Abstract
Introduction
COVID-19 infections are associated with a high prevalence of venous thromboembolism, particularly pulmonary embolism (PE). It is suggested that COVID-19 associated PE represents in situ immunothrombosis rather than venous thromboembolism, although the origin of thrombotic lesions in COVID-19 patients remains largely unknown.
Methods
In this study, we assessed the clinical and computed tomography (CT) characteristics of PE in 23 consecutive patients with COVID-19 pneumonia and compared these to those of 100 consecutive control patients diagnosed with acute PE before the COVID-19 outbreak. Specifically, RV/LV diameter ratio, pulmonary artery trunk diameter and total thrombus load (according to Qanadli score) were measured and compared.
Results
We observed that all thrombotic lesions in COVID-19 patients were found to be in lung parenchyma affected by COVID-19. Also, the thrombus load was lower in COVID-19 patients (Qanadli score −8%, 95% confidence interval [95%CI] −16 to −0.36%) as was the prevalence of the most proximal PE in the main/lobar pulmonary artery (17% versus 47%; −30%, 95%CI −44% to −8.2). Moreover, the mean RV/LV ratio (mean difference −0.23, 95%CI −0.39 to −0.07) and the prevalence of RV/LV ratio >1.0 (prevalence difference −23%, 95%CI −41 to −0.86%) were lower in the COVID-19 patients.
Conclusion
Our findings therefore suggest that the phenotype of COVID-19 associated PE indeed differs from PE in patients without COVID-19, fuelling the discussion on its pathophysiology.
Keywords: Pulmonary embolism, COVID-19, Computed tomography, Thrombosis
Highlights
-
•
COVID-19 pneumonia is associated with high incidence of pulmonary embolism (PE).
-
•
The origin of COVID-19 associated PE is debated.
-
•
We compared radiological PE parameters in COVID-19 patients with control patients.
-
•
In COVID-19 patients, thrombus load and prevalence of RV dysfunction was lower.
-
•
Our findings support the concept of in situ immunothrombosis in COVID-19 patients
1. Introduction
COVID-19 infections are associated with frequent activation of the coagulation system. This so-called COVID-19 coagulopathy has been shown to be predictive of poor outcome and excess mortality [[1], [2], [3], [4], [5], [6]]. Moreover, COVID-19 illness is associated with high rates of venous thromboembolism, particularly acute pulmonary embolism (PE) [[7], [8], [9], [10], [11], [12], [13], [14]]. This high rate of venous thromboembolic complications is likely related to the COVID-19 coagulopathy, in combination with well-known strong thrombotic risk factors including inflammation, hypoxia and immobilisation, which all become more pronounced in critically ill patients. Based on autopsy studies, it has been proposed that the inflammatory process in the microcirculation of the lung may cause in situ immunothrombosis [15,16]. This suggests an alternative explanation to the conventional thromboembolic pathomechanism of PE [17]. To investigate this hypothesis, we set out to determine the clinical and computed tomography (CT) characteristics of acute PE in COVID-19 patients, and compare these to the characteristics of acute PE in patients without COVID-19 pneumonia.
2. Methods
2.1. Patients and design
We included all adult patients admitted to the Leiden University Medical Center (LUMC) with polymerase chain reaction (PCR) proven COVID-19 infection and computed tomography pulmonary angiography (CTPA) proven acute PE between March 19th and April 14th 2020. Additionally, we studied a convenience control cohort, in whom all radiological parameters had been assessed as part of an ongoing observational study. This control group consisted of 100 consecutive adult patients with CTPA confirmed acute PE, diagnosed between July 2017 and October 2019 in the LUMC, before the COVID-19 outbreak. In both cases and controls, CTPA was ordered only upon clinically suspected acute PE. In patients not admitted to the intensive care unit (ICU) and with suspected acute PE, the YEARS algorithm was applied and CTPA was performed only if the D-dimer level was above the threshold [18]. In patients admitted to the ICU and with suspected acute PE, CTPA was directly ordered without prior clinical probability scoring. This study was approved by the Institutional Review Board of the LUMC for observational studies.
2.2. Aims of the study
We aimed to determine the clinical and CT parameters of patients with COVID-19 associated PE, i.e. the reasons for clinical suspicion of PE, the location of the pulmonary emboli in the pulmonary artery tree in general and in relation to COVID-19 affected pulmonary segments, total thrombus load, right to left ventricular diameter ratio (RV/LV ratio) and pulmonary artery trunk diameter. We compared the clinical and CT characteristics of acute PE in COVID-19 patients to those assessed in the control cohort.
2.3. Image acquisition and analysis
CTPA examinations were performed on a 320-multislice detector row CT scanner (Canon) after iodinated contrast administration. RV/LV ratio, pulmonary artery trunk diameter and total thrombus load for both COVID-19 cases and controls were evaluated by an expert thoracic radiologist (LK) with over 20 years of experience in CTPA reading. The maximum diameters of both the RV and LV were measured in the standard axial view in which the maximal distance between the ventricular endocardium and the interventricular septum perpendicular to the long axis of the heart were assessed. The pulmonary artery trunk was measured at its largest transverse diameter. The total thrombus load was assessed by using the Qanadli CT pulmonary artery obstruction index, including 10 lung segments for each lung [19]. For each PE location, ground glass opacities or consolidations were reported as being present or not present for each affected lung segment. Also, the extent of COVID-19 lung lesions by ground-glass opacities and consolidations was visually assessed as percentage of affected lung volume.
Clinical and CT characteristics of PE are described as mean with standard deviation (SD) or median with interquartile range (IQR). We calculated absolute differences in these characteristics between COVID-19 patients and controls with corresponding 95% confidence interval (95%CI). All statistical analyses were performed in SPSS version 25 (IBM, Armonk, NY, USA).
3. Results
A total of 23 patients with proven COVID-19 pneumonia and symptomatic CTPA proven PE were included in the study. Their baseline characteristics are shown in Table 1 . All received pharmacological thromboprophylaxis. The main reason for PE suspicion was lack of clinical improvement in ventilated patients (n = 13, 57%), sudden unexpected respiratory deterioration (n = 7, 30%), hemodynamic collapse (n = 2, 8.7%) and persistent fever of unknown origin (n = 1, 4.3%).
Table 1.
Characteristics of pulmonary embolism (PE) patients with and without COVID-19.
| PE patients with COVID-19 (n = 23) |
PE patients without COVID-19 (n = 100) |
|
|---|---|---|
| Characteristics and venous thromboembolism (VTE) risk factors | ||
| Mean age (±SD) – years | 63 (6.4) | 62 (16) |
| Male sex – n (%) | 16 (70) | 53 (53) |
| Previous VTE – n (%) | 1 (4) | 17 (17) |
| Active malignancy – n (%) | 1 (4) | 27 (27) |
| Trauma/surgery during the past 4 weeks – n (%) | 0 (0) | 22 (22) |
| Clinical presentation | ||
| Chest tightness – n (%) | 0/4 (0)* | 25 (25) |
| Pleural pain – n (%) | 0/4 (0)* | 55 (55) |
| Dyspnea – n (%) | 3/4 (75)* | 82 (82) |
| Hemoptysis – n (%) | 0 (0) | 6 (6.0) |
| Clinical signs of deep vein thrombosis | 0 (0) | 6 (6.0) |
| D-dimer results (ng/mL) – median (IQR) | 7551 (3852–10,005) | 2637 (3345–4998) |
| Hemodynamic unstable at diagnosis – n (%) | 2 (8.7) | 6 (6.0) |
| Reperfusion therapy – n (%) | 1 (4.3) | 5 (5.0) |
| >24 hour supplemental oxygen therapy– no (%) | 23 (100) | 25 (25) |
| Inording to Qanadli score) were measured antensive care admission – no (%) | 20 (87) | 8 (8.0) |
| Radiological presentation | ||
| Most proximal anatomic location | ||
| Main/lobar | 4 (17) | 38 (38) |
| Segmental | 16 (70) | 41 (41) |
| Subsegmental | 3 (13) | 11 (11) |
| Qanadli score (%) – mean (SD) | 23 (18) | 31 (17) |
| Right ventricle diameter (mm) – mean (SD) | 43 (8.0) | 45 (9.9) |
| Left ventricle diameter (mm) – mean (SD) | 44 (7.0) | 41 (8.9) |
| RV/LV ratio – mean (SD) | 0.97 (0.15) | 1.2 (0.38) |
| Pulmonary artery trunk diameter (mm) – mean (SD) | 29 (4.6) | 28 (4.7) |
Note: *Only for non-sedated non-intubated patients.
In the COVID-19 cohort, the most proximal PE was located in the main/lobar pulmonary artery in 4 patients (17%), and the most proximal PE was in a segmental artery in 16 patients (70%) (Fig. 1 ). In 3 patients (13%), the PE was limited to subsegmental arteries and 1 patient (4.3%) had a single subsegmental PE. The mean Qanadli score was 23% (SD 18%). Overall, 426/460 (93%) of the anatomical lung segments in the 23 patients were affected by COVID-19 and the mean percentage of affected lung was 58% (SD 22%). A total of 178/460 (39%) pulmonary artery segments were affected by PE; all pulmonary artery segments with PE were in lung parenchyma with radiological signs of COVID-19 pneumonia. The mean RV/LV ratio was 0.97 (SD 0.15) and the mean pulmonary arterty trunk diameter was 29 mm (SD 4.6 mm). Six patients had a RV/LV ratio > 1.0 (26%).
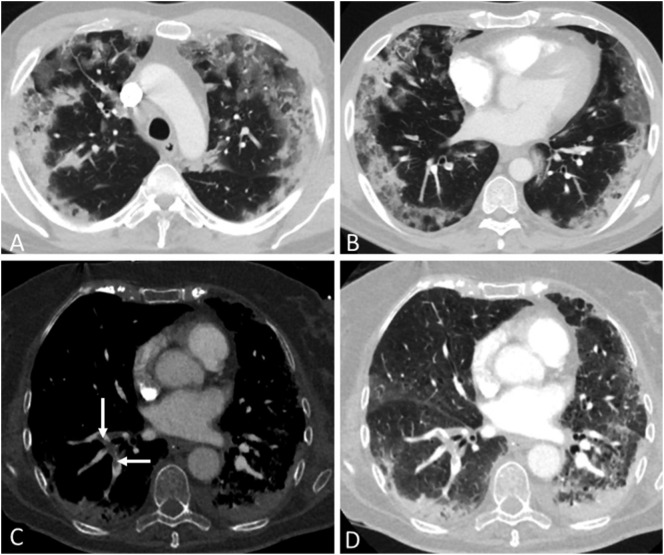
Fig. 1.
Computed tomography (CT) pulmonary angiography: A and B: 54-year old male patient with COVID-19 pneumonia, axial CT images in 3 mm reconstructions at upper and lower lung levels showing typical COVID-19 lesions with bilateral patchy ground-glass opacities and consolidations in predominantly peripheral distribution. The pulmonary involvement of COVID-19 lesions was 50% of lung volume. C and D: 69-year old female patient with COVID-19 pneumonia, axial CT images in 1 mm reconstructions. Soft-tissue setting showing thrombus in the right lower lobe segmental pulmonary arteries (arrows, C). Lung setting showing extensive pulmonary interstitial- and subpleural consolidations in both lungs (D), predominantly in the dependent areas but not related with presence (right lung) or absence (left lung) of visible pulmonary thrombus.
The control cohort consisted of 100 patients (Table 1). The location of the most proximal PE in the COVID-19 patients was less often the main/lobar pulmonary artery than in the control patients (17% versus 47%; −30%, 95%CI −44 to −8.2%). The Qanadli score was lower in the COVID-19 patients (mean difference −8%, 95%CI −16 to −0.13) as was the mean RV/LV ratio (mean difference −0.23, 95%CI −0.39 to −0.07) and the prevalence of RV/LV ratio >1.0 (26% versus 49%; −23%, 95%CI −41 to −0.86%). The mean pulmonary artery trunk diameter was comparable between cases and controls.
4. Discussion
Our findings suggest that the PE phenotype in patients with COVID-19 is different from PE patients without COVID-19 pneumonia. Specifically, in COVID-19 patients, the thrombotic lesions were more distributed in the peripheral arteries of the lung, total clot burden was lower and the mean RV/LV ratio and prevalence of RV/LV ratio > 1 were lower.
Differences between patients with COVID-19 associated PE and the control patients related to thrombosis risk (e.g. sex or BMI) may explain our observations. The relatively small sample size did not allow performing multivariate analyses to adjust for these differences. Nevertheless, sex and BMI have been shown not to be associated with clot burden or distribution in previous studies so we feel this explanation is less likely [20]. It is however possible that prophylactic anticoagulation, which was prescribed in all COVID-19 patients, and a shorter diagnostic delay may have influenced the radiological parameters of the PE. An alternative explanation is that in situ immunothrombosis indeed plays a role in the pathophysiology of COVID-19 associated PE. Alveolar injury and the inflammatory storm caused by COVID-19 pneumonia as well as disruption of the thrombo-protective state of the pulmonary vascular endothelial cells contribute to profound small vessel thrombus formation [15,16,21]. Even so, since 93% of lung segments was affected by infection, a ‘true’ association between location of the thrombotic and COVID-19 lesions could not be established, simply because unaffected segments were hardly present. The markedly higher D-dimer levels in COVID-19 patients with PE nonetheless underline the extreme coagulopathy and pro-thrombotic state associated with COVID-19 infection. The absence of clinical signs of deep vein thrombosis (DVT) in the COVID-19 patients may also support the concept of in situ immunothrombosis, although the prevalence of clinical signs of DVT was equally low in the control group. Importantly, the fact that the majority of PEs was located in the segmental arteries and 17% even centrally in the pulmonary artery tree is strongly compatible with the conventional thromboembolic origin of PE. This was also seen in previous publications in which COVID-19 associated PE was located in the central/lobar pulmonary artery in 44–56% of the cases [22,23]. Our findings are in line with histologic findings in autopsy studies were multiple thrombi in small to mid-sized pulmonary arteries were observed supporting the concept of immunothrombosis [21,24]. The observation by others that the incidence of DVT in COVID-19 patients was high upon screening also suggests that the conventional thromboembolic origin of PE plays indeed a role in COVID-19 associated PE [9,25]. Of note, increased prevalence of PE and not of DVT has been described before in pulmonary conditions such as COPD and pneumonia [26].
The main limitations of our study include the relatively small sample size and the lack of screening ultrasonograms of the leg veins. Furthermore, selection bias may have occurred as the threshold for PE screening may have been lower in COVID-19 patients than in the control group resulting in less extensive PE in the COVID-19 patients. Another limitation may be that blinded CT assessment by the radiologist was not possible as typical COVID-19 lesions can be seen in patients with COVID-19 infection.
In conclusion, our data suggest that the phenotype of PE in COVID-19 patients may be different from the PE phenotype in patients without COVID-19 pneumonia. Since pulmonary emboli in COVID-19 associated PE patients are more located in the peripheral lung segments and are less extensive compared to PE in patients without COVID-19, COVID-19 associated PE more likely represents a combination of thromboembolic disease and in situ immunothrombosis. Further investigations, including laboratory studies, are needed to explore the true causal relation between COVID-19 pneumonia and PE or in situ immunothrombosis. Exact knowledge of the origin of PE in COVID-19 patients has important therapeutic consequences, since the effect of (prophylactic) anticoagulation on in situ thrombosis is largely unknown.
Acknowledgments
Acknowledgements
None.
Author contributions
FAK, LJMK and LFvD designed the study and gathered data. They performed the analyses and drafted the first version of the manuscript. All authors revised the manuscript critically for important intellectual content and agreed with the final version.
Declaration of competing interest
Frederikus Klok reports research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, MSD and Actelion, the Dutch Heart Foundation and the Dutch Thrombosis Foundation, all outside the submitted work. Menno Huisman reports grants from ZonMW Dutch Healthcare Fund, and grants and personal fees from Boehringer-Ingelheim, Pfizer-BMS, Bayer Health Care, Aspen, Daiichi-Sankyo, all outside the submitted work. Jeroen Eikenboom reports grants from the Dutch Thrombosis Foundation, the Landsteiner Foundation for Blood Transfusion Research, and CSL Behring, all outside the submitted work. The other authors having nothing to disclose.
References
- 1.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;4:1–10. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. (https://doi.org/101016/jthromres202004024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erkens P.M., Gandara E., Wells P., Shen A.Y., Bose G., Le Gal G. Safety of outpatient treatment in acute pulmonary embolism. J. Thromb. Haemost. 2010;8(11):2412–2417. doi: 10.1111/j.1538-7836.2010.04041.x. [DOI] [PubMed] [Google Scholar]
- 11.Hendriks S.V., Huisman M.V., Eikenboom J.C.J., Fogteloo J., Gelderblom H., van der Meer F.J.M. Home treatment of patients with cancer-associated venous thromboembolism - an evaluation of daily practice. Thromb. Res. 2019;184:122–128. doi: 10.1016/j.thromres.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Ani F., Chehade S., Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb. Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. 2020. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series From New Orleans. (2020.04.06.20050575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huisman M.V., Barco S., Cannegieter S.C., Le Gal G., Konstantinides S.V., Reitsma P.H. Pulmonary embolism. Nat. Rev. Dis. Prim. 2018;4:18028. doi: 10.1038/nrdp.2018.28. [DOI] [PubMed] [Google Scholar]
- 18.van der Hulle T., Cheung W.Y., Kooij S., Beenen L.F.M., van Bemmel T., van Es J. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390(10091):289–297. doi: 10.1016/S0140-6736(17)30885-1. [DOI] [PubMed] [Google Scholar]
- 19.Qanadli S.D., El Hajjam M., Vieillard-Baron A., Joseph T., Mesurolle B., Oliva V.L. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am. J. Roentgenol. 2001;176(6):1415–1420. doi: 10.2214/ajr.176.6.1761415. [DOI] [PubMed] [Google Scholar]
- 20.El-Menyar A., Nabir S., Ahmed N., Asim M., Jabbour G., Al-Thani H. Diagnostic implications of computed tomography pulmonary angiography in patients with pulmonary embolism. Ann. Thorac. Med. 2016;11(4):269–276. doi: 10.4103/1817-1737.191868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poyiadji N., Cormier P., Patel P.Y., Hadied M.O., Bhargava P., Khanna K. Acute pulmonary embolism and COVID-19. Radiology. 2020;201955 doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;201561 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann. Intern. Med. 2020:20–2566. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020:20–2566. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Langevelde K., Flinterman L.E., van Hylckama Vlieg A., Rosendaal F.R., Cannegieter S.C. Broadening the factor V Leiden paradox: pulmonary embolism and deep-vein thrombosis as 2 sides of the spectrum. Blood. 2012;120(5):933–946. doi: 10.1182/blood-2012-02-407551. [DOI] [PubMed] [Google Scholar]