Summary
Around the world, recommendations for cancer treatment are being adapted in real time in response to the pandemic of COVID-19. We, as a multidisciplinary team, reviewed the standard management options, according to the Barcelona Clinic Liver Cancer classification system, for hepatocellular carcinoma. We propose treatment recommendations related to COVID-19 for the different stages of hepatocellular carcinoma (ie, 0, A, B, and C), specifically in relation to surgery, locoregional therapies, and systemic therapy. We suggest potential strategies to modify risk during the pandemic and aid multidisciplinary treatment decision making. We also review the multidisciplinary management of intrahepatic cholangiocarcinoma as a potentially curable and incurable diagnosis in the setting of COVID-19.
Introduction
Since early 2020, global recommendations for cancer treatment have been adapted in real time in response to the COVID-19 pandemic. Cancer continues to result in a substantial number of deaths on average per day;1 thus, maintaining cancer treatment while minimising the risk of exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) must be balanced carefully. Multidisciplinary cancer collaborations are occurring worldwide,2, 3, 4, 5, 6, 7, 8, 9 with the shared goal of developing short-term to medium-term treatment strategies to circumvent procedural, staffing, and resource shortages, while limiting the potential negative outcomes for patients10 and staff.11 Therefore, treatment strategies must align with region-specific resource limitations. Currently, many centres are at different timepoints along the pandemic curve (eg, rises, peaks or surges, declines, and second waves), with each timepoint presenting its own specific challenges. During the rise, departments prepare for the peak stage by reducing cancer care services and treatments and planning for potential staff shortages due to illness and redeployment. As the pandemic peaks and surges, priority might be given to cancer emergencies, such as spinal cord compression, and patients for whom cancer therapy is likely to be curative and therefore the benefits of treatment outweigh the possible risk of SARS-CoV-2 infection and the use of the health-care system's resources. As the decline of the pandemic begins for many centres, the return of routine diagnostics will result in an increase in new cancer diagnoses and the return of existing patients with cancer whose treatments might have been deferred. The decline phase might also be a challenging time for many departments who are continuing to manage ongoing staff shortages and the risk of SARS-CoV-2 infection, in addition to anticipating a potential second wave. As we navigate these difficult times, the point at which each centre is located along this curve and the resources available must be considered when deciding on the most appropriate treatments for patients with cancer. However, considering the downstream implications of treatment choices is also an imperative. To guide our own departments and other cancer centres and clinics, multidisciplinary representatives from academic hospitals in multiple countries at different stages of the COVID-19 pandemic, including some hospitals in the epicentres of the pandemic, have collaborated to synthesise these recommendations on the safe and effective management of patients with primary hepatic malignancies during the pandemic.
Methods
This Rapid Review was done as part of an international collaborative effort to combine and develop guidelines for the management of patients with liver cancer during the COVID-19 pandemic. This collaboration consisted of 19 multidisciplinary liver specialists from high-volume liver malignancy academic centres in seven countries (ie, Canada, the USA, the UK, Italy, Australia, South Korea, and Chile) and five continents (ie, North America, Europe, Australia, Asia, and South America) at different stages of the pandemic. A Delphi-type methodology was not feasible because of the timeliness of response that was needed.12 Instead, institutional guidelines from the authors' centres were used to form the basis of the collaborative recommendations. We rapidly reviewed the published literature and comprehensively searched professional society guidelines, including recommendations related to COVID-19 and patients with liver cancer (appendix), to ensure that the evidence and recommendations incorporated into the initial draft of the manuscript were up to date.3, 4, 5, 6, 13, 14 Each specialist was invited to provide their opinions and recommendations regarding hepatocellular carcinoma, staged by the Barcelona Clinic Liver Cancer (BCLC)15 classification system as either 0, A, B, or C, and intrahepatic cholangiocarcinoma, whether potentially curable or incurable. Once all opinions were collected, specialists were invited to provide individual feedback at least four more times, which was incorporated into this Rapid Review.
Recommendations
The management of primary intrahepatic malignancies, such as hepatocellular carcinoma and intrahepatic cholangiocarcinoma, requires a multidisciplinary approach involving hepatology, surgical oncology, transplant surgery, medical oncology, diagnostic and interventional radiology, and radiation oncology. Worldwide, primary hepatic malignancies are the fourth leading cause of deaths from cancer.16 Tumour burden (eg, size, location, number of lesions, and vascular invasion) and patient factors (eg, age, underlying liver disease, liver function, and portal hypertension) are taken into consideration when clinicians decide on appropriate treatment pathways for primary hepatic malignancies.
Each discipline involved in this treatment pathway is uniquely and adversely affected by the COVID-19 pandemic. In addition to the effect on cancer services, including access to the operating room, chemotherapy day care, radiation therapy, diagnostic imaging, staffing, and shortages of personal protective equipment, patients with cancer are undergoing rigorous, individual risk–benefit assessments of their treatment options with the scarce and rapidly changing data available on the effect of COVID-19. Despite the paucity of evidence-based data, it has become routine to discuss with patients the risk of infection with SARS-CoV-217 because the combination of cancer diagnoses and comorbidities (eg, underlying liver disease, diabetes,18 cardiovascular disease, and immunosuppressive states) might result in a possible increase in the morbidity and mortality related to COVID-19.10 Liver disease is commonly associated with comorbidities; therefore, these patients are particularly susceptible.19 Published data have shown that patients with comorbidities, such as diabetes, a high body-mass index (eg, >30 kg/m2), and malignancies, are at an increased risk for admission to intensive care units, the need for mechanical ventilatory support, and death if infected with SARS-CoV-2.20, 21, 22 Notably, chronic viral hepatitis has not been shown to be a particular risk factor for negative outcomes after SARS-CoV-2 infection;23 however, patients with non-alcoholic fatty liver disease might have hypertension, diabetes, and obesity, and are probably at an increased risk of negative outcomes.5 Recipients of solid organ transplants might be at an increased risk of developing severe SARS-CoV-2 infections,24 or secondary bacterial coinfections, or both, and might see higher rates of complications and mortality due to COVID-19 compared with immunocompetent patients.25, 26 However, transplant recipients do not seem to require a reduction in immunosuppression to protect against complications from SARS-CoV-2 infection.5
Many of the treatment options available for intrahepatic malignancies involve invasive procedures. Screening guidelines for COVID-19 among the institutions involved in this Rapid Review vary; however, all patients with concerning symptoms and risk factors for COVID-19 are being screened. Additionally, all patients have a nasopharyngeal swab tested for SARS-CoV-2 24–72 h before planned surgery. Surgeons in some institutions are only operating on patients who are negative for SARS-CoV-2, and when patients are positive for SARS-CoV-2, after delays of a minimum of 7–14 days, they require at least one negative swab for SARS-CoV-2 before surgery. In many centres, asymptomatic patients undergoing interventional radiology procedures will have SARS-CoV-2 testing done, if they require general anaesthesia, or even if they require conscious sedation, before having locoregional therapies to mitigate the risk of exposure to staff. In most chemotherapy and radiotherapy departments, all patients are screened and swabbed before treatment. For patients who test positive for SARS-CoV-2, continuation or initiation of chemotherapy or radiotherapy should be considered if required for the urgent control of the cancer, or, when possible, deferred until the patient has had at least two tests negative for SARS-CoV-2.27 Regardless of the variability in testing for SARS-CoV-2, all institutions had individual recommendations. As the COVID-19 pandemic continues to evolve, we recommend that all centres routinely review their own policies on the basis of the rapidly changing data on SARS-CoV-2 infection and the availability of personal protective equipment.
For many patients, the COVID-19 pandemic has also affected the availability of clinical trials. Patients should continue to be reassessed and considered for trials when trials are available; modifications to clinical trial assessments and follow-up procedures might be required to allow for physical distancing when possible (eg, telemedicine follow-up visits).
Treatment of BCLC 0 and BCLC A stage hepatocellular carcinoma during the COVID-19 pandemic
Surgery
For patients with hepatocellular carcinoma, we have made our recommendations on the basis of the commonly used BCLC classification system (table 1 , figure ).15 Surgical intervention (ie, resection and transplantation) for hepatocellular carcinoma confined to the liver is associated with the best outcomes, is the most probable chance for a cure, and, in general, is the treatment of choice for well selected patients.28, 29, 30, 31 Liver transplants and hepatobiliary surgical programmes require substantial resources to provide presurgical assessments and care, to do the surgeries, and to provide care after surgery, including the supply of personal protective equipment. During the COVID-19 pandemic, these essential cancer care services have been substantially affected, with reduced (or suspended) transplant activity at most institutions and scarce operating room resources for liver-related surgeries. These reductions are mainly to facilitate staff redeployment to areas of more acute need, such as intensive care units and so-called COVID wards, and to ensure the increased access and availability of much needed intensive care beds. We should, however, continue to advocate for surgery in selected patients who are most likely to be cured, when resources and personnel are available, and when aligned with the overall priorities of the cancer centre. This recommendation is particularly relevant in the setting of smaller, unifocal disease that is amenable to straightforward resection with the anticipation that intensive care units will hardly be used. The surgeon should select patients for whom they have no preoperative expectation of prolonged inpatient hospitalisation, requirement of blood transfusion, or prolonged admission to intensive care units, because ventilators and blood products are already in short supply in many areas.32 For centres with experience of minimally invasive hepatectomy, the use of laparoscopic or robotic approaches to achieve these goals can be considered,33 but must be balanced against the theoretical aerosol dissemination of SARS-CoV-2 to staff. Preoperatively screening patients for SARS-CoV-2 and universal personal protective equipment might mitigate this risk. Postoperative follow-up should use telemedicine options as allowable by local regulatory bodies. As resources become more scarce, surgery might not be an immediate option for many patients with primary hepatic malignancies in many regions during the COVID-19 pandemic. Regardless, we would encourage patients to be referred to tertiary liver cancer centres to allow a process of treatment optimisation based on expert multidisciplinary rounds, an awareness of the best evidence-based care available during the COVID-19 pandemic, and the ability to provide patient consultations via telehealth.
Table 1.
Recommendations for the treatment of hepatocellular carcinoma during the COVID-19 pandemic by the BCLC classification system
| Standard-of-care treatment recommendations before the COVID-19 pandemic | Proposed treatment recommendations during the COVID-19 pandemic* | |
|---|---|---|
| BCLC 0 or BCLC A | Liver transplant (with a cadaveric or living donor); surgical resection; locoregional ablation | If a liver transplant or surgical resection is unavailable, consider bridging with locoregional therapies (eg, radiofrequency ablation, microwave ablation, stereotactic body radiotherapy, proton beam therapy, TACE, or TARE); if surgical resection is unavailable, consider surveillance†; consider locoregional ablation with radiofrequency ablation, microwave ablation, stereotactic body radiotherapy, proton beam therapy, TACE, or TARE (by use of a same-day model that does not use technetium-99-labelled macroaggregated albumin), and surveillance† |
| BCLC B | Locoregional therapies (eg, TACE, TAE, or TARE); liver transplant if within the transplant criteria of the institution | Consider (1) locoregional therapies (eg, TACE, TAE, or TARE); (2) radiotherapy (eg, stereotactic body radiotherapy, proton beam therapy, or systemic radiotherapy); and (3) surveillance† |
| BCLC C | If the patient has portal vein thrombosis and no extrahepatic disease, use systemic therapy or a combination of TACE and radiotherapy (45 Gy in 15 fractions); if the patient has extrahepatic disease, use systemic therapy | Consider (1) systemic therapy; (2) a combination of TACE and radiotherapy (45 Gy in 15 fractions); (3) for patients with hepatocellular carcinoma and portal vein thrombosis, stereotactic body radiotherapy; (4) for patients with hepatocellular carcinoma and portal vein thrombosis, TARE; (5) best supportive care; and (6) palliative radiotherapy in a single 8 Gy fraction for symptomatic disease (whether local or metastatic) |
Recommendations are presented in the order that they should be considered. BCLC=Barcelona Clinic Liver Cancer. TACE=transarterial chemoembolisation. TAE=transarterial embolisation. TARE=transarterial radioembolisation.
These recommendations are to be considered when standard therapies are not available.
Surveillance involves blood tests every 1–3 months (eg, for α-fetoprotein in secreting tumours) and diagnostic imaging every 3 months.
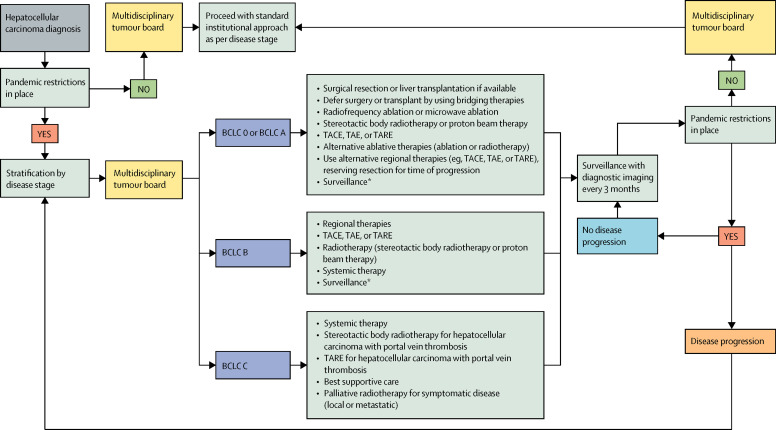
Figure.
Proposed treatment pathway for hepatocellular carcinoma during the COVID-19 pandemic
BCLC=Barcelona Clinic Liver Cancer. TACE=transarterial chemoembolisation. TAE=transarterial embolisation. TARE=transarterial radioembolisation. *Surveillance involves blood tests every 1–3 months (eg, for α-fetoprotein in secreting tumours) and diagnostic imaging every 3 months.
For patients whose cancers are resectable but resection is not available, alternative local therapies, such as thermal ablation (ie, radiofrequency ablation and microwave ablation), stereotactic body radiotherapy, or proton beam therapy, can be used instead as upfront, definitive, or neoadjuvant therapies to bridge patients while they wait for surgery, reserving resection for the time of progression or after the pandemic. For patients on the transplant list, the aforementioned local therapies, together with regional therapies, such as transarterial chemoembolisation, transarterial embolisation, or transarterial radioembolisation, can be more frequently used to bridge patients, attempting to ensure disease control while waiting times become further delayed.34 For patients who received transarterial chemoembolisation, radiofrequency ablation, or stereotactic body radiotherapy in the bridging setting, Sapisochin and colleagues35 described no difference in waiting list drop-out rates, postoperative complications, or 5-year overall survival from the time of listing, between the bridging methods used. Patients who would have had their cancers resected or been considered for a liver transplant before the COVID-19 pandemic, but who are treated with alternative local therapies, systemic therapies, or both during the pandemic, should undergo restaging and be considered once again for surgical resection or transplantation when operating facilities are re-established. Alternatively, further local and regional therapies can be reconsidered at the time of follow-up.
Local ablative therapies
In patients with solitary or a few, small, accessible tumour masses, the use of locally ablative therapies, such as radiofrequency ablation, microwave ablation,36, 37 and yittrium-90 in the form of radiation segmentectomy, stereotactic body radiotherapy, or proton beam therapy, will depend on the centre's local expertise and the availability of particular technologies. When recommended for patients with hepatocellular carcinoma, each method can be considered as an alternative to the other. Stereotactic body radiotherapy and proton beam therapy have shown sustained local control benefit for patients with early stage hepatocellular carcinoma.38, 39, 40, 41
In many centres, procedures, such as thermal ablation and transarterial embolisation, require specialisation in anaesthetics, and the need for anaesthesia introduces a greater likelihood of patients undergoing aerosol generating procedures (as opposed to those that generate respiratory droplets). This likelihood places patients and health-care workers at greater risk of exposure to SARS-CoV-2. In centres that do these procedures with consciously sedated patients, an aerosol generating procedure is avoided, which is valuable in reducing the possible exposure of health-care workers to SARS-CoV-2. Many centres screen for COVID-19 before the procedure to detect potentially infectious asymptomatic carriers of SARS-CoV-2.42 Still, other local methods, such as stereotactic body radiotherapy and proton beam therapy, have their own disadvantages. Radiotherapy treatments generally require patients to attend multiple outpatient visits, potentially increasing the risk of SARS-CoV-2 exposure. Reducing the number of radiotherapy fractions delivered and the use of breathing control devices,43 and the use of non-invasive tumour motion techniques (eg, avoiding insertion on fiducial markers) should strongly be considered.
Systemic therapies
In a resource-limited setting with no access to locoregional therapies and surgery, the use of (preferably outpatient) oral systemic therapies as a bridge to more definitive therapy could be considered if the pace of disease progression in a surveillance strategy would result in a tumour no longer amenable to locoregional therapies at the time these therapies became available. There are no strong data to support this recommendation. A small case series reported the ability of sorafenib to downstage advanced hepatocellular carcinoma to a resectable state, but typically, sorafenib yields stable disease at best.44 However, if stable disease would be adequate, first-line, oral tyrosine kinase inhibitors, such as sorafenib or lenvatinib, could be used. At the American Society of Clinical Oncology's annual meeting in 2019, early results from a phase 2 trial of perioperative checkpoint inhibitor therapy with nivolumab monotherapy or nivolumab–ipilimumab combination therapy for resectable hepatocellular carcinoma showed that 6 weeks of checkpoint inhibitor therapy before resection was able to produce a pathological complete response in three of the eight patients treated thus far.45 However, this is not standard care and the combination of nivolumab and ipilimumab has been associated with high requirements for corticosteroids; thus, this combination is not recommended as a treatment of choice when alternative therapies are available.46 In the COVID-19 era, oral systemic therapies are preferable to those requiring infusion or day care because oral therapies are well suited for telemedicine, allowing virtual outpatient assessments and fewer visits to the hospital or clinic. Presently, shortages in chemotherapeutic and targeted agents because of import and travel restrictions, and increased patient referrals because of shifting treatment frameworks, have placed undue pressure on medical oncologists. Additionally, there are increased pressures to consider alternatives to standard-of-care systemic therapy during the COVID-19 pandemic because factors such as drug-induced immunosuppression and in-hospital visits might potentially increase the risk of COVID-19 infection. We suggest, when systemic therapy is required, using oral and out-of-hospital therapies while leveraging telemedicine resources to assess toxicity at the discretion of the medical oncologist.
Surveillance
After doing a multidisciplinary review of patients who are candidates for definitive surgery, and depending on the availability of locoregional therapies, deferred treatment and surveillance is a further potential option during the COVID-19 pandemic. Access to, and the availability of, diagnostic imaging and pathology at this time might substantially affect standard surveillance guidelines.47 For patients who could potentially be cured and who are not treated with upfront local therapy, follow-up blood tests (eg, α-fetoprotein in secreting disease, liver function tests, and platelet count) every 1–3 months and imaging (eg, ultrasound, triphasic liver CT, or triphasic liver MRI) every 3 months should be considered for those with very early or early stage disease (ie, BCLC 0 or BCLC A). Frequent surveillance imaging and blood tests might identify patients in whom there is tumour progression (and who therefore require earlier treatment) and those in which the tumour burden is stable and can be safely watched.48 Patients whose treatments are deferred must be carefully monitored to ensure that their treatment options are subsequently reviewed. For patients who are treated upfront, the frequency of follow-up can be reduced to every 4–6 months to reduce resource use. However, the frequency of these tests and procedures will probably vary according to the phase of the pandemic that a centre is experiencing (eg, the tests and procedures should not overlap with the estimated peak or surge of the pandemic whenever possible) and should be related to the suitability and availability of salvage or next-line therapies. Follow-up visits should be led by a single discipline and done with the use of telemedicine whenever feasible to reduce travel and in-person hospital visits.49 We also recommend the regular continuation of virtual tumour boards and multidisciplinary team meetings to help review patient care, especially for the review of patients who have had their definitive therapy deferred.
Treatment of BCLC B stage hepatocellular carcinoma during the COVID-19 pandemic
Locoregional therapies
BCLC15 B stage hepatocellular carcinoma, defined as intermediate stage disease with Child-Pugh A or B status and multiple nodules without vascular invasion or extrahepatic metastases, is a very heterogenous cohort for whom numerous treatment options are considered. Options will be based on the availability of resources and expertise when the risk of SARS-CoV-2 infection is high. In some centres, patients with BCLC B disease might fulfil transplant or resection criteria. Continuing to refer these patients for transplant assessments when appropriate is important, even in centres that have temporarily put their transplant programme on hold. Although transplant assessments might be delayed because of the COVID-19 pandemic, if assessments are completed this patient group might be in an optimal position when transplantation activity increases. Locoregional therapies remain the standard of care and include transarterial chemoembolisation, transarterial embolisation, transarterial radioembolisation, and in selected patients, local ablation or radiotherapy (ie, stereotactic body radiotherapy, proton beam therapy, and external beam radiotherapy; table 1). For patients with intermediate stage disease who require locoregional therapies, interventional radiology expertise might be reduced or deployed, but such interventions (eg, transarterial chemoembolisation and transarterial embolisation) are highly effective for local tumour control; these interventions are still considered the standard of care and involve a single day case procedure (table 2 ). In locations where interventional radiology services are available during the COVID-19 pandemic (ie, major cancer centres), a model that does not use technetium-99-labelled macroaggregated albumin should be implemented where appropriate.50 Because a low risk of radiation pneumonitis has been reported, macroaggregated albumin scans to estimate lung shunt fractions and lung dose are not required.50 The availability of these procedures might also depend on the stage of the pandemic each centre is at, and if the centres do such procedures as inpatient or outpatient visits. Therefore, if certain interventional resources are diminished or unavailable, the use of radiotherapy in tumours equal to and less than 10 cm in diameter (ie, those most likely to be controlled)51 could be considered, so long as liver dose-volume constraints are met.
Table 2.
Specific considerations for non-surgical locoregional therapy for hepatocellular carcinoma during the COVID-19 pandemic
| Inpatient or outpatient | Anaesthesia requirement | Number of visits to a hospital or clinic | Other considerations | SARS-CoV-2 screening | |
|---|---|---|---|---|---|
| Thermal ablation (ie, radiofrequency ablation and microwave ablation) | Outpatient | Local sedation | One | Select patients at low risk of treatment complications due to tumour position; further ablation might be needed | Consider testing for SARS-CoV-2 24–48 h before admission if using aerosol generating procedures or general anaesthetic; if the patient is positive for SARS-CoV-2, delay the procedure for 7–14 days until the patient has at least one test negative for SARS-CoV-2; in cases of pending or positive SARS-CoV-2 testing, standard personal protective equipment and respiratory protocols should be instituted |
| TAE or TACE | Outpatient (a day case) or inpatient (a stay of 1 day) | Local sedation or conscious sedation | One visit or up to three visits for bilobar disease | Consider postponing procedures for older adults (>80 years) and for patients with comorbidities; for TACE, consider alternatives (ie, TAE, DEB-TACE, or TARE) to reduce the risk of immunosuppression | Consider testing for SARS-CoV-2 24–48 h before admission if using aerosol generating procedures or general anaesthetic; if the patient is positive for SARS-CoV-2, delay the procedure for 7–14 days until the patient has at least one test negative for SARS-CoV-2; in cases of pending or positive SARS-CoV-2 testing, standard personal protective equipment and respiratory protocols should be instituted |
| TARE | Outpatient | Conscious sedation | One visit for angiogram mapping followed by treatment (up to two visits for bilobar disease) | Consider postponing procedures for older adults (>80 years) and for patients with comorbidities | Consider testing for SARS-CoV-2 24–48 h before admission if using aerosol generating procedures or general anaesthetic; if the patient is positive for SARS-CoV-2, delay the procedure for 7–14 days until the patient has at least one test negative for SARS-CoV-2; in cases of pending or positive SARS-CoV-2 testing, standard personal protective equipment and respiratory protocols should be instituted |
| External beam radiotherapy (ie, stereotactic body radiotherapy, proton beam therapy, or hypofractionated radiotherapy) | Outpatient | None | One visit for radiotherapy simulation followed by visits for treatment; stereotactic body radiotherapy will require 1–6 visits; proton beam therapy will require 5–15 visits; and hypofractionated radiotherapy will require 15 visits | Consider alternatives to liver fiducial markers in areas where people are at high risk of COVID-19, whenever possible; breathing and motion management should be done as per institutional guidelines; free breathing, abdominal compression, and active breathing control in patients positive for SARS-CoV-2 can be considered, ensuring the use of personal protective equipment and respiratory protocols; 3–5 fractions are preferable | Consider testing for SARS-CoV-2 24–48 h before the radiotherapy simulation is done; if the patient is not urgent and tests positive for SARS-CoV-2, delay the procedure for 7–14 days until the patient has at least one test negative for SARS-CoV-2; in cases of pending or positive SARS-CoV-2 testing, standard personal protective equipment and respiratory protocols should be instituted |
DEB=drug-eluting beads. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. TACE=transarterial chemoembolidation. TAE=transarterial embolisation. TARE=transarterial radioembolisation.
Systemic therapies
Identifying patients with intermediate hepatocellular carcinoma refractory to locoregional therapies but with preserved liver function has been increasingly recognised as being important and allows these patients access to systemic therapies.52 Furthermore, in patients who are unsuitable for locoregional therapies, systemic treatment is a first-line option. The REFLECT study53 showed that lenvatinib resulted in higher responses rates than sorafenib; therefore, in a resource-limited setting, lenvatinib could be considered a bridge to locoregional therapies, such as transarterial chemoembolisation or transarterial radioembolisation, if these therapies are not immediately available. In these circumstances, virtual appointments for monitoring tolerance are feasible and could be spaced to one appointment every 4 weeks, depending on tolerance. Oral therapy with tyrosine kinase inhibitors is often complicated by side-effects, the management of which might require more contact with health-care staff than would be desired during the COVID-19 pandemic. To limit the risk of side-effects from oral tyrosine kinase inhibitors while bridging to locoregional therapies, reducing the dose of the inhibitor can be considered.54
Treatment of BCLC C stage hepatocellular carcinoma during the COVID-19 pandemic
Locoregional therapies
For patients with BCLC C stage hepatocellular carcinoma, defined as advanced stage disease with Child-Pugh A or B stage liver disease, vascular invasion by the hepatocellular carcinoma or extrahepatic metastases, or any stage hepatocellular carcinoma with cancer-related symptoms (ie, performance status 1–2), the recommended therapies can be divided according to the presence or absence of macrovascular invasion or extrahepatic metastases. Systemic therapy is considered the standard of care. Although resection has been used in selected patients with macrovascular invasion (of whom some have survived in the long term),55 we do not recommend resection during the COVID-19 pandemic because the surgical procedures are extensive, require admission to intensive care units, and result in a high risk of prolonged hospitalisation because of liver decompensation. Radiotherapy has also been shown to lead to the recanalisation of hepatocellular carcinomas with macrovascular invasion, but this recanalisation can take months.56 Despite excellent cancer control with local therapies, most patients with macrovascular invasion will ultimately develop intrahepatic progression. Macrovascular invasion is associated with diffuse occult vascular invasion and a high risk of diffuse hepatocellular carcinoma that will ultimately progress when only local therapy is used.
Systemic therapies
The combination of atezolizumab and bevacizumab19 is now recognised as the preferred first-line treatment option for advanced hepatocellular carcinoma over sorafenib; however, during the COVID-19 pandemic, oral tyrosine kinase inhibitors are the preferred therapeutic strategy.53 As previously emphasised, these strategies minimise hospital exposure and the use of infusion clinics, especially with the use of telemedicine and, for patients with a poor performance status, substantial comorbidities, or both, upfront dose reductions. Furthermore, the use of atezolizumab–bevacizumab would require patients to have upper endoscopies to assess varices, given the risk of bleeding association with the drug combination shown by the IMbrave150 trial.19 However, upper endoscopies are considered to be high-risk, aerosol generating procedures and have been restricted during the COVID-19 pandemic, requiring full personal protective equipment in some countries.57 The use of immune checkpoint inhibitors comes with the risk of immune-related adverse events, most notably pneumonitis, which might present a diagnostic challenge in communities with a high prevalence of SARS-CoV-2 infection. Moreover, there is theoretical concern that immune-mediated pneumonitis and lung injury induced by SARS-CoV-2 could have synergistic lung toxicity. Whether immune checkpoint inhibitors, through their mechanism of action, amplify the immune hyperactivation and cytokine storm characteristic of severe COVID-19 disease is unknown.58 For these reasons, choosing an oral tyrosine kinase inhibitor rather than an immune checkpoint inhibitor in the first-line setting might be prudent. In the second-line setting, after disease progression on sorafenib or lenvatinib, there are several potential considerations. For those patients who tolerated their first-line tyrosine kinase inhibitor and who have Child-Pugh class A disease, the oral tyrosine kinase inhibitors regorafenib and cabozantinib are a treatment option. For those patients intolerant to their first-line tyrosine kinase inhibitor and who have an α-fetoprotein concentration of equal to or more than 400 ng/mL, ramucirumab has an excellent toxicity profile, but requires intravenous administration every 2 weeks and so uses more resources. El Kouheiry and colleagues46 showed the efficacy of administering 1 mg/kg nivolumab and 3mg/kg ipilimumab every 3 weeks for four doses, followed by nivolumab (240 mg every 2 weeks or 480 mg every 4 weeks), in patients who had progressed on sorafenib. Although this regimen has been granted approval by the US Food and Drug Administration, 25 (51%) of 49 patients required systemic corticosteroids for adverse events related to treatment, and this regimen would therefore not be recommended if there is a high risk of COVID-19 exposure. Best supportive care or palliation are alternative options, especially for patients at high risk of having drug-related toxic effects, including patients with Child-Pugh B, or worse, liver function (table 1).
Combined modality therapy
Combined modality therapies, such as external beam radiotherapy and transarterial chemoembolisation, or sorafenib and transarterial radioembolisation, have been studied in patients with hepatocellular carcinoma and macrovascular invasion with some success.59, 60 Other combinations are being investigated in randomised trials, including sorafenib with or without stereotactic body radiotherapy (NCT01730937). However, combined modality treatment might not be practical during the COVID-19 pandemic, and systemic therapy alone is generally preferred. Although not standard of care, in selected patients who are not good candidates for systemic therapy or who are refractory to systemic therapy, radiotherapy or transarterial radioembolisation alone can be used, deferring systemic therapy until the time of progression.61
Treatment of intrahepatic cholangiocarcinoma during the COVID-19 pandemic
Treatment of potentially curable intrahepatic cholangiocarcinoma
In patients diagnosed with intrahepatic cholangiocarcinoma, resection is standard of care when possible, and neoadjuvant systemic therapy has been used to reduce tumour size to make the cancer resectable (table 3 ).62 When resource availability permits, surgery should be considered in the setting of small, easily resectable lesions with minimal anticipated postoperative morbidity and resource use. However, given the decreased access to operating theatres seen during the COVID-19 pandemic, alternative strategies might be necessary. For patients requiring downstaging for resectability, neoadjuvant systemic therapy can be used for extended periods until resources become available. For patients who do not require downstaging before definitive therapy, but for whom surgery is not an option because of scarce resources, we would favour the use of other available locoregional therapies rather than neoadjuvant chemotherapy.
Table 3.
Recommendations for the treatment of intrahepatic cholangiocarcinoma during the COVID-19 pandemic
| Standard-of-care treatment recommendations before the COVID-19 pandemic | Proposed treatment recommendations during the COVID-19 pandemic* | |
|---|---|---|
| Potentially curatable | Surgical resection and lymph node dissection with or without adjuvant systemic therapy | Consider the following alternatives only if resection is unavailable: (1) systemic therapy; (2) local ablative therapies (eg, stereotactic body radiotherapy, hypofractionated radiotherapy, radiofrequency ablation, or microwave ablation); (3) TARE (by use of a same-day model that does not use technetium-99-labelled macroaggregated albumin) with deferred resection; (4) surveillance† |
| Incurable, metastatic, or both | First, consider systemic therapy; but, for localised, non-metastatic disease, a combination of systemic therapy and radiotherapy, or TARE, can be used | Consider (1) systemic therapy; (2) surveillance†; (3) best supportive care; (4) palliative radiotherapy (with a single fraction) for symptomatic liver disease or metastatic disease; and (5) targeted therapies for selected subgroups |
Recommendations are presented in the order that they should be considered. TARE=transarterial radioembolisation.
These recommendations are to be considered when standard therapies are not available.
Surveillance involves blood tests every 1–3 months (eg, for α-fetoprotein in secreting tumours) and diagnostic imaging every 3 months.
As an alternative to resection for small, unresectable intrahepatic cholangiocarcinoma, radiofrequency ablation or microwave ablation can be considered; retrospective cohort studies have suggested that ablation provides good local tumour control in patients with lesions less than or equal to 5 cm in diameter that are located away from segmental bile ducts, the liver surface, and major vessels.63, 64, 65 Stereotactic body radiotherapy and proton beam therapy can be considered for peripheral lesions and hypofractionated radiotherapy (ie, with 15 fractions) can be considered for more centrally located disease. For larger, localised intrahepatic cholangiocarcinoma, neoadjuvant chemotherapy, radiotherapy, or transarterial radioembolisation66 can be considered when surgery is not possible. Tao and colleagues67 reported long-term survival rates with definitive radiotherapy in localised, inoperable intrahepatic cholangiocarcinoma (median tumour size 7·9 cm [range 2·2–17·0]) that compare favourably with resection. Close surveillance is also an option for such patients upon multidisciplinary review and frequent surveillance is particularly needed if a plan is made to treat only if there is evidence of disease progression. In settings where surgery and locoregional therapies are unavailable, bridging chemotherapy is a reasonable option until resources are renewed. The most commonly used neoadjuvant regimens include intravenous gemcitabine-based chemotherapy (typically gemcitabine–cisplatin) and are not ideal to administer during the COVID-19 pandemic because of the added risks of immunosuppression and exposure to health-care settings (eg, multiple visits to infusion centres and to clinics or hospitals to manage toxic effects). Unfortunately, where surgery and locoregional therapies are unavailable, there is not enough evidence to recommend the use of any oral chemotherapies. In the adjuvant setting, oral capecitabine, as reported by the BILCAP trial,68 has shown an overall survival advantage compared with resection alone and we would recommend that adjuvant capecitabine continues to be instituted for all patients that undergo resection for intrahepatic cholangiocarcinoma. In the BILCAP trial,68 adjuvant capecitabine was either started within 12 weeks after surgery or within 16 weeks after surgery. Therefore, in times of scarce resources or elevated COVID-19 prevalence, it might be reasonable to postpone adjuvant chemotherapy for up to 16 weeks after surgery to minimise the patient's exposure to health-care settings. For patients with margin-positive disease after resection, we typically recommend the addition of chemoradiation in the course of adjuvant therapy; however, chemoradiation can be done 5–6 months after adjuvant capecitabine monotherapy, thereby delaying the intensive exposure to health-care settings and the use of resources needed for radiotherapy.
Treatment of incurable intrahepatic cholangiocarcinoma
A regimen of cisplatin–gemcitabine is the current standard-of-care for palliative chemotherapy for intrahepatic cholangiocarcinoma. In the midst of serious resource constraints and a high prevalence of COVID-19 in the community, the cisplatin–gemcitabine regimen could be modified from dosing on day 1 and day 8 of a 21 day cycle to dosing on day 1 and day 14 of a 28 day cycle.69 This modification could reduce the number of visits to infusion centres, the immunosuppressive effects of the chemotherapy, and the development of cytopenias when blood transfusions are in short supply. There is less justification for the use of second-line chemotherapies when the benefit is more modest.70 For specific patient cohorts selected on a molecular basis, oral targeted therapies are both effective and non-immunosuppressive and can be considered.71 Ghassan K Abou-Alfa and colleagues72 published a phase 3 randomised, controlled study of ivosidenib versus placebo in IDH1-mutant chemotherapy-refractory cholangiocarcinoma. The study showed a significant improvement in progression-free survival in the ivosidenib group compared with placebo (median 2·7 months [95% CI 1·6–4·2] vs 1·4 months [1·4–1·6]; hazard ratio 0·37 [95% CI 0·25–0·54]; one-sided p<0·0001).72 During this time of drug shortages and the potential for SARS-CoV-2 transmission to patients, consideration can also be given to surveillance and best supportive care or palliative, single-fraction radiotherapy for symptomatic disease, if needed.73
For patients with intrahepatic cholangiocarcinoma who have or are at high risk of biliary obstruction requiring decompression, the method of biliary stenting should be individualised on the basis of tumour and patient factors. Both endoscopically assisted procedures and percutaneous biliary drainage are possible, but additional risk factors, such as the use of aerosol generating procedures, should be considered. When possible, in patients who are not candidates for surgical resection, metal stents would be preferable to plastic stents to avoid the need for potential restenting in the future. Given the challenges in doing endoscopic procedures, for patients positive for SARS-CoV-2 who require stenting, a percutaneous transhepatic drain can be placed while the patient is consciously sedated. The position along the COVID-19 pandemic curve where each institution lies will influence the timing of when biliary stents are replaced.
Conclusion
COVID-19 is directly affecting all multidisciplinary aspects of cancer care in institutions at all stages of the pandemic curve and could potentially negatively affect patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma quite substantially. During these challenging times, clinicians must continue to work together, advocate for our patients with liver cancer, be flexible in considering alternative interventions that carry less of a risk to patients and health-care workers, and use telemedicine and virtual tumour board resources when needed. These recommendations are based on the most up-to-date available evidence, aim to provide guidance for centres across the world in the management of intrahepatic malignancies during the COVID-19 pandemic in the short-term and medium-term, and should be used flexibly according to the dynamic state of COVID-19 in each centre.
Search strategy and selection criteria
We searched MEDLINE and PubMed using the search terms “COVID-19, coronavirus, novel coronavirus, SARS-CoV-2”, “cancer, tumour, malignancy”, and “liver, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, intrahepatic*, cholangio*” to identify articles for this Rapid Review published between Dec 1, 2019, and April 28, 2020. We also identified publications through searches of our own files, providing additional references on the basis of specialist interests. We reviewed only articles published in English. We selected up-to-date and evolving management guidelines related to the liver, original research articles, letters, and reviews on the basis of their clinical relevance to each section of this Rapid Review.
Contributors
AB, LAD, and MAH drafted the outline and planned this Rapid Review. AB, SA, LAD, and MAH contributed to the initial drafting of the manuscript. All authors who contributed opinions based on their individual expertise and the expertise and policies of their centres critically reviewed the manuscript. AB, SA, LAD, MAH, and PMS drafted the figures and the tables. All authors agreed to submit the final version of the manuscript.
Declaration of interests
RS is a consultant for Boston Scientific, Cook, Sirtex, Eisai, Genentech, and Becton Dickinson. PMS is on the Advanced Radiotherapy Committee for the International Association for the Study of Lung Cancer. LAD has a licensing agreement with Raysearch for image registration software in a system for radiation treatment planning. GMK is a consultant for Eisai. GS declares research funding from Roche and Bayer, outside the submitted work. AD declares funding from the Clinical Research Division at Fred Hutchinson Cancer Research Center, outside the submitted work. MAH and JB declare funding from the NIHR Biomedical Research Centre at University College London Hospitals National Health Service Foundation Trust, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Martin R. Covid vs. US daily average cause of death. April 21, 2020. https://public.flourish.studio/visualisation/1712761/
- 2.American Society of Clinical Oncology ASCO coronavirus resources. https://www.asco.org/asco-coronavirus-information
- 3.American Society for Radiation Oncology COVID-19 recommendations and information. Summary. https://www.astro.org/Daily-Practice/COVID-19-Recommendations-and-Information/Summary
- 4.American Association for the Study of Liver Diseases COVID-19 resources. https://www.aasld.org/about-aasld/covid-19-resources
- 5.Boettler T, Newsome PN, Mondelli MU. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Liver Cancer Association COVID-19 and liver cancer. https://ilca-online.org/covid19andlivercancer
- 7.European Society for Medical Oncology ESMO management and treatment adapted recommendations in the COVID-19 era: hepatocellular carcinoma (HCC) 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/gastrointestinal-cancers-hepatocellular-carcinoma-hcc-in-the-covid-19-era
- 8.Iavarone M, Sangiovanni A, Carrafiello G, Rossi G, Lampertico P. Management of hepatocellular carcinoma in the time of COVID-19. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.04.007. published online April 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao R, Liang J, Wu K-C, Chen M-H. Responding to COVID-19: perspectives from the Chinese Society of Gastroenterology. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.046. published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang W, Guan W, Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayor S. COVID-19: impact on cancer workforce and delivery of care. Lancet Oncol. 2020;21:633. doi: 10.1016/S1470-2045(20)30240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalkey N, Helmer O. An experimental application of the DELPHI method to the use of experts. Manage Sci. 1963;9:458–467. [Google Scholar]
- 13.American Society of Clinical Oncology COVID-19 provider and practice information. 2020. https://www.asco.org/asco-coronavirus-information/provider-practice-preparedness-covid-19
- 14.Meyers BM, Knox J, Cosby R. Non-surgical management advanced hepatocellular carcinoma. May 5, 2019. https://www.cancercareontario.ca/en/content/non-surgical-management-advanced-hepatocellular-carcinoma
- 15.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 16.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. published online March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BH, Park J-W. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol. 2018;24:1–9. doi: 10.3350/cmh.2017.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn RS, Qin S, Ikeda M. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 20.Guan W-J, Liang W-H, Zhao Y. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020 doi: 10.1183/13993003.00547-2020. published online March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow N, Fleming-Dutra K, Gierke R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020 doi: 10.1038/s41574-020-0364-6. published online April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W-j, Ni Z-y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:643–644. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S-H, Ko J-H, Park GE. Atypical presentations of MERS-CoV infection in immunocompromised hosts. J Infect Chemother. 2017;23:769–773. doi: 10.1016/j.jiac.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ison MG, Hirsch HH. Community-acquired respiratory viruses in transplant patients: diversity, impact, unmet clinical needs. Clin Microbiol Rev. 2019;32:e00042–e00119. doi: 10.1128/CMR.00042-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence COVID-19 rapid guideline: delivery of systemic anticancer treatments. March 20, 2020. https://www.nice.org.uk/guidance/ng161 [PubMed]
- 28.Baccarani U, Isola M, Adani GL. Superiority of transplantation versus resection for the treatment of small hepatocellular carcinoma. Transpl Int. 2008;21:247–254. doi: 10.1111/j.1432-2277.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 29.Mazzaferro V, Llovet JM, Miceli R. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 30.Sapisochin G, Goldaracena N, Laurence JM. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: a prospective validation study. Hepatology. 2016;64:2077–2088. doi: 10.1002/hep.28643. [DOI] [PubMed] [Google Scholar]
- 31.Endo I, Gonen M, Yopp AC. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration Coronavirus (COVID-19) update: blood donations. March 19, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-blood-donations
- 33.Levi Sandri GB, Ettorre GM, Aldrighetti L. Laparoscopic liver resection of hepatocellular carcinoma located in unfavorable segments: a propensity score-matched analysis from the I Go MILS (Italian Group of Minimally Invasive Liver Surgery) Registry. Surg Endosc. 2019;33:1451–1458. doi: 10.1007/s00464-018-6426-3. [DOI] [PubMed] [Google Scholar]
- 34.Sandroussi C, Dawson LA, Lee M. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int. 2010;23:299–306. doi: 10.1111/j.1432-2277.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 35.Sapisochin G, Barry A, Doherty M. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92–99. doi: 10.1016/j.jhep.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Lee DH, Lee JM, Lee JY. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 37.Chen S-W, Lin L-C, Kuo Y-C, Liang J-A, Kuo C-C, Chiou J-F. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1041–1047. doi: 10.1016/j.ijrobp.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Yoon SM, Lim Y-S, Park MJ. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuong MD, Kaiser A, Khan F. Consensus report from the Miami Liver Proton Therapy conference. Front Oncol. 2019;9:457. doi: 10.3389/fonc.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim N, Cheng J, Jung I. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.03.005. published online March 10. [DOI] [PubMed] [Google Scholar]
- 41.Wahl DR, Stenmark MH, Tao Y. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai Y, Yao L, Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. published online Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song A, Manukian G, Taylor A, Anne PR, Simone NL. Concerns for activated breathing control (ABC) with breast cancer in the era of COVID-19: maximizing infection control while minimizing heart dose. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.03.009. published online April 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbier L, Muscari F, Le Guellec S, Pariente A, Otal P, Suc B. Liver resection after downstaging hepatocellular carcinoma with sorafenib. Int J Hepatol. 2011;2011 doi: 10.4061/2011/791013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaseb A, Vence L, Blando J. Randomized, open-label, perioperative phase II study evaluating nivolumab alone versus nivolumab plus ipilimumab in patients with resectable HCC. J Clin Oncol. 2019;37(suppl 4):185. [Google Scholar]
- 46.El-Khoueiry AB, Hsu C, Kang YK, et al. Safety profile of nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients with advanced hepatocellular carcinoma (HCC) in the CheckMate 40 study. 2019 ILCA Annual Conference; Chicago, IL; Sept 20–22, 2019 (abstr 0–13).
- 47.Harris PS, Hansen RM, Gray ME, Massoud OI, McGuire BM, Shoreibah MG. Hepatocellular carcinoma surveillance: an evidence-based approach. World J Gastroenterol. 2019;25:1550–1559. doi: 10.3748/wjg.v25.i13.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rich NE, John BV, Parikh ND. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multi-center cohort of patients with cirrhosis. Hepatology. 2020 doi: 10.1002/hep.31159. published online Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Medicare & Medicaid Services President Trump expands telehealth benefits for medicare beneficiaries during COVID-19 outbreak. March 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
- 50.Gabr A, Ranganathan S, Mouli SK. Streamlining radioembolization in UNOS T1/T2 hepatocellular carcinoma by eliminating the lung shunt study. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.02.024. published online March 5. [DOI] [PubMed] [Google Scholar]
- 51.Jang WI, Kim MS, Bae SH. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8:250. doi: 10.1186/1748-717X-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kudo M, Raoul J-L, Lee HC, Cheng A-L, Nakajima K, Peck-Radosavljevic M. Deterioration of liver function after transarterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): an exploratory analysis of OPTIMIS—an international observational study assessing the use of sorafenib after TACE. J Clin Oncol. 2018;36(suppl 4):368. [Google Scholar]
- 53.Kudo M, Finn RS, Qin S. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 54.Reiss KA, Yu S, Mamtani R. Starting dose of sorafenib for the treatment of hepatocellular carcinoma: a retrospective, multi-institutional study. J Clin Oncol. 2017;35:3575–3581. doi: 10.1200/JCO.2017.73.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kokudo T, Hasegawa K, Matsuyama Y. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938–943. doi: 10.1016/j.jhep.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 56.Lin C-S, Jen Y-M, Chiu S-Y. Treatment of portal vein tumor thrombosis of hepatoma patients with either stereotactic radiotherapy or three-dimensional conformal radiotherapy. Jpn J Clin Oncol. 2006;36:212–217. doi: 10.1093/jjco/hyl006. [DOI] [PubMed] [Google Scholar]
- 57.British Society of Gastroenterology Endoscopy activity and COVID-19: BSG and JAG guidance. https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/
- 58.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269–273. doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon SM, Ryoo B-Y, Lee SJ. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vilgrain V, Pereira H, Assenat E. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 61.Schuffenegger PM, Barry AS, Atenafu E. Stereotactic body radiation therapy for hepatocellular carcinoma with macrovascular invasion. Int J Radiat Oncol Biol Phys. 2019;105:S157. [Google Scholar]
- 62.Le Roy B, Gelli M, Pittau G. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839–847. doi: 10.1002/bjs.10641. [DOI] [PubMed] [Google Scholar]
- 63.Koay EJ, Odisio BC, Javle M, Vauthey J-N, Crane CH. Management of unresectable intrahepatic cholangiocarcinoma: how do we decide among the various liver-directed treatments? Hepatobiliary Surg Nutr. 2017;6:105–116. doi: 10.21037/hbsn.2017.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim JH, Won HJ, Shin YM, Kim K-A, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196:W205–W219. doi: 10.2214/AJR.10.4937. [DOI] [PubMed] [Google Scholar]
- 65.Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol. 2015;26:943–948. doi: 10.1016/j.jvir.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 66.Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015;41:120–127. doi: 10.1016/j.ejso.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tao R, Krishnan S, Bhosale PR. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol. 2016;34:219–226. doi: 10.1200/JCO.2015.61.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Primrose JN, Fox RP, Palmer DH. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 69.Ahn DH, Reardon J, Ahn CW. Biweekly cisplatin and gemcitabine in patients with advanced biliary tract cancer. Int J Cancer. 2018;142:1671–1675. doi: 10.1002/ijc.31144. [DOI] [PubMed] [Google Scholar]
- 70.Lamarca A, Palmer DH, Wasan HS. ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol. 2019;37(suppl 15) [Google Scholar]
- 71.Ghassan K Abou-Alfa, Sahai V, Hollebecque A. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abou-Alfa GK, Macarulla T, Javle MM. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30157-1. published online May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soliman H, Ringash J, Jiang H. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol. 2013;31:3980–3986. doi: 10.1200/JCO.2013.49.9202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.