Abstract
Extra-renal expression of Cytochrome P450 Family 27 Subfamily B Member 1 (CYP27B1) has been well recognized and reflects the importance of intracrine/paracrine vitamin D signaling in different tissues under physiological and pathological conditions. In a prior RNA sequencing project, we identified CYP27B1 mRNA as upregulated in muscle samples from patients with amyotrophic lateral sclerosis (ALS) compared to normal controls. Our aims here were: (1) to validate this finding in a larger sample set including disease controls, (2) to determine which cell type is expressing CYP27B1 protein in muscle tissue, (3) to correlate CYP27B1 mRNA expression with disease progression in the SOD1G93A ALS mouse and in ALS patients. We assessed CYP27B1 expression by qPCR, western blot, and immunohistochemistry in a repository of muscle samples from ALS, disease controls (myopathy and non-ALS neuropathic disease), normal subjects, and muscle samples from the SOD1G93A mouse. Eight ALS patients were studied prospectively over 6-12 months with serial muscle biopsies. We found that CYP27B1 mRNA and protein levels were significantly increased in ALS versus normal and myopathy muscle samples. Neuropathy samples had increased CYP27B1 mRNA and protein expression but at a lower level than the ALS group. Immunohistochemistry showed that CYP27B1 localized to myofibers, especially those with features of denervation. In the SOD1G93A mouse, CYP27B1 mRNA and protein were detected in skeletal muscle in early pre-symptomatic stages and increased through end-stage. In the human study, increases in CYP27B1 mRNA in muscle biopsies correlated with disease progression rates over the same time period. In summary, we show for the first time that CYP27B1 mRNA and protein expression are elevated in muscle fibers in denervating disease, especially ALS, where mRNA levels can potentially serve as a surrogate marker for tracking disease progression. Its upregulation may reflect a local perturbation of vitamin D signaling, and further characterization of this pathway may provide insight into underlying molecular processes linked to muscle denervation.
Keywords: CYP27B1, muscle biopsy, muscle denervation, amyotrophic lateral sclerosis, SOD1G93A mouse, disease progression
1. Introduction
CYP27B1 is the major enzyme driving production of 1,25(OH)2D, the active form of vitamin D. Although concentrated in the kidney, it has become clear over time that CYP27B1 is expressed under physiological and/or pathological conditions in many extra-renal sites including bone, brain, colon, skin, lymphoid tissues, macrophages and monocytes [1–3]. We first identified CYP27B1 as being elevated in muscle through RNA sequencing of biopsies from patients with amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disease of motor neurons [4, 5]. Skeletal muscle is the “end-organ” affected in ALS and denervation atrophy is a prominent and nearly universal finding by muscle histology [6]. In ALS, the earliest detectable pathology occurs in skeletal muscle even prior to changes in the cell body of the motor neuron [7, 8]. This includes breakdown of the neuromuscular junction, retraction of motor neuron termini, and mitochondrial dysfunction. Muscle denervation as a result of the degenerative process leads to progressive and ultimately fatal muscle weakness. Our long-term goal with the RNA sequencing project is to identify potential biomarkers that could aid in diagnosing ALS earlier and/or in tracking disease progression. ALS has extensive heterogeneity with respect to clinical progression, with some patients rapidly deteriorating over months and others surviving for more than 10 years. Markers that can assist with tracking disease progression would be quite useful in the design of clinical trials [9, 10]. The aims of this study were to: (1) Validate our prior RNA sequencing results by assessing CYP27B1 mRNA expression in a large collection of muscle samples from patients with ALS and disease controls, (2) Determine if CYP27B1 protein levels were similarly elevated and in which cells within muscle tissue, and (3) Correlate CYP27B1 mRNA expression with disease progression in the SOD1G93A ALS mouse and in patients with ALS.
2. Materials and methods
2.1. Animals
B6.Cg-Tg (SOD1_G93A) 1 Gur/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). This mouse model has a later clinical onset and prolonged survival compared to the original ALS model on the B6/SJL background [11, 12]. Transgenic mice were maintained in the hemizygous state by mating SOD1G93A males with C57BL/6J females. Non-transgenic littermates were used as controls. For SOD1G93A mice, clinical progression was evaluated by weight determination and performance on the rotarod as described previously [5]. All animal procedures were reviewed and approved by the UAB Institutional Animal Care and Use Committee in compliance with the National Research Council Guide for the Care and Use of Laboratory Animals.
2.2. Tissue Collection and Clinical correlation
Human ALS and control tissue collections were approved by the UAB Institutional Review Board. Muscle biopsy samples from the Neuromuscular Division at UAB were identified in a database of archived samples stored at −80° C or in paraffin blocks. Biopsy sites varied and included tibialis anterior, gastrocnemius, vastus lateralis, biceps and deltoid muscles. Some ALS and normal samples were obtained post-mortem on patients evaluated and followed in the UAB Neuromuscular Division as part of a tissue collection program (samples were obtained by PHK and YS within 4–6 hours of death). All ALS patients had definite ALS by El Escorial criteria as previously detailed and most were cared for at our institution [5, 13]. For the RNA and protein analyses, normal muscle samples were obtained from two sources: (1) a pool of patients with non-specific muscle symptoms (e.g. pain) whose biopsies were read out as normal by a neuromuscular pathologist, and (2) autopsy samples from patients with no neuromuscular disease or muscle symptoms. Non-ALS neuropathic and myopathy samples were chosen based on histological evidence (denervation or myopathic changes) as determined by the neuromuscular pathologist. In the prospective study, patients with definite or probable ALS (laboratory supported) based on El Escorial criteria were consented and enrolled following approval by the Institutional Review Board at UAB. After detailed examination, a muscle in an affected limb was selected for serial biopsy based on having a power grade of at least 4 using the Medical Research Council (MRC) scale for muscle strength. Muscle biopsies (performed by MK) were collected at baseline, 6 and 12 months by percutaneous needle biopsy using a 5-mm Bergstrom biopsy needle under suction as previously described [14]. ALS functional rating scale-revised (ALSFRS-R) was measured at study entry and at 6 and 12 months. Genetic testing was not performed on study participants. The control population for the prospective study consisted of normal subjects without neurological disease who participated in an exercise resistance training program (mean age, 38 years, range 20–66 years) and had sequential biopsies from the vastus lateralis [15]. For mouse samples, animals were sacrificed by CO2 inhalation followed by cervical dislocation. Hindlimb muscle tissues were dissected from SOD1G93A and littermate controls at post-natal (PN) day 40, 60, 105, 125 and 150. These time points were chosen as they represent the full range of disease stages: early pre-symptomatic (40 d and 60 d), late pre-symptomatic (105 d), early symptomatic (125 d), and late symptomatic (150 d). Mouse samples were briefly rinsed in phosphate buffered saline (PBS), and frozen in liquid nitrogen and stored at - 80°C prior to molecular analysis. For immunohistochemical studies, samples were fixed in 4% PFA followed by paraffin embedding.
2.3. RNA Isolation and qPCR
RNA was extracted from frozen tissues with Trizol Reagent (Invitrogen) according to the manufacturer’s instructions. Two micrograms of RNA were reverse transcribed according to the manufacturer’s specifications (Applied Biosystems). Multiplex PCR was performed using as previously described [5]. GAPDH served as the internal control for each PCR reaction.
2.4. Western Blot and Immunohistochemistry
For western blot, frozen tissues were homogenized in T-Per (Thermo Fisher) and quantitated with a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher). Sixty micrograms of protein were electrophoresed in an SDS-polyacrylamide gel, blotted and probed with antibodies to the following targets: CYP27B1 (sc-67261, Santa Cruz Biotechnology) and GAPDH (Cell Signaling). Densitometry was done with the VersaDoc Imaging System (Bio-Rad) and quantified using Image Lab (Bio-Rad). Densitometry values for CYP27B1 were normalized to those of the housekeeping control (GAPDH) for each lane. Three normal controls were run on each blot and CYP27B1 values for disease samples were expressed as a fold-difference from the mean value of the normal samples. For immunohistochemistry, 10 micron paraffin sections were used. Deparaffinized sections were immersed in 10 mM citrate buffer (pH 6.0) heated at 100°C for 30 min, and allowed to cool to room temperature. After antigen retrieval, all sections were treated with 3% H2O2 for 10min. After blocking, sections were incubated with CYP27B1 antibodies (ab95047, Abcam) overnight at 4°C. Slides were incubated for an hour at room temperature with OneStep polymer HRP anti-rabbit secondary antibody (GTX83399, Genetex, Irvine). After rinsing with PBS, slides were developed with a DAB peroxidase kit (Vector Laboratories) and counterstained with hematoxylin.
2.5. Statistical Analyses
Comparisons between human ALS and control groups for qPCR and protein densitometry were made using a one-way analysis of variance with a Dunnett’s multiple comparisons test (performed in Graphpad, v8.1). A Student’s t-test was used for analysis of mouse qPCR comparisons and for the human study. A Pearson correlation was used for analysis of CYP27B1 and ALSFRS results.
3. Results
3.1. Validation of CYP27B1 upregulation in ALS muscle
Upon review of RNA sequencing data originally reported by our group, CYP27B1 was identified as being significantly elevated in ALS compared to normal muscle samples [5]. To validate this finding, we broadened our assessment to a larger number of ALS and control muscle samples (Table 1). The demographics of the ALS cohort were in line with what we and others have reported including an average age of 59 years, male to female ratio of 1.3, and a predominance (77%) of spinal onset ALS [4, 16]. Diseased controls consisted of muscle biopsy samples from patients with primary myopathic disease and non-ALS neuropathic disease (Table 1).
Table 1:
Muscle samples1
| Normal | ALS | Myopathy | Neuropathy | |
|---|---|---|---|---|
| Number | 22 | 39 | 14 | 15 |
| Age (y) | ||||
| Mean (± SD) | 54 ± 11 | 59 ± 12 | 56 ± 14 | 60 ± 11 |
| Range | 32–74 | 27–82 | 38–74 | 33–88 |
| Gender (M:F) | 2.6:1 | 1.2:1 | 1.1:1 | 3.3:1 |
| Diagnosis | -- | Spinal (77%) | Inflammatory (7) | Axonal (6) |
| Bulbar (23%) | Mitochondrial (4) | Plexopathy (3) | ||
| Necrotizing (1) | CIDP (1) | |||
| Non-specific (1) | Axonal GBS (1) | |||
| Critical Illness (1) | Non-specific (4) |
Patient samples were used for either qPCR or western blot analysis. CIDP, Chronic inflammatory demyelinating polyradiculoneuropathy; GBS, Guillain Barre syndrome
The former included inflammatory and mitochondrial processes, while the latter mainly consisted of peripheral axonal and a few demyelinating neuropathies. The average age for all three control groups was similar to the ALS group. We performed qPCR to measure CYP27B1 mRNA expression levels in the muscle samples (Figure 1A). With the mean value of normal control samples set at 1.0, we observed a 19.3-fold increase in ALS samples (P < 0.0001). This increase was significantly greater (P < 0.005) than the myopathy group which showed a non-significant increase over normal controls (P = 0.19). The neuropathy control samples showed an increase over normal controls by 13-fold (P < 0.05). Although ALS and neuropathy groups were not significantly different, the ALS group had some samples as high as 60-fold greater than controls. As shown in the figure, muscle types biopsied were fairly well distributed among groups except for myopathy samples which included only proximal muscles. We next assessed ALS muscle samples by western blot for CYP27B1 expression (Figure 1B). In ALS patients we observed strong protein expression, as determined by band intensity, compared to normal muscle. Neuropathy samples showed increased intensity but myopathy samples were similar to normal controls. Interestingly, the protein is approximately 56 kDa, but in ALS and other disease control samples we observed a doublet, with the second band migrating slightly lower than the expected size. The origin of this band is unclear, but may represent an alternatively spliced variant of CYP27B1 which can occur in a tissue and cell-specific manner [17, 18]. To obtain quantitative data on protein expression, we performed densitometry using GAPDH as a loading control. The number of samples for each disease category was expanded to get statistical power (Figure 1C). Normal samples were run on each gel and used as comparators, with values set at one. CYP27B1 expression for ALS samples was 3.9-fold higher than normal controls (P < 0.0001) and was significantly higher than neuropathy (P < 0.05) and myopathy (P < 0.001) controls. Although neuropathy samples trended higher than myopathy and normal controls (1.8 versus 1.1 and 1.0 respectively), the differences were not statistically significant. Taken together, CYP27B1 expression is increased in muscle in denervation diseases with highest levels detected in ALS.
Figure 1: CYP27B1 is increased in ALS and neuropathy muscle tissues.

(A) RNA levels of CYP27B1 and the endogenous control, GAPDH, were quantitated using qPCR to calculate a normalized expression level as described in the methods. The average CYP27B1 value for the normal control samples (n = 9) was set at 1. Mean values (± SD) for the ALS (n = 28), myopathy (n = 8), and neuropathy (n = 13) cohorts were then expressed as a fold-increase over the normal control group. (B) Protein lysates from muscle samples were quantitated by western blot for CYP27B1 expression. A representative blot of ALS and control samples shows an immunoreactive band (indicated by arrow) consistent with the size estimated for CYP27B1. The blot was stripped and probed with GAPDH as the loading control. Estimated sizes of each protein are shown to the right. (C) CYP27B1 was quantitated by densitometry after normalizing to GAPDH as described in the methods section. Sample numbers are shown in parentheses. Data points represent the mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005. BI, biceps; DL, deltoid; GC, gastrocnemius; TA, tibialis anterior; VL, vastus lateralis.
3.2. CYP27B1 protein is expressed in myofibers
Having established that CYP27B1 is increased in ALS and to a lesser extent other denervating disease, we next sought to determine the cell of origin for this upregulation. Immunohistochemistry with an anti-CYP27B1 antibody was performed on ALS and normal control muscle samples. In the ALS samples, we observed immunostaining of myofibers, often in groups (Figure 2). Some of the fibers were angular and atrophic, a morphology consistent with denervation, whereas others appeared normal (Figure 2A, B, and D). In some areas there were collections of endomysial mononuclear cells. The normal control sections, on the other hand, showed little to no immunostaining (Figure 2C and E). We next looked at muscle samples from diseased controls (Figure 3). As with the ALS samples, there was increased immunostaining of myofibers in the neuropathy samples, often in smaller groups, with some being angular and atrophic and others having normal morphology (Figure 3A, B, and C). The overall intensity of immunostaining appeared less in neuropathy samples compared to ALS samples although this was not quantified. Little to no immunostaining was observed in a patient with inflammatory myopathy (Figure 3D). Note that the perivascular infiltrates of mononuclear cells are consistent with the inflammatory nature of the myopathy.
Figure 2: CYP27B1 is detected in myofibers in ALS but not normal control muscle.

Sections from vastus lateralis muscle were immunostained with a CYP27B1 antibody and counterstained with hematoxylin. (A) Low power view of an ALS muscle section shows positive immunostaining (brown color) of myofibers. (B) High-powered view of boxed area in (A) shows myofiber staining and surrounding mononuclear infiltrate. Here the muscle fibers range between 5-50 μM in diameter. One group of atrophic angular fibers contains up to 20 muscle fibers. (C) Muscle section from a normal control shows no immunostaining. (D) Muscle section from another ALS patient again shows immunostaining of some, but not all, myofibers. Likewise, there is an increase in mononuclear cells surrounding some of the fibers. (E) A second normal control muscle section shows no immunostaining. Scale bars, 50 μM.
Figure 3: CYP27B1 is detected in myofibers of neuropathy but not myopathy controls.
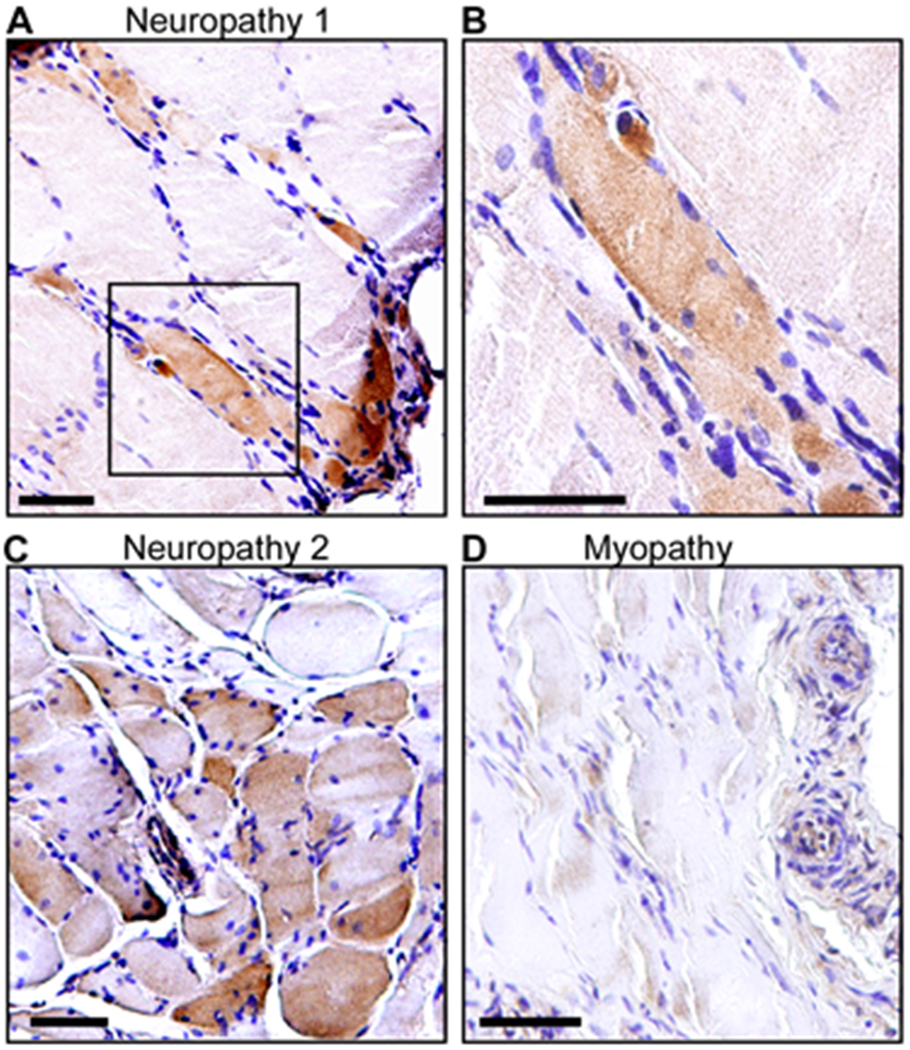
(A) A tibialis anterior muscle section from a patient with neuropathy showing positive immunostaining for CYP27B1. (B) High-power view of boxed area in (A). (C) A tibialis anterior muscle section from a second patient with neuropathy shows CYP27B1 immunoreactivity. (D) A vastus lateralis muscle section from a patient with inflammatory myopathy showing no immunoreactivity. All muscle sections were counterstained with hematoxylin. Scale bars, 50 μM.
3.3. CYP27B1 tracks disease progression in the SOD1G93A mouse
We next determined whether CYP27B1 is elevated in muscle from the SOD1G93A mouse and whether it can track disease progression. We assessed CYP27B1 mRNA levels by qPCR in gastrocnemius muscle samples at pre-symptomatic and symptomatic stages as previously determined in our mouse colony [5] (Figure 4A). CYP27B1 was elevated compared to littermate controls by postnatal day 60 which is an early pre-symptomatic stage. The lack of significant changes at day 40 is consistent with the time frame observed with other muscle biomarkers we have reported on including Smads and TGF-β [5, 19]. As the mouse advanced to clinically symptomatic stages, there was a progressive increase in CYP27B1 which peaked at nearly 6-fold over controls by end stage (day 150). CYP27B1 protein was also assessed and found to be progressively elevated but starting at day 105 (Figure 4B). Histochemical assessment of muscle sections showed CYP27B1 immunostaining in myofibers as with the human ALS samples (Figure 4C). Mononuclear cells were also seen in the vicinity of the densely stained myofibers.
Figure 4: CYP27B1 is increased in the SOD1G93A mouse.

(A) Upper panel shows schematic of the clinical timeline for the SOD1G93A mouse at different postnatal ages. Lower panel shows results of qPCR quantification of CYP27B1 at different ages in SOD1G93A mice and littermate controls (mean ± SD; n = 6 mice per data point). *P < 0.05, **P < 0.005, ***P < 0.0005. (B) Western blot of muscle lysates of SOD1G93A mice (M) and littermate controls (W) at different ages. Antibodies are shown to the right. Densitometry values for CYP27B1, after adjusting to the GAPDH loading control, are shown below. Control values were set at 1. (C) Immunostaining of a section from the gastrocnemius muscle of a SOD1G93A and control mouse at age 115 days. Scale bars, 25 μM.
3.4. Changes in CYP27B1 mRNA levels correlate with rate of clinical progression in human ALS
We prospectively studied 8 ALS patients over 6-12 months with serial biopsies of the same muscle to determine whether CYP21B1 mRNA levels change over time and whether these changes correlate with clinical progression as determined by changes in the revised ALS functional rating scale (ALSFRS-R). The average age of the study population was 55 years old (range 41 – 66). Clinical features of these patients are summarized in Table 2. Based on monthly decline in scores of the ALSFRS-R (ΔFRS) during the study period, two patients (ALS1 and 2) were classified as fast progressors (ΔFRS of 2.5), two as intermediate (ALS3 and ALS4 with a ΔFRS of 1.0 and 0.9 respectively) and four as slow progressors (ALS5–8, with ΔFRS ranging between 0.2 and 0.6) [20–22]. In general the pre-study ΔFRS decline (calculated based on symptom onset [21]) was similar with the exception of patient ALS8 whose ΔFRS rate dropped from 1.2 to 0.2 points per month. Interestingly, this patient received edaravone during the study period. The two fast progressors expired prior to the 12-month time point, and ALS4 declined a 12-month biopsy. To assess changes in CYP27B1 mRNA, we chose a muscle in an affected limb which had an MRC power grade of at least 4 at the beginning of the study. After measuring CYP27B1 mRNA levels at each time point, we estimated the rate of change by calculating the slope of the line generated by the data points (using linear regression). In 5 out the 8 patients (ALS 1–4 and ALS6), CYP27B1 progressively increased over time as indicated by a positive slope (Figure 5A). The other three patients (ALS5, 7, and 8) had essentially no change with a slope of 0. This latter group consisted of patients with clinically slow progression whereas four of the five patients in the former group were intermediate to fast progressors (ALS1, 2, 3, and 4). The degree of CYP27B1 changes correlated with ΔFRS over the course of the study (Figure 5B). In contrast, the control group, which consisted of healthy subjects who underwent two biopsies an average of 9 months apart [15], showed a modest 28% decrease in CYP27B1 levels (Figure 5C). Overall, CYP27B1 mRNA levels were 3-fold higher in ALS patients than controls at study entry and more than 14-fold at study completion (Figure 5D). Taken together, these results suggest that changes in CYP27B1 mRNA levels may track disease progression in humans as in the ALS mouse.
Table 2:
Demographic and clinical data on ALS study patients
| Sex | Age | Onset | 1Duration (m) | 2Pre-study ΔFRS | Study duration (m) |
3ALSFRS-R |
4ΔFRS | 5Muscle |
6MRC grade |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | End | Entry | End | |||||||||
| ALS1 | M | 59 | Bulbar | 7 | 2.9 | 6* | 28 | 13 | 2.5 | DL | 4 | 4 |
| ALS2 | M | 64 | Bulbar | 8 | 1.5 | 6* | 36 | 21 | 2.5 | DL | 5 | 4 |
| ALS3 | M | 41 | Spinal | 15 | 1.6 | 12 | 24 | 12 | 1.0 | TA | 4 | 2 |
| ALS4† | M | 44 | Spinal | 17 | 1.1 | 12 | 30 | 19 | 0.9 | DL | 5 | 2 |
| ALS5 | M | 54 | Spinal | 47 | 0.3 | 12 | 34 | 27 | 0.6 | BI | 4 | 1 |
| ALS6 | M | 66 | Spinal | 17 | 0.2 | 12 | 44 | 42 | 0.2 | DL | 4 | 4 |
| ALS7† | F | 53 | Spinal | 26 | 0.2 | 12 | 43 | 40 | 0.3 | DL | 5 | 4 |
| ALS8† | M | 62 | Spinal | 16 | 1.2 | 12 | 29 | 27 | 0.2 | DL | 5 | 5 |
From symptom onset to study entry.
Estimated monthly decline of the revised ALS Functional Rating Scale (ALSFRS-R) score prior to study entry (19).
ALSFRS-R score at entry and end of study.
Estimated monthly decline of FRS during the study period.
Muscle biopsied for CYP27B1 mRNA measurement. BI, biceps; DL, deltoid; TA, tibialis anterior.
Medical Research Council (MRC) power grade of biopsied muscle at entry and end of study.
Expired before the 12-month visit.
Received edaravone treatment during the study period.
Figure 5: Increases in muscle CYP27B1 mRNA in ALS patients correlate with rate of disease progression.

(A) CYP27B1 mRNA levels were determined by qPCR in serial muscle biopsy samples from 8 ALS patients over a 6 to 12-month time period (see Table 3). ALS4 declined biopsy at the 12-month interval and ALS 5 had insufficient tissue for RNA analysis at 6 months. The monthly decline in the ALSFRS-R (ΔFRS) is shown. The change in CYP27B1 mRNA over time was estimated by the slope (m). In patients that had 3 data points, the slope was estimated using linear regression. (B) Correlation plot of CYP27B1 slopes from (A) versus ΔFRS for the study period. (C) Mean (± SD) CYP27B1 mRNA levels in two serial muscle biopsies from seven neurologically normal controls over an average of 9 months. (D) Mean (± SD) values of CYP27B1 mRNA levels of ALS patients and controls at baseline and at the end of the study. *P < 0.05.
4. Discussion
In this report we found that CYP27B1 is broadly upregulated in myofibers of patients with muscle denervating disease, particularly in ALS. In skeletal muscle of the ALS mouse, CYP27B1 mRNA and protein levels increased as the disease advanced, suggesting that it can track disease progression. This possibility was supported in a small prospective human ALS study where increases in muscle CYP27B1 mRNA expression over time correlated with the rate of disease progression.
CYP27B1 is integral to the normal development and functioning of a wide range of tissues and cell types [1–3]. Our report is the first to describe CYP27B1 upregulation in denervated muscle fibers. One other report described an increase in CYP27B1 in regenerating muscle fibers using an experimental mouse model of chemically induced muscle injury [23]. Our finding suggests a perturbation of local vitamin D signaling in muscle denervating disease, perhaps in response to aberrant extra- or intra-cellular signals. Based on studies looking at extra-renal CYP27B1, factors controlling expression differ from that of the kidney where tight transcriptional regulation of CYP27B1 is maintained by parathyroid hormone (positive), FGF23 (negative) and active vitamin D (negative) [1–3, 24]. Inflammatory modulators such as LPS, for example, can selectively induce CYP27B1 in non-renal tissues [25]. TGF-β, a cytokine which we have shown to be abundant in mononuclear cells surrounding myofibers in ALS muscle tissue, can induce CYP27B1 in non-renal cells [19, 26, 27]. The microenvironment in ALS muscle and other denervating states often includes the presence of inflammatory mononuclear cells which can produce other CYP27B1-inducing cytokines such as TNF-α and IFN-ϒ [1, 6, 28]. Our recent report, for example, demonstrated the presence of activated mast cells and neutrophils (including extracellular neutrophil traps) adjacent to myofibers in muscle from human ALS subjects and in a rat model of ALS [29]. Both cell types produce these CYP27B1-inducing cytokines [30, 31]. Consistent with these prior reports, we frequently observed mononuclear cell infiltrates in the vicinity of CYP27B1-positive myofibers in both ALS and neuropathy sections (Figs. 2 and 3).
The downstream effect of upregulated CYP27B1 is not clear but may relate to vitamin D signaling if there is a concomitant increase in active vitamin D. There is growing evidence that vitamin D regulates many other functions besides bone mineralization, and vitamin D receptors (VDR) have been identified in a large number of human tissues, including muscle [32]. Myocytes express VDRs which, when bound to active Vitamin D, exert genomic and non-genomic effects that can modulate cellular physiology and maintain normal muscle function [32]. Genomic effects include upregulation of gene programs promoting myoblast differentiation, muscle regeneration, neuromuscular junction maintenance and calcium/phosphate homeostasis [33, 34]. These programs may be in play in denervating disease as the muscle attempts to compensate for atrophy, myofiber death, and loss of muscle power. Likewise, non-genomic effects of active vitamin D on calcium flux or mitochondrial metabolism may impact muscle contractility or energy production [34, 35]. The importance of vitamin D and muscle health has clearly been established in both human and animals where systemic vitamin D deficiency leads to muscle weakness and atrophy preferentially involving type II fibers [36]. Interestingly, type 2 muscle fibers are selectively affected early in the course of ALS [37]. From our data we cannot determine whether CYP27B1 upregulation in myofibers is beneficial or detrimental in denervating disease. This could be tested by assessing the impact of selective CYP27B1 deletion in skeletal muscle of the ALS mouse.
Since CYP27B1 was upregulated in non-ALS neuropathic processes, the utility of CYP27B1 as a potential clinical biomarker in ALS lies in its capacity to track disease progression. This could apply to pre-clinical stages as the elevated expression began at a very early age in the SOD1G93A mouse (between 40 and 60 d), well before clinical symptoms develop. Such a biomarker, for example, might be useful in detecting phenoconversion of patients with hereditary forms of ALS [38]. The temporal pattern observed with CYP27B1 in the SOD1G93A mouse parallels that of other muscle biomarkers we have previously described including Smad1, 2, 5 and 8, TGF-β1–3, and certain miRNAs (e.g. miR-206, 133a, and 133b) [5, 19, 39]. The striking overlap of these molecular responses supports prior observations that the earliest pathological changes in ALS occur in skeletal muscle. A prior report, for example, described extensive end-plate denervation in fast-fatigable fibers in this time frame which preceded ventral root or cell body loss [7]. Our prospective study on serial muscle samples from ALS patients provided a unique opportunity to validate observations in the ALS mouse. Although the study was small, there was good representation of the range in progression rates seen with this disease [9]. The observed correlation between changes in CYP27B1 mRNA and changes in clinical status are consistent with the pattern observed in the ALS mouse.
Since this enzyme is localized within mitochondria and not typically secreted, it is unlikely to be readily measured in plasma or urine, limiting its potential as a readily accessible biomarker in ALS or other muscle denervating disease. It is possible that a PET or SPECT tracer could be developed to image this enzyme’s activity as has been done for other metabolic pathways [40]. Circulating vitamin D has been investigated as a potential biomarker in ALS with mixed results. Some reports link low vitamin D levels with a significantly faster rate of decline, shorter lifespan or poor mobility whereas other studies show no impact of vitamin D levels on survival or even possibly a worse prognosis with higher levels [41–45]. These studies typically looked at 25(OH)D3, the precursor to active vitamin D, which is produced mainly in the liver. However, these measurements do not reflect local production of active vitamin D that occurs in peripheral tissues to produce tissue-specific autocrine or paracrine effects [46]. Thus, it is conceivable that muscle tissue levels of active vitamin D may be a more accurate biomarker in ALS and other denervating diseases. This possibility could be tested when reliable and non-invasive methods become available for measuring active vitamin D levels in muscle fibers.
There were limitations to this study. It is possible that CYP27B1 upregulation may vary among the muscle types, possibly related to different proportions of fiber types [47, 48]. Because of the clinical nature of myopathies, for example, muscle types were limited to proximal muscles (vastus lateralis, biceps, and deltoid) whereas ALS and non-ALS neuropathic samples included more distal muscles (tibialis anterior and gastrocnemius). The number of myopathy and neuropathy samples was also relatively small and not representative of all subtypes. Thus, it is possible that CYP27B1 may be induced in some forms of myopathy such as inclusion body myositis which was not represented in the test group. Likewise, certain neuropathy subtypes may have higher CYP27B1 induction than others. Finally, considering the heterogeneous nature of ALS, this prospective study was small and will require a larger cohort of patients to validate whether CYP27B1 can track disease progression.
In summary, we have identified CYP27B1 as a novel muscle marker of denervation. Our findings open different avenues for future investigation including the potential role of CYP27B1 in tracking ALS and possibly other progressive denervating diseases, the identification of underlying mechanism(s) for CYP27B1 induction, and the characterization of its function in muscle denervation.
ACKNOWLEDGEMENTS
We wish to thank Dr. Andrzej Slominski (Dept. of Dermatology, University of Alabama at Birmingham) for kindly providing a CYP27B1 antibody and for advice on experimental design. We wish to thank Terry Lewis, PhD, and the UAB Neuroscience Molecular Detection Core (P30 NS47466) for assistance with immunohistochemistry. This work was supported by NINDS R01NS092651 and R21NS111275-01 (PHK) and a Merit Review BX001148 from the Department of Veterans Affairs (PHK). Finally, we would like to extend gratitude toward our patients who participated in this study and those who generously donated their tissues post-mortem to help advance ALS research.
FUNDING
This work was supported by the National Institute of Neurological Disorders and Stroke, R01NS092651 and R21NS111275–01 (PHK), and by the Dept. of Veterans Affairs, BX001148 (PHK)
Footnotes
Conflict of Interest: none.
REFERENCES
- [1].Bikle DD, Patzek S, Wang Y, Physiologic and pathophysiologic roles of extra renal CYP27B1: Case report and review, Bone reports, 8 (2018) 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Adams JS, Rafison B, Witzel S, Reyes RE, Shieh A, Chun R, Zavala K, Hewison M, Liu PT, Regulation of the extrarenal CYP27B1-hydroxylase, J Steroid Biochem Mol Biol, 144 (2014) 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jones G, Prosser DE, Kaufmann M, The Activating Enzymes of Vitamin D Metabolism (25- and 1α-Hydroxylases), in: Feldman D (Ed.) Vitamin D (4th Ed), Academic Press; 2018, pp. 57–79. [Google Scholar]
- [4].Hardiman O, van den Berg LH, Kiernan MC, Clinical diagnosis and management of amyotrophic lateral sclerosis, Nat Rev Neurol, 7 (2011) 639–649. [DOI] [PubMed] [Google Scholar]
- [5].Si Y, Cui X, Kim S, Wians R, Sorge R, Oh SJ, Kwan T, AlSharabati M, Lu L, Claussen G, Anderson T, Yu S, Morgan D, Kazamel M, King PH, Smads as muscle biomarkers in amyotrophic lateral sclerosis, Ann Clin Transl Neurol, 1 (2014) 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Al-Sarraj S, King A, Cleveland M, Pradat P-F, Corse A, Rothstein JD, Leigh PN, Abila B, Bates S, Wurthner J, Meininger V, Mitochondrial abnormalities and low grade inflammation are present in the skeletal muscle of a minority of patients with amyotrophic lateral sclerosis; an observational myopathology study, Acta Neuropathol Com, 2 (2014) 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD, Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man, Experimental Neurology, 185 (2004) 232–240. [DOI] [PubMed] [Google Scholar]
- [8].Moloney EB, de Winter F, Verhaagen J, ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease, Front. Neurosci., 8 (2014) 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bradley WG, Biological markers in amyotrophic lateral sclerosis: help or hindrance?, J Neurol 246 (1999) III13–III15. [DOI] [PubMed] [Google Scholar]
- [10].Benatar M, Boylan K, Jeromin A, Rutkove SB, Berry J, Atassi N, Bruijn L, ALS biomarkers for therapy development: State of the field and future directions, Muscle Nerve, 53 (2016) 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, a. et, Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation, Science, 264 (1994) 1772–1775. [DOI] [PubMed] [Google Scholar]
- [12].Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, Byers N, Toman I, Alexander GM, Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS, J. Neurol. Sci, 236 (2005) 1–7. [DOI] [PubMed] [Google Scholar]
- [13].Brooks BR, Miller RG, Swash M, Munsat TL, World D Federation of Neurology Research Group on Motor Neuron, El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis, Amyotroph. Lateral Scler., 1 (2000) 293–299. [DOI] [PubMed] [Google Scholar]
- [14].Evans WJ, Phinney SD, Young VR, Suction applied to a muscle biopsy maximizes sample size, Med Sci Sports Exerc, 14 (1982) 101–102. [PubMed] [Google Scholar]
- [15].Bickel CS, Cross JM, Bamman MM, Exercise dosing to retain resistance training adaptations in young and older adults, Med Sci Sports Exerc, 43 (2011) 1177–1187. [DOI] [PubMed] [Google Scholar]
- [16].Kazamel M, Cutter G, Claussen G, Alsharabati M, Oh SJ, Lu L, King PH, Epidemiological features of amyotrophic lateral sclerosis in a large clinic-based African American population, Amyotroph Lateral Scler Frontotemporal Degener, 14 (2013) 334–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brozyna AA, Jozwicki W, Jochymski C, Slominski AT, Decreased expression of CYP27B1 correlates with the increased aggressiveness of ovarian carcinomas, Oncol. Rep, 33 (2015) 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu S, Ren S, Nguyen L, Adams JS, Hewison M, Splice Variants of the CYP27B1 Gene and the Regulation of 1,25-Dihydroxyvitamin D3 Production, Endocrinology, 148 (2007) 3410–3418. [DOI] [PubMed] [Google Scholar]
- [19].Si Y, Kim S, Cui X, Zheng L, Oh SJ, Anderson T, AlSharabati M, Kazamel M, Volpicelli-Daley L, Bamman MM, Yu S, King PH, Transforming Growth Factor Beta (TGF-beta) Is a Muscle Biomarker of Disease Progression in ALS and Correlates with Smad Expression, PLoS One, 10 (2015) e0138425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gomeni R, Fava M, Amyotrophic lateral sclerosis disease progression model, Amyotroph Lateral Scler Frontotemporal Degener, 15 (2014) 119–129. [DOI] [PubMed] [Google Scholar]
- [21].Kimura F, Fujimura C, Ishida S, Nakajima H, Furutama D, Uehara H, Shinoda K, Sugino M, Hanafusa T, Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS, Neurology, 66 (2006) 265–267. [DOI] [PubMed] [Google Scholar]
- [22].Tortelli R, Ruggieri M, Cortese R, D’Errico E, Capozzo R, Leo A, Mastrapasqua M, Zoccolella S, Leante R, Livrea P, Logroscino G, Simone IL, Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression, Eur J Neurol, 19 (2012) 1561–1567. [DOI] [PubMed] [Google Scholar]
- [23].Srikuea R, Zhang X, Park-Sarge OK, Esser KA, VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation, Am. J. Physiol. Cell Physiol, 303 (2012) C396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Meyer MB, Pike JW, Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of CYP27B1 and Cyp24a1 expression, J Steroid Biochem Mol Biol, 196 (2020) 105500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Meyer MB, Benkusky NA, Kaufmann M, Lee SM, Onal M, Jones G, Pike JW, A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene CYP27B1 essential for vitamin D3 activation, Journal of Biological Chemistry, 292 (2017) 17541–17558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang J, Liu X, Wang H, Li Y, Lan N, Yuan X, Wu M, Liu Z, Li G, Allergen specific immunotherapy enhanced defense against bacteria via TGF-beta1-induced CYP27B1 in asthma, Oncotarget, 8 (2017) 68681–68695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL, Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism, J Clin Invest, 117 (2007) 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mochizuki Y, Ojima K, Uezumi A, Masuda S, Yoshimura K, Takeda S.i, Participation of bone marrow-derived cells in fibrotic changes in denervated skeletal muscle, Am J Pathol, 166 (2005) 1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Trias E, King PH, Si Y, Kwon Y, Varela V, Ibarburu S, Kovacs M, Moura IC, Beckman JS, Hermine O, Barbeito L, Mast cells and neutrophils mediate peripheral motor pathway degeneration in ALS, JCI Insight, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mukai K, Tsai M, Saito H, Galli SJ, Mast cells as sources of cytokines, chemokines, and growth factors, Immunological reviews, 282 (2018) 121–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tecchio C, Micheletti A, Cassatella MA, Neutrophil-derived cytokines: facts beyond expression, Front. Immunol, 5 (2014) 508–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pojednic RM, Ceglia L, The emerging biomolecular role of vitamin D in skeletal muscle, Exerc Sport Sci Rev, 42 (2014) 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gifondorwa DJ, Thompson TD, Wiley J, Culver AE, Shetler PK, Rocha GV, Ma YL, Krishnan V, Bryant HU, Vitamin D and/or calcium deficient diets may differentially affect muscle fiber neuromuscular junction innervation, Muscle Nerve, 54 (2016) 1120–1132. [DOI] [PubMed] [Google Scholar]
- [34].Ceglia L, Harris SS, Vitamin D and its role in skeletal muscle, Calcif Tissue Int, 92 (2013) 151–162. [DOI] [PubMed] [Google Scholar]
- [35].Silvagno F, Pescarmona G, Spotlight on vitamin D receptor, lipid metabolism and mitochondria: Some preliminary emerging issues, Molecular and Cellular Endocrinology, 450 (2017) 24–31. [DOI] [PubMed] [Google Scholar]
- [36].Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE, The Roles of Vitamin D in Skeletal Muscle: Form, Function, and Metabolism, Endocr. Rev, 34 (2013) 33–83. [DOI] [PubMed] [Google Scholar]
- [37].Nijssen J, Comley LH, Hedlund E, Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis, Acta neuropathologica, 133 (2017) 863–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Benatar M, Turner MR, Wuu J, Defining pre-symptomatic amyotrophic lateral sclerosis, Amyotroph Lateral Scler Frontotemporal Degener, (2019) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Si Y, Cui X, Crossman DK, Hao J, Kazamel M, Kwon Y, King PH, Muscle microRNA signatures as biomarkers of disease progression in amyotrophic lateral sclerosis, Neurobiol Dis, 114 (2018) 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rempel BP, Price EW, Phenix CP, Molecular Imaging of Hydrolytic Enzymes Using PET and SPECT, Molecular Imaging, 16 (2017) 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Camu W, Tremblier B, Plassot C, Alphandery S, Salsac C, Pageot N, Juntas-Morales R, Scamps F, Daures JP, Raoul C, Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis, Neurobiol Aging, 35 (2014) 1198–1205. [DOI] [PubMed] [Google Scholar]
- [42].Paganoni S, Macklin EA, Karam C, Yu H, Gonterman F, Fetterman KA, Cudkowicz M, Berry J, Wills AM, Vitamin D levels are associated with gross motor function in amyotrophic lateral sclerosis, Muscle Nerve, 56 (2017) 726–731. [DOI] [PubMed] [Google Scholar]
- [43].Yang J, Park J-S, Oh K-W, Oh S.-i, Park H-M, Kim SH, Vitamin D levels are not predictors of survival in a clinic population of patients with ALS, J. Neurol. Sci, 367 (2016) 83–88. [DOI] [PubMed] [Google Scholar]
- [44].Blasco H, Madji Hounoum B, Dufour-Rainfray D, Patin F, Maillot F, Beltran S, Gordon PH, Andres CR, Corcia P, Vitamin D is Not a Protective Factor in ALS, CNS Neurosci Ther, 21 (2015) 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Libonati L, Onesti E, Gori MC, Ceccanti M, Cambieri C, Fabbri A, Frasca V, Inghilleri M, Vitamin D in amyotrophic lateral sclerosis, Functional neurology, 32 (2017) 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dirks-Naylor AJ, Lennon-Edwards S, The effects of vitamin D on skeletal muscle function and cellular signaling, J Steroid Biochem Mol Biol, 125 (2011) 159–168. [DOI] [PubMed] [Google Scholar]
- [47].Saltin B, Gollnick PD, Skeletal Muscle Adaptability: Significance for Metabolism and Performance, Comprehensive Physiology, John Wiley & Sons, Inc; 2010. [Google Scholar]
- [48].Gollnick PD, Sjödin B, Karlsson J, Jansson E, Saltin B, Human soleus muscle: A comparison of fiber composition and enzyme activities with other leg muscles, Pflügers Archiv, 348 (1974) 247–255. [DOI] [PubMed] [Google Scholar]


