Abstract
SARS-CoV-2 or COVID-19 is representing the major global burden that implicated more than 4.7 million infected cases and 310 thousand deaths worldwide in less than 6 months. The prevalence of this pandemic disease is expected to rise every day. The challenge is to control its rapid spread meanwhile looking for a specific treatment to improve patient outcomes. Hesperidin is a classical herbal medicine used worldwide for a long time with an excellent safety profile. Hesperidin is a well-known herbal medication used as an antioxidant and anti-inflammatory agent. Available shreds of evidence support the promising use of hesperidin in prophylaxis and treatment of COVID 19. Herein, we discuss the possible prophylactic and treatment mechanisms of hesperidin based on previous and recent findings. Hesperidin can block coronavirus from entering host cells through ACE2 receptors which can prevent the infection. Anti-viral activity of hesperidin might constitute a treatment option for COVID-19 through improving host cellular immunity against infection and its good anti-inflammatory activity may help in controlling cytokine storm. Hesperidin mixture with diosmin co-administrated with heparin protect against venous thromboembolism which may prevent disease progression. Based on that, hesperidin might be used as a meaningful prophylactic agent and a promising adjuvant treatment option against SARS-CoV-2 infection.
Keywords: SARS-CoV-2, COVID-19, Hesperidin, Prophylaxis, Treatment, Viral entry, Anti-viral activity, Immunity
COVID-19 a public ongoing health disease
At the end of December 2019, pneumonia of unknown origin was detected in the hospitals of Wuhan city, China, and reported to the WHO country office for the first time [1], [2], [3]. After a few days, the Chinese government has confirmed the human-to-human transmission of the new infectious respiratory disease [4]. At the end of January 2020, the WHO declared the outbreak of severe acute respiratory syndrome (SARS), caused by a novel coronavirus (SARS-CoV-2), as an international public health emergency. The disease termed coronavirus 19 (COVID-19) rapidly transmitted from China to all over the world and subsequently the WHO declared it a global pandemic disease. The virulent virus structure is closely related to (SARS-CoV) strain with a single-stranded positive-sense RNA composition [5].
This pandemic disease is particularly of major importance to the whole world and especially to countries with a heavy population like Egypt. There is a critical need for emergent, continuous, and cost-effective health care delivery to infected people. Early detection and strategies for prevention of progression of COVID-19 would make a major difference for infected patients and would also be economically beneficial for a resource-constrained country.
People infected with COVID-19 may have no symptoms but still, act as a source of infection to other surrounding persons. The most common clinical manifestations following infection range from mild symptoms of (generalized fatigue, dry cough, low-grade fever, and sore throat) to severe symptoms of (typical severe acute respiratory distress syndrome (ARDS) and pneumonia) [6]. Although the tremendous scientific research effort is focusing mainly on the use of antiviral drugs, certain drug repurposing, and vaccine production for the treatment of COVID-19 patients, there is no specific cure or vaccine for treatment up till now. New drug development is a time-consuming process so that drug repositioning may be the optimum solution to control this pandemic infection.
Hesperidin
Hesperidin is a common flavone glycoside found in citrus fruit such as lemons and sweet oranges [7], [8]. Hesperidin has several pharmacological activities such as anti-atherogenic, antihyperlipidemic, antidiabetic, venotonic, cardioprotective, anti-inflammatory, and antihypertensive actions. The anti-inflammatory activity of hesperidin was mainly attributed to its antioxidant defense mechanism and suppression of pro-inflammatory cytokine production [7]. Hesperidin exhibited anti-viral activity against the influenza virus through a significant reduction of virus replication. Treatment of infected cells with hesperidin enhanced cell-autonomous immunity via activation and upregulation of p38 and JNK expression which is essential for cell defense mechanisms against influenza virus [9].
Hesperidin has been used as an herbal medicine for a long time. The safety of hesperidin was confirmed by FASEB (Federation of American Societies of Experimental Biology) upon request of the FDA. Toxicity studies have confirmed the high safety profile of hesperidin after oral intake. Results from oral toxicity studies showed the absence of adverse side effects after oral hesperidin ingestion of more than 2 g /kg [10].
Daflon 500 mg is a marketed tablet dosage form containing a micronized flavonoid mixture of 50 mg of hesperidin and 450 mg of diosmin which used as vasoprotective venotonic agent [10]. This hesperidin mixture is characterized by its high safety profile. Continues oral administration for hesperidin mixture to rats for 13 and 26 weeks, using a very high dose of 35-fold of the daily dosage showed no toxicity with a high LD50 value of more than 3 g/kg body weight. Clinical trials used more than 2850 patients treated with the hesperidin mixture for a period of 6 weeks to 1 year showed normal hematological parameters, hepatic and renal functions with no signs of toxicity [11].
Hesperidin role in prevention and treatment of COVID 19
Unraveling host cellular receptors used for cellular entry of COVID-19 will provide possible lines for attack. Cell entry of COVID-19 depends on two consecutive steps, firstly binding of the viral spike (S-protein) to host cellular receptors followed by priming of S-protein by cell proteases. Recently, researchers showed that COVID-19 uses the ACE-2 receptor for entry [12] and the serine protease TMPRSS2 for priming of S-protein. Camostat mesylate, a serine protease inhibitor drug blocked virus entry and was used as a COVID-19 treatment in Japan [13].
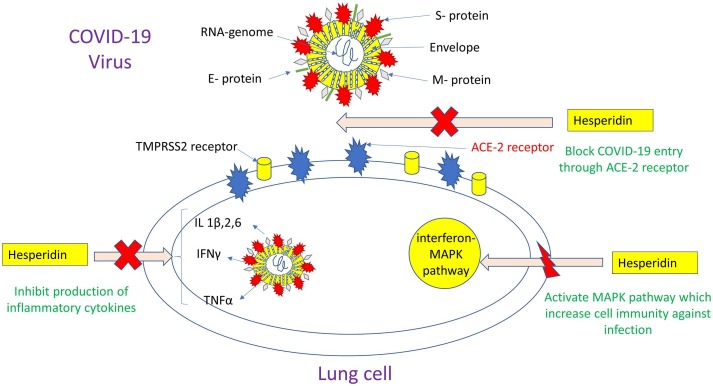
COVID-19 binds to the ACE-2 receptor through its specific Spike-receptor binding domain (RBD) sequence to form the SARS-CoV-2-RBD-ACE-2 complex. The proposed computational activity of 78 anti-viral drugs against the human ACE2 receptor was screened using homology modeling. This study showed that hesperidin is the only compound that could target the binding interface between SARS-CoV-2 Spike and ACE2 human receptors. Based on virtual screening, hesperidin may disrupt the interaction of ACE2 with RBD of SARS-CoV-2 thus block its entry into the lung cells (Fig. 1 ) [14]. Therefore, hesperidin can be used as a promising prophylactic agent against COVID-19 infection.
Fig. 1.
Effect of hesperidin on prophylaxis and treatment of COVID-19.
Host antiviral responses against COVID-19 infection depend on the activation of both the immune systems and cellular self-defense mechanisms. Immunity plays a major role in the protection of the host against viral infection. The occurrence of immune over-response or immune deficiency is responsible for the condition of infected patients becoming critical or severe [15]. The anti-viral activity of hesperidin against the influenza virus involves its role in the activation of the mitogen-activated protein kinase (MAPK) pathway. The MAPK host defense cascade contributes to efficiently restraining viral replication, spread, and minimizing tissue damage [9]. A recent study showed that the interferon-MAPK pathway played an important role in the COVID-19 immune response [15]. Therefore, hesperidin by its activation to host immunity my help against COVID-19 viral replication and hence its progression which will improve the patient outcome (Fig. 1).
Patients infected with COVID-19 exhibited what is called “cytokine storm” which initiated primarily as an inflammatory response and resulted in uncontrolled over-production of soluble markers of inflammation. Available evidence showing that cytokine storm, is a major cause for the development of ARDS. Cytokine storm involves the release of various immune-active molecules such as Interferons (e.g. IFNγ), interleukins (e.g. IL-1β, IL-2, IL-6), chemokines, and tumor necrosis factor-alpha (TNF-α) [16].
Hesperidin with its high anti-inflammatory activity inhibited the secretion of pro-inflammatory cytokines such as IFN-γ and IL-2 [17]. Besides, hesperidin inhibited IL‑1β‑stimulated inflammatory responses by inhibiting the activation of the NF‑κB signaling cascade [18]. It also played a major rule in suppressing the release of inflammatory markers such as (TNFα and IL-6) in type 2 diabetic patients [19]. Therefore, it can be used as adjuvant therapy to control the severe inflammatory reaction against COVID-19 (Fig. 1).
Activation of coagulation pathways following the immune response to COVID-19 infection promotes clot formation. The proposed mechanism of formation of micro thrombosis involves the occurrence of procoagulant–anticoagulant imbalance, platelet activation, and converting fibrinogen to fibrin. Disseminated intravascular coagulation predisposes to the development of multiorgan failure especially in severe infected cases [20].
A prophylactic dose of heparin (with low molecular weight, LMWH) is recommended for protection against venous thromboembolism in COVID-19 hospitalized patients [20]. In this context, it is essential to highlight the role of concomitant administration of hesperidin and diosmin mixture with heparin for protection against thromboembolism. Results from previous clinical trials that used Daflon 500 mg with LMWH confirmed the significant effect of this combination compared to LMWH alone in preventing the incidence of pulmonary embolism and deep vein thrombosis. Therefore, co-administration of LMWH and Daflon 500 mg can significantly inhibit clot formation and prevent disease progression [21].
Conclusions
Hesperidin is an old herbal medicine which has a long history of eating. Fortunately, it is a commonly available drug all over the world. Hesperidin used to treat vascular diseases in Europe and Australia and distributed with vitamin C as a dietary supplement in the USA. A drink powder of hesperidin was approved for health use in China and Japan. Hesperidin is a promising drug candidate for the prevention and treatment of COVID-19. To sum it up, hesperidin interferes with viral entry through ACE2 receptors, improves the host cellular immunity, minimizes the release of inflammatory mediators and its mixture protects against venous thromboembolism. We are planning to register a clinal trail on ClinicalTrials.gov to evaluate the clinical efficacy of hesperidin against COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. novel coronavirus in Wuhan China. Lancet. 2019;395(2020):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;382(2020):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2019;395(2020):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;104787 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mani Mishra P., Uversky V.N., Nandi C.K. Serum albumin-mediated strategy for the effective targeting of SARS-CoV-2. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanwar A.A., Badole S.L., Shende P.S., Hegde M.V., Bodhankar S.L. Chapter 76 – Cardiovascular effects of hesperidin: a flavanone glycoside. In: Watson R.R., Preedy V.R., Zibadi S., editors. Polyphenols in Human Health and Disease. Academic Press; San Diego: 2014. pp. 989–992. [Google Scholar]
- 8.Jadeja R.N., Devkar R.V. Chapter 47 – Polyphenols and flavonoids in controlling non-alcoholic steatohepatitis. In: Watson R.R., Preedy V.R., Zibadi S., editors. Polyphenols in Human Health and Disease. Academic Press; San Diego: 2014. pp. 615–623. [Google Scholar]
- 9.Dong W., Wei X., Zhang F., Hao J., Huang F., Zhang C. A dual character of flavonoids in influenza A virus replication and spread through modulating cell-autonomous immunity by MAPK signaling pathways. Sci Rep. 2014;4:7237. doi: 10.1038/srep07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagasako-Akazome Y. Chapter 58 - Safety of high and long-term intake of polyphenols. In: Watson R.R., Preedy V.R., Zibadi S., editors. Polyphenols in Human Health and Disease. Academic Press; San Diego: 2014. pp. 747–756. [Google Scholar]
- 11.Meyer O.C. Safety and security of daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology. 1994;45:579–584. doi: 10.1177/000331979404500614. [DOI] [PubMed] [Google Scholar]
- 12.Bao L., Deng W., Huang B., Gao H., Liu J., Ren L. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020 doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.L. Huang, Y. Shi, B. Gong, L. Jiang, X. Liu, J. Yang, J. Tang, C. You, Q. Jiang, B. Long, T. Zeng, M. Luo, F. Zeng, F. Zeng, S. Wang, X. Yang, Z. Yang, Blood single cell immune profiling reveals the interferon-MAPK pathway mediated adaptive immune response for COVID-19, medRxiv, (2020) 2020.2003.2015.20033472.
- 16.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The Cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao S., Liu W., Bi J., Liu S., Zhao H., Gong N. Anti-inflammatory effect of hesperidin enhances chondrogenesis of human mesenchymal stem cells for cartilage tissue repair. J. Inflamm. (London, England) 2018;15:14. doi: 10.1186/s12950-018-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Z., Chen Z., Xie Q., Lei H., Xiang S. Hesperidin protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes. Exp. Therap. Med. 2018;16:3721–3727. doi: 10.3892/etm.2018.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homayouni F., Haidari F., Hedayati M., Zakerkish M., Ahmadi K. Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes; a randomized double-blind controlled clinical trial. Phytotherapy Res. PTR. 2018;32:1073–1079. doi: 10.1002/ptr.6046. [DOI] [PubMed] [Google Scholar]
- 20.R.J. Jose, A. Manuel, COVID-19 cytokine storm: the interplay between inflammation and coagulation, The Lancet Respiratory Medicine, (2020). [DOI] [PMC free article] [PubMed]
- 21.Tsimoyiannis E.C., Floras G., Antoniou N., Papanikolaou N., Siakas P., Tassis A. Low-molecular-weight heparins and Daflon for prevention of postoperative thromboembolism. World J Surg. 1996;20:968–971. doi: 10.1007/s002689900145. discussion 972. [DOI] [PubMed] [Google Scholar]