To the Editor:
Critically ill COVID-19 patients frequently present with profound hypoxemia with ARDS requiring mechanical ventilation (MV).1 According to recently published Covid-19 guidelines,2 ventilatory support aims at increasing Pao 2 with noninvasive methods and eventually MV. Ventilator settings are optimized to recruit collapsed alveoli and reduce ventilator-induced lung injury.
However, in Sars-Cov-2 ARDS, recruitment strategies may be hazardous because of a preserved compliance3 and a poor response to positive end-expiratory pressure (PEEP),4 whereas physiological measurements rather show increased intrapulmonary shunt.5 Abnormal pulmonary vascular dilation and increased perfusion surrounding areas of lung opacity have been identified with dual-energy CT imaging, suggesting that insufficient hypoxic pulmonary vasoconstriction (HPV) may play a major role in the onset of hypoxemia.6
Almitrine, a drug that used to decrease intrapulmonary shunt by enhancing HPV, improves gas exchange in ARDS.7 We hypothesized that almitrine could improve hypoxemia in patients with Sars-Cov-2 ARDS.
Materials and Methods
Study Design
This monocenter retrospective study aimed to evaluate the association between almitrine introduction and improvement of oxygenation in Sars-Cov-2 ARDS. The study was conducted in a 36-bed ICU (Hôpital Lariboisière, Paris, France) fully dedicated to the Covid-19 outbreak. The medical records of all patients admitted between March 14, 2020 and April 11, 2020 were reviewed.
Inclusion criteria in the study were: admission for respiratory failure, a diagnosis of ARDS according to Berlin criteria,8 laboratory-confirmed Sars-Cov-2 infection, almitrine infusion in ICU. The primary end point was the Pao 2 to Fio 2 ratio between baseline value and peak value during the 1st through 6th hour timeframe after introduction of almitrine. The Pao 2/Fio 2 ratio was measured with Fio 2 1. The other endpoints were incidence of treatment failure at 24, 48, 72, and 96 hours, and safety. Treatment failure was defined as death, or the need for additional rescue therapy. Increase in right atrial pressure (RAP) and lactate during the first 6 hours, and peak values for liver tests during the first 48 hours were reported as safety data.
This study was approved by an institutional ethics committee: Institutional Review Board (IRB 00006477) of HUPNVS, Paris 7 University.
Patients’ Management
Patients were managed according to local protocol based on international guidelines.9 Intrapulmonary shunt is confirmed after exclusion of a patent foramen ovale. Pleural effusions are considered for drainage. Hemodynamic optimization is performed to address low mixed venous oxygen tension (Pvo 2 effect). Early respiratory management includes limitation of tidal volume and plateau pressure, systematic neuromuscular blockade, and prone positioning session of at least 16 hours. PEEP is individualized to improve oxygenation without deteriorating compliance and cardiac output. In case of persistent refractory hypoxemia, we usually consider almitrine infusion (initial dose, 2 μg/kg/min) or inhaled nitric oxide. Almitrine and inhaled nitric oxide use are decided after collegial discussion on a case-by-case basis. If severe hypoxemia persists despite the latter treatments, the extracorporeal membrane oxygenation team is called to evaluate the indication of the device.
Statistical Analysis
Continuous variables before and after almitrine infusion were compared by a Wilcoxon rank-sum test for paired data. All statistical analyses were performed using R statistical software version 3.6.1.
Results
Patients
Eighty-six patients were admitted to our ICU during the studied period. Nineteen of the 20 patients that met inclusion criteria had complete data and were analyzed (age 63 [54-67] years, sex ratio 2.8, BMI 28 [26-32]). Median time from ICU admission was 4 (2-6) days.
At the time of almitrine infusion, 15 of 19 (79%) were under MV with neuromuscular blockade. PEEP was set at 10 (10-12) cm H2O, and static compliance was 32 (28-35) mL/cm H2O. Eighteen patients (95%) had at least one session of prone positioning before almitrine.
Efficacy of Almitrine
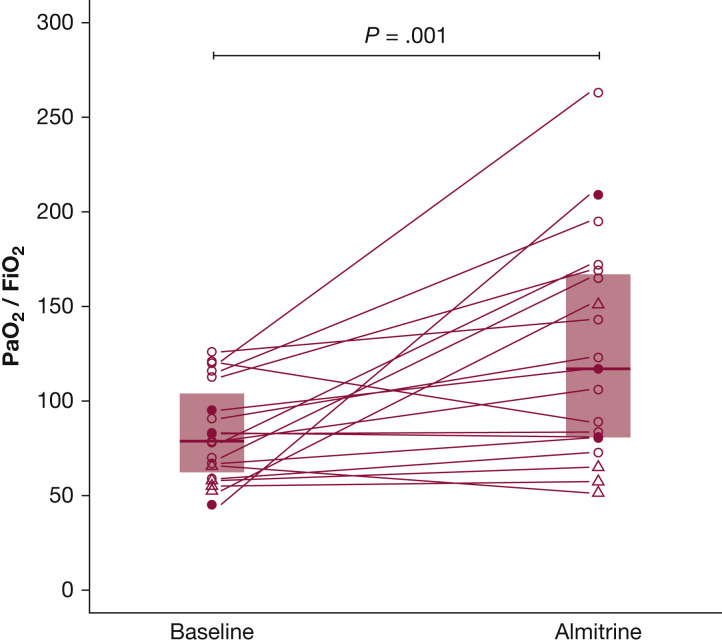
The median Pao 2/Fio 2 ratio increased from 79 (64-100) at baseline to 117 (81-167) after almitrine (P = .001) (Fig 1 ).
Figure 1.
Evolution of PaO2/Fio2 ratio following almitrine infusion. Circles (○) represent individual PaO2/Fio2 ratio at FiO2 1 of patients under mechanical ventilation. Triangles (△) are for patients who are spontaneously breathing. Solid circles (●) are for the three mechanically ventilated patients that had concomitant inhaled nitric oxide started along with almitrine. Segments connect baseline and almitrine value for each individual patient. Boxes represent interquartile range, and horizontal lines inside the boxes represent median values. The closest available PaO2/Fio2 ratio up to 3 hours before almitrine infusion was taken as baseline value. Peak PaO2/Fio2 ratio measured from the first to the sixth hour after the start of the infusion was taken as almitrine value. During this period, there was no change in patient positioning, and no additional rescue therapy was started.
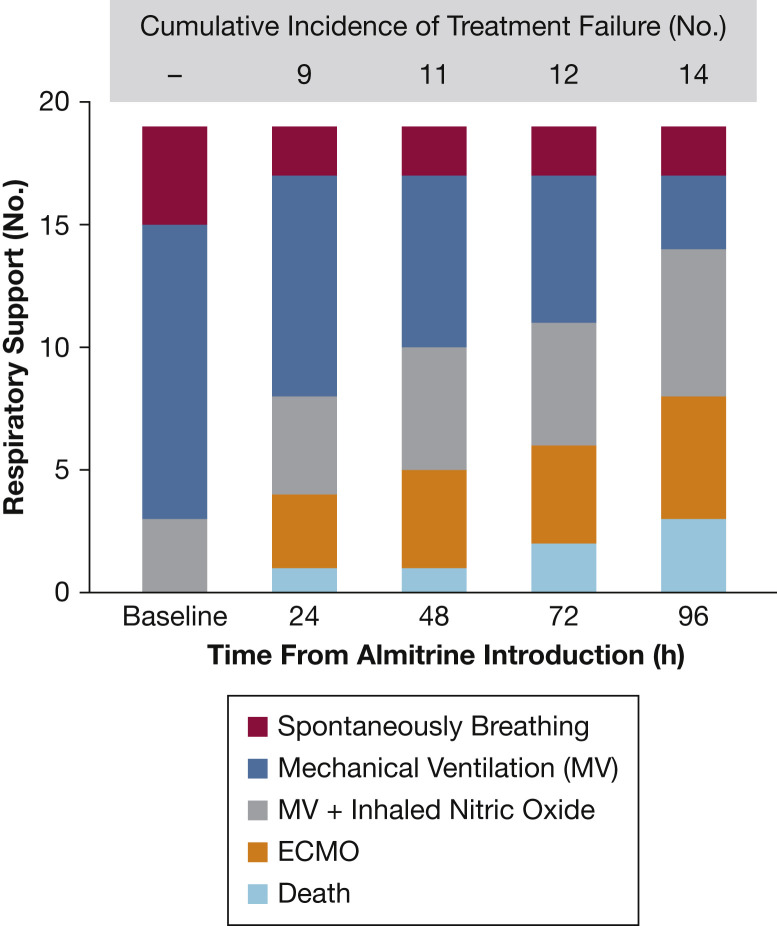
Figure 2 shows that almitrine infusion remained associated with an incremental need for supportive treatments in the following 96 hours.
Figure 2.
Type of respiratory support and cumulative incidence of almitrine failure during the first 96 hours. Treatment failure is defined as death, or the need for any of the following additional rescue therapies: new endotracheal intubation if the patient was not under mechanical ventilation, administration of inhaled nitric oxide in a patient that was not treated with it, or extracorporeal membrane oxygenation cannulation. Cumulative incidence reports the absolute number of patients with reported treatment failure from the start of the almitrine infusion to the evaluation time.
Safety of Almitrine
The median arterial lactate level increased from 1.2 (0.9-1.4) to 1.5 (1.1-1.7) mmol/L after almitrine infusion (P = .002). Right atrial pressure did not change after almitrine infusion (9 [8-11] to 10 [9-11] mm Hg, P = .174).
Alanine transaminase, alkaline phosphatase, and gamma glutamyl transpeptidase did not change during the 48 hours after almitrine infusion. Peak values were 60 (37-78), 111 (76-142), and 116 (103-154) IU/L.
Discussion
This is the first study describing therapeutic effects of almitrine in Sars-Cov-2 ARDS. In our observational study, almitrine was associated with an increase in Pao 2/Fio 2 ratio after treatment. However, this improvement of hypoxemia seems to be heterogenous amongst patients. Furthermore, despite an associated improvement in Pao 2/Fio 2 ratio, most patients receiving almitrine went on to needing additional rescue interventions or died. This may be explained by the fact that, in our study, almitrine has been used as a rescue therapy in severe patients with worsening hypoxemia and very low Pao 2/Fio 2 ratio.
Even though this is a small sample study without control group, our data show that enhancing HPV is an encouraging strategy to reduce hypoxemia in Sars-Cov-2 ARDS. This could at least be helpful to secure intrahospital or interhospital transfers in the most severe patients, or to gain precious time until a more invasive life support method is available.
The ideal time to start almitrine also remains to be determined. Almitrine could be used earlier in the treatment of Sars-Cov-2 hypoxemia to reduce the need for or duration of MV, a scarce resource in the setting of a worldwide outbreak. However, improvement in hypoxemia is not necessarily associated with improved outcome. Conversely, almitrine has been identified as a potential candidate against targeted proteins of SARS-Cov-2 with expected inhibitory effect.10 Altogether, we believe that almitrine should be evaluated in clinical trials aiming at improving patient-centered outcome for Covid-19 patients.
Conclusion
In this monocenter retrospective study, we found that almitrine at the dose of 2 μg/kg/min was associated with an increased Pao 2/Fio 2 ratio in the following 6 hours in Sars-Cov-2 ARDS patients.
Footnotes
Drs Barthélémy and Blot contributed equally to this manuscript.
FINANCIAL/NONFINANCIAL DISCLOSURES: The authors have reported to CHEST the following: A. M. received speaker’s honoraria from Novartis, Orion, and Servier and fees as a member of the advisory board or steering committee from Adrenomed, Sanofi, Roche, Abbott, and 4TEEN4. B. G. C. received fees as a member of an advisory board from Roche Diagnostics. None declared (R. B., P.-L. B., A. T., A. L. G., C. M., S. G., L. M., E. G.).
References
- 1.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhazzani W., Møller M.H., Arabi Y.M. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L., Chiumello D., Caironi P. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan C., Chen L., Lu C. Lung recruitability in SARS-CoV-2 associated acute respiratory distress syndrome: a single-center, observational study. Respir Crit Care Med. 2020;201(10):1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang M., Som A., Mendoza D.P. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30367-4. [Published online ahead of print April 30, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payen D., Muret J., Beloucif S. Inhaled nitric oxide, almitrine infusion, or their coadministration as a treatment of severe hypoxemic focal lung lesions. Anesthesiology. 1998;89(5):1157–1165. doi: 10.1097/00000542-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 8.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Papazian L., Aubron C., Brochard L. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C., Liu Y., Yang Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]