorectal Disease 2015;17:225–34. [DOI] [PubMed] [Google Scholar]
- 14.Eckert KG, Abbasi-Neureither I, Koppel M, et al. Structured physical activity interventions as a complementary therapy for patients with inflammatory bowel disease - a scoping review and practical implications. BMC Gastroenterol 2019;19:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heger P, Probst P, Wiskemann J, et al. A Systematic Review and Meta-analysis of Physical Exercise Prehabilitation in Major Abdominal Surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg 2019. [DOI] [PubMed]
- 16.Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: A systematic review and meta-analysis. Surgery 2016;160:1189–1201. [DOI] [PubMed] [Google Scholar]
- 17.Kurnool S, Nguyen NH, Proudfoot J, et al. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther 2018;47:1472–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bultman E, de Haar C, van Liere-Baron A, et al. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn’s disease patients. Alimentary Pharmacology & Therapeutics 2012;35:335–41. [DOI] [PubMed] [Google Scholar]
- 19.Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti-tumor necrosis factor-alpha agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS One 2018;13:e0195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh S, Proudfoot J, Xu R, et al. Obesity and Response to Infliximab in Patients with Inflammatory Bowel Diseases: Pooled Analysis of Individual Participant Data from Clinical Trials. Am J Gastroenterol 2018;113:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makino T, Shukla PJ, Rubino F, et al. The Impact of Obesity on Perioperative Outcomes After Laparoscopic Colorectal Resection. Annals of Surgery 2012;255:228–236. [DOI] [PubMed] [Google Scholar]
- 22.McKenna NP, Mathis KL, Khasawneh MA, et al. Obese Patients Undergoing Ileal Pouch-Anal Anastomosis: Short-and Long-term Surgical Outcomes. Inflamm Bowel Dis 2017;23:2142–2146. [DOI] [PubMed] [Google Scholar]
- 23.Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond) 2015;39:1197–202. [DOI] [PubMed] [Google Scholar]
- 24.Di Minno MN, Peluso R, Iervolino S, et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis 2014;73:1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aminian A, Andalib A, Ver MR, et al. Outcomes of Bariatric Surgery in Patients with Inflammatory Bowel Disease. Obes Surg 2016;26:1186–90. [DOI] [PubMed] [Google Scholar]
- 26.Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis 2011;17:1882–9. [DOI] [PubMed] [Google Scholar]
- 27.Ranjbaran Z, Keefer L, Farhadi A, et al. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol 2007;22:1748–53. [DOI] [PubMed] [Google Scholar]
- 28.Ananthakrishnan AN, Long MD, Martin CF, et al. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol 2013;11:965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson RG, Stevens BW, Guo AY, et al. High C-Reactive Protein Is Associated with Poor Sleep Quality Independent of Nocturnal Symptoms in Patients with Inflammatory Bowel Disease. Dig Dis Sci 2015;60:2136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol 2013;13:190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakradeo PS, Keshavarzian A, Singh S, et al. Chronotype, social jet lag, sleep debt and food timing in inflammatory bowel disease. Sleep Med 2018;52:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borren NZ, Tan W, Colizzo FP, et al. Longitudinal trajectory of fatigue with initiation of biologic therapy in inflammatory bowel diseases: A prospective cohort study. J Crohns Colitis 2019. [DOI] [PubMed]
- 33.Ali T, Orr WC. Sleep disturbances and inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1986–95. [DOI] [PubMed] [Google Scholar]
- 34.Borren NZ, van der Woude CJ, Ananthakrishnan AN. Fatigue in IBD: epidemiology, pathophysiology and management. Nat Rev Gastroenterol Hepatol 2019;16:247–259. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein CN. The Brain-Gut Axis and Stress in Inflammatory Bowel Disease. Gastroenterol Clin North Am 2017;46:839–846. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen NH, Koola J, Dulai PS, et al. Rate of Risk Factors for and Interventions to Reduce Hospital Readmission in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed]
- 37.Lerebours E, Gower-Rousseau C, Merle V, et al. Stressful life events as a risk factor for inflammatory bowel disease onset: A population-based case-control study. Am J Gastroenterol 2007;102:122–31. [DOI] [PubMed] [Google Scholar]
- 38.Tocchi A, Lepre L, Liotta G, et al. Familial and psychological risk factors of ulcerative colitis. Ital J Gastroenterol Hepatol 1997;29:395–8. [PubMed] [Google Scholar]
- 39.Bernstein CN, Singh S, Graff LA, et al. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol 2010;105:1994–2002. [DOI] [PubMed] [Google Scholar]
- 40.Duffy LC, Zielezny MA, Marshall JR, et al. Lag time between stress events and risk of recurrent episodes of inflammatory bowel disease. Epidemiology 1991;2:141–5. [DOI] [PubMed] [Google Scholar]
- 41.Langhorst J, Hofstetter A, Wolfe F, et al. Short-term stress, but not mucosal healing nor depression was predictive for the risk of relapse in patients with ulcerative colitis: a prospective 12-month follow-up study. Inflamm Bowel Dis 2013;19:2380–6. [DOI] [PubMed] [Google Scholar]
- 42.Mardini HE, Kip KE, Wilson JW. Crohn’s disease: a two-year prospective study of the association between psychological distress and disease activity. Dig Dis Sci 2004;49:492–7. [DOI] [PubMed] [Google Scholar]
- 43.Targownik LE, Sexton KA, Bernstein MT, et al. The Relationship Among Perceived Stress, Symptoms, and Inflammation in Persons With Inflammatory Bowel Disease. Am J Gastroenterol 2015;110:1001–12; quiz 1013. [DOI] [PubMed] [Google Scholar]
- 44.Bitton A, Dobkin PL, Edwardes MD, et al. Predicting relapse in Crohn’s disease: a biopsychosocial model. Gut 2008;57:1386–92. [DOI] [PubMed] [Google Scholar]
- 45.Levenstein S, Prantera C, Varvo V, et al. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol 2000;95:1213–20. [DOI] [PubMed] [Google Scholar]
- 46.Sexton KA, Walker JR, Graff LA, et al. Evidence of Bidirectional Associations Between Perceived Stress and Symptom Activity: A Prospective Longitudinal Investigation in Inflammatory Bowel Disease. Inflamm Bowel Dis 2017;23:473–483. [DOI] [PubMed] [Google Scholar]
- 47.Gracie DJ, Irvine AJ, Sood R, et al. Effect of psychological therapy on disease activity, psychological comorbidity, and quality of life in inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:189–199. [DOI] [PubMed] [Google Scholar]
- 48.Cosnes J, Carbonnel F, Beaugerie L, et al. Effects of cigarette smoking on the long-term course of Crohn’s disease. Gastroenterology 1996;110:424–31. [DOI] [PubMed] [Google Scholar]
- 49.Picco MF, Bayless TM. Tobacco consumption and disease duration are associated with fistulizing and stricturing behaviors in the first 8 years of Crohn’s disease. Am J Gastroenterol 2003;98:363–8. [DOI] [PubMed] [Google Scholar]
- 50.Sutherland LR, Ramcharan S, Bryant H, et al. Effect of cigarette smoking on recurrence of Crohn’s disease. Gastroenterology 1990;98:1123–8. [DOI] [PubMed] [Google Scholar]
- 51.Nunes T, Etchevers MJ, Domènech E, et al. Smoking does influence disease behaviour and impacts the need for therapy in Crohn′s disease in the biologic era. Alimentary Pharmacology & Therapeutics 2013;38:752–760. [DOI] [PubMed] [Google Scholar]
- 52.To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment Pharmacol Ther 2016;43:549–61. [DOI] [PubMed] [Google Scholar]
- 53.Alexakis C, Saxena S, Chhaya V, et al. Smoking Status at Diagnosis and Subsequent Smoking Cessation: Associations With Corticosteroid Use and Intestinal Resection in Crohn’s Disease. Am J Gastroenterol 2018;113:1689–1700. [DOI] [PubMed] [Google Scholar]
- 54.Poon SS, Asher R, Jackson R, et al. Body Mass Index and Smoking Affect Thioguanine Nucleotide Levels in Inflammatory Bowel Disease. J Crohns Colitis 2015;9:640–6. [DOI] [PubMed] [Google Scholar]
- 55.Domenech E, Carrion S, Garcia-Planella E, et al. Smoking status and response to thiopurines in steroid-dependent inflammatory bowel disease. Inflamm Bowel Dis 2011;17:971–5. [DOI] [PubMed] [Google Scholar]
- 56.Mowat C, Arnott I, Cahill A, et al. Mercaptopurine versus placebo to prevent recurrence of Crohn’s disease after surgical resection (TOPPIC): a multicentre, double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol 2016;1:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inamdar S, Volfson A, Rosen L, et al. Smoking and early infliximab response in Crohn’s disease: a meta-analysis. J Crohns Colitis 2015;9:140–6. [DOI] [PubMed] [Google Scholar]
- 58.Dulai PS, Singh S, Jiang X, et al. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am J Gastroenterol 2016;111:1147–55. [DOI] [PubMed] [Google Scholar]
- 59.Blackwell J, Saxena S, Alexakis C, et al. The impact of smoking and smoking cessation on disease outcomes in ulcerative colitis: a nationwide population-based study. Aliment Pharmacol Ther 2019;50:556–567. [DOI] [PubMed] [Google Scholar]
- 60.To N, Ford AC, Gracie DJ. Systematic review with meta-analysis: the effect of tobacco smoking on the natural history of ulcerative colitis. Aliment Pharmacol Ther 2016;44:117–26. [DOI] [PubMed] [Google Scholar]
- 61.Dias CC, Rodrigues PP, da Costa-Pereira A, et al. Clinical predictors of colectomy in patients with ulcerative colitis: systematic review and meta-analysis of cohort studies. J Crohns Colitis 2015;9:156–63. [DOI] [PubMed] [Google Scholar]
- 62.Cosnes J, Carbonnel F, Carrat F, et al. Effects of current and former cigarette smoking on the clinical course of Crohn’s disease. Aliment Pharmacol Ther 1999;13:1403–11. [DOI] [PubMed] [Google Scholar]
- 63.Biedermann L, Fournier N, Misselwitz B, et al. High Rates of Smoking Especially in Female Crohn’s Disease Patients and Low Use of Supportive Measures to Achieve Smoking Cessation--Data from the Swiss IBD Cohort Study. J Crohns Colitis 2015;9:819–29. [DOI] [PubMed] [Google Scholar]
- 64.Nunes T, Etchevers MJ, Merino O, et al. High smoking cessation rate in Crohn’s disease patients after physician advice--the TABACROHN Study. J Crohns Colitis 2013;7:202–7. [DOI] [PubMed] [Google Scholar]
- 65.Beaugerie L, Massot N, Carbonnel F, et al. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol 2001;96:2113–6. [DOI] [PubMed] [Google Scholar]
- 66.Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med 1994;330:811–5. [DOI] [PubMed] [Google Scholar]
- 67.Thomas GA, Rhodes J, Mani V, et al. Transdermal nicotine as maintenance therapy for ulcerative colitis. N Engl J Med 1995;332:988–92. [DOI] [PubMed] [Google Scholar]
- 68.Thomas GA, Rhodes J, Ragunath K, et al. Transdermal nicotine compared with oral prednisolone therapy for active ulcerative colitis. Eur J Gastroenterol Hepatol 1996;8:769–76. [PubMed] [Google Scholar]
- 69.Ingram JR, Thomas GA, Rhodes J, et al. A randomized trial of nicotine enemas for active ulcerative colitis. Clin Gastroenterol Hepatol 2005;3:1107–14. [DOI] [PubMed] [Google Scholar]
- 70.Nikfar S, Ehteshami-Ashar S, Rahimi R, et al. Systematic review and meta-analysis of the efficacy and tolerability of nicotine preparations in active ulcerative colitis. Clin Ther 2010;32:2304–15. [DOI] [PubMed] [Google Scholar]
- 71.Bergmann MM, Hernandez V, Bernigau W, et al. No association of alcohol use and the risk of ulcerative colitis or Crohn’s disease: data from a European Prospective cohort study (EPIC). Eur J Clin Nutr 2017;71:512–518. [DOI] [PubMed] [Google Scholar]
- 72.Yang Y, Xiang L, He J. Beverage intake and risk of Crohn disease: A meta-analysis of 16 epidemiological studies. Medicine (Baltimore) 2019;98:e15795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khasawneh M, Spence AD, Addley J, et al. The role of smoking and alcohol behaviour in the management of inflammatory bowel disease. Best Pract Res Clin Gastroenterol 2017;31:553–559. [DOI] [PubMed] [Google Scholar]
- 74.Mantzouranis G, Fafliora E, Saridi M, et al. Alcohol and narcotics use in inflammatory bowel disease. Ann Gastroenterol 2018;31:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nie JY, Zhao Q. Beverage consumption and risk of ulcerative colitis: Systematic review and meta-analysis of epidemiological studies. Medicine (Baltimore) 2017;96:e9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swanson GR, Sedghi S, Farhadi A, et al. Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol 2010;44:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vagianos K, Clara I, Carr R, et al. What Are Adults With Inflammatory Bowel Disease (IBD) Eating? A Closer Look at the Dietary Habits of a Population-Based Canadian IBD Cohort. JPEN J Parenter Enteral Nutr 2016;40:405–11. [DOI] [PubMed] [Google Scholar]
- 78.Hey H, Schmedes A, Nielsen AA, et al. Effects of five different alcoholic drinks on patients with Crohn’s disease. Scand J Gastroenterol 2007;42:968–72. [DOI] [PubMed] [Google Scholar]
- 79.Magee EA, Edmond LM, Tasker SM, et al. Associations between diet and disease activity in ulcerative colitis patients using a novel method of data analysis. Nutr J 2005;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut 2004;53:1479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swanson GR, Tieu V, Shaikh M, et al. Is moderate red wine consumption safe in inactive inflammatory bowel disease? Digestion 2011;84:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swaminath A, Berlin EP, Cheifetz A, et al. The Role of Cannabis in the Management of Inflammatory Bowel Disease: A Review of Clinical, Scientific, and Regulatory Information. Inflamm Bowel Dis 2019;25:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ravikoff Allegretti J, Courtwright A, Lucci M, et al. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Storr M, Devlin S, Kaplan GG, et al. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis 2014;20:472–80. [DOI] [PubMed] [Google Scholar]
- 85.Naftali T, Bar-Lev Schleider L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol 2013;11:1276–1280 e1. [DOI] [PubMed] [Google Scholar]
- 86.Naftali T, Mechulam R, Marii A, et al. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn’s Disease, a Randomized Controlled Trial. Dig Dis Sci 2017;62:1615–1620. [DOI] [PubMed] [Google Scholar]
- 87.Irving PM, Iqbal T, Nwokolo C, et al. A Randomized, Double-blind, Placebo-controlled, Parallel-group, Pilot Study of Cannabidiol-rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm Bowel Dis 2018;24:714–724. [DOI] [PubMed] [Google Scholar]
- 88.Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med 2014;370:2219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flores A, Burstein E, Cipher DJ, et al. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Digestive Diseases & Sciences 2015;60:2436–45. [DOI] [PubMed] [Google Scholar]
- 90.Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis 2015;21:2857–63. [DOI] [PubMed] [Google Scholar]
- 91.Pringle PL, Stewart KO, Peloquin JM, et al. Body Mass Index, Genetic Susceptibility, and Risk of Complications Among Individuals with Crohn’s Disease. Inflamm Bowel Dis 2015;21:2304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hass DJ, Brensinger CM, Lewis JD, et al. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin Gastroenterol Hepatol 2006;4:482–8. [DOI] [PubMed] [Google Scholar]