Abstract
Background:
TCEB1-mutant renal cell carcinoma (RCC) is a rare variant of RCC with clear-cell features. Owing to its unique morphological and molecular features it has recently been proposed as a separate entity. Initial series suggested an indolent, early-stage phenotype. Here we expand our clinical cohort and describe a more detailed genomic analysis looking for potential drivers of aggressiveness.
Design, setting, and participants:
We identified five new cases in our institutional sequencing cohort, four of whom were found to have high-stage disease (American Joint Committee on Cancer stage III/IV). Twelve previously reported cases were pooled for comparison purposes (Sato, The Cancer Genome Atlas, TRACERx Renal).
Outcome measures and statistical analysis:
We used our previously validated pipeline to analyze somatic mutations and copy number alterations (CNAs) in seven tumor samples with available data and estimated the number of cancer cells bearing each somatic mutation. The oncogenic potential of mutations was assessed using OncoKB and two other algorithms. Mann-Whitney U tests were used to evaluate differences in genomic markers between stage groups.
Results and limitations:
All tumors showed biallelic inactivation of the TCEB1 gene according to a combination of somatic mutation and CNA analyses. Mutations were always found in residues involved in hydrophobic interactions with VHL. We found that high-stage tumors had additional oncogenic mutations (1, interquartile range [IQR] 1–1 vs 2, IQR 2–2; median difference 1, 95% confidence interval [CI] 1–1; p = 0.002) and had whole-genome doubling events. They also seemed to have a higher burden of somatic CNAs (fraction CNA genome 0.10, IQR 0.10–0.15 vs 0.63, IQR 0.58–0.68), but this finding did not reach statistical significance (median difference 0.49, 95% CI 0.33–0.63; p = 0.052).
Conclusions:
TCEB1-mutant RCC can show variable behavior ranging from very indolent to aggressive. Specific molecular events leading to high genomic instability seem to be associated with aggressiveness. This study expands the clinical spectrum of TCEB1-mutant RCC.
Keywords: DNA mutational analysis, Genomic instability, Metastasis, Renal cell carcinoma, Tumor suppressor genes
Patient summary:
We present four cases of aggressive TCEB1-mutant renal cell carcinoma, a rare type of kidney cancer. In-depth analysis of the genomes of these tumors revealed certain abnormalities that might explain this aggressive behavior.
1. Introduction
TCEB1-mutant renal cell carcinoma (RCC) is a rare variant of RCC previously regarded as related to clear-cell RCC (ccRCC). Owing to its unique morphological characteristics, TCEB1-mutant RCC has been recently proposed as a separate entity. Its features include thick fibromuscular bands and variable architecture (that can range from branching tubular to papillary), mimicking in some cases other RCC histologic subtypes such as clear-cell papillary RCC [1,2]. These tumors have been described as having a low nuclear grade and a distinct immunohistochemical (IHC) profile characterized by a mix of clear-cell and non– clear-cell markers (CA-IX and CD10+/CK7+) [1].
Molecularly, these tumors are quite distinct from ccRCCs, which are defined by near-ubiquitous biallelic inactivation of the VHL gene (3p21) [3]. Instead, TCEB1-mutant RCCs are VHL wild-type (WT) and exhibit somatic mutations in the TCEB1 gene (recently renamed ELOC) as well as loss of the alternate allele (8q21) [4]. The product of this gene is elongin C, which forms part of the VHL protein complex responsible for ubiquitination of HIF1a and its subsequent degradation [5,6]. It has been shown that specific mutations in the VHL-binding regions of TCEB1 can impair VHL-complex assembly, leading to HIF1a overexpression [4].
Previous series have only reported on patients presenting with low-grade and low-stage disease. This led investigators to hypothesize that TCEB1-mutant RCC might be a more indolent variant of RCC. Notably, our group previously reported on 11 TCEB1-mutant RCCs that were treated surgically and had no evidence of recurrence at median follow-up of 48 mo [1].
In this study we describe five new patients with TCEB1-mutant RCC and variable clinical courses, including two individuals who developed metastatic disease. These new cases challenge the previous paradigm that TCEB1-mutant RCC is an indolent entity, and instead suggest that it has malignant potential. Since our last report, genomic studies have shed more light on the molecular drivers of disease aggressiveness in ccRCC [7]. Therefore, we used next-generation sequencing (NGS) data to interrogate the genomes of these tumors and identify molecular events that could potentially be associated with this aggressive disease course.
2. Patients and methods
2.1. Patients and samples
Five TCEB1-mutant RCC cases were identified retrospectively from our institutional sequencing database (MSK-IMPACT) on the basis of the presence of TCEB1 (ELOC) mutations. The corresponding hematoxylin and eosin (H&E) slides for the MSK cases were retrieved and the defining histological features were confirmed by two independent genitourinary pathologists. Data for 12 previously reported cases were retrieved for analysis. All these tumors, with the exception of one sample from the TRACERx study (K114R), had already been described by our group and presented in a previous publication. These tumors were initially reported as ccRCCs on the basis of their morphological appearance and the diagnosis of TCEB1-mutant RCC was made retrospectively after performing DNA sequencing and confirming the distinct pathologic features [1]. The cohort consisted of eight cases from the Sato study, of whom only five underwent NGS [4], three from The Cancer Genome Atlas (TCGA) [3], and one from the TRACERx Renal study [7]. Only two of these samples had raw sequencing data available for analysis: K114_R (from TRACERx Renal, sequenced using a targeted gene panel) and TCGA-B8–5545 (from the KIRC cohort, for which whole-exome sequencing [WES] was performed).
2.2. Sequencing and initial data processing
The NGS assay used consisted of a targeted panel of 410 canonical cancer genes and tiling of heterozygous single-nucleotide polymorphisms (SNPs) across the genome for determination of somatic copy number (CN) variants [8]. Each tumor sample profiled was matched to a normal control (the patient’s blood). Primary tumor samples were used in four of the five cases, except for P-0009010, for which only a lung metastasis was available. Mean coverage of 566× and 382× was obtained across tumor and normal samples, respectively. Raw sequencing data were aligned to the human reference genome (b37) and somatic variant calling was performed using a combination of four variant calling tools (Mutect2,Varscan, Strelka2, and Platypus). Only mutations called by two or more tools were considered. Additional filters were applied to obtain high-confidence mutations only; these included a variant allele frequency (vAF) in the tumor sample of ≥0.05, vAF in the normal sample of ≤0.02, tumor coverage of ten or more reads, and variant allele coverage in the tumor sample five or more reads. Finally, variants were reviewed manually by the investigators for accuracy [9]. To evaluate the functional impact of mutational events, we queried the OncoKB database for any evidence of oncogenicity [10]. In addition, we assessed the impact of these mutations using both the Mutation Assessor [11] and FATHMM-MKL algorithms [12].
2.3. Structural analysis of TCEB1-VHL interactions
Structural analysis and visualization of the interaction between TCEB1 and VHL were performed with the UCSF Chimera software [13]. The X-ray crystallographic structure of the TCEB1/VHL complex was downloaded from the Protein Data Bank (PDB:4WQO) [13,14]. Hydrophobic interactions between TCEB1 and VHL were determined using the Find Clashes/Contacts function in Chimera to search for carbon atoms of hydrophobic residues in TCEB1 closer than 4 Å from carbon atoms of hydrophobic residues in VHL [15]. Pairs of residues with three or more contacts were taken to have hydrophobic interactions. Molecules other than TCEB1 and VHL were removed from the structure for clarity.
2.4. CN analysis
Analysis of CN alterations (CNAs) was performed using FACETS v0.5.6 (critical value = 100), which allows allele-specific CN analysis and determination of copy-neutral loss of heterozygosity (LOH) [16]. Only autosomal chromosomes were included. Tumor purity estimates were obtained computationally from this algorithm via deconvolution of the relative tumor/normal abundances in the sample. To estimate the fraction of the genome with CNAs, which has been shown to be a surrogate for genomic instability, we considered any genomic segment at a nondiploid level (ie, integer CN ≠ 2:1) [17,18].
2.5. Cancer-cell fraction estimation
To estimate the relative timing of mutations during tumor evolution, we calculated the cancer-cell fraction (CCF) for each somatic mutation as described by McGranahan et al [19]. This value represents the fraction of tumor cells bearing the somatic mutation of interest. The approach uses the allelic frequency of mutations (vAF) and renormalizes it taking tumor purity and locus-specific ploidy into account as follows:
2.6. Statistical analysis
Descriptive statistics such as median and interquartile range (IQR) were used to summarize results. Mann-Whitney U tests were used to evaluate differences in genomic markers between stage groups. Point estimates and 95% confidence intervals (CIs) are also reported. All tests were two-sided and analyses were performed using the R platform v3.5.0.
3. Results
3.1. Pathologic and molecular description
After querying our institutional sequencing database we identified five RCC cases with TCEB1 mutations. The corresponding H&E slides were reviewed independently by two genitourinary pathologists and all cases were found to have the pathognomonic histologic features of TCEB1-mutant RCC (Fig. 1). Two of the patients identified developed disseminated disease; both of these tumors showed high-grade nuclear features in the specimens evaluated. For one of them, only a sample from the lung metastasis was available for review (no primary, P-0009010); for the other, none of the distant disease sites were available for profiling (P-0006239). In the lung metastasis, prominent papillations were observed; however, clear-cell features were only seen focally and some necrotic areas were also observed (Fig. 1H).
Fig. 1 –
Distinct morphologic features of TCEB1-mutant renal cell carcinoma. (A) Low-magnification view of a low-stage case showing distinct features. (B) Higher-magnification of a low-stage tumor; note the clear-cell cytology. (C) Predominantly branching tubular growth pattern with the characteristic cytological features of cells, with abundant, clear cytoplasm. (D) Low-magnification view showing the prominent fibromuscular septations imparting a vaguely nodular configuration to the tumor. (E) Tubular, branching tubular, and microcystic architecture, with two nodules of tumor cells separated by a fibromuscular septum. (F) This tumor showed predominantly high-grade cytology with nuclear pleomorphism, prominent nucleoli, and multifocal tumor necrosis. Fibromuscular stroma was focally present in these high-grade areas. (G) Only small areas (approx. 5%) of the tumor showed the more typical histology. (H) Only the lung metastasis was available for histological evaluation. This metastasis had prominent papillations and only focal clear-cell cytology. Legend: fibromuscular bands are denoted by white, papillations by yellow, clear-cell cytology by green, and necrosis by black asterisks.
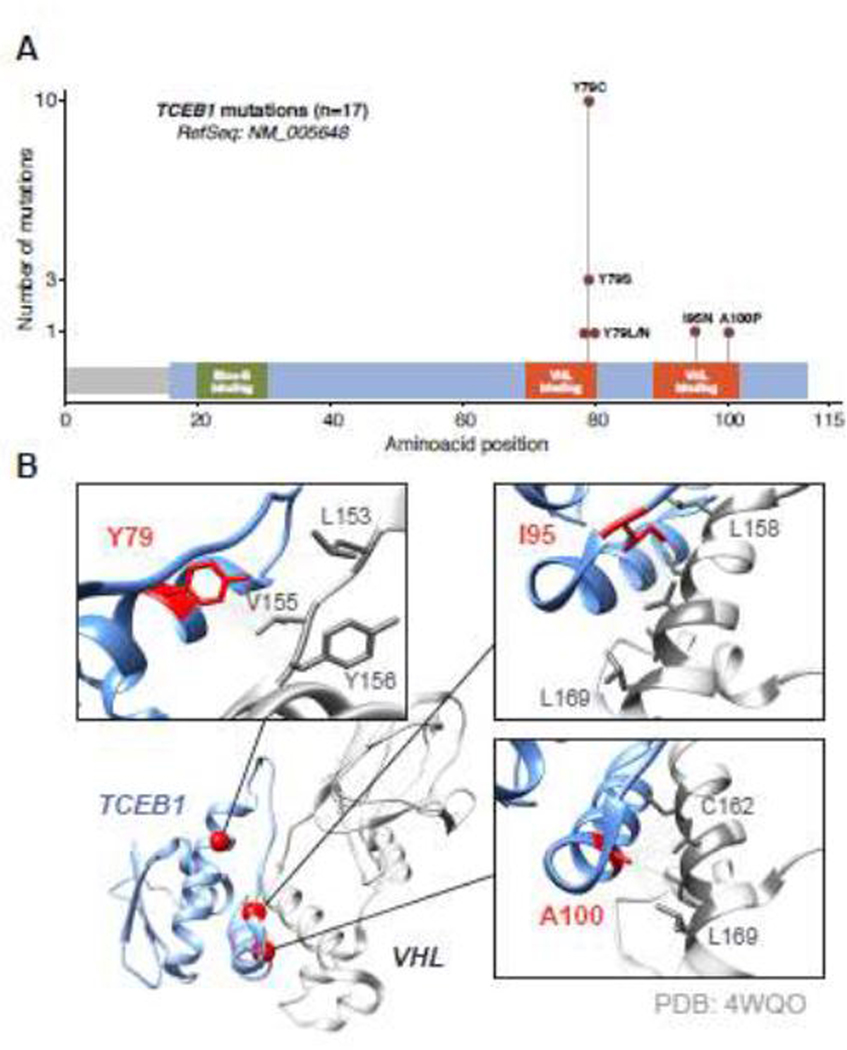
After performing NGS using our institutional sequencing platform (MSK-IMPACT) we were able to detect a total of 18 nonsynonymous mutations (MSK cohort, n = 5). Additional mutations identified in the Sato (n = 5) [4], TCGA KIRC (n = 3) [20], and TRACERx Renal (n = 1) [7] cohorts were pooled for comparison purposes. None of the missense mutations seen in the low-stage cases were found to be oncogenic (except for a KMT2C frameshift insertion), while high-grade/stage cases contained other potential oncogenic drivers. These included TERT promoter hotspot mutations and other genes commonly affected in ccRCC such as SETD2, PIK3CA, and TP53 (Fig. 2). Of the 17 TCEB1-mutant tumors identified in all cohorts, 15 harbored mutations of the Y79 residue, while two cases had mutations of other residues also located in the VHL-binding region (I95N and A100P; Fig. 3A). Assessment of the VHL-elongin C complex revealed that all mutated residues were closely associated with VHL.
Fig. 2 –
Overview of clinical and genomic features. Overview of the somatic mutations identified, including single-nucleotide variants and indels; only the 410 cancer genes included in the MSK-IMPACT panel are shown. The top panel represents the cohort in which the tumors were originally identified. Additional pathologic and clinical characteristics are displayed in the bottom tiles. Higher-stage cases (stage III/IV) showed a higher number of oncogenic mutations (median 2, IQR 2–2) compared to stage I tumors (median 1, IQR 1–1; Mann-Whitney U, p = 0.002). * One sample (P-0009010) had only a metastatic lung lesion available for pathology review; primary tumor features not shown. IQR = interquartile range.
Fig. 3 –
Somatic mutations identified in TCEB1. (A) Somatic mutations identified in the TCEB1 (ELOC) gene; all the events were found in the VHL-binding domains. (B) Graphical representation of the interaction between the VHL and elongin C proteins. The residues in which somatic mutations were found correspond to sites of hydrophobic interaction between the two proteins.
3.2. Genomic analyses: drivers of aggressiveness
CN data were analyzed for the five MSK cases as well as the TRACERx Renal case and a TCGA KIRC case (n = 7). All samples showed LOH at the TCEB1 locus (8q21.11) with different CN patterns (Fig. 4A). In addition, all high-stage cases showed whole-genome doubling (WGD) events and a higher burden of CN aberrations (Supplementary Fig. 1). Overall, high-stage cases seemed to have a higher fraction of CN-altered genome (FCNAg) value than lower-stage cases (median 0.10, IQR 0.10–0.15 vs 0.63, IQR 0.58–0.68), although this result did not reach statistical significance (median difference 0.49, 95% CI 0.33–0.63; p = 0.052; Fig. 4B). In addition, stage III/IV tumors showed a higher frequency of oncogenic cancer-gene mutations (median 1, IQR 1–1 vs 2, IQR 2–2; median difference 1, 95% CI 1–1; p = 0.002) The number of oncogenic mutations was also assessed with two additional tools and was significant with any tool/combination except FATHMM-MKL (Supplementary Fig. 2). One of the stage III cases (P-0018555) also showed a potentially oncogenic intragenic fusion in PPP6C leading to deletion of this tumor suppressor.
Fig. 4 –
Copy-number profiles at the TCEB1 locus (8q21). (A) Graphical representation of the copy-number state at 8q21 showing the mutant chromosomes (green/solid) and lost chromosomes (white/dashed). The numbers at the bottom represent the total number of 8q21 copies and the number of wild-type copies. Only low-stage cases (n = 3) did not show whole-genome doubling (WGD) events. (B) Fraction of copy-number–altered genome (FCNAg) by overall stage. Stage III/IV cases showed a higher FCNAg (median 0.63, IQR 0.58–0.68) compared to their stage I counterparts (median 0.10, IQR 0.10–0.15; Mann-Whitney U, p = 0.052), representing greater genomic instability. IQR = interquartile range.
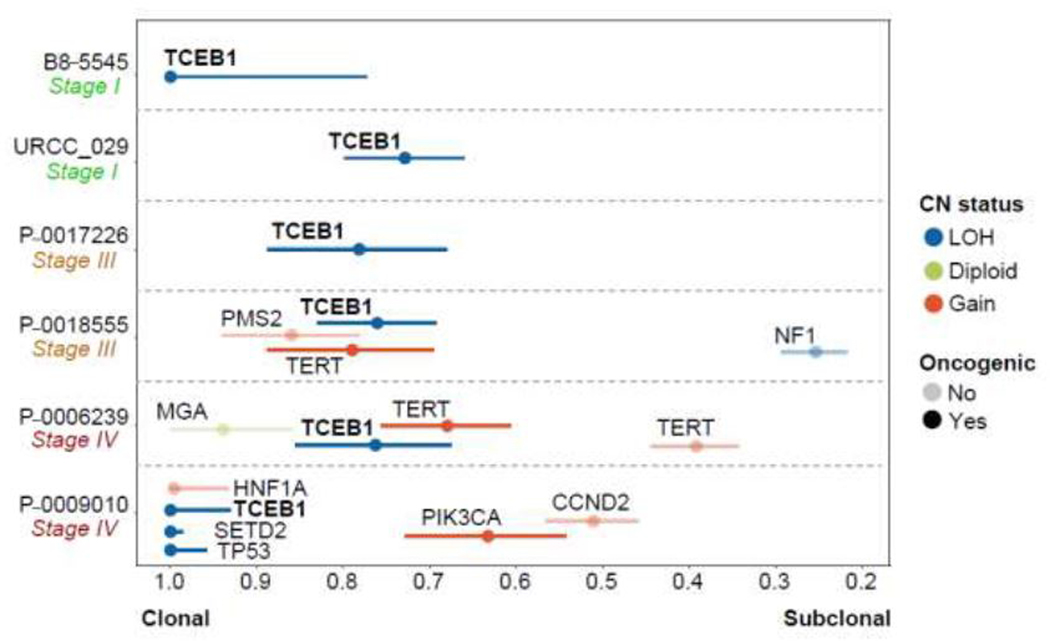
We estimated the CCF for all nonsynonymous mutational events identified in the cases with CN data available. TCEB1 was found at an average CCF of 0.86 (95% CI 0.77–0.95), indicating that TCEB1 mutations are virtually clonal in all cases. In one case, a nononcogenic mutation in MGA was found to be at a higher CCF than TCEB1 (Fig. 5). Notably, the metastatic lesion was found to have other oncogenic mutations at high CCF in TP53 and SETD2 as well as a subclonal PIK3CA hotspot mutation. Regarding the sample with multiregional sequencing data (from TRACERx Renal), we observed the same TCEB1 mutation (I95N) and heterozygous loss of chromosome 8 in all the regions of the primary tumor, suggesting early (clonal) biallelic inactivation (Supplementary Fig. 3).
Fig. 5 –
Mutational clonality analysis using next-generation DNA sequencing. Cancer-cell fraction (CCF) estimates were computed using tumor purity, locus-specific ploidy, and variant allele frequency. Somatic mutation with CCF of ~1.0 are present in all tumor cells in the sample profiled. High-stage cases showed other clonal oncogenic mutations in addition to TCEB1. CN = copy number; LOH = loss of heterozygosity.
3.3. Clinical course TCEB1-mutant RCC
Two cases identified at our institution developed metastatic disease. One (P-0009010) was a patient with a history of Hodgkin’s lymphoma 40 yr previously (treated with chemotherapy/radiotherapy) and chronic kidney disease who presented with an incidental 3.5-cm mass in a solitary kidney. A decision was made to follow the patient because of his high surgical risk and poor kidney function. At the next follow-up visit (after 12 mo), imaging showed multiple pleural, lung, and mediastinal nodules; one of the lesions was resected and a diagnosis of metastatic RCC was made (only sample available, P-0009010). After confirmation of this diagnosis, the patient was started on pazopanib and had stable disease while on treatment. He later developed multiple gastrointestinal and respiratory complications, including a pyloric perforation, which eventually led to his death.
The second metastatic case (P-0006239) presented with a pleural effusion that was found to contain atypical cells. After draining the pleural fluid, a lung nodule was biopsied and a diagnosis of metastatic RCC was made (sample unavailable). This patient was administered a combination of atezolizumab and bevacizumab and developed a profound objective response. At this point a decision was made to perform cytoreductive nephrectomy with thrombectomy and node dissection (sample available, P-0006239). The patient was deemed a complete responder according to Response Evaluation Criteria in Solid Tumors v1.1 and remained with no evidence of disease (NED) for approximately 2 yr while on treatment. At this point a decision was made to discontinue the treatment (bevacizumab was discontinued first because of an adverse reaction). The patient still has NED after total follow-up of 48.5 mo (Fig. 6).
Fig. 6 –
Clinical course of high-stage cases from the MSKCC cohort. Swimmer plot depicting the clinical course of four high-stage cases identified in the Memorial Sloan Kettering Cancer Center (MSKCC) cohort. One stage III case (P-0017226) received adjuvant pazopanib after initial nephrectomy as part of a clinical trial. The metastatic case P-0009010 initially presented with a small renal mass in a solitary kidney and was followed up for 1 yr (not shown). NED = no evidence of disease; CR = complete response; TKI = tyrosine kinase inhibitor.
The remaining patients from the MSK cohort (n = 3) had localized disease at presentation and were rendered disease-free after initial nephrectomy. None of them have been found to have local or distant recurrence during follow-up visits. Two of these patients have been followed for more than 5 yr, while one (URCC_029) has had follow-up of only 14 mo. Only one patient with localized disease received adjuvant pazopanib as part of a clinical trial and currently has NED (Fig. 6). The previously reported cases all had localized tumors that were fully resected during nephrectomy. The patients were reported to have NED at median follow-up of 48 mo [1].
4. Discussion
We report on five new TCEB1-mutant RCC cases with variable clinical course, including two patients who had metastatic disease and received systemic therapy. Genomic analyses performed on these rare tumors provide additional evidence of genomic drivers of aggressiveness that might transcend RCC histology. In addition, we compared these high-stage tumors to previously reported cases from Sato, TCGA KIRC, and TRACERx Renal cohorts, making this series the largest description of its kind and (to our knowledge) the first report of metastatic TCEB1-mutant RCC.
The fundamental event giving rise to TCEB1-mutant RCC is biallelic inactivation of the TCEB1 gene [1,4]. We confirmed this via allele-specific CN analysis that allowed us to detect absolute losses as well as copy-neutral LOH at the TCEB1 locus. Three cases showed a total CN of ≥2 in 8q21 and loss of the WT allele (with concurrent evidence of WGD events). This demonstrates the behavior of TCEB1 as a classical tumor suppressor, whereby inactivation of both copies is necessary for tumorigenesis. Furthermore, we report the largest summary to date of TCEB1 mutations, including an I95N mutation initially described in the TRACERx Renal cohort [7]. All three mutant residues have been shown to be highly conserved across species and are located in the VHL-binding domains [5]. By measuring the distances between interacting carbon molecules in VHL and elongin C, we could confirm that all three mutated amino acids (Y69, I95, and A100) are involved in hydrophobic interactions with VHL, strengthening evidence on the role of elongin C in tumor suppression through this mechanism. Additional TCEB1 mutations have recently been described in a case series of RCCs with leiomyomatous stroma that included S23L and A106D [21]. These also correspond to highly conserved residues and are predicted to impact elongin C functions (high and medium functional impact, respectively, according to Mutation Assessor); however, S23 does not seem to be implicated in VHL binding, suggesting that TCEB1 mutations might induce oncogenesis through more than one mechanism. It has been shown that through interaction with elongin A and B, the TCEB1 gene product acts as a positive regulator of a heterotrimeric RNA polymerase II elongation factor (elongin SIII), promoting mRNA elongation [22,23]. Recognition of this entity with certainty via pathologic review alone is very difficult and confirmation of the diagnosis currently requires molecular evidence. In all our cases, a final diagnosis was established based on evidence of TCEB1 mutations in the sequencing data. At our institution, without molecular corroboration we refer to these tumors as ccRCCs with prominent fibromuscular septations and CK7-positivity. We acknowledge that this terminology is nonspecific (as these features can also be present in rare TSC1/2-driven RCCs, MiTF-translocation–associated RCC, or carcinomas with no known molecular alterations, for example); however, we believe that reporting these findings can provide additional clues about the potential tumor etiology and perhaps even direct towards further testing on the pathology specimens.
In addition, using the mutation and CN data we were able to calculate the CCF for these mutations and draw an inference about the order in which they arose [19]. This measure represents the percentage of tumor cells bearing a particular mutation; as expected, TCEB1 alterations were always present in the majority of cells (average CCF 0.86). In all cases, TCEB1 was among the oncogenic mutations with the highest CCF, and another mutation at a higher CCF than TCEB1 was found in only one sample (Fig. 5, P-0006239). We think that this is probably a false positive due to inaccuracies when computing CCF estimates (discussed below) and that both events are likely to be clonal. Interestingly, in the metastatic lung lesion (P-0009010), TCEB1 was found at a CCF of 1.0 together with other oncogenic mutations. This could represent either seeding of the metastatic site by a subclone from the primary tumor (harboring all the mutations) or the presence of a primary tumor with multiple clonal drivers. Both the presence of multiple drivers and deleterious mutations in key genes such as TP53 (such as the R248W hotspot mutation identified in this sample [24,25]) have been associated with poor prognosis in RCC and could potentially explain the aggressive behavior displayed by this tumor [20,26]. However, we do not have definitive proof of clonality in the primary tumor (owing to tissue unavailability) and both evolutionary patterns could potentially explain the poor clinical course experienced by this patient (3.5-cm small renal mass that became metastatic during 1 yr of active surveillance).
High-stage (American Joint Committee on Cancer stage III/IV) TCEB1-mutant RCCs showed a higher burden of oncogenic mutations overall when compared to their lower-stage counterparts. This was true even after excluding nonprimary samples (P-0009010, lung) from the analysis (Mann-Whitney U, p = 0.004). The number of oncogenic driver events has been reported as a feature of poor prognosis in RCC [7]. Regarding the additional oncogenic mutations with potential prognostic significance, a TERT hotspot mutation was also identified, albeit at a lower CCF in the second metastatic case (P-0006239, Fig. 5). Although the role of TERT promoter mutations in RCC is far from clear, previous reports have shown that they can be associated with poor prognosis and disease spread in ccRCC and non-ccRCC [27]. High-stage TCEB1-mutant RCCs also exhibited the presence of WGD events and a higher CNA burden across the genome compared to low-stage tumors (Mann-Whitney U, p = 0.052). This measure has been proposed as a surrogate for chromosomal instability and recent genomic studies have demonstrated its role in RCC prognosis [7]. Although values are probably inflated by the presence of WGD, which itself has been reported to be associated with poor pan-cancer survival [18], even after accounting for this, higher-stage samples show higher FCNAg values than lower-stage ones. This provides yet another line of evidence on the role of CNAs in the aggressiveness of RCC.
The present series has certain limitations that preclude definitive conclusions about potential drivers of aggressiveness in TCEB1-mutant RCC. First, only a single sample for each tumor was profiled for both localized and metastatic cases. To properly resolve the underlying molecular heterogeneity of these tumors, multiregional sequencing would be preferable [28]; however, other disease sites were not available. Second, owing to the relatively low resolution of heterozygous SNPs in targeted sequencing panels, this is not the most accurate NGS assay for CN analysis. Therefore, CCF estimates might be less accurate compared to other platforms such as WES or whole-genome sequencing. In addition, although this is the biggest series of TCEB1-mutant RCCs presented to date, the data used for validation purposes (Sato, TCGA and TRACERx cohorts) were highly heterogeneous and consisted of a wide variety of sequencing approaches and variant-calling algorithms, which could pose certain limitations when interpreting the results. Regarding the mutation data identified on WES, to allow comparability between cohorts we downsampled the mutations identified to the 410 cancer genes included in our institutional panel. We must acknowledge, however, that this approach does not address the heterogeneity that arises from the use of different mutation-calling tools between cohorts. On the contrary, CN analysis was performed using the same analytical pipeline on raw sequencing data, which therefore limited the possibility that these findings could be a result of differences in sequencing approaches. Finally, owing to the rarity of this entity we only identified two patients with metastatic disease. The evidence is thus still limited and these analyses should be expanded as more data become available.
5. Conclusions
TCEB1-mutant RCC is a rare variant of RCC with variable clinical course; its behavior can range from very indolent to metastatic and aggressive. Specific molecular events leading to high genomic instability seem to drive this phenotype.
Supplementary Material
Acknowledgments
Financial disclosures: A. Ari Hakimi certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was supported by The Sidney Kimmel Center for Prostate and Urologic Cancers and in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The sponsors played a role in data collection and management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hakimi AA, Tickoo SK, Jacobsen A, et al. TCEB1-mutated renal cell carcinoma: a distinct genomic and morphological subtype. Mod Pathol 2015;28:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Reznik E, Lee H-J, et al. Abnormal oxidative metabolism in a quiet genomic background underlies clear cell papillary renal cell carcinoma. eLife 2019;8:38986. 10.7554/elife.38986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43–9. 10.1038/nature12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet 2013;45:860–7. 10.1038/ng.2699 [DOI] [PubMed] [Google Scholar]
- 5.Stebbins CE, Kaelin WG Jr, Pavletich NP. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science 1999;284:455–61. [DOI] [PubMed] [Google Scholar]
- 6.Krek W. VHL takes HIF’s breath away. Nat Cell Biol 2000;2:E121–3. [DOI] [PubMed] [Google Scholar]
- 7.Turajlic S, Xu H, Litchfield K, et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx Renal. Cell 2018;173:595–610.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT). J Mol Diagn 2015;17:251–64. 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnell EK, Ronning P, Campbell KM, et al. Standard operating procedure for somatic variant refinement of sequencing data with paired tumor and normal samples. Genet Med 2019;21:972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol 2017;1:1–16. 10.1200/PO.17.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res 2011;39:e118. 10.1093/nar/gkr407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shihab HA, Rogers MF, Gough J, et al. An integrative approach to predicting the functional effects of non-coding and coding sequence variation. Bioinformatics 2015;31:1536–43. 10.1093/bioinformatics/btv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettersen EF, Goddard TD, Huang CC, et al. UCSF chimera? A visualization system for exploratory research and analysis. J Comput Chem 2004;25:1605–12. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen HC, Yang H, Fribourgh JL, Wolfe LS, Xiong Y. Insights into Cullin-RING E3 ubiquitin ligase recruitment: structure of the VHL-EloBC-Cul2 complex. Structure 2015;23:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira de Freitas R, Schapira M. A systematic analysis of atomic protein-ligand interactionsin the PDB. MedChemComm 2017;8:1970–81. 10.1039/C7MD00381A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res 2016;44:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endesfelder D, Burrell R, Kanu N, et al. Chromosomal instability selects gene copy-number variants encoding core regulators of proliferation in ER+ breast cancer. Cancer Res 2014;74:4853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bielski CM, Zehir A, Penson AV, et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat Genet 2018;50:1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGranahan N, Favero F, de Bruin EC, Birkbak NJ, Szallasi Z, Swanton C. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med 2015;7:283ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricketts CJ, De Cubas AA, Fan H, et al. The Cancer Genome Atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep 2018;23:3698. [DOI] [PubMed] [Google Scholar]
- 21.Parilla M, Alikhan M, Al-Kawaaz M, et al. Genetic underpinnings of renal cell carcinoma with leiomyomatous stroma. Am J Surg Pathol 2019;43:1135–44. [DOI] [PubMed] [Google Scholar]
- 22.Takagi Y, Pause A, Conaway RC, Conaway JW. Identification of elongin C sequences required for interaction with the von Hippel-Lindau tumor suppressor protein. J Biol Chem 1997;272:27444–9. [DOI] [PubMed] [Google Scholar]
- 23.Aso T, Lane WS, Conaway JW, Conaway RC. Elongin (SIII): a multisubunit regulator of elongation by RNA polymerase II. Science 1995;269:1439–43. [DOI] [PubMed] [Google Scholar]
- 24.Chang MT, Asthana S, Gao SP, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol 2016;34:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J, Chang MT, Johnsen HC, et al. 3D clusters of somatic mutations in cancer reveal numerous rare mutations as functional targets. Genome Med 2017;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voss MH, Reising A, Cheng Y, et al. Genomically annotated risk model for advanced renal-cell carcinoma: a retrospective cohort study. Lancet Oncol 2018;19:1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casuscelli J, Becerra MF, Manley BJ, et al. Characterization and impact of TERT promoter region mutations on clinical outcome in renal cell carcinoma. Eur Urol Focus 2019;5:642–9. 10.1016/j.euf.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turajlic S, Sottoriva A, Graham T, Swanton C. Resolving genetic heterogeneity in cancer. Nat Rev Genet 2019;20:404–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.