Abstract
The Coronavirus Disease 2019 (COVID-19) is now a global pandemic with millions affected and millions more at risk for contracting the infection. The COVID-19 virus, SARS-CoV-2, affects multiple organ systems, especially the lungs and heart. Elevation of cardiac biomarkers, particularly high-sensitivity troponin and/or creatine kinase MB, is common in patients with COVID-19 infection. In our review of clinical analyses, we found that in 26 studies including 11,685 patients, the weighted pooled prevalence of acute myocardial injury was 20% (ranged from 5% to 38% depending on the criteria used). The plausible mechanisms of myocardial injury include, 1) hyperinflammation and cytokine storm mediated through pathologic T-cells and monocytes leading to myocarditis, 2) respiratory failure and hypoxemia resulting in damage to cardiac myocytes, 3) down regulation of ACE2 expression and subsequent protective signaling pathways in cardiac myocytes, 4) hypercoagulability and development of coronary microvascular thrombosis, 5) diffuse endothelial injury and ‘endotheliitis’ in several organs including the heart, and, 6) inflammation and/or stress causing coronary plaque rupture or supply-demand mismatch leading to myocardial ischemia/infarction. Cardiac biomarkers can be used to aid in diagnosis as well as risk stratification. In patients with elevated hs-troponin, clinical context is important and myocarditis as well as stress induced cardiomyopathy should be considered in the differential, along with type I and type II myocardial infarction. Irrespective of etiology, patients with acute myocardial injury should be prioritized for treatment. Clinical decisions including interventions should be individualized and carefully tailored after thorough review of risks/benefits. Given the complex interplay of SARS-CoV-2 with the cardiovascular system, further investigation into potential mechanisms is needed to guide effective therapies. Randomized trials are urgently needed to investigate treatment modalities to reduce the incidence and mortality associated with COVID-19 related acute myocardial injury.
Abbreviations and acronyms: ACE, Angiotensin converting enzyme; ACEI, Angiotensin converting enzyme inhibitor; ACS, Acute coronary syndrome; ARB, Angiotensin receptor blocker; AT, 1 angiotensin II receptor type 1; COVID, 19 Coronavirus disease 2019; CV, cardiovascular; HF, heart failure; IL, interleukin; MI, myocardial infarction; SARS, severe acute respiratory syndrome; STEMI, ST segment elevation myocardial infarction
Keywords: COVID-19, Myocardial injury, Prognosis, Biomarkers, Management
The Coronavirus Disease 2019 (COVID-19) is now a global pandemic with over five million confirmed cases and millions more at risk for contracting the infection. The virus shares close resemblance with SARS-CoV that caused the severe acute respiratory syndrome (SARS) epidemic of 2002–2003. The COVID-19 virus, SARS-CoV-2, affects multiple organ systems particularly the lungs and heart. The cardiac manifestations of the infection place an overwhelmed health care system under considerable stress due to the substantial resources and potential intensive care support required for these patients. In this concise review, we will focus on acute myocardial injury in COVID-19 infection, its prevalence, plausible pathophysiologic mechanisms, guidance on the use of cardiac biomarkers, and general management strategies.
Acute myocardial injury
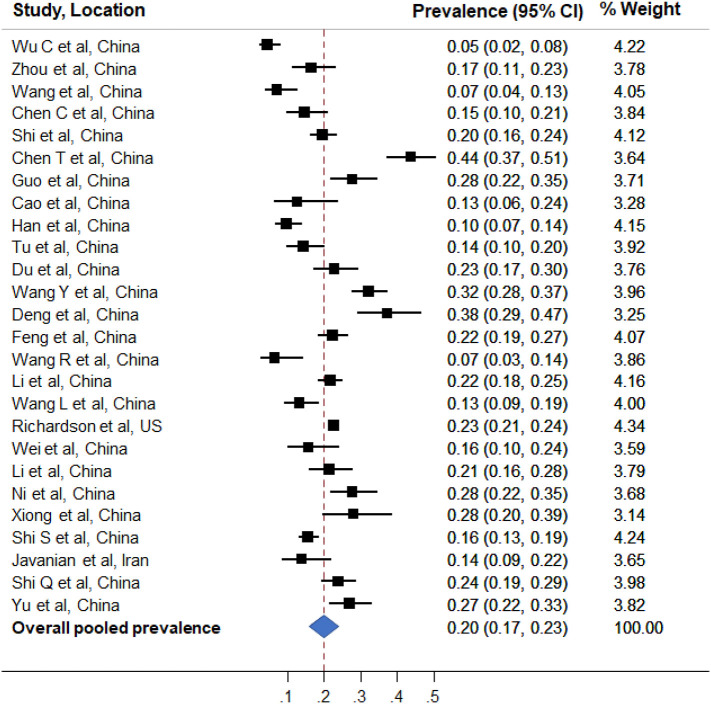
Elevation of cardiac biomarkers, particularly high-sensitivity cardiac troponin (hs-troponin) and/or creatinine kinase MB, is a marker of myocardial injury. Elevation of cardiac biomarkers is common in patients with COVID-19 infection. In our review of clinical studies with at least 100 COVID-19 patients (published until May 20th, 2020), we found that in 26 studies1., 2., 3., 4., 5., 6., 7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26. including 11,685 patients, the overall prevalence of acute myocardial injury ranged from 5% to 38% depending on the criteria used (Table 1 ). The overall crude prevalence of acute myocardial injury was 21.4% (1961/9164). Using meta-analytic approach,27 the overall weighted pooled prevalence estimate of acute myocardial injury was found to be 20% (95% confidence interval 17% to 23%) (Fig 1 ). In the study by Zhou et al.28 including 191 COVID-19 patients, 17% patients had elevated hs-troponin. One of the interesting findings from this study was that in non-survivors, hs- troponin increased rapidly from day 16 after disease onset, which coincided with other markers of inflammation, thrombosis and injury, such as interleukin (IL)-6, D-dimer, and lactate dehydrogenase. In another seminal study of 182 COVID-19 patients by Li et al.20 markers of cellular and immune dysregulation were found to be associated with myocardial injury. On multivariate adjusted analysis, age, WBC count, neutrophil percentage, lymphocyte percentage, CD3+ T cell counts, CD4+ T cell counts, CD8+ T cell counts, CD16 + CD56+ NK cell counts, hs-C-reactive protein, and procalcitonin were independently associated with myocardial injury in patients with COVID-19.
Table 1.
Select studies (with sample size ≥100 patients) reporting cardiac biomarkers and acute myocardial injury in patients hospitalized with confirmed COVID-19 infection.
| Study, publication date | Location | Study period | Patients | Age | Cardiovascular comorbidities | Acute myocardial injury, criteria and prevalence |
|---|---|---|---|---|---|---|
| Wang D et al.1, February 7 | Zhongnan Hospital, China | Jan 1 to 28, 2020 | 138 | 56 | HTN 31% DM 10% CVD 15% |
hs Troponin I > 28 pg/ml or new EKG/echo changes, 7.2% |
| Chen C et al.2, March 6 | Hankou Headquarters, Sino-French New City Campus and Optics Valley Campus of Tongji Hospital, China | Jan 2019 to Feb 2020 | 150 | 59 | HTN 33% DM 13% CVD 6% |
Troponin I > 26.3 ng/l, 15% |
| Zhou F et al.3, March 11 | Jinyintan Hospital and Wuhan Pulmonary Hospital, China | Dec 29, 2019 to Jan 31, 2020 | 191 (145) | 56 | HTN 30% DM 19% CVD 8% |
hs Troponin I > 28 pg/ml, 17% |
| Wu C et al.4, March 13 | JinYintan Hospital, China | Dec 25, 2019 to Jan 26, 2020 | 201 (198) | 51 | HTN 19% DM 11% CVD 4% |
Creatine Kinase MB > 24 U/l, 4.5% |
| Shi S et al.5, March 25 | Renmin Hospital, China | Jan 20 to Feb 10, 2020 | 416 | 64 | HTN 31% DM 14% CVD 16% |
hs Troponin I > 99th percentile μg/l, 19.7% |
| Chen T et al.6, March 26 | Tongji Hospital, China | Jan 13 to Feb 12, 2020 | 274 (203) | 62 | HTN 34% DM 17% CVD 8% |
Troponin I > 99th percentile or new EKG/echo changes, 44% |
| Guo T et al.7, March 27 | Seventh Hospital of Wuhan City, China | Jan 23 to Feb 23, 2020 | 187 | 59 | HTN 33% DM 15% CVD 16% |
Troponin T > 99-percentile ng/ml, 27.8% |
| Han et al.8, March 31 | Renmin Hospital, China | Jan 1 to Feb 18, 2020 | 273 | 58 | NR | hs Troponin I ≥ 0.04 ng/ml, 10% |
| Cao J et al.9, April 2 | Zhongnan Hospital, China | Jan 3 to Feb 1, 2020 | 102 (55) | 54 | HTN 28% DM 11% CVD 11% |
hs Troponin I > 26 pg/ml, 12.7% |
| Tu et al.10, April 6 | Zhongnan Hospital, China | Jan 3 to Feb 24, 2020 | 174 | 60 | HTN 21% DM 10% CVD 9% |
Troponin I > 99th percentile or new EKG/echo changes, 14.4% |
| Du et al.11, April 7 | Wuhan Pulmonary Hospital, China | Dec 25, 2019 to Feb 7, 2020 | 179 | 58 | HTN 32% DM 18% CVD 16% |
Troponin I ≥ 0.05 ng/ml, 22.9% |
| Wang Y et al.12, April 8 | Tongji hospital, China | Jan 25 to Feb 25, 2020 | 344 | 64 | HTN 41% DM 19% CVD 12% |
Elevated Troponin I or new EKG/echo changes, 32.3% |
| Deng et al.13, April 8 | Renmin Hospital, China | Jan 6 to Feb 20, 2020 | 112 | 65 | HTN 32% DM 17% CVD 13% |
Troponin I > 0.04 ng/ml, 37.5% Troponin I > 0.12 ng/ml, 28.6% |
| Feng et al.14, April 10 | Jinyintan Hospital in Wuhan, Shanghai Public Health Clinical Center in Shanghai and Tongling People's Hospital in Anhui Province, China | Jan 1 to Feb 15, 2020 | 476 (384) | 53 | HTN 24% DM 10% CVD 8% |
Troponin I > 0.04 ng/ml or troponin T > 28 pg/ml, 22.4% |
| Wang R et al.15, April 11 | No.2 People's Hospital of Fuyang City, China | Jan 20 to Feb 9, 2020 | 125 (76) | 39 | CVD 14% | Creatine Kinase MB > 24 U/l, 6.6% |
| Li et al.16, April 12 | Sino-French New City Branch of Tongji Hospital, China | Jan 26 to Feb 5, 2020 | 548 | 60 | HTN 30% DM 15% CVD 6% |
hs Troponin I > 15.6 pg/ml, 21.7% |
| Wang L et al.17, April 14 | People's Hospital of Wuhan University | Jan 31 to Feb 5, 2020 | 202 | 63 | HTN 30% DM 11% CVD 8% |
hs Troponin I > 0.04 ng/ml, 13.4% |
| Richardson et al.18, April 22 | 12 hospitals in New York City, Long Island, and Westchester County, US | March 1 to April 4, 2020 | 5700 (3533)⁎ | 63 | HTN 60% DM 11% CVD 7% |
Troponin I, T (including hs-troponin) above the upper limit of normal reference limit, 22.6% |
| Wei et al.19, April 30 | Public Health Clinical Centre of Chengdu and West China Hospital, Sichuan University, China | January 16 to March 10, 2020 | 101 | 49 | HTN 21% DM 14% CVD 5% |
hs Troponin T > 14 pg/ml, 16% |
| Li et al.20, May 7 | West China Hospital, and Disaster Medical Center, China | NR | 182 | 65 | NR | hs Troponin I > 0.04 ng/ml, 21.4% |
| Ni et al.21, May 8 | Central Hospital of Wuhan, China | Jan 28 to March 16, 2020 | 176 | 67 | HTN 49% DM 26% CVD 14% |
Troponin I > 99th percentile, 27.8% |
| Xiong et al.22, May 8 | Wuhan Hemodialysis Quality Control Center, China |
Jan 1 to March 10, 2020 | 131 (85)⁎ | 63 | HTN 26% DM 23% CVD 69% |
Elevated Troponin I or new EKG/echo changes, 28.2% |
| Shi S et al.23, May 11 | Renmin Hospital, China | Jan 1 to Feb 23, 2020 | 671 | 63 | HTN 30% DM 15% CVD 9% |
Troponin I > 99th percentile, 15.8% |
| Javanian et al.24, May 11 | Hospitals affiliated to Babol University of Medical Sciences, Iran | Feb 25 to March 12, 2020 | 100 | 60 | HTN 32% DM 37% CVD 20% |
Not specified, 14% |
| Shi Q et al.25, May 14 | Renmin Hospital of Wuhan University and Zhongnan Hospital, China |
January 1 to March 8, 2020 | 306 | 65 | HTN 43% DM 50% CVD 16% |
hs Troponin I > 99-percentile ng/ml, 23.9% |
| Yu et al.26, May 14 | 19 intensive care units of 16 hospitals in Wuhan, China | Feb 26 to 27, 2020 | 226 | 64 | HTN 43% DM 21% CVD 10% |
hs-TnI > 28 ng/l or TnI > 0.3 ng/ml, 27% |
Patients with cardiac biomarker data reported. Abbreviations: CVD: cardiovascular disease, DM: diabetes mellitus, EKG: electrocardiogram, HTN: hypertension, ICU: intensive care unit, NR: not reported.
Fig 1.
Forest plot of pooled analysis of prevalence of acute myocardial injury in hospitalized patients with COVID-19 infection.
Figure shows prevalence estimates of acute myocardial injury (boxes) with 95% confidence limits (bars) for each study selected; pooled prevalence estimate is represented by diamond in this forest plot.
It appears that the magnitude of elevation of cardiac troponin may be associated with severity of disease and prognosis.29 Shi et al.23 studied 671 patients with confirmed COVID-19, the prevalence of myocardial injury defined as hs-troponin I above the 99th percentile was 15.8%. Both, CK-MB >2.2 ng/ml (hazards ratio, 6.62, p < .001) and cardiac troponin I > 0.026 ng/ml (hazards ratio, 4.56, p = .02) were found to be independently associated with increased in-hospital mortality. In a prospective cohort study by Du et al11 of 179 patients with COVID-19 pneumonia, troponin I ≥ 0.05 ng/ml was independently associated with mortality in addition to age ≥ 65 years, pre-existing cardiovascular (CV) or cerebrovascular diseases and CD3 + CD8+ T-cells ≤75 cells/μl. Further large-scale prospective studies are needed to thoroughly investigate these findings.
Other CV manifestations in COVID-19
The proinflammatory milieu and increased sympathetic stimulation in COVID-19 can increase the risk for other CV complications, such as cardiac arrythmias, worsening of existing heart failure (HF), or development of new-onset HF. In patients with severe disease, hypoxia and electrolyte disturbances can further potentiate the risk for arrythmias. In a study1 by Wang and colleagues including 138 consecutive hospitalized patients with COVID-19, the incidence of arrythmia was found to be 17%. In a recent study by Goyal et al.,30 examining the clinical characteristics of first 393 consecutive patients with COVID-19 admitted in 2 hospitals in Manhattan, 7.4% of patients had a cardiac arrythmia during hospitalization. Although the type of arrythmia was not described in these reports, both tachy- and brady-arrythmias can occur. In another study7 of 187 COVID-19 patients by Guo et al., the overall incidence of ventricular tachycardia/fibrillation was 5.9% and was notably more common in patients with myocardial injury compared with those without (17.3% vs 1.4%, p < 0.001). In the study of 191 COVID-19 patients by Zhou et al.,3 HF was observed in 23.0% of patients, however, the etiology of heart failure was not reported. Acute myocardial injury, arrythmia, and HF can manifest either alone or can occur in combination based on the clinical course.
Pathophysiology of acute myocardial injury
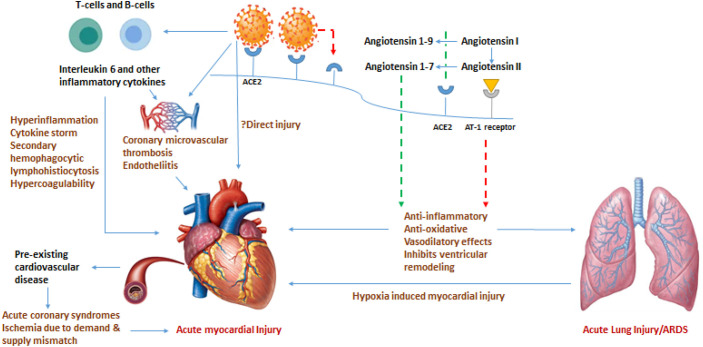
The COVID-19 virus (SARS-CoV-2), uses the angiotensin converting enzyme (ACE) 2 for entry into target cells. ACE2 is predominantly expressed by epithelial cells of the lung, intestine, kidney, heart, and blood vessels. While ACE cleaves angiotensin I to angiotensin II and leads to vasoconstrictive, pro-inflammatory, and pro-oxidative effects through the angiotensin II receptor type 1 (AT-1) receptor, ACE2 leads to anti-inflammatory, anti-oxidative and vasodilatory effects through the angiotensin 1-9-Mas receptor complex. The protective effect of ACE2 in lung is well defined, and therefore down regulation of ACE2 due to viral binding to this receptor plays a key role in acute lung injury and acute respiratory distress syndrome. Our understanding of the pathophysiology of COVID-19 and host immune responses is still evolving, however, immune-mediated inflammation plays a key role in the pathogenesis of COVID-19.31 , 32 On one hand, the innate and adaptive anti-viral immune response is vital in fighting the invading virus, on the other hand a robust and persistent anti-viral immune response may elicit an intense hyperinflammatory response akin to cytokine storm and cause damage to the host cells (Fig 2 ). Acute myocardial injury is typically seen in advanced stages of disease and is associated with worse prognosis. The mechanism of myocardial injury in COVID-19 is not well understood. The plausible mechanisms of myocardial injury include (Fig 3 ): 1) hyperinflammation and cytokine storm mediated through pathologic T cells and monocytes leading to myocarditis,33 2) respiratory failure and hypoxemia resulting in damage to cardiac myocytes,34 3) down regulation of ACE2 expression and subsequent protective signaling pathways in cardiac myocytes, 4) hypercoagulability and development of coronary microvascular thrombosis,35 5) diffuse endothelial injury and ‘endotheliitis’ in several organs, including the heart as a direct consequence of SARS-CoV-2 viral involvement and/or resulting from host inflammatory response,36 and, 6) inflammation and/or stress causing coronary plaque rupture or supply-demand mismatch leading to myocardial ischemia/infarction (MI). In the study by Oudit et al.37 the SARS-CoV viral RNA was detected in 35% (7/20) of autopsied human heart samples obtained from patients who died during the SARS outbreak. Due to presence of ACE2 receptors on cardiac myocytes, direct infiltration of cardiac myocardium by SARS-CoV-2 virus is also a possibility.38
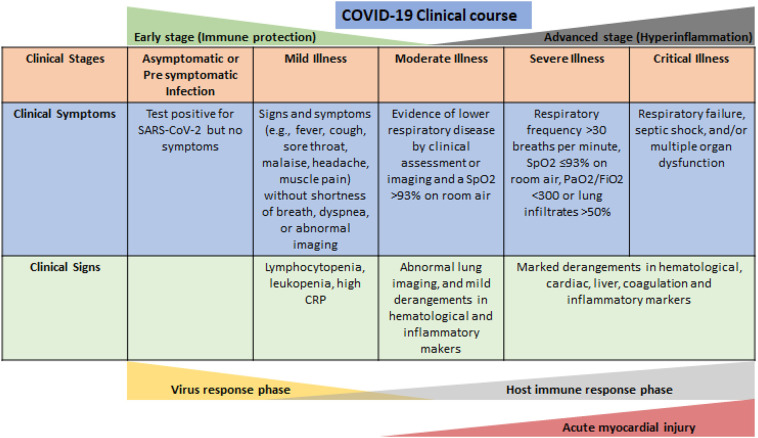
Fig 2.
Clinical stages of COVID-19 infection and proposed pathophysiological changes. Clinical stages are based on National Institute of Health treatment guidelines.31 Acute myocardial injury is typically seen in advanced stages of disease and is associated with worse prognosis.
Fig 3.
Schematic diagram on possible pathophysiological mechanisms of acute myocardial injury in COVID-19 infection. Abbreviations: ACE: angiotensin converting enzyme, ARDS: acute respiratory distress syndrome. Green broken lines represent positive effect, red broken lines represent negative effect. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Cardiac biomarkers in COVID-19 infection
Acute myocardial injury in COVID-19 can range from asymptomatic elevation of cardiac troponins to fulminant myocarditis and circulatory shock. The Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition) recommends to evaluate myocardial enzymes in patients admitted with COVID-19 infection.39 Similarly, the World Health Organization document40 - Clinical management of severe acute respiratory infection when COVID-19 disease is suspected (version 1.2, March 13, 2020) also recommends laboratory testing for acute myocardial injury at admission and as clinically indicated. The American College of Cardiology states ‘to only measure troponin if the diagnosis of acute MI is being considered on clinical grounds’.41 Guidelines from various professional societies39., 40., 41. and expert groups42 , 43 on testing of cardiac biomarkers in patients hospitalized with COVID-19 is presented in Table 2 . Treatment protocols on in-patient management of COVID-19 from several US hospitals have included hs-troponin as a part of the routine laboratory assessment.44 , 45 In patients with elevated hs-troponin, clinical context is important and myocarditis as well as stress induced cardiomyopathy should be considered in the differential, along with type I and type II MI. Evaluation of NT-proBNP should be considered if clinically indicated. COVID-19 induced myocarditis has been reported in case reports from China and elsewhere that usually requires aggressive management.46., 47., 48., 49. New onset cardiomyopathy and arrythmias with elevated troponins should raise the suspicion of myocarditis. The initial diagnostic modality includes echocardiography and right and left cardiac catheterization with placement of a pulmonary artery catheter for continuous hemodynamic monitoring in critically ill shock patients. Cardiac MRI and endomyocardial biopsy are more definite tests. However, in the face of COVID-19 pandemic, when resources are limited and one of the primary goals is to minimize health care personnel's exposure, imaging modalities may not be readily available. Conceivably, cardiac biomarkers can be used to aid in diagnosis as well as risk stratification.
Table 2.
Recommendations on testing of cardiac biomarkers in patients hospitalized with COVID-19 infection.
| Recommendation | |
|---|---|
| Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition) | In admitted patients |
| World Health Organization document - Clinical management of severe acute respiratory infection when COVID-19 disease is suspected (version 1.2, March 13, 2020) | At admission and as clinically indicated |
| American College of Cardiology | Only if clinically indicated |
| Handbook of COVID-19 Prevention and Treatment, The First Affiliated Hospital, Zhejiang University School of Medicine | In admitted patients |
| Asian Critical Care Clinical Trials Group | In admitted patients |
| BMJ Best Practice | In patients with severe illness |
General management strategies
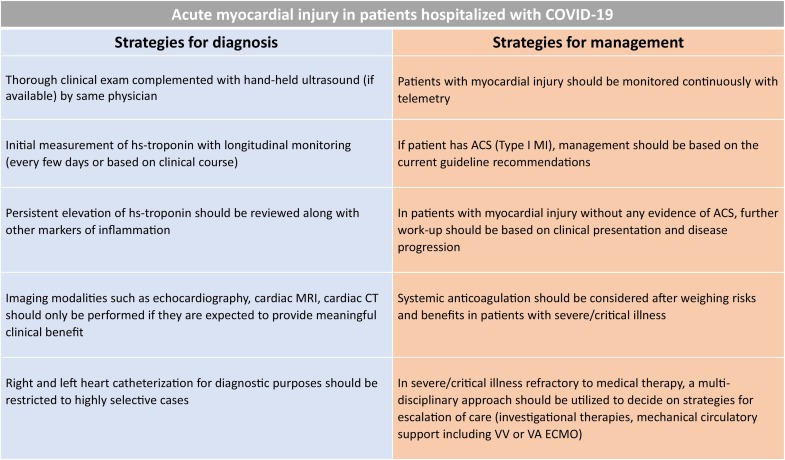
The COVID-19 pandemic poses unique challenges in terms of availability of appropriate personal protective equipment (PPE) and utilization of health care personnel and resources without compromising patient care. Although respiratory illness is the dominant clinical manifestation of COVID-19 infection, for severe and critically ill patients in addition to treatment focused on respiratory support, evaluation of multiorgan failure and management is vital. In a multicenter study50 analyzing 68 fatal cases of COVID-19, myocardial injury with or without respiratory failure was noted to be the cause of death in 40% of cases. Bedside clinical exam complemented with hand-held ultrasound, if available, by the same physician can help diagnose early signs of cardiac and respiratory decompensation and can potentially reduce further downstream testing. Initial measurement of hs-troponin at the time of hospitalization with longitudinal monitoring (every few days or based on clinical course) during the hospital stay may help identify a subset of patients with evidence of acute myocardial injury and worse prognosis. Echocardiography and other imaging modalities including cardiac magnetic resonance imaging and cardiac computed tomography should only be performed if they are expected to provide meaningful clinical benefit. Patients with acute myocardial injury may need to be prioritized for treatment and require continuous monitoring with telemetry.
Patients with risk factors or existing CV disease have a heightened risk of developing an acute coronary syndrome (ACS) during acute infections, including viral illnesses and other acute inflammatory conditions. Patients with ACS should be managed based on the current guideline recommendations. However, in the face of an ongoing major public health crisis, treatment protocols may be adjusted based on clinical acuity and local resource availability.51 The Society for Cardiovascular Angiography and Interventions (SCAI) have put forward useful recommendations for triage and management strategies of these patients.52 SCAI and other expert groups52 , 53 recommend to consider fibrinolytic therapy in select patients with ‘low-risk’ ST-elevation MI (STEMI). However, it is important to note, that patients with COVID-19 infection and STEMI may not have thrombotic or occlusive coronary artery as the cause of STEMI.54 Clinical decisions including interventions should be individualized and carefully tailored based on thorough review of risks/benefits. In patients who require interventional therapies, all health care personnel involved should follow best practices for the use of PPE. In patients where the pre-test probability of an acute coronary event is low, elevated troponins are usually a marker of systemic critical illness. Persistent elevation of hs-troponin should be reviewed in conjunction with other markers of inflammation such as serum ferritin, IL-6, liver enzymes, coagulation panel and treatment should be intensified to address the underlying etiology. Based on the Chinese experience and recommendations, consideration should be given to start systemic anticoagulation after weighing risks and benefits, due to high prevalence of hypercoagulability in COVID-19 patients, with several reports of deep vein thrombosis, pulmonary embolism, and autopsy-confirmed coronary microvascular thrombosis leading to STEMI.55 Several anti-viral and anti-inflammatory agents are used/under investigation in patients with COVID-19, however, conclusive evidence regarding their efficacy and safety is awaited.56 The combination of hydroxychloroquine with azithromycin has been advocated as one of the potential treatment options based on the antiviral properties as well as immunomodulatory effects and initial reports of efficacy in small studies.57 , 58 However, subsequent studies have shown that treatment with hydroxychloroquine, azithromycin, or both, was not associated with improved survival, and may be associated with increased risk for ventricular arrythmias in patients hospitalized with COVID-19.59 Remdesivir, an inhibitor of the viral RNA with potent inhibitory activity against SARS-CoV-2 virus, has shown encouraging results. In the ACCT-1 double-blind, randomized, placebo-controlled trial,60 intravenous remdesivir was superior to placebo in shortening the time to recovery in adults hospitalized with COVID-19 and evidence of lower respiratory tract infection. The 14 -day mortality was 7.1% with remdesivir and 11.9% with placebo (hazard ratio 0.70; 95% CI, 0.47 to 1.04) suggesting that remdesivir may improve survival in patients hospitalized with COVID-19. Clinicians should be aware of drug-drug interactions since many of these investigational agents are known to interfere with cardiac medications. In patients with severe or critical illness who are refractory to medical therapy, a multidisciplinary approach should be used to decide on strategies for escalation of care (investigational therapies, mechanical circulatory support including veno-venous or veno-arterial extracorporeal membrane oxygenation). A summary of these general management recommendations in presented in Fig 4 . Patients with acute myocardial injury who are managed conservatively and those who recover from COVID-19 infection, should be followed closely to ensure adherence to guideline directed medical therapy. At the earliest time feasible, every effort should be made to ensure that these patients get appropriate work-up including imaging and/or invasive testing, if not performed during the hospitalization.61
Fig. 4.
General management strategies for management of acute myocardial injury in patients hospitalized with COVID-19 infection. ACS: acute coronary syndrome, CT: computerized tomography, ECMO: extracorporeal membrane oxygenation, MI: myocardial infarction, MRI: magnetic resonance imaging, VA: veno-arterial, VV: veno-venous.
The use of ACE inhibitors (ACEI) and angiotensin receptor blockers (ARB) in patients with COVID-19 infection is a matter of considerable debate.62 The SARS-CoV-2 virus binds to the ACE2 receptor to gain entry into the host cells. Both ACEI/ARBs upregulate expression of ACE2 in various tissues, including cardiomyocytes, and some experts think that this can potentially increase the risk of developing or worsening COVID-19 infection. However, to date, no clinical data have emerged to support these concerns. At the same time, the risks of discontinuing these therapies are well known. Several leading professional societies recommend continuing ACEI/ARBs in patients with COVID-19 infection unless stopping is clinically indicated. In a small retrospective study63 of 51 patients with COVID-19 and hypertension, patients taking ACEI/ARBs had lower levels of IL-6 and peak viral load compared to those taking other anti-hypertensives. Recently, several observational studies64 , 65 have been published that showed no association with the use of ACEI/ARBs and increased susceptibility for COVID-19 infection as well as risk for mortality or severe disease in patients with COVID-19. As in any retrospective analysis, the findings from these studies are limited by residual confounding however, it is unlikely that use of ACEI/ARBs is detrimental in patients with COVID-19 and it is possible that ACEI/ARBs may exert a protective effect on pulmonary outcome of viral pneumonia.66 Currently, several prospective multicenter studies and randomized controlled trials evaluating the role of ACEI/ARBs in COVID-19 patients are underway.
Given the complex interplay of SARS-CoV-2 with the CV system, further investigation into potential mechanisms is needed to guide effective therapies. Epidemiological studies and randomized trials are urgently needed to investigate treatment modalities regulating immune function and inhibiting inflammatory responses to reduce the incidence and mortality associated with COVID-19 related acute myocardial injury.
Funding
None.
All authors had access to the data and a role in writing the manuscript.
Statement of conflict of interest
Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda.
References
- 1.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han H., Xie L., Liu R. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92(7):819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao J., Tu W.J., Cheng W. Clinical features and Short-term outcomes of 102 patients with Corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu W.J., Cao J., Yu L., Hu X., Liu Q. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med. 2020;46(6):1117–1120. doi: 10.1007/s00134-020-06023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du R.H., Liang L.R., Yang C.Q. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Lu X., Chen H. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201(11):1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Q., Hu B., Zhang Y. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y., Ling Y., Bai T. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R., Pan M., Zhang X. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421–428. doi: 10.1016/j.ijid.2020.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. S0091-6749(20)30495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L., He W.B., Yu X.M., Liu H.F., Zhou W.J., Jiang H. Prognostic value of myocardial injury in patients with COVID-19. Zhonghua Yan Ke Za Zhi. 2020;56:E009. doi: 10.3760/cma.j.cn112148-20200313-00202. [DOI] [PubMed] [Google Scholar]
- 18.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J.F., Huang F.Y., Xiong T.Y. Acute myocardial injury is common in patients with covid-19 and impairs their prognosis. Heart. 2020 doi: 10.1136/heartjnl-2020-317007. heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D., Chen Y., Jia Y. SARS-CoV-2-induced immune dysregulation and myocardial injury risk in China: insights from the ERS-COVID-19 study. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317070. [DOI] [PubMed] [Google Scholar]
- 21.Ni W., Yang X., Liu J. Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.007. S0735-1097(20)35225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong F., Tang H., Liu L. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020 doi: 10.1681/ASN.2020030354. ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S., Qin M., Cai Y. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javanian M., Bayani M., Shokri M. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol north of Iran: a retrospective cohort study. Rom J Intern Med. 2020 doi: 10.2478/rjim-2020-0013. /j/rjim.ahead-of-print/rjim-2020-0013/rjim-2020-0013.xml. [DOI] [PubMed] [Google Scholar]
- 25.Shi Q., Zhang X., Jiang F. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y., Xu D., Fu S. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 28.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of Coronavirus Disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 30.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed May 22, 2020. [PubMed]
- 32.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y., Fu B., Zheng X. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. March 13, 2020:nwaa041. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubasiak L.A., Hernandez O.M., Bishopric N.H., Webster K.A. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han H., Yang L., Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 36.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. April 20, 2020 doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.http://kjfy.meetingchina.org/msite/news/show/cn/3337.html
- 40.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 41.https://www.acc.org/latest-in-cardiology/articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid19
- 42.Phua J., Weng L., Ling L. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.https://bestpractice.bmj.com/topics/en-gb/3000168/investigations
- 44.Brigham and Women's Hospital COVID-19 critical care clinical guidelines. http://www.covidprotocols.org/
- 45.Massachusetts General Hospital COVID-19 treatment guidance. Version 1.351. https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/MGH%20ID%20COVID19%20Here%20and%20Now%20Treatment%20Guidance%20V1.351%2003272020.pdf
- 46.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. March 27, 2020 doi: 10.1001/jamacardio.2020.1096. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim I.C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41(19):1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41(19):1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X., Bhatt D.L. COVID-19: an unintended force for medical revolution? J Invasive Cardiol. 2020;32:E81–E82. [PubMed] [Google Scholar]
- 52.Szerlip M., Anwaruddin S., Aronow H.D. Considerations for cardiac catheterization laboratory procedures during the COVID-19 pandemic perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM) members and graduates. Catheter Cardiovasc Interv. 2020 doi: 10.1002/ccd.28887. [DOI] [PubMed] [Google Scholar]
- 53.Daniels M.J., Cohen M.G., Bavry A.A., Kumbhani D.J. Reperfusion of STEMI in the COVID-19 era - business as usual? Circulation. 2020;141(24):1948–1950. doi: 10.1161/CIRCULATIONAHA.120.047122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9:687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 57.Chen J., Liu D., Liu L. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beigel J.H., Tomashek K.M., Dodd L.E. ACTT-1 study group members. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. May 22. [DOI] [PubMed] [Google Scholar]
- 61.Bhatt A.S., Moscone A., McElrath E.E. Declines in hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic: a multicenter tertiary care experience. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bavishi C., Maddox T.M., Messerli F.H. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1282. [DOI] [PubMed] [Google Scholar]
- 63.Meng J., Xiao G., Zhang J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehta N., Kalra A., Nowacki A.S. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Messerli F.H., Siontis G.C.M., Rexhaj E. COVID-19 and renin angiotensin blockers: current evidence and recommendations. Circulation. 2020;141(25):2042–2044. doi: 10.1161/CIRCULATIONAHA.120.047022. [DOI] [PMC free article] [PubMed] [Google Scholar]