Abstract
Supplemental arginine has shown promise as a safe therapeutic option to improve endogenous nitric oxide (NO) regulation in cardiovascular diseases associated with endothelial dysfunction. L-arginine, an endogenous amino acid, was reported in clinical studies in adults to improve cardiovascular function in hypertension, pulmonary hypertension, pre-eclampsia, angina, and mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome. L-citrulline, a natural precursor of L-arginine, is more bioavailable than L-arginine because of hepatic first-pass metabolism avoidance and longer circulation time. Although not yet well studied, arginine/citrulline has immense therapeutic potential in some life-threatening diseases of children. However, optimal clinical development of arginine or citrulline in children is dependent on more information about pharmacokinetics and exposure-response relationships at appropriate ages and under relevant disease states. This article summarizes the pre-clinical and clinical studies of arginine/citrulline in both adults and children, including currently available pharmacokinetic information. The pharmacology of arginine/citrulline is confounded by several patient-specific factors such as baseline variation of arginine/citrulline due to developmental ages and disease states. Currently available pharmacokinetic studies are not enough to inform the optimal design of clinical studies, especially those in children. Successful bench to bedside clinical translation of arginine supplementation awaits information from well-designed pharmacokinetic-pharmacodynamic studies, along with pharmacometric approaches.
Keywords: Citrulline, Arginine, Pharmacokinetics, Pediatrics, Nitric Oxide
Introduction
L-arginine, a semi-essential amino acid that plays critical physiological roles in muscle development and ammonia detoxification [1, 2], has been investigated for therapeutic use in a wide variety of pathological conditions [3-5], in addition to its widespread use as an ergogenic nutrition supplement [5]. Therapeutic arginine supplementation, as it improves endogenous nitric oxide (NO) production, was mostly investigated for clinical use in the ailments of the cardiovascular system that include hypertension, pulmonary hypertension, angina, nitrate tolerance, and pre-eclampsia. L-arginine supplementation was reported to restore normal endothelial function in both normotensive and hypertensive patients with microvascular angina [6, 7]. Tolerance to the organic nitrates, a widely used NO donor for the treatment of coronary artery diseases and chronic heart failure, remains as a long-standing therapeutic problem for which the nitrate-free treatment intervals remains as the only option to prevent nitrate tolerance in the current clinical practice [8, 9]. Arginine supplementation, as it restores the normal function of NO, has been useful in preventing rapid nitrate tolerance from transdermal nitroglycerine use in patients with stable angina [10]. Altered NO production is also contributing to the pathophysiology of pre-eclampsia and intrauterine growth restriction [11]. Supplemental arginine during pregnancy was found to reduce the incidence of pre-eclampsia in the high-risk pregnant population [12].
Arginine is also a promising therapy for pulmonary hypertension (PH), a devastating cardio-pulmonary disease with a high mortality rate [13]. Clinical studies in adults showed that arginine supplementation, administered either in the form of arginine or its pre-cursor, citrulline, improves pulmonary hemodynamics in PH-afflicted adults [14-16]. Although aspects of the underlying pathophysiology for PH may differ from the adult disease, use of arginine/citrulline supplementation for PH in children (idiopathic or disease-associated) is also receiving attention [17-21] .
There is also evidence that arginine may be therapeutically useful in some life-threatening inherited diseases in children that are accompanied by cardiovascular complications. Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome, a progressively fatal mitochondrial disease, is caused by genetic mutations in the mitochondrial DNA. In MELAS syndrome, endothelial dysfunction, because of uncontrolled mitochondrial proliferation, leads to alterations in cerebral hemodynamics and stroke-like episodes. Published literature shows that supplemental arginine can replenish NO deficiency and thus can improve blood circulation in the cerebral microvasculature [22, 23]. A recently published clinical study suggests that arginine supplementation, either in the form of arginine or citrulline, appears to have therapeutic benefits in children with MELAS syndrome [24].
Urea cycle disorders (UCD) are another group of diseases that affect young children. In UCD, inherited deficiencies of some key enzymes and transporters of the urea cycle pathway results in the accumulation of ammonia and some precursor metabolites causing severe neurological damages [18, 25]. For the last two decades, supplemental arginine was useful in improving clinical outcomes in children with UCD [26, 27]. Arginine therapy replenishes the deficient urea cycle intermediates by activating alternative enzymatic pathways [27, 28]. In clinical studies, supplemental arginine therapy has been used either as direct L-arginine or in the form of arginine precursor—citrulline. Citrulline, an alpha-amino acid that is metabolized to L-arginine, when given orally, is more effective than L-arginine in improving arginine plasma concentration because it is not the substrate of hepatic or intestinal arginases [5].
Although arginine supplementation possesses tremendous therapeutic potential in some life-threatening diseases of children, the clinical pharmacology of arginine/citrulline therapy— particularly pharmacokinetics, exposure-response relationship, and safety— has not been investigated extensively in the pediatric population. In this review, we summarize the molecular basis of arginine/citrulline supplementation in different diseases, pharmacokinetics, and pharmacodynamics of arginine/citrulline in their potential therapeutic uses in adult and pediatric diseases. We discuss the relationship between pharmacokinetic exposure and therapeutic response of arginine/citrulline in pre-clinical studies with its possible translation towards clinical studies. This article will serve as a ready reference as there is a lack of review of arginine/citrulline supplementation regarding its pharmacokinetics and potential therapeutic uses in pediatric diseases.
L-arginine deficiency in vascular endothelial cell dysfunction and the use of citrulline as an efficient arginine supplement
Regulation of vascular tone and contractility is critically dependent on the availability of L-arginine, a significant substrate for NO production in the vascular endothelium. Inadequate supply of L-arginine resulted in increased superoxide generation by eNOS and altered L-arginine metabolism within the vascular endothelial cells. Prolonged deficiency of L-arginine further causes elevated oxidative stress, sequestration of intracellular L-arginine, and slower recycling of L-arginine from its precursor–L-citrulline. As a result, vasoconstriction and vascular remodeling occur as hyperactive eNOS fails to produce enough NO [29-31]. Published studies have shown that supplemental arginine can prevent endothelial dysfunction and thus can restore the normal vasodilatory capacity of the vascular endothelium. However, due to the presence of arginase in intestinal enterocytes and the high first-pass metabolism of L-arginine to ornithine and urea by the liver arginases, oral arginine supplementation cannot sufficiently elevate the plasma levels of L-arginine [32, 33]. Moreover, higher levels of circulating L-arginine induce arginases in most of the tissues resulting in rapid L-arginine clearance.
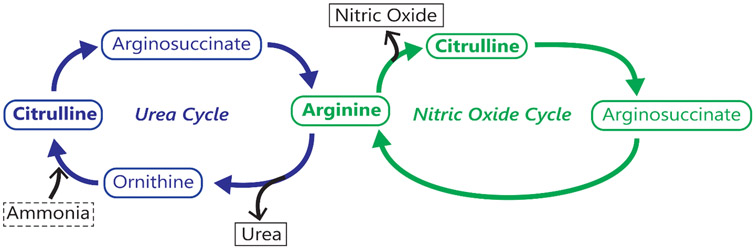
L-citrulline, a natural precursor of L-arginine that takes part in both urea and NO cycles, can be a better substitute of L-arginine supplementation because it bypasses hepatic first-pass metabolism and can be converted to L-arginine specifically within the tissues (Fig.1). L-citrulline is an intermediate product of urea cycle in which L-ornithine, a metabolite of L-glutamine, is converted to L-citrulline by ornithine carbamoyltransferase. With development, L-citrulline becomes a useful product of small intestine in human as compared to L-arginine and, the kidney, the primary organ of L-citrulline metabolism, gradually becomes more efficient in converting L-citrulline to L-arginine in the post-natal period. In other words, L-citrulline, when taken orally, can effectively improve L-arginine plasma levels as it is transported into enterocytes without metabolic loss by arginase and because it then bypasses hepatic metabolism and is transported to the kidneys where it is metabolized to L-arginine [33]. These elements of L-citrulline processing provide pharmacokinetic advantages over L-arginine. Consequently, orally administered L-citrulline is expected to result in comparatively higher concentrations of L-arginine and hence, can bring about more significant therapeutic benefits than orally administered L-arginine. Thus, L-citrulline supplementation is considered as a better alternative to L-arginine supplementation in a wide variety of diseases caused by L-arginine deficiency.
Figure 1:
Arginine and citrulline: two significant players in the urea and nitric oxide cycles
Preclinical studies exploring the use of L-arginine supplementation in various diseases
Pre-clinical studies are necessary when investigating novel drugs/supplements to establish safety and proof of concept for efficacy before initiating clinical trials in humans, especially in vulnerable populations such as pediatric or pregnant populations. The use of in vivo (whole animal) or ex vivo (isolated organ) models for assessing safety and efficacy provide a basis for understanding the potential effects size in humans as well as the possible side effect profile.
The use of citrulline and L-arginine has been investigated pre-clinically in some diseases and different animal models. This section focuses on atherosclerosis (vascular wall and vascular function), systemic hypertension, pulmonary hypertension, and preeclampsia. Table 1 summarizes the pre-clinical studies conducted in these diseases as well as the outcome of arginine or citrulline supplementation.
Table 1.
Summary of different pre-clinical studies with arginine/citrulline supplementation
| Disease | Animal model | The outcome of L-arginine or citrulline supplementation |
|---|---|---|
| Atherosclerosis | Hypercholesterolemic rabbit | - ↓ lesion surface area[110, 34, 38, 35] - ↓intima thickness[34-36, 45] - Absence of adherent monocytes or tissue macrophages[45] - Prevents xanthoma formation[110] |
| LDL receptor knockout mouse | ||
| Vascular endothelial function | Hypercholesterolemic rabbit | - ↑ Endothelial dependent relaxation [34-39] - ↑NO production/superoxide radical release[34, 39, 38, 111] |
| Pulmonary hypertension | Hypoxia-induced pulmonary hypertension (rat and newborn pig) Monocrotaline-induced pulmonary hypertension [43] |
- ↑NO production [40, 42, 44, 53] - ↓Pulmonary vascular resistance[40-44] |
| Systemic hypertension | Salt-sensitive hypertensive (rats) Spontaneous hypertension (young rat) |
-↑NO production[48, 47] |
| Preeclampsia | Insulin induced hypertension (pregnant rat) | - ↓Blood pressure[49, 51, 50, 52] - ↓urinary metabolites or nitrate[49, 51, 50, 52] - ↓urine protein excretion[51, 52] |
| Adriamycin-induced nephropathy (pregnant rat) |
↓=decrease; ↑= increase; LDL= Low Density Lipoprotein; NO= Nitric Oxide; PAH=Pulmonary Arterial Hypertension
As described in the previous section, NO is vital to maintain vascular endothelial function. As such, the significant effect of arginine/citrulline supplementation is to improve endothelial function in both [34-39] systemic and pulmonary vasculature [40-44] Notably, the improvement in endothelial function results in downstream benefits such as decreased vascular/pulmonary pressure or normalization of vascular wall intimal thickness [34-36, 45]. As an example, the vascular wall effects of L-arginine supplementation are summarized in the review by Preli et al. [46]. The dominant animal model used in this setting is the hypercholesteremic rabbit (high cholesterol chow). Briefly, L-arginine supplementation reduces the surface area of atherosclerotic lesions and reduces the intima thickness compared to untreated animals (Table 1) Furthermore, another study used LDL receptor knockout mice and showed that L-arginine prevented the formation of xanthomas. The authors of this paper concluded that L-arginine supplementation might be of benefit to the patients with familial hypercholesterolemia. L-arginine supplementation also reduced systemic blood pressure in both salt-sensitive hypertension [47] and spontaneous hypertension rat models[48] , as well as in different animal models for preeclampsia, including insulin-induced hypertension [49, 50] and adriamycin-induced nephropathy models [51, 52]. The link between arginine/citrulline supplementation improved NO production, and vascular function has been shown in ofseveral animal models investigating pulmonary hypertension. Intact animal as well as ex vivo models for pulmonary hypertension were developed either with environmental injury (hypoxia) [40-42, 53], or drug-toxin-induced (monocrotaline) injury [44]. L-arginine supplementation, when tested in these models, increased NO production [41, 40, 47, 48, 53, 42, 44] that ultimately resulted in a reduction of pulmonary vascular resistance [40-44] (Table 1). Similarly, L-citrulline supplementation was effective in improving pulmonary vascular NO production and ameliorating pulmonary hypertension when investigated in newborn/juvenile animal models, for example, newborn pigs [40, 41, 53] and young rats [48].
The effect of arginine supplementation was also evaluated in some preclinical models representing systemic hypertension. L-arginine or L-citrulline intake improved NO synthesis and prevented salt-sensitive hypertension in rats [47]. A recently published study showed that L-citrulline supplementation prevents spontaneous hypertension in rats [48].
These preclinical studies, although demonstrating the efficacy of arginine/citrulline supplementation in a wide variety of pathologic conditions, suffer limitations. One limitation is the dearth of studies using age or physiologically appropriate (i.e., pediatric/juvenile or pregnant animal models. Also, many of the studies involved short-term exposures and have not sufficiently addressed safety concerns. One animal study investigated the toxicity of L-arginine over 13 weeks in rats; overall, the side effects were minimal and generally transient [54]. Theoretically, an overdose of arginine/citrulline may result in hypotension due to its vasodilatory effects. Published articles show that large doses of L-arginine are used in preclinical models to induce acute pancreatitis [55], and an accidental overdose of arginine hydrochloride was fatal [56]. No adverse side effects have been reported in studies with citrulline, although only a limited range of doses have been used. Thus, the safety of arginine supplementation remains of concern, and mandates that safety issues be carefully considered, and adverse effects closely monitored for when a clinical translation is done.
Clinical use of arginine supplementation
Therapeutic effects of L-citrulline are mostly dependent on the action of L-arginine. L-arginine is generated from L-citrulline by argininosuccinate synthase as part of the urea cycle (Figure 1). As a substrate for nitric oxide synthases (NOS), L-arginine is integral to the formation of NO, and reformation of the precursor L-citrulline. NO is a cofactor for soluble guanylate cyclase, catalyzing the formation of cyclic guanosine monophosphate (cGMP), a regulator, mediator, and messenger in the nervous, immunologic, and cardiovascular systems. In the vasculature, cGMP is a regulator for vasodilation. As dysfunctional NO signaling causes a wide variety of diseases, supplemental arginine in the form of L-arginine or L-citrulline was evaluated in different clinical studies as summarized in Table 2.
Table 2.
Clinical studies on arginine/citrulline supplementation in different diseases
| Disease | Study population |
Clinical outcome | ||
|---|---|---|---|---|
| Age (yrs.) |
n | Dosing | ||
| Inborn errors of ureagenesis [26] | <0.5 | 4 | Arginine/citrulline 0.2 mmol/kg/day | - Arginine supplementation improved symptomatic hyperammonemia |
| Angina pectoris [6] | Angina=57±9 Control=59±8 |
16; microvascular angina=8, control=8 | Arginine 500 mg as 10-minute infusion | - Arginine supplementation improved endothelium-dependent vasodilation in the coronary arteries |
| Angina pectoris [7] | Angina=57±9 Control=59±8 |
13 | Arginine 2g, tid oral | - Arginine supplementation improved endothelial function in hypertensive patients with microvascular angina |
| Preeclampsia [12] | Placebo=28.2±5.1 Arginine=28.0±6.1 |
450; Placebo=222, Arg=228 | Arginine 6.6g/day oral | - Arginine supplementation prolongs the latency to development of pre-eclampsia |
| Nitrate tolerance in patients with angina [10] | 14; arginine=7, placebo=7 | Arginine 700 mg oral | - Arginine supplementation modified or prevented the development of nitrate tolerance during continuous transdermal nitroglycerine therapy | |
| Sickle cell disease [87] | 10-18 | 5 | Oral Cit 0.1 g/kg bid | - ↓Total leukocyte & segmental neutrophil count - Symptomatic improvement - No controls used |
| Postoperative pulmonary hypertension [88] | < 6 | 40; Placebo=20, Cit=20 | 5 post-operative doses of Cit 1g/m2 | - Citrulline supplementation elevated plasma arginine/citrulline concentration - Elevated citrulline in plasma prevented post-operative pulmonary hypertension |
| MELAS syndrome [24] | 6-14 | 10; Placebo=5, Cit/Arg=5 | 3 g/kg/day; Cit/Arg | - Citrulline supplementation is more effective than arginine in improving NO production |
| MELAS syndrome [23] | 20-46 | 20; Placebo=10, Cit/Arg=10 | 0.8 g/kg/day; Cit/Arg | - Citrulline supplementation substantially increased NO production than that observed with arginine supplementation |
| Acute myocardial infarction [85] | >30 | 153; Placebo=75, Cit/Arg=78 | 1-3gm tid for six months | - No improvement in vascular stiffness or ejection fraction - Might be associated with higher postinfarction mortality - L-arginine supplementation should not be recommended in acute MI |
| Tuberculosis [86] | 15-73 | 200; Arg+vitD=50, Arg+placebo=49, placebo+vitD=51, all placebo=50 | Arg=6 g daily as oral tablet; 1250 mcg vitD3 as oral tablet | - L-arginine supplementation has no effect on tuberculosis outcome |
| Pulmonary hypertension [79] | 52±5 | Pulmonary hypertension=10 Heart failure without PH=5 |
30-minute infusion of arginine (500 mg/kg) | - Exogenous L-arginine is beneficial in some patients with pulmonary hypertension in short-term |
| Cystic fibrosis associated airway obstruction [84] | 14-45 | 13 patients with cystic fibrosis | 18 mL of 7% arginine HCl solution given through an electronic nebulizer | - Single inhalation of arginine results in an acute and transient improvement of pulmonary function and oxygenation |
‘>‘= greater than; ‘<‘= less than; Arg=arginine; Cit= citrulline; g= gram; kg= kilogram; m2= meter squared; mcg= microgram; mg=milligram; mmol= millimole; PAH=Pulmonary Arterial Hypertension; tid= three-times daily; vit = vitamin
Supplemental arginine/citrulline in adult clinical studies
Clinical studies in adults have evaluated the effect of dietary supplementation with citrulline or citrulline-rich watermelon, in improving exercise outcomes [57, 58] and cardiovascular health, including pulmonary hypertension [59-77]. Although clinical studies in the mid-1990s were not in agreement on the benefits of arginine supplementation in pulmonary hypertension [78-81], recent studies have more consistently supported the beneficial effects of arginine/citrulline in adults with cardiovascular problems [76, 82]. Oral L-citrulline administration for two weeks was associated with improvement in the quality of life of patients with idiopathic pulmonary hypertension [15]. Three studies assessed the effect of arginine supplementation in angina pectoris related complications. Egashira et al. showed that intracoronary infusion of L-arginine improved coronary microcirculation in patients with angina pectoris but not in control subjects who had atypical chest pain and normal coronary angiogram [6]. L-arginine supplementation in this study was associated with the improvement in endothelium-dependent vasodilation. Eight years later, in another clinical study, Palloshi et al. found oral L-arginine supplementation to be a useful way to improve endothelial function in hypertensive patients with angina [7]. Supplemental arginine, in a separate study, effectively prevented nitrate tolerance, a significant limitation of organic nitrates that are used as first-line therapies for managing angina pectoris. In this randomized, placebo-controlled double-blind study, oral L-arginine therapy showed beneficial effects in preventing nitrate tolerance during continuous transdermal nitroglycerin study [10]. Arginine/citrulline supplementation was effective in managing symptoms associated with MELAS syndrome [83] and in cystic fibrosis-associated airway obstruction[84]. However, arginine supplementation was not effective in improving acute myocardial infarction or tuberculosis outcome [85, 86].
Supplemental arginine/citrulline in pediatric clinical studies
Brusilow et al. first showed that arginine supplementation could ameliorate symptomatic hyperammonemia in a pediatric patient with inborn errors in urea synthesis [26]. In 2001, Waugh et al. reported a pilot phase-II clinical trial with five pediatric participants (ages 10–18 years) with sickle cell disease. This study found that administration of 0.09 to 0.13 g/kg of oral L-citrulline twice daily for four weeks increased plasma arginine concentrations by 65%, and normalized leukocyte and neutrophil counts. Based on these results and positive perceptions by the patients, the authors suggested L-citrulline be used as palliative therapy although pharmacokinetic exposure of L-citrulline was not studied in this study. This pilot study, although showed encouraging results, suffered through significant limitations such as small sample size, the absence of controls, was not definitive on a dose, and measured outcomes within the designated 4-week period. In recognition of these limitations, the authors recommended additional well-controlled, long-term studies to be conducted [87].
In 2006, Smith et al. reported a randomized, placebo-controlled trial of 40 children undergoing surgery with cardiopulmonary bypass given oral L-citrulline supplementation in preventing postoperative pulmonary hypertension. Administration of a total of 9.5 g/m2 L-citrulline perioperatively was associated with ~85% increases in mean arginine and L-citrulline plasma concentrations 12 hours after surgery. This is the largest of the reported pediatric studies. Study observations included citrulline concentrations below the age-specific norms for participants that developed postoperative pulmonary hypertension [88]. Determination of optimized L-citrulline dosing was outside the study scope, and the study revealed that controls for baseline citrulline concentrations are of potential consequence in future studies.
In 2016, El-Hattab et al. reported a controlled crossover study of 5 pediatric patients with MELAS syndrome, with five healthy pediatric participants serving as controls, given citrulline and arginine. Participants with a bodyweight of at least 20 kg were given 10 g/m2/day of the supplement. Supplementation was 3 g/kg/day in participants with weights below 20 kg. In comparison to arginine supplementation, supplementation with L-citrulline resulted in higher increases in nitric oxide production rate, arginine and citrulline flux, plasma arginine and citrulline concentrations, and de novo arginine synthesis rates [24].
One additional study in 2017 by Stepanova et al. showed that treatment with citrulline maleate improved serum concentrations of L-arginine, nitrite, and NO metabolites in pediatric patients who presented signs of endothelial dysfunction [89].
Overall, recent clinical studies in both adult and pediatric populations provide evidence for supplemental arginine/citrulline associated improvement in efficacy outcomes, although the exposure-response correlation and dose-ranging effects are yet to be well evaluated. These studies create the ground of confidence for future well-designed, randomized clinical trials that will establish efficacy and will inform dosing decisions for arginine supplementation in both pediatric and adult diseases. However, the optimal design of future clinical trials depends on accurate information about the pharmacokinetics of arginine/citrulline.
Evaluation of arginine and citrulline pharmacokinetics in pre-clinical studies
The pharmacokinetics of supplemental arginine is complex because this endogenous molecule shows variable basal levels and contributes to multiple biochemical pathways. Arginine exposure and clearance after supplementation are influenced by the patients’ age and the types and stages of the targeted diseases. Wu et al. evaluated the pharmacokinetics of arginine in different animal models [90]. Regardless of the physiologic, pathologic and species differences in these animals, intravenous supplementation of arginine increased plasma arginine levels, but it returned to the baseline with 4-5 hours after administration. Higher clearance of arginine in intravenously administered animals was observed in pregnant, neonatal, and lean animals when compared to that of their counterparts–non-pregnant, adult and obese animals, respectively. Oral supplementation results in variable absorption kinetics of arginine due to the interplay of intestinal absorption and gut metabolism. However, clearance of arginine followed a similar trend as observed in the case of intravenous dosing. Circulating arginine undergoes rapid clearance in animals of different stages of physiological maturation.
Disease states can influence the basal levels of arginine and citrulline. Effect of arginine supplementation, either by arginine or citrulline, on its PK, was evaluated in different animal disease models. Alloxan-induced diabetic rats showed reduced basal levels of arginine that can be restored to the normal levels with arginine supplementation [91]. To evaluate the effects of NO in different CNS disorders, Heinzen et al. reported a pharmacokinetic (PK)-pharmacodynamic (PD) model of exogenous arginine supplements that can successfully predict arginine levels in the brain [92]. PK-PD model obtained from this study suggests that exogenous arginine supplementation increased hippocampal arginine concentration in a dose-dependent fashion. Another study evaluated the effect of oral arginine plus citrulline therapy in augmenting NO-dependent responses in animal models of cardiovascular diseases [93]. Oral combination of arginine and citrulline showed a shorter Tmax, the time to reach maximum arginine concentration in plasma, than that of the single L-citrulline therapy in human suggesting an advantage of short-acting effects if interspecies differences in absorption between rat and human are negligible. While citrulline-only supplements support the long-acting enhancement of arginine availability, the benefits of citrulline plus arginine therapy as a rapid-acting arginine supplement requires validation through human studies. Choice of supplements can also be dependent on the pathologic conditions. Infectious conditions such as endotoxemic sepsis can severely reduce citrulline bioavailability, although arginine pharmacokinetics remains largely unaltered [94].
Although preclinical research is generating impressive evidence supporting the therapeutic efficacy of supplemental arginine/citrulline in a wide variety of pathological conditions, studies reporting arginine pharmacokinetics are minimal in number, sporadic regarding study objectives, and challenging to translate to human. Significant challenges in studying arginine exposure after supplementation include the estimation of highly variable baseline and the prediction of disease-associated alterations in arginine biosynthesis. Ultimately, for accurate translation to clinical trials, PK information must be generated in humans.
Pharmacokinetics of arginine and citrulline in human
To date, most of the clinical studies that explored the pharmacokinetics of supplemental arginine were conducted in a healthy adult population. Clinical studies that evaluated the pharmacokinetics of arginine or citrulline are summarized in Table 3.
Table 3.
Clinical studies reporting the pharmacokinetics of arginine/citrulline
| Study type | Subjects | Dosage | PK outcome |
|---|---|---|---|
| Randomized placebo-controlled study [14] | 19 subjects with precapillary pulmonary hypertension | Oral 50 mg/kg L-arginine | - Plasma L-arginine: from 90±4 (baseline) to 274±34 (statistically significant change from baseline) |
| Phase 1 dose escalation [19] | 5 infants and children undergoing cardiac surgery for congenital problems | Stepwise dose escalation: 50 mg/kg, 100 mg/kg and 150 mg/kg; two doses were administered-- one before the surgery and one immediate after the surgery. | - Citrulline plasma clearance, distribution-volume and half-life was 0.6 Lhr−1kg−1, 0.9 Lkg−1 and 1 hour, respectively |
| Controlled study on subjects with MELAS syndrome [23] | 20 subjects (10 with MELAS syndrome and 10 healthy subjects) | L-arginine or L-citrulline: 10 g per m2 body surface area per day for two days. | - Arginine supplementation: plasma arginine increased from 57.1±3.2 (baseline) to. 143.8±9.9 (statistically significant change from baseline) - Citrulline supplementation: plasma arginine increased from 57.6±2.1 (baseline) to. 182±14.4 (statistically significant change from baseline) |
| Controlled crossover study [24] | Five pediatric patients with MELAS syndrome and five healthy children as control | Citrulline/Arginine dose: Patients weighing < 20 kg: 500 mg/kg/dose given orally every 4 hours for 48 hours. Patients weighing ≥ 20 kg: 10 g/m2/day. |
- Citrulline supplementation increased plasma arginine concentration from 64±5.7 to 257±21 μmol/L; - Arginine supplementation increased plasma arginine concentration from 59±5 to 184±14 μmol/L |
| Pilot study in patients with pulmonary hypertension [79] | 10 subjects with pulmonary hypertension | 500 mg/kg of l-arginine as 30-minute infusion | Plasma arginine increased from 59±6 μmol/L to 10,726±868 μmol/L (statistically significant change) |
| Pilot phase-II clinical trial [87] | Five sickle cell disease patients (ages 10-18) | 0.09 to 0.13 g/kg twice daily for four weeks | - Increase of 65% (from 77±9.1 to 127±18) in plasma Arginine |
| Randomized controlled trial of oral citrulline [88] | 40 children undergoing cardiopulmonary bypass | 5 doses (1.9 g per m2 body surface area) | - Plasma L-arginine in citrulline group was significantly higher than placebo group (36 μmol /L vs 26 μmol /L in immediately after operation; 37 μmol /L vs 20 μmol /L at 12 h after operation) |
| A randomized study [98] | Ten healthy volunteers | 0.18 g/kg/d | - Citrulline: increase of 448±92% (from 39±4 to 225±44)- Arginine: increase of 92±57% (from 134±33 to 247 ±62)- Increase in urine and RBC Citrulline- No change in urinary Arginine, nor plasma urea, urinary urea nitrogen excretion thus enhanced nitrogen balance |
| Pharmacokinetic study after watermelon ingestion [99] | Six healthy adults | 3.3 kg wet weight red fruit of ripe watermelon | - Citrulline: increase from 22 μmol/L to 593 μmol/L (range 386 - 1069) 1 h after ingestion - Arginine: increase from 65 μmol/L to 199 μmol/L (range 128 - 251) 2 h after ingestion |
| Pharmacokinetic study after watermelon ingestion [100] | 23 healthy adults | 780 g (i.e., 1 g Citrulline), 1560 g (i.e., 2g Citrulline) watermelon juice for three weeks | - Low ingestion of watermelon juice:12% increase in plasma Arginine - High ingestion of watermelon juice:22% increase in plasma Arginine, 18% increase in plasma Ornithine. |
| Pilot study in patients with pulmonary hypercholesterolemia [101] | 10 subjects | Oral L-arginine (5 or 7 g) followed by intravenous L-arginine (10 or 30 g) after 8 hours at each of the four different visits | - Mean Cmax range after oral dose: 42.8 to 46.5 μg/mL - Mean AUC range after oral dose: 651 to 894 μg.min−1.ml−1.g−1 - Mean AUC range after IV dose: 1762 to 1926 μg.min−1.ml−1.g−1 - Mean non-renal clearance: 564.4 to 586 mL/min - Mean bioavailability: 0.37 to 0.52 |
| Randomized pilot study in patients with moderately severe malaria [103] | 78 subjects; 48 in the observational group and 30 received L-arginine | 3, 6, or 12 g L-arginine infused over 30 minutes | - Follows a two-compartment pharmacokinetic model - Clearance from central compartment: 31 Lhr−1 - Clearance from peripheral compartment: 74 Lhr−1 - Distribution volume (central): 27 L - Distribution volume (peripheral): 21 L |
| Randomized pilot study in patients with severe malaria [104] | 8 subjects: two received saline and 6 received L-arginine | 12 g L-arginine infused over 8 hours | - Follows a two-compartment pharmacokinetic model - Clearance from central compartment: 61.8 Lhr−1 - Clearance from peripheral compartment: 21.4 Lhr−1 - Distribution volume (central): 115 L - Distribution volume (peripheral): 70.9 L |
| Phase 1 dose finding study in patients with sickle cell disease [105] | 8 subjects | Step 1: Bolus L-citrulline 20 mg/kg Step 2: 7 mg/kg per hour continuous infusion of L-citrulline |
- Peak plasma concentration of L-citrulline after bolus dose: 257 μmol/l - Clearance and distribution volume after bolus administration were 0.52±0.13 L.h−1.kg-1 and 0.50±0.14 L.kg-1, respectively - Clearance and distribution volume after continuous infusion were 0.52±0.17 L.h−1.kg-1 and 0.41±0.02 L.kg-1, respectively |
| Single-blind crossover study [97] | 8 healthy adults | 2, 5, 10 or 15g of Citrulline | - After ingestion of 10 g of Citrulline- - Citrulline Tmax = 0.72±0.08 h, Cmax = 2756±70μmol/l - Arginine Tmax = 1.67±0.05h, Cmax = 280 ±1043μmol/l |
| Double-blind randomized placebo-controlled crossover study [96] | 20 healthy volunteers | Citrulline: 0.75, 1.5 and 3g twice daily Arginine: immediate release (IR) 1g tid, sustained release (SR) 1.6 twice daily for 1 week | - After load of 3 g Citrulline- - Citrulline: Tmax = 0.7±0.1 h, Cmax = 846±45μmol/l- - Arginine: Tmax = 1.4±0.1h, Cmax = 149 ±42μmol/l |
| Randomized placebo-controlled trial [88] | 40 children undergoing surgery to correct congenital heart lesions | Citrulline 1.9 g/m2/dose x 5 The total dose of 9.5g /m2 |
12h postoperative: - Citrulline: 37 (18-83) vs 20± (15-29) (placebo) - Arginine: 36±24 vs. 23±1357 and 85% increases in mean plasma levels of Arginine and Citrulline, respectively |
‘≥’ = greater than or equal to; ‘<‘ = less than; Arg=arginine; Cmax = maximum plasma concentration; Cit= citrulline; d= day; g= gram; h= hour; kg= kilogram; L= liter; m2= meter squared; mcg= microgram; MELAS= mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes; mg=milligram; mmol= millimole; PAH=Pulmonary Arterial Hypertension; tid= three-times daily; vit = vitamin; μmol= micromole; Tmax = time after dose to observe maximum plasma concentration
Arginine/citrulline supplementation in a healthy adult population
Tangphao et al. tested exogenous arginine in healthy humans as an initial effort to understand the pharmacokinetics of L-arginine in patients with cardiovascular diseases [95]. Basal L-arginine levels in untreated healthy adults showed a substantial diurnal variation. Baseline corrected plasma L-arginine concentrations measured after oral or intravenous L-arginine administrations showed a bi-phasic elimination pattern [95]. Intravenous L-arginine administration triggered an initial concentration-dependent rapid renal elimination followed by a slower elimination mainly guided by non-renal elimination. A 10g single dose of oral L-arginine having a ~20% bioavailability caused a three-fold increase in plasma L-arginine concentration from the baseline [95]. Schwedhelm et al. reported that chronic oral administration of citrulline dose-dependently increased the concentration of L-arginine in healthy adults [96]. In this randomized, double-blind, placebo-controlled study, different doses of L-citrulline were compared with two different formulations of L-arginine. L-citrulline, with a one-half the dosage strength of L-arginine, was associated with similar plasma exposure of L-arginine suggesting an efficient way of L-arginine supplementation. Like previous studies, determination of plasma L-arginine half-life was not possible due to irregular baseline values. They reported Tmax and Cmax for both citrulline and arginine after a 3 g dose of citrulline: Tmax = 0.7±0.1 h and 1.4±0.1 h, and Cmax = 846±45μmol/l and 149±42 μmol/l, respectively [96]. Another study that characterized the pharmacokinetics of citrulline in healthy adults for four oral doses (2, 5, 10, and 15 g). A dose-dependent significant decrease in clearance was observed for 2, 5, and 10 gm doses, although mean distribution volumes for each dose was similar [97]. L-citrulline doses of 10 gm or greater were associated with ~50% decrease in clearance than that of the 2-gm dose. Plasma half-life and exposure of citrulline also increased dose-dependently and the Tmax and Cmax were determined for both citrulline (Tmax = 0.72±0.08 h, Cmax = 2756±70 μmol/l) and arginine (Tmax = 1.67±0.05 h, Cmax = 280±1043 μmol/l) [97]. In 2007, Rouge et al. reported results from a randomized study of 10 healthy volunteers. Supplementation with 0.18 g/kg/day citrulline resulted in increases of citrulline and arginine plasma concentrations. Plasma concentrations of citrulline increased from 39±4 to 225±44 μmol/L, and arginine increased from 134±33 to 247±62 μmol/L. This supplementation enhanced nitrogen balance, increased levels of RBC-bound citrulline as well as the renal clearance of citrulline [98]. Few studies evaluated whether citrulline-rich dietary intervention can improve plasma citrulline/arginine levels. Mandel et al. observed significant increases in both arginine and citrulline within the first two hours after ingestion of 3.3 kg (wet weight) of watermelon by six healthy adults. Citrulline increased from 22 μmol/L to 593 μmol/L (range 386 - 1069) 1 hour after ingestion and Arginine increased from 65 μmol/L to 199 μmol/L (range 128 - 251) 2 h after ingestion [99]. In 2007, Collins et al. also reported increases in plasma arginine in 23 healthy adults given watermelon juice: 780 g or 1560 g for three weeks, resulting in 12% and 22% increases in plasma arginine concentrations, respectively [100].
Arginine/citrulline supplementation in adult patients with disease states
As a step forward to their previous study in healthy adults, Tangphao et al. evaluated pharmacokinetics of L-arginine in hypercholesterolemic patients who are reported to have impaired nitric oxide signaling in the endothelium and platelets [101]. Long-term (~12 weeks) administration of oral or intravenous L-arginine was associated with a significant increase in plasma L-arginine level in patients with hypercholesterolemia. A previous study in rabbit suggested that long-term treatment with exogenous arginine caused a gradual decline in plasma L-arginine levels [102]. However, this study showed a sustained increased level of plasma L-arginine after oral or intravenous administration. Although authors were not able to calculate two key parameters—distribution volume and elimination half-life of L-arginine due to diurnal variation of basal L-arginine levels. Exogenous L-arginine treatment did not show pharmacodynamic tolerance to plasma L-arginine induced growth hormone release [101].
L-arginine pharmacokinetics was also evaluated in severe malaria-affected patients who showed lower than normal plasma L-arginine levels due to malaria-associated endothelial dysfunction [103]. In a prospective observational study in healthy and malaria-afflicted subjects, dose-ranging effect of intravenous L-arginine infusion in replenishing plasma L-arginine level was determined by using a population pharmacokinetic approach. Plasma concentration-time profiles of a 30-minute infusion, for all the doses of 3, 6, or 12 g, supported a two-compartment linear model with first-order elimination. With an estimated clearance of 733 mL/min, exogenous L-arginine showed a slightly shorter half-life in malaria afflicted patients than that in healthy subjects. Simulations of plasma profiles for 12 g dose of L-arginine at different dosing frequencies-- 6, 8, and 12 hr.-- predicted a 60%, 75%, and 90% increase, respectively, above the required half-maximal saturating concentration at L-arginine transporter for optimal NO production [103]. In a separate open-label pilot study, a 12g dose of L-arginine given every 8-hourly was associated with lower than the previous model predicted concentrations and a 40% increased L-arginine clearance in patients with severe malaria than that in moderately-severe malaria patients [104, 103].
In 2012, El-Hattab et al. reported a controlled crossover study of 20 participants: 10 patients with MELAS syndrome and ten healthy participants given 10 g/m2/day oral citrulline or oral arginine. Supplementation with citrulline resulted in more significant increases in nitric oxide production rate, arginine and citrulline flux, and plasma arginine and citrulline concentrations. Citrulline supplementation increased plasma arginine concentration from 57.6±2.1 to 182.0±14.4 μmol/L; arginine supplementation increased plasma arginine concentration from 57.1±3.2 to 143.8±9.9 μmol/L. De novo arginine synthesis was observed to decrease with arginine supplementation and increase with citrulline supplementation [23] .
The pharmacokinetic and safety profile of intravenously administered L-citrulline has recently been evaluated in adults, ages 18-24, with sickle cell disease [105]. In this dose-finding study, a bolus dose of 20 mg/kg followed by a 7 mg/kg/h continuous infusion of citrulline was able to maintain a trough plasma arginine concentration of 100 μmol/l. IV bolus administration of citrulline resulted in a mean maximum plasma citrulline concentration of 259 μmol/l [105]. .
Arginine/citrulline supplementation in pediatric patients
The PK of intravenously administered L-citrulline was evaluated in children undergoing congenital cardiac surgery [19]. In the first group of patients, intravenous bolus doses ranging between 50 mg/kg and 150 mg/kg were implemented to achieve a 4-hr trough citrulline plasma concentration of 80-100 μmol/L. Pharmacokinetic simulations based on this data suggested a bolus dose of 150 mg/kg followed by an intravenous infusion (9 mg/kg.hr) after 4 hours to maintain optimum citrulline levels. The half-life of citrulline was reported to be approximately 60 minutes with a distribution volume ranging between 0.8 and 1.0 L/kg [19]. However, the effect of citrulline administration in increasing the plasma level of the active compound L-arginine was not measured. In pediatric patients with MELAS, citrulline supplementation showed a 4-fold increase in plasma arginine concentration (64±5.7 to 257±21 μmol/L) from baseline. Arginine supplementation also showed a modest increase in plasma arginine concentration (59±5 to 184±14 μmol/L)[24].
All the clinical studies that report the pharmacokinetics of arginine/citrulline after exogenous arginine supplementation in the form of arginine or citrulline or citrulline-rich diet showed an increase in plasma arginine because of the intervention. Clearance of arginine/citrulline is dependent on the dose of citrulline/arginine, age, and the disease state although high variation in baseline arginine/citrulline precludes the accurate estimation of distribution volumes and clearances. These variables must be considered when designing clinical trials that evaluate the efficacy of arginine/citrulline in different age groups suffering from various disease states.
Pharmacometric approaches to evaluate arginine pharmacokinetics
There has been minimal work published for assessing the population pharmacokinetics of arginine and citrulline [103]. Yeo et al. investigated the L-arginine pharmacokinetics in adult patients with moderately severe malaria and developed a two-compartment model to describe the concentration-time profiles. Bodyweight and ethnicity were found to influence the PK in patients with malaria disease, although these covariates were not identified in previous analysis with healthy volunteers. Additionally, an empirical second-order polynomial equation was used to describe the increase in endogenous L-arginine levels over time in patients who did not receive exogenous arginine. For baseline arginine description, an alternative turnover model parameterizing the endogenous production of arginine was also evaluated but not retained, due to the model sensitivity to initial estimates. In another study with healthy Chinese volunteers, Wang et al. applied a similar two-compartment model structure to describe the L-arginine PK [106]. The final parameter estimates were comparable between the two modeling attempts. However, in the latter study, weight was identified as a significant covariate on peripheral volume, and the baseline was not specifically described in the model. By the time of this review, there were no published studies on citrulline population PK.
The limited number of publications on arginine and citrulline population modeling and inconsistency in published models has indicated the gap of knowledge in understanding the exposure-response relationship of these drugs in patients. More studies are needed to establish PK and PD characteristics of arginine and citrulline, their population variability, and potentially influential factors from patient features. Especially considering the endogenous functionality of these molecules, study design should accommodate appropriate sampling strategies to describe the natural progression of baseline arginine/citrulline levels in patients. Metabolism of arginine and production of nitric oxide has been shown to influence by disease status [107-109]. Therefore, when developing quantitative models for these molecules, disease status should also be evaluated as potential covariates for both exogenous and endogenous molecule kinetics.
Conclusion
Supplemental arginine, primarily because of its crucial role as an endogenous amino acid substrate in nitric oxide regulation, has shown potential for therapeutic use in different diseases in both adult and pediatric age groups and in both pre-clinical and clinical studies. Currently, supplemental arginine is available as intravenous or oral dosage forms of arginine. The arginine precursor, citrulline, is available in oral forms and an IV preparation is being investigated in clinical trials of children undergoing cardiopulmonary bypass surgery but is not yet available. Ingestion of watermelon, a citrulline rich fruit, has also been used in clinical studies to improve arginine levels. Citrulline appears to be more efficient than arginine in enhancing systemic arginine concentration. Although in several clinical studies, arginine/citrulline supplementation effectively improved some life-threatening conditions in the pediatric population, the widespread clinical application is limited because PK data and the dose-response relationship of arginine/citrulline requires further evaluation. Arginine follows complex pharmacology that depends on several confounding factors, e.g., baseline variation of arginine due to developmental and disease state, age, disease condition, pregnancy, etc. Pharmacokinetic studies of arginine/citrulline in diseased or special populations are inadequate to inform appropriate dosing guidance. Research directed towards understanding the pharmacokinetic-pharmacodynamic relationship of arginine, given either as arginine or citrulline supplements, in different age groups and disease conditions is critically essential in its bench-to-bedside translation.
Key points.
Arginine/citrulline has immense therapeutic potential in some life-threatening diseases of children including cardiovascular function in hypertension and pulmonary hypertension.
The pharmacology of arginine/citrulline is impacted by several patient-specific factors such as baseline variation of arginine/citrulline due to developmental ages and disease states.
Acknowledgments:
This study was supported by a grant awarded to Principal Investigator: C.D. Fike, R34HL142995 from NIH/NHLBI. The authors have no other potential conflicts of interest.
Reference
- 1.Meijer AJ, Lamers WH, Chamuleau RA. Nitrogen metabolism and ornithine cycle function. Physiol Rev. 1990;70(3):701–48. [DOI] [PubMed] [Google Scholar]
- 2.Morris SM Jr., Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 3.Wu G, Meininger CJ, Knabe DA, Bazer FW, Rhoads JM. Arginine nutrition in development, health and disease. Curr Opin Clin Nutr Metab Care. 2000;3(1):59–66. [DOI] [PubMed] [Google Scholar]
- 4.Bahri S, Zerrouk N, Aussel C, Moinard C, Crenn P, Curis E et al. Citrulline: from metabolism to therapeutic use. Nutrition. 2013;29(3):479–84. [DOI] [PubMed] [Google Scholar]
- 5.Romero MJ, Platt DH, Caldwell RB, Caldwell RW. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc Drug Rev. 2006;24(3-4):275–90. doi: 10.1111/j.1527-3466.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 6.Egashira K, Hirooka Y, Kuga T, Mohri M, Takeshita A. Effects of L-arginine supplementation on endothelium-dependent coronary vasodilation in patients with angina pectoris and normal coronary arteriograms. Circulation. 1996;94(2):130–4. [DOI] [PubMed] [Google Scholar]
- 7.Palloshi A, Fragasso G, Piatti P, Monti LD, Setola E, Valsecchi G et al. Effect of oral L-arginine on blood pressure and symptoms and endothelial function in patients with systemic hypertension, positive exercise tests, and normal coronary arteries. Am J Cardiol. 2004;93(7):933–5. doi: 10.1016/j.amjcard.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 8.DeMots H, Glasser SP. Intermittent transdermal nitroglycerin therapy in the treatment of chronic stable angina. J Am Coll Cardiol. 1989;13(4):786–95. [DOI] [PubMed] [Google Scholar]
- 9.Ferratini M Risk of rebound phenomenon during nitrate withdrawal. Int J Cardiol. 1994;45(2):89–96. [DOI] [PubMed] [Google Scholar]
- 10.Parker JO, Parker JD, Caldwell RW, Farrell B, Kaesemeyer WH. The effect of supplemental L-arginine on tolerance development during continuous transdermal nitroglycerin therapy. J Am Coll Cardiol. 2002;39(7):1199–203. [DOI] [PubMed] [Google Scholar]
- 11.Morris NH, Eaton BM, Dekker G. Nitric oxide, the endothelium, pregnancy and pre-eclampsia. Br J Obstet Gynaecol. 1996;103(1):4–15. [DOI] [PubMed] [Google Scholar]
- 12.Vadillo-Ortega F, Perichart-Perera O, Espino S, Avila-Vergara MA, Ibarra I, Ahued R et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ (Clinical research ed). 2011;342:d2901. doi: 10.1136/bmj.d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–56. [DOI] [PubMed] [Google Scholar]
- 14.Nagaya N, Uematsu M, Oya H, Sato N, Sakamaki F, Kyotani S et al. Short-term oral administration of L-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. Am J Respir Crit Care Med. 2001;163(4):887–91. doi: 10.1164/ajrccm.163.4.2007116. [DOI] [PubMed] [Google Scholar]
- 15.Sharif Kashani B, Tahmaseb Pour P, Malekmohammad M, Behzadnia N, Sheybani-Afshar F, Fakhri M et al. Oral l-citrulline malate in patients with idiopathic pulmonary arterial hypertension and Eisenmenger Syndrome: a clinical trial. J Cardiol. 2014;64(3):231–5. doi: 10.1016/j.jjcc.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Morris CR, Morris SM, Hagar W, Warmerdam Jv, Claster S, Kepka-Lenhart D et al. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease. Am J Resp Crit Care Med. 2003;168:63–9. [DOI] [PubMed] [Google Scholar]
- 17.Rashid A, Dunbar Ivy D. Pulmonary hypertension in children. Current Paediatrics. 2006;16(4):237–47. doi: 10.1016/j.cupe.2006.05.012. [DOI] [Google Scholar]
- 18.Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A et al. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. 2001;344(24):1832–8. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- 19.Barr FE, Tirona RG, Taylor MB, Rice G, Arnold J, Cunningham G et al. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery: potential therapy for postoperative pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;134:319–26. [DOI] [PubMed] [Google Scholar]
- 20.Canter JA, Summar ML, Smith HB, Rice BD, Hall LD, Ritchie MD et al. Genetic variation in the mitochondrial enzyme carbamyl-phosphate synthetase I predisposes children to increased pulmonary artery pressure following surgical repair of congenital heart difects: a validated genetic association study. Mitochondrion. 2007;7:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S, So TY. The Use of Citrulline for Pediatric Pulmonary Hypertension. Research Journal of Clinical Pediatrics. 2017;1(1):1–6. [Google Scholar]
- 22.Koga Y, Akita Y, Junko N, Yatsuga S, Povalko N, Fukiyama R et al. Endothelial dysfunction in MELAS improved by l-arginine supplementation. Neurology. 2006;66(11):1766–9. doi: 10.1212/01.wnl.0000220197.36849.1e. [DOI] [PubMed] [Google Scholar]
- 23.El-Hattab AW, Hsu JW, Emrick LT, Wong LJ, Craigen WJ, Jahoor F et al. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Molecular genetics and metabolism. 2012;105(4):607–14. doi: 10.1016/j.ymgme.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Hattab AW, Emrick LT, Hsu JW, Chanprasert S, Almannai M, Craigen WJ et al. Impaired nitric oxide production in children with MELAS syndrome and the effect of arginine and citrulline supplementation. Molecular genetics and metabolism. 2016;117(4):407–12. doi: 10.1016/j.ymgme.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gropman AL, Summar M, Leonard JV. Neurological implications of urea cycle disorders. J Inherit Metab Dis. 2007;30(6):865–79. doi: 10.1007/s10545-007-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brusilow SW. Arginine, an indispensable amino acid for patients with inborn errors of urea synthesis. J Clin Invest. 1984;74(6):2144–8. doi: 10.1172/JCI111640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adam S, Almeida MF, Assoun M, Baruteau J, Bernabei SM, Bigot S et al. Dietary management of urea cycle disorders: European practice. Molecular genetics and metabolism. 2013;110(4):439–45. doi: 10.1016/j.ymgme.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Batshaw ML, Brusilow S, Waber L, Blom W, Brubakk AM, Burton BK et al. Treatment of inborn errors of urea synthesis: activation of alternative pathways of waste nitrogen synthesis and excretion. N Engl J Med. 1982;306(23):1387–92. doi: 10.1056/NEJM198206103062303. [DOI] [PubMed] [Google Scholar]
- 29.Cynober L, Le Boucher J, Vasson M-P. Arginine metabolism in mammals. The Journal of Nutritional Biochemistry. 1995;6(8):402–13. [Google Scholar]
- 30.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17(9):571–88. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108(16):2000–6. [DOI] [PubMed] [Google Scholar]
- 32.Boelens PG, van Leeuwen PA, Dejong CH, Deutz NE. Intestinal renal metabolism of l-citrulline and l-arginine following enteral or parenteral infusion of l-alanyl-l-[2, 15 N] glutamine or l-[2, 15 N] glutamine in mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005;289(4):G679–G85. [DOI] [PubMed] [Google Scholar]
- 33.Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S et al. Almost all about citrulline in mammals. Amino acids. 2005;29(3):177. [DOI] [PubMed] [Google Scholar]
- 34.Boger RH, Bode-Boger SM, Brandes RP, Phivthong-ngam L, Bohme M, Nafe R et al. Dietary L-arginine reduces the progression of atherosclerosis in cholesterol-fed rabbits: comparison with lovastatin. Circulation. 1997;96(4):1282–90. [DOI] [PubMed] [Google Scholar]
- 35.Cooke JP, Singer AH, Tsao P, Zera P, Rowan RA, Billingham ME. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90(3):1168–72. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer AH, Tsao PS, Wang BY, Bloch DA, Cooke JP. Discordant effects of dietary L-arginine on vascular structure and reactivity in hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 1995;25(5):710–6. [DOI] [PubMed] [Google Scholar]
- 37.Rossitch E Jr., Alexander E 3rd, Black PM, Cooke JP. L-arginine normalizes endothelial function in cerebral vessels from hypercholesterolemic rabbits. J Clin Invest. 1991;87(4):1295–9. doi: 10.1172/JCI115132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boger RH, Bode-Boger SM, Phivthong-ngam L, Brandes RP, Schwedhelm E, Mugge A et al. Dietary L-arginine and alpha-tocopherol reduce vascular oxidative stress and preserve endothelial function in hypercholesterolemic rabbits via different mechanisms. Atherosclerosis. 1998;141(1):31–43. [DOI] [PubMed] [Google Scholar]
- 39.Boger RH, Bode-Boger SM, Mugge A, Kienke S, Brandes R, Dwenger A et al. Supplementation of hypercholesterolaemic rabbits with L-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis. 1995;117(2):273–84. [DOI] [PubMed] [Google Scholar]
- 40.Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, Cunningham G et al. L-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fike CD, Dikalova A, Kaplowitz MR, Cunningham G, Summar M, Aschner JL. Rescue treatment with L-citrulline inhibits hypoxia-induced pulmonary hypertension in newborn pigs. Am J Resp Cell Mol Biol. 2015;53:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin Y, Chen B, Calvert TJ, Chicoine LG, Liu Y, Nelin LD. Chronic hypoxia decreases arterial and venous compliance in isolated perfused rat lungs: an effect that is reversed by exogenous L-arginine. American journal of physiology Heart and circulatory physiology. 2013;304(2):H195–205. doi: 10.1152/ajpheart.00188.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitani Y, Maruyama K, Sakurai M. Prolonged administration of L-arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation. 1997;96(2):689–97. [PubMed] [Google Scholar]
- 44.Ou ZJ, Wei W, Huang DD, Luo W, Luo D, Wang ZP et al. L-arginine restores endothelial nitric oxide synthase-coupled activity and attenuates monocrotaline-induced pulmonary artery hypertension in rats. Am J Physiol Endocrinol Metab. 2010;298(6):E1131–9. doi: 10.1152/ajpendo.00107.2010. [DOI] [PubMed] [Google Scholar]
- 45.Wang BY, Singer AH, Tsao PS, Drexler H, Kosek J, Cooke JP. Dietary arginine prevents atherogenesis in the coronary artery of the hypercholesterolemic rabbit. J Am Coll Cardiol. 1994;23(2):452–8. [DOI] [PubMed] [Google Scholar]
- 46.Preli RB, Klein KP, Herrington DM. Vascular effects of dietary L-arginine supplementation. Atherosclerosis. 2002;162(1):1–15. [DOI] [PubMed] [Google Scholar]
- 47.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88(5):1559–67. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chien SJ, Lin KM, Kuo HC, Huang CF, Lin YJ, Huang LT et al. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: l-citrulline and nitrate. Transl Res. 2014;163(1):43–52. doi: 10.1016/j.trsl.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Bursztyn M, Podjarny E, Dahan R, Raz I, Bernheim J. Insulin-induced hypertension, L-arginine, and endothelial nitric oxide synthase in pregnant rats. Hypertens Pregnancy. 2003;22(3):267–74. doi: 10.1081/PRG-120024030. [DOI] [PubMed] [Google Scholar]
- 50.Podjarny E, Bursztyn M, Rashed G, Benchetrit S, Katz B, Green J et al. Chronic exogenous hyperinsulinaemia-induced hypertension in pregnant rats: effect of chronic treatment with l-arginine. Clin Sci (Lond). 2001;100(6):667–71. [PubMed] [Google Scholar]
- 51.Podjarny E, Ben-Chetrit S, Rathaus M, Korzets Z, Green J, Katz B et al. Pregnancy-induced hypertension in rats with adriamycin nephropathy is associated with an inadequate production of nitric oxide. Hypertension. 1997;29(4):986–91. [DOI] [PubMed] [Google Scholar]
- 52.Podjarny EP A; Rathus M; Green J; Gonen O; Shamir R; Bernheim J Effect of L-Arginine Treatment in Pregnant Rats with Adriamycin Nephropathy. Hypertension in Pregnancy. 1993;12(3):517–24. doi: 10.3109/10641959309042870. [DOI] [Google Scholar]
- 53.Fike CD, Kaplowitz MR, Rehorst-Paea LA, Nelin LD. L-arginine increases nitric oxide production in isolated lungs of chronically hypoxic newborn pigs. J Appl Physiol. 2000;88:1797–803. [DOI] [PubMed] [Google Scholar]
- 54.Tsubuku S, Hatayama K, Mawatari K, Smriga M, Kimura T. Thirteen-week oral toxicity study of L-arginine in rats. Int J Toxicol. 2004;23(2):101–5. doi: 10.1080/10915810490435622. [DOI] [PubMed] [Google Scholar]
- 55.Kui B, Balla Z, Vasas B, Vegh ET, Pallagi P, Kormanyos ES et al. New insights into the methodology of L-arginine-induced acute pancreatitis. PLoS One. 2015;10(2):e0117588. doi: 10.1371/journal.pone.0117588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerard JM, Luisiri A. A fatal overdose of arginine hydrochloride. J Toxicol Clin Toxicol. 1997;35(6):621–5. [DOI] [PubMed] [Google Scholar]
- 57.Tedesco TA, Benford SA, Foster RC, Barness LA. Free amino acids in Citrullus vulgaris (watermelon). Pediatrics. 1984;73(6):879. [PubMed] [Google Scholar]
- 58.Akashi K, Mifune Y, Morita K, Ishitsuka S, Tsujimoto H, Ishihara T. Spatial accumulation pattern of citrulline and other nutrients in immature and mature watermelon fruits. J Sci Food Agric. 2017;97(2):479–87. doi: 10.1002/jsfa.7749. [DOI] [PubMed] [Google Scholar]
- 59.Figueroa A, Wong A, Jaime SJ, Gonzales JU. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr Opin Clin Nutr Metab Care. 2017;20(1):92–8. doi: 10.1097/MCO.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 60.Martinez-Sanchez A, Ramos-Campo DJ, Fernandez-Lobato B, Rubio-Arias JA, Alacid F, Aguayo E. Biochemical, physiological, and performance response of a functional watermelon juice enriched in L-citrulline during a half-marathon race. Food Nutr Res. 2017;61(1):1330098. doi: 10.1080/16546628.2017.1330098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellis AC, Dudenbostel T, Locher JL, Crowe-White K. Modulating Oxidative Stress and Inflammation in Elders: The MOXIE Study. J Nutr Gerontol Geriatr. 2016;35(4):219–42. doi: 10.1080/21551197.2016.1250693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez-Sanchez A, Alacid F, Rubio-Arias JA, Fernandez-Lobato B, Ramos-Campo DJ, Aguayo E. Consumption of Watermelon Juice Enriched in l-Citrulline and Pomegranate Ellagitannins Enhanced Metabolism during Physical Exercise. J Agric Food Chem. 2017;65(22):4395–404. doi: 10.1021/acs.jafc.7b00586. [DOI] [PubMed] [Google Scholar]
- 63.Takahara K, Akashi K, Yokota A. Purification and characterization of glutamate N-acetyltransferase involved in citrulline accumulation in wild watermelon. FEBS J. 2005;272(20):5353–64. doi: 10.1111/j.1742-4658.2005.04933.x. [DOI] [PubMed] [Google Scholar]
- 64.Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA et al. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr. 2007;137(12):2680–5. [DOI] [PubMed] [Google Scholar]
- 65.Figueroa A, Sanchez-Gonzalez MA, Perkins-Veazie PM, Arjmandi BH. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: a pilot study. Am J Hypertens. 2011;24(1):40–4. doi: 10.1038/ajh.2010.142. [DOI] [PubMed] [Google Scholar]
- 66.Figueroa A, Sanchez-Gonzalez MA, Wong A, Arjmandi BH. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am J Hypertens. 2012;25(6):640–3. doi: 10.1038/ajh.2012.20. [DOI] [PubMed] [Google Scholar]
- 67.Figueroa A, Wong A, Hooshmand S, Sanchez-Gonzalez MA. Effects of watermelon supplementation on arterial stiffness and wave reflection amplitude in postmenopausal women. Menopause. 2013;20(5):573–7. doi: 10.1097/GME.0b013e3182733794. [DOI] [PubMed] [Google Scholar]
- 68.Tarazona-Diaz MP, Alacid F, Carrasco M, Martinez I, Aguayo E. Watermelon juice: potential functional drink for sore muscle relief in athletes. J Agric Food Chem. 2013;61(31):7522–8. doi: 10.1021/jf400964r. [DOI] [PubMed] [Google Scholar]
- 69.Figueroa A, Wong A, Kalfon R. Effects of watermelon supplementation on aortic hemodynamic responses to the cold pressor test in obese hypertensive adults. Am J Hypertens. 2014;27(7):899–906. doi: 10.1093/ajh/hpt295. [DOI] [PubMed] [Google Scholar]
- 70.Soteriou GA, Kyriacou MC, Siomos AS, Gerasopoulos D. Evolution of watermelon fruit physicochemical and phytochemical composition during ripening as affected by grafting. Food Chem. 2014;165:282–9. doi: 10.1016/j.foodchem.2014.04.120. [DOI] [PubMed] [Google Scholar]
- 71.Cutrufello PT, Gadomski SJ, Zavorsky GS. The effect of l-citrulline and watermelon juice supplementation on anaerobic and aerobic exercise performance. J Sports Sci. 2015;33(14):1459–66. doi: 10.1080/02640414.2014.990495. [DOI] [PubMed] [Google Scholar]
- 72.Bailey SJ, Blackwell JR, Williams E, Vanhatalo A, Wylie LJ, Winyard PG et al. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide. 2016;59:10–20. doi: 10.1016/j.niox.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Massa NM, Silva AS, de Oliveira CV, Costa MJ, Persuhn DC, Barbosa CV et al. Supplementation with Watermelon Extract Reduces Total Cholesterol and LDL Cholesterol in Adults with Dyslipidemia under the Influence of the MTHFR C677T Polymorphism. J Am Coll Nutr. 2016;35(6):514–20. doi: 10.1080/07315724.2015.1065522. [DOI] [PubMed] [Google Scholar]
- 74.Massa NM, Silva AS, Toscano LT, Silva JD, Persuhn DC, Goncalves Mda C. Watermelon extract reduces blood pressure but does not change sympathovagal balance in prehypertensive and hypertensive subjects. Blood Press. 2016;25(4):244–8. doi: 10.3109/08037051.2016.1150561. [DOI] [PubMed] [Google Scholar]
- 75.Shanely RA, Nieman DC, Perkins-Veazie P, Henson DA, Meaney MP, Knab AM et al. Comparison of Watermelon and Carbohydrate Beverage on Exercise-Induced Alterations in Systemic Inflammation, Immune Dysfunction, and Plasma Antioxidant Capacity. Nutrients. 2016;8(8). doi: 10.3390/nu8080518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown MB, Kempf A, Collins CM, Long GM, Owens M, Gupta S et al. A prescribed walking regimen plus arginine supplementation improves function and quality of life for patients with pulmonary arterial hypertension: a pilot study. Pulm Circ. 2018;8(1):2045893217743966. doi: 10.1177/2045893217743966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahboobi S, Tsang C, Rezaei S, Jafarnejad S. Effect of L-citrulline supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Journal of Human Hypertension. 2019;33:10–21. [DOI] [PubMed] [Google Scholar]
- 78.Shiraishi Y, Lee JR, Laks H, Waters PF, Meneshian A, Blitz A et al. L-arginine administration during reperfusion improves pulmonary function. The Annals of thoracic surgery. 1996;62(6):1580–6; discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 79.Mehta S, Stewart DJ, Langleben D, Levy RD. Short-term pulmonary vasodilation with L-arginine in pulmonary hypertension. Circulation. 1995;92(6):1539–45. [DOI] [PubMed] [Google Scholar]
- 80.Surdacki A, Zmudka K, Bieron K, Kostka-Trabka E, Dubiel JS, Gryglewski RJ. Lack of beneficial effects of L-arginine infusion in primary pulmonary hypertension. Wiener klinische Wochenschrift. 1994;106(16):521–6. [PubMed] [Google Scholar]
- 81.Boger RH, Mugge A, Bode-Boger SM, Heinzel D, Hoper MM, Frolich JC. Differential systemic and pulmonary hemodynamic effects of L-arginine in patients with coronary artery disease or primary pulmonary hypertension. International journal of clinical pharmacology and therapeutics. 1996;34(8):323–8. [PubMed] [Google Scholar]
- 82.Papadia C, Osowska S, Cynober L, Forbes A. Citrulline in health and disease. Review on human studies. Clin Nutr 2017. doi: 10.1016/j.clnu.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 83.El Hattab AW, Hsu JW, Emrick LT, Wong L-JC, Craigen WJ, Jahoor F et al. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Molecular genetics and metabolism. 2012;105:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grasemann H, Kurtz F, Ratjen F. Inhaled L-arginine improves exhaled nitric oxide and pulmonary function in patients with cystic fibrosis. Am J Respir Crit Care Med. 2006;174(2):208–12. doi: 10.1164/rccm.200509-1439OC. [DOI] [PubMed] [Google Scholar]
- 85.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S et al. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295(1):58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 86.Ralph AP, Waramori G, Pontororing GJ, Kenangalem E, Wiguna A, Tjitra E et al. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013;8(8):e70032. doi: 10.1371/journal.pone.0070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waugh WH, Daeschner CW, 3rd, Files BA, McConnell ME, Strandjord SE. Oral citrulline as arginine precursor may be beneficial in sickle cell disease: early phase two results. J Natl Med Assoc. 2001;93(10):363–71. [PMC free article] [PubMed] [Google Scholar]
- 88.Smith HAB, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman bW et al. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:58–65. [DOI] [PubMed] [Google Scholar]
- 89.Stepanova YI, Kolpakov IY, Zygalo VM, Vdovenko VY, Kondrashova VH, Leonovich OS et al. Experience of correcting endothelial dysfunction in children-residents of radioactively contaminated areas by nitric oxide potential donator citrulline. Probl Radiac Med Radiobiol. 2017;22:463–75. [PubMed] [Google Scholar]
- 90.Wu G, Bazer FW, Cudd TA, Jobgen WS, Kim SW, Lassala A et al. Pharmacokinetics and safety of arginine supplementation in animals. J Nutr. 2007;137(6 Suppl 2):1673s–80s. [DOI] [PubMed] [Google Scholar]
- 91.Escudero A, Petzold G, Moreno J, Gonzalez M, Junod J, Aguayo C et al. Supplementation with apple enriched with L-arginine may improve metabolic control and survival rate in alloxan-induced diabetic rats BioFactors (Oxford, England: ). 2013;39(5):564–74. doi: 10.1002/biof.1103. [DOI] [PubMed] [Google Scholar]
- 92.Heinzen EL, Pollack GM. Pharmacokinetics and pharmacodynamics of L-arginine in rats: a model of stimulated neuronal nitric oxide synthesis. Brain Res. 2003;989(1):67–75. [DOI] [PubMed] [Google Scholar]
- 93.Morita M, Hayashi T, Ochiai M, Maeda M, Yamaguchi T, Ina K et al. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochemical and biophysical research communications. 2014;454(1):53–7. doi: 10.1016/j.bbrc.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 94.Elwafi F, Curis E, Zerrouk N, Neveux N, Chaumeil JC, Arnaud P et al. Endotoxemia affects citrulline, arginine and glutamine bioavailability. European journal of clinical investigation. 2012;42(3):282–9. doi: 10.1111/j.1365-2362.2011.02581.x. [DOI] [PubMed] [Google Scholar]
- 95.Tangphao O, Grossmann M, Chalon S, Hoffman BB, Blaschke TF. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br J Clin Pharmacol. 1999;47(3):261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol. 2008;65(1):51–9. doi: 10.1111/j.1365-2125.2007.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moinard C, Nicolis I, Neveux N, Darquy S, Benazeth S, Cynober L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutrition. 2009;99:855–62. [DOI] [PubMed] [Google Scholar]
- 98.Rouge C, Des Robert C, Robins A, Le Bacquer O, Volteau C, De La Cochetiere MF et al. Manipulation of citrulline availability in humans. Am J Physiol Gastrointest Liver Physiol. 2007;293(5):G1061–7. doi: 10.1152/ajpgi.00289.2007. [DOI] [PubMed] [Google Scholar]
- 99.Mandel H, Levy N, Izkovitch S, Korman SH. Elevated plasma citrulline and arginine due to consumption of Citrullus vulgaris (watermelon). J Inherit Metab Dis. 2005;28(4):467–72. doi: 10.1007/s10545-005-0467-1. [DOI] [PubMed] [Google Scholar]
- 100.Collins JK, Wu G, Perkins-Veazie P, Spears K, Claypool PL, Baker RA et al. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition. 2007;23(3):261–6. doi: 10.1016/j.nut.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 101.Tangphao O, Chalon S, Moreno H Jr., Hoffman BB, Blaschke TF. Pharmacokinetics of L-arginine during chronic administration to patients with hypercholesterolaemia. Clin Sci (Lond). 1999;96(2):199–207. [PubMed] [Google Scholar]
- 102.Jeremy RW, McCarron H, Sullivan D. Effects of Dietary L-Arginine on Atherosclerosis and Endothelium-Dependent Vasodilatationin the Hypercholesterolemic Rabbit. Response According to Treatment Duration, Anatomic Site, and Sex. 1996;94(3):498–506. doi: 10.1161/01.Cir.94.3.498. [DOI] [PubMed] [Google Scholar]
- 103.Yeo TW, Rooslamiati I, Gitawati R, Tjitra E, Lampah DA, Kenangalem E et al. Pharmacokinetics of L-arginine in adults with moderately severe malaria. Antimicrob Agents Chemother. 2008;52(12):4381–7. doi: 10.1128/AAC.00421-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yeo TW, Lampah DA, Rooslamiati I, Gitawati R, Tjitra E, Kenangalem E et al. A randomized pilot study of L-arginine infusion in severe falciparum malaria: preliminary safety, efficacy and pharmacokinetics. PLoS One. 2013;8(7):e69587. doi: 10.1371/journal.pone.0069587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Majumdar S, Tirona R, Mashegu H, Desai J, Shannon NT, Summar M et al. A phase 1 dose-finding study of intravenous L-citrulline in sickle cell disease: a potential novel therapy for sickle cell pain crises. Br J Haemotology. 2019;184:634–96. [DOI] [PubMed] [Google Scholar]
- 106.Wang J, Zheng H, Wang K, Wang Z, Ding Y. Population pharmacokinetics of arginine glutamate in healthy Chinese volunteers. Xenobiotica. 2017:1–9. doi: 10.1080/00498254.2017.1370745. [DOI] [PubMed] [Google Scholar]
- 107.Argaman Z, Young VR, Noviski N, Castillo-Rosas L, Lu XM, Zurakowski D et al. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med. 2003;31(2):591–7. doi: 10.1097/01.CCM.0000050291.37714.74. [DOI] [PubMed] [Google Scholar]
- 108.Villalpando S, Gopal J, Balasubramanyam A, Bandi VP, Guntupalli K, Jahoor F. In vivo arginine production and intravascular nitric oxide synthesis in hypotensive sepsis. Am J Clin Nutr. 2006;84(1):197–203. [DOI] [PubMed] [Google Scholar]
- 109.Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204(11):2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aji W, Ravalli S, Szabolcs M, Jiang XC, Sciacca RR, Michler RE et al. L-arginine prevents xanthoma development and inhibits atherosclerosis in LDL receptor knockout mice. Circulation. 1997;95(2):430–7. [DOI] [PubMed] [Google Scholar]
- 111.Bode-Boger SM, Boger RH, Kienke S, Junker W, Frolich JC. Elevated L-arginine/dimethylarginine ratio contributes to enhanced systemic NO production by dietary L-arginine in hypercholesterolemic rabbits. Biochemical and biophysical research communications. 1996;219(2):598–603. doi: 10.1006/bbrc.1996.0279. [DOI] [PubMed] [Google Scholar]